Introduction
Breast cancer is the most frequently diagnosed
cancer type in females, with an estimated 1.68 million new cases
worldwide in 2012 (1). Breast cancer
is also the leading cause of cancer-associated cases of mortality
in females, accounting for ~520,000 mortalities per year worldwide
(1). In China, breast cancer ranks
first among cancer types diagnosed in females, with 270,000 new
cases per year (2). Despite the
declining trend in the mortality rate of breast cancer, advanced
breast cancer is predominantly an incurable malignancy, with an
overall survival (OS) ranging from 2 to 3 years (3). The primary goals of treatment are
symptomatic palliation and disease control. Treatment options for
advanced breast cancer include chemotherapy, hormone therapy and
human epidermal growth factor receptor 2 (HER2)-targeted therapy,
however, the majority of patients eventually develop drug
resistance (4). Novel therapeutic
approaches for such patients are urgently required.
Angiogenesis serves an important role in tumor
growth, invasion and metastasis in breast cancer (5–7).
Vascular endothelial growth factor (VEGF) and VEGF receptors
(VEGFRs) are key proteins regulating vascular development during
angiogenesis (7). Previously,
combinations of modern chemotherapeutic agents with targeted
biologic agents, including anti-angiogenic agents, have led to
marked improvements in the clinical efficacy of advanced breast
cancer. Bevacizumab is a monoclonal antibody that binds to VEGF and
inhibits the development of tumor vasculature (8). Bevacizumab has been demonstrated to
significantly improve the response rate and increase
progression-free survival (PFS) when combined with paclitaxel in
first-line treatment of metastatic breast cancer (9). Several anti-angiogenic tyrosine kinase
inhibitors (TKIs), including sorafenib (10,11) and
sunitinib (12–15), have also been evaluated in the
treatment of advanced breast cancer. The combination of
anti-angiogenic TKIs with specific chemotherapeutic agents,
particularly sorafenib combined with capecitabine, has demonstrated
promising results in the treatment of advanced breast cancer
(16–19). In clinical practice, treatment with
anti-angiogenic agents combined with chemotherapy is recommended
for patients with advanced breast cancer following failure of prior
standard therapy (20).
Apatinib is an orally administered, novel,
small-molecule VEGFR TKI. By selectively binding to VEGFR-2,
apatinib inhibits subsequent VEGFR-2 autophosphorylation, leading
to decreased VEGF-mediated endothelial cell migration,
proliferation and tumor microvascular density (21). Apatinib monotherapy has demonstrated
objective efficacy and acceptable toxicity for pretreated advanced
breast cancer in previous phase II clinical trials (22,23).
Apatinib combined with chemotherapeutic agents may provide greater
clinical benefit for patients with advanced breast cancer following
prior standard therapy. However, to the best of our knowledge, no
study has documented the efficacy of this combined therapy in
actual clinical practice. Therefore, the current study sought to
evaluate the efficacy and safety of apatinib combined with
chemotherapeutic agents in advanced breast cancer following
multiple lines of treatment and to explore the predictive or
prognostic factors associated with apatinib efficacy. To the best
of our knowledge, this is the first study reporting the outcome of
apatinib treatment combined with chemotherapeutic agents in
advanced breast cancer.
Patients and methods
Ethics statement
The present study was approved by the National
Cancer Center/National Clinical Research Center for Cancer/Cancer
Hospital, Chinese Academy of Medical Sciences and Peking Union
Medical College (Beijing, China). Additionally, due to the
retrospective design of the current study and patient
anonymization, the review board determined that informed consent
was not required. All methods were performed in accordance with
relevant guidelines and regulations.
Patients
The current study retrospectively reviewed the
medical data of 85 Chinese patients (83 females and 2 males) with
pretreated metastatic or locally advanced breast cancer who
received apatinib combined with chemotherapy at the National Cancer
Center/National Clinical Research Center for Cancer/Cancer
Hospital, Chinese Academy of Medical Sciences and Peking Union
Medical College between July 2015 and May 2017. The median age of
the patients included in the study was 54 years (range, 30–71
years).
Eligible patients had to: i) Be ≥18 years of age;
ii) have been treated with at least one prior chemotherapeutic
regimen for advanced disease; and iii) have received anthracycline-
or taxane-containing neoadjuvant or adjuvant therapies. If patients
had a history of other malignancies within the previous 5 years,
abnormal laboratory findings or severe comorbid illness, they were
not included in the current study. Patients were also excluded if
they were enrolled in clinical trials that had an impact on their
daily clinical practice.
Treatment
Patients received apatinib combined with
plant-derived chemotherapeutic agents, including vinorelbine,
etoposide and paclitaxel, or non-plant-derived chemotherapeutic
agents, including gemcitabine, cyclophosphamide, capecitabine and
platinum. Apatinib was administered daily with an initial dose of
450 or 500 mg depending on the patient's age and disease status and
at the discretion of their physician. Adverse events (AEs) were
graded according to the Common Terminology Criteria for AEs
(version 4.03) (24). Treatment was
discontinued in the case of disease progression, unacceptable
toxicity or mortality. Computed tomography was performed at
baseline and following every two cycles of combined treatment. The
tumor response was evaluated according to the Response Evaluation
Criteria in Solid Tumors (RECIST; version 1.1) (25). The PFS, OS, objective response rate
(ORR), clinical benefit rate (CBR) and incidence of AEs were
calculated.
Data collection
Demographic and baseline clinical information of
patients was described using standard descriptive and analytical
methods. PFS was defined as the time from the start of combined
treatment to the date of documented disease progression or
mortality from any cause. OS was defined as the time from the start
of combined treatment to the date of mortality from any cause or
the most recent date patients were known to be alive. ORR was
defined as the proportion of patients who achieved a PR or a
confirmed complete response (CR). CBR was defined as the proportion
of evaluable subjects with CR, PR or stable disease (SD) for ≥24
weeks (26).
Statistical analyses
All statistical analyses were completed using SPSS
software (IBM Corp., Armonk, NY, USA; version 20.0). PFS and OS
were estimated using the Kaplan-Meier method. In addition, the
Kaplan-Meier method and log-rank test were used to analyze the
univariate discrimination of PFS and OS by demographic data,
baseline clinical information and toxicities. Furthermore, the
combined effects of these variables on both PFS and OS were
examined in multivariate analysis using Cox proportional hazards
regression models. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
A total of 85 patients with pretreated distantly
metastatic or locally advanced breast cancer received apatinib
combined with chemotherapy at the National Cancer Center/National
Clinical Research Center for Cancer/Cancer Hospital, Chinese
Academy of Medical Sciences and Peking Union Medical College
between July 2015 and May 2017. The baseline patient
characteristics are presented in Table
I. The majority of patients (68.2%) had an Eastern Cooperative
Oncology Group (27) performance
status of 0–1. For breast cancer molecular type, 35 patients
(41.2%) were diagnosed with triple-negative breast cancer (TNBC),
42 patients (49.4%) had hormone receptor-positive breast cancer and
16 patients (18.8%) had HER2-positive breast cancer. Furthermore,
35 (41.2%) patients exhibited histological grade I–II tumors, 34
(40.0%) patients exhibited grade III tumors, while the remaining 16
patients exhibited unknown tumor grade. A total of 39 patients
(45.9%) had stage I–II disease and 36 patients (42.3%) had stage
III disease. Tumors >2 cm were detected in 55.3% of the
patients.
 | Table I.Patient characteristics at
baseline. |
Table I.
Patient characteristics at
baseline.
| Characteristic | n (%) |
|---|
| Age, years |
|
|
<55 | 43 (50.6) |
|
≥55 | 42 (49.4) |
| ECOG performance
status |
|
|
0–1 | 58 (68.2) |
| 2 | 2 (2.4) |
|
Unknown | 25 (29.4) |
| Molecular type |
|
|
Triple-negative breast
cancer | 35 (41.2) |
| Hormone
receptor-positive breast cancer | 42 (49.4) |
|
HER2-positive breast
cancer | 16 (18.8) |
| Histopathologic
grade |
|
|
I–II | 35 (41.2) |
|
III | 34 (40.0) |
|
Unknown | 16 (18.8) |
| TNM stage |
|
|
I–II | 39 (45.9) |
|
III | 36 (42.3) |
|
Unknown | 10 (11.8) |
| Tumor size, cm |
|
|
≤2.0 | 27 (31.8) |
|
>2.0 | 47 (55.3) |
|
Unknown | 11 (12.9) |
| Local
recurrence | 15 (17.6) |
| Metastatic
sites |
|
| Lymph
node | 64 (75.3) |
|
Regional lymph node | 50 (58.8) |
| Distant
lymph node | 41 (48.2) |
|
Lung | 38 (44.7) |
|
Bone | 33 (38.8) |
|
Liver | 32 (37.6) |
| Chest
wall | 30 (35.3) |
|
Pleura | 14 (16.5) |
|
Brain | 11 (12.9) |
|
Skin | 9 (10.6) |
|
Muscle | 4 (4.7) |
|
Metastasis ≥3 sites | 44 (51.8) |
| DFS duration,
months |
|
|
≤24 | 35 (41.2) |
|
>24 | 40 (47.0) |
|
Unknown | 10 (11.8) |
| Lines of combined
treatment, lines |
|
| ≥3 | 40 (47.1) |
|
>3 | 45 (52.9) |
| Chemotherapeutic
agents |
|
|
Plant-derived agents | 56 (65.9) |
|
Non-plant-derived agents | 29 (34.1) |
All 85 patients had received at least one
chemotherapeutic regimen for advanced disease. Patients with
hormone receptor-positive disease had previously been administered
at least one regimen of endocrine treatment. Following prior
treatment, local recurrence occurred in 15 patients (17.6%). For
tumor metastasis, the most common metastatic site was the lymph
nodes (64 patients, 75.3%); 50 patients (58.8%) were identified to
have regional lymph node metastases and 41 patients (48.2%) were
identified to have distant lymph node metastases. Other metastatic
sites included the lung (44.7%), bone (38.8%), liver (37.6%), chest
wall (35.3%), pleura (16.5%), brain (12.9%), skin (10.6%) and
muscle (4.7%). A total of 44 patients (51.8%) had more than three
metastatic sites.
Additionally, all patients had previously received
anthracycline- or taxane-based chemotherapy and 52.9% of patients
had been treated with more than three lines of chemotherapy.
Furthermore, 40 patients (47.0%) had a disease-free survival (DFS)
of >24 months following initial treatment.
Efficacy outcomes
Among 85 patients, 56 (65.9%) received apatinib
combined with plant-derived chemotherapy and the remaining 29
patients (34.1%) were administered apatinib combined with
non-plant-derived chemotherapy (Table
I). During the combined treatment, 27 patients experienced
transient discontinuation or dose modification due to AEs. In
addition, treatment was discontinued in 5 cases due to severe AEs,
with a median treatment period of 1.2 months (range=0.5–7.0
months).
With a median follow-up of 9.7 months
(range=2.3–25.8 months), 73 of 85 patients had progressive disease
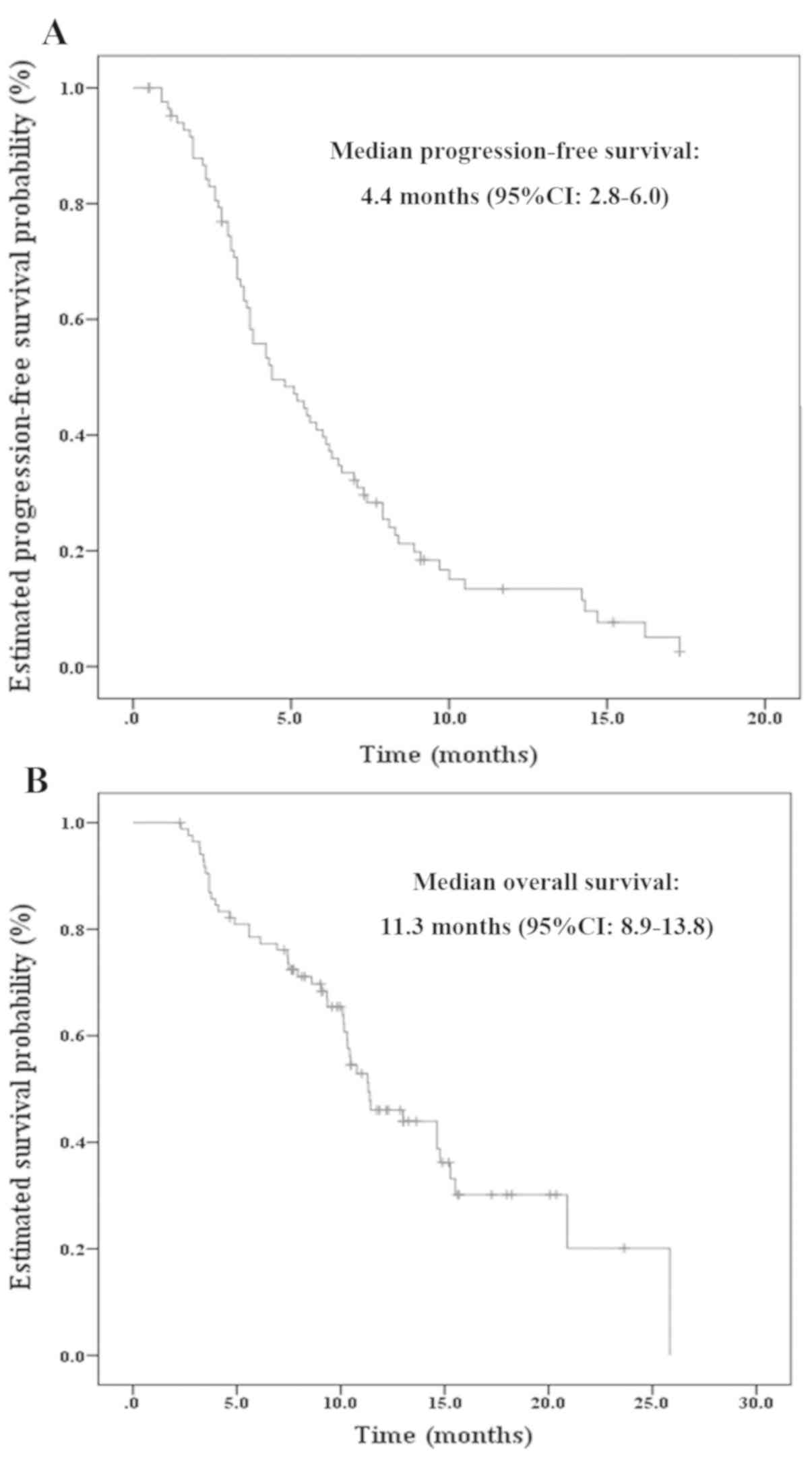
(PD) and 48 mortalities occurred. As demonstrated in Fig. 1, the median PFS was 4.4 months [95%
confidence interval (CI)=2.8–6.0 months] and the median OS was 11.3
months (95% CI=8.9–13.8 months). In addition, during combined
treatment, 6 patients changed to apatinib monotherapy due to severe
myelosuppression or gastrointestinal reaction. The patients who
received apatinib combined with chemotherapy followed by apatinib
maintenance treatment exhibited a median PFS of 14.7 months
(range=7.3–17.3 months).
Among 85 patients, 82 were evaluable for response
assessment. A total of 19 patients achieved a PR and 53 patients
achieved SD, with an ORR of 23.2% at the best response.
Additionally, 39 patients had a PR or SD for ≥24 weeks,
demonstrating a CBR of 47.6%.
Although the PFS was longer for patients who
achieved remission (comprising patients with CR or PR; n=19)
compared with those that did not (n=63), a significant difference
was not identified [7.0 months (95% CI=5.6–8.4 months) vs. 3.8
months (95% CI=2.9–4.7 months); P=0.157; Fig. 2A]. Furthermore, no significant
difference was identified in OS between these patients [11.4 months
(95% CI=5.8–17.0 months) vs. 11.3 months (95% CI=8.7–14.0 months);
P=0.827; Fig. 2B]. The PFS and OS
were also compared between patients who gained a clinical benefit
(referring to patients with CR, PR or SD for ≥24 weeks; n=39) with
those who did not gain a clinical benefit (n=43). A significantly
longer PFS and OS were identified in patients who gained a clinical
benefit compared with those that did not [PFS=8.1 months (95%
CI=6.7–9.5) vs. 3.2 months (95% CI=2.8–3.6 months), P<0.001;
OS=15.3 months (95% CI=14.2–16.4) vs. 10.1 months (95% CI=8.2–12.0
months), P<0.001, Fig. 2C and
D].
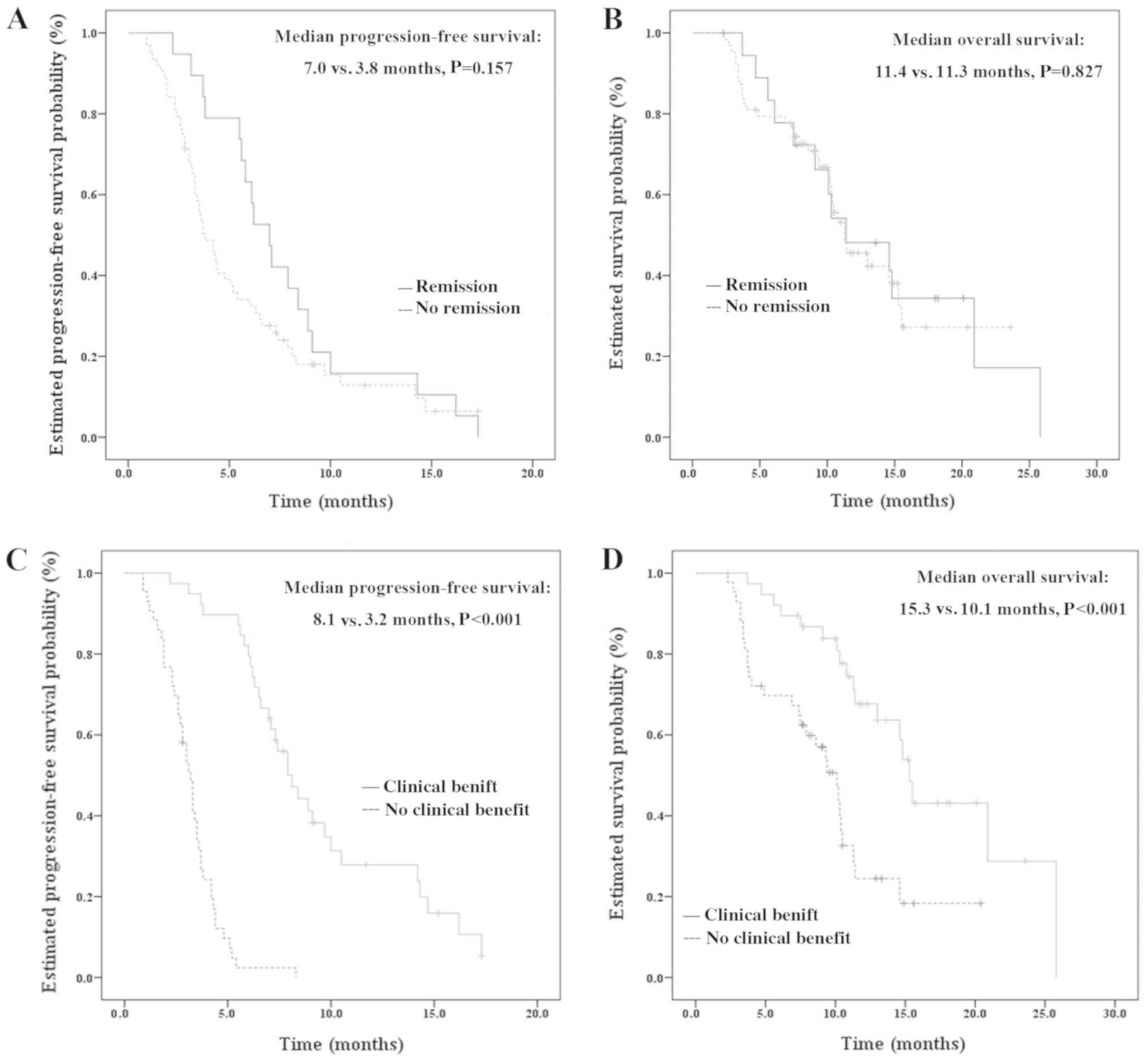 | Figure 2.Kaplan-Meier curves of PFS and OS in
subgroup analysis. (A) Kaplan-Meier curve of PFS comparing patients
who achieved remission following apatinib combined with
chemotherapy, with a median PFS of 7.0 months, and those who did
not, with a median PFS of 3.8 months. No significant difference was
identified between these patients (P=0.157). (B) Kaplan-Meier curve
of OS comparing patients who achieved remission following apatinib
combined with chemotherapy, with a median PFS of 11.4 months, and
those who did not, with a median PFS of 11.3 months. No significant
difference was identified between these patients (P=0.827). (C)
Kaplan-Meier curve of PFS comparing patients who achieved a
clinical benefit following apatinib combined with chemotherapy,
with a median PFS of 8.1 months, and those who did not, with a
median PFS of 3.2 months. A statistically significant difference
was identified between these patients (P<0.001). (D)
Kaplan-Meier curve of OS comparing patients who achieved a clinical
benefit following apatinib combined with chemotherapy, with a
median PFS of 15.3 months, and those who did not, with a median PFS
of 10.1 months. A significant difference was identified between
these patients (P<0.001). PFS, progression-free survival; OS,
overall survival. |
Safety
No treatment-associated mortalities occurred. A
total of 5 patients discontinued apatinib due to severe AEs,
including myelosuppression (3 cases), gastrointestinal reaction (1
case) and mucositis (1 case). The top ten AEs for all grades are
presented in Table II. The most
common AEs for all grades included myelosuppression (49.4%),
gastrointestinal reaction (45.9%), fatigue (43.5%), hypertension
(37.6%), hand-foot skin reaction (25.9%), pain (20.0%) and
proteinuria (16.5%). Myelosuppression (31.8%), gastrointestinal
reaction (8.2%) and hypertension (8.2%) were the most common AEs
for grade III or IV. Due to severe myelosuppression or
gastrointestinal reaction, 6 patients changed to apatinib
monotherapy as maintenance therapy. Most toxicities were limited to
patients with grade I or II and were therefore tolerable and
manageable.
 | Table II.Summary of top ten adverse
events. |
Table II.
Summary of top ten adverse
events.
| Adverse events | All grades (n=85) n
(%) | Grade III or IV
(n=37) n (%) | Grade I or II
(n=78) n (%) |
|---|
|
Myelosuppression | 42 (49.4) | 27 (31.8) | 15 (17.6) |
| Gastrointestinal
reaction | 39 (45.9) | 7 (8.2) | 32 (37.6) |
| Fatigue | 37 (43.5) | 1 (1.1) | 36 (42.4) |
| Hypertension | 32 (37.6) | 7 (8.2) | 25 (29.4) |
| Hand-foot skin
reaction | 22 (25.9) | 3 (3.5) | 19 (22.4) |
| Pain | 17 (20.0) | 3 (3.5) | 14 (6.5) |
| Proteinuria | 14 (16.5) | 1 (1.1) | 13 (15.3) |
| Mucositis | 13 (15.3) | 3 (3.5) | 10 (11.8) |
| Elevated
transaminase | 13 (15.3) | 0 (0.0) | 13 (15.3) |
| Hemorrhage | 10 (11.8) | 0 (0.0) | 10 (11.8) |
Univariate and multivariate
analysis
By univariate analysis, the difference in PFS and OS
between patients with different demographic data, baseline clinical
information and toxicities was first assessed using Kaplan-Meier
analysis and a log-rank test. As presented in Table III, a significantly longer PFS was
identified in patients who had received combined treatment with ≤3
lines of therapy (P=0.038) or who exhibited proteinuria during
combined treatment (P=0.047). Furthermore, the following factors
were identified to be significantly associated with OS: Lines of
combined treatment, number of metastatic sites, hypertension,
hand-foot skin reaction and proteinuria (P<0.05).
 | Table III.Subgroup analysis to compare median
PFS and OS between patients with different characteristics. |
Table III.
Subgroup analysis to compare median
PFS and OS between patients with different characteristics.
|
| Progression-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
| Characteristic | Median PFS, months
(95% CI) | P-value | Median OS, months
(95% CI) | P-value |
|---|
| Age, years |
| 0.105 |
| 0.918 |
|
<55 | 4.2 (3.4–5.0) |
| 14.6
(9.0–20.2) |
|
|
≥55 | 6.0 (4.9–7.1) |
| 11.3
(10.1–12.5) |
|
| Molecular type |
| 0.961 |
| 0.458 |
|
TNBC | 5.2 (3.4–7.0) |
| 11.4
(8.0–14.8) |
|
|
Non-TNBC | 4.3 (2.5–6.1) |
| 11.3
(9.9–12.7) |
|
| Molecular type |
| 0.844 |
| 0.272 |
| Hormone
receptor-positive | 3.7 (2.1–5.3) |
| 11.4
(6.2–16.6) |
|
| Hormone
receptor-negative | 5.2 (3.3–7.1) |
| 11.3
(9.7–12.9) |
|
| Molecular type |
| 0.846 |
| 0.82 |
|
HER2-positive | 5.8 (3.5–8.1) |
| 11.3
(8.4–14.2) |
|
|
HER2-negative | 4.4 (2.9–5.9) |
| 11.4
(8.0–14.8) |
|
| Lines of combined
treatment, lines |
| 0.038 |
| 0.036 |
| ≤3 | 5.8 (2.0–9.6) |
| 14.6
(9.9–19.3) |
|
|
>3 | 4.2 (3.2–5.2) |
| 10.3
(7.9–12.7) |
|
| Histological
grade |
| 0.562 |
| 0.45 |
|
I–II | 4.8 (3.3–6.3) |
| 11.3
(9.9–12.7) |
|
|
III | 4.3 (2.3–6.3) |
| 11.4
(7.9–14.9) |
|
| TNM stage |
| 0.111 |
| 0.481 |
|
I–II | 6.0 (4.0–8.0) |
| 13.0
(9.7–16.3) |
|
|
III | 3.8 (2.8–4.8) |
| 10.2
(9.0–11.4) |
|
| Tumor size, cm |
| 0.585 |
| 0.357 |
| ≤2 | 4.8 (2.7–6.9) |
| 14.6
(9.9–19.3) |
|
|
>2 | 4.4 (2.6–6.2) |
| 10.5
(9.6–11.4) |
|
| Visceral
metastasis |
| 0.53 |
| 0.955 |
| No | 3.8 (3.2–4.4) |
| 11.4
(5.5–17.3) |
|
|
Yes | 5.2 (3.7–6.7) |
| 11.3
(8.5–14.1) |
|
| Chest wall
metastasis |
| 0.365 |
| 0.392 |
| No | 4.8 (3.3–6.3) |
| 11.4
(8.4–14.4) |
|
|
Yes | 4.4 (2.1–6.7) |
| 10.3
(7.8–12.8) |
|
| Lymph node
metastasis |
| 0.207 |
| 0.766 |
| No | 5.5 (2.6–8.4) |
| 11.3
(9.9–12.7) |
|
|
Yes | 4.2 (2.8–5.6) |
| 11.4
(7.1–15.7) |
|
| Number of
metastatic sites, n |
| 0.407 |
| 0.02 |
|
<3 | 5.5 (2.5–8.5) |
| 14.6
(10.1–19.2) |
|
| ≥3 | 4.3 (3.4–5.2) |
| 10.3
(9.4–11.2) |
|
| Hypertension |
| 0.959 |
| 0.016 |
| No | 4.3 (2.5–6.1) |
| 10.4
(9.2–11.6) |
|
|
Yes | 5.2 (3.4–7.0) |
| 25.8 (NE-NE) |
|
| Hand-foot skin
reaction |
| 0.419 |
| 0.046 |
| No | 4.2 (3.4–5.0) |
| 10.5
(9.3–14.0) |
|
|
Yes | 5.5 (4.0–7.0) |
| NE (NE-NE) |
|
| Proteinuria |
| 0.047 |
| 0.001 |
| No | 4.2 (3.0–5.5) |
| 10.5
(9.4–11.6) |
|
|
Yes | 7.4 (2.5–12.4) |
| 25.8 (NE-NE) |
|
| Chemotherapeutic
agents |
| 0.611 |
| 0.283 |
|
Plant-derived agents | 4.2 (3.1–5.3) |
| 10.4
(9.5–11.3) |
|
|
Non-plant-derived agents | 5.4 (3.3–7.5) |
| 14.6
(10.9–18.3) |
|
In addition, a multivariate model containing all
these variables was established (Table
IV). In multivariate analysis, the presence of proteinuria
during combined treatment was associated with a significantly
longer PFS [hazard ratio (HR)=0.398; 95% CI=0.173–0.915; P=0.030].
Additionally, a significantly longer OS was identified in patients
with ≤3 lines of combined treatment (HR=0.419; 95% CI=0.202–0.869;
P=0.019) and in patients who exhibited proteinuria during combined
treatment (HR=0.160; 95% CI=0.031–0.826; P=0.029).
 | Table IV.Multivariate Cox proportional hazard
models predicting PFS and OS for patients receiving apatinib
combined with chemotherapeutic agents. |
Table IV.
Multivariate Cox proportional hazard
models predicting PFS and OS for patients receiving apatinib
combined with chemotherapeutic agents.
|
| PFS | OS |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
|
|
|
|
| <55
vs. ≥55 | 1.498
(0.864–2.598) | 0.150 | 0.684
(0.337–1.387) | 0.292 |
| Molecular type |
|
|
|
|
| TNBC
vs. non-TNBC | 1.582
(0.491–5.099) | 0.491 | 0.497
(0.090–2.753) | 0.423 |
| Hormone
receptor-positive vs. negative | 1.617
(0.574–4.551) | 0.363 | 0.390
(0.086–1.769) | 0.222 |
|
HER2-positive vs.
negative | 1.321
(0.434–4.026) | 0.624 | 0.320
(0.069–1.495) | 0.147 |
| Lines of combined
treatment, lines |
|
|
|
|
| ≤3 vs.
>3 | 0.573
(0.327–1.004) | 0.052 | 0.419
(0.202–0.869) | 0.019 |
| Histological
grade |
|
|
|
|
| I–II
vs. III | 0.985
(0.566–1.712) | 0.956 | 0.967
(0.483–1.934) | 0.924 |
| TNM stage |
|
|
|
|
| I–II
vs. III | 0.536
(0.283–1.015) | 0.056 | 0.575
(0.263–1.257) | 0.165 |
| Tumor size, cm |
|
|
|
|
| ≤2 vs.
>2 | 1.058
(0.588–1.903) | 0.851 | 0.822
(0.395–1.712) | 0.601 |
| Visceral
metastasis |
|
|
|
|
| Yes vs.
no | 1.236
(0.616–2.477) | 0.551 | 0.848
(0.342–2.101) | 0.722 |
| Chest wall
metastasis |
|
|
|
|
| Yes vs.
no | 1.848
(0.912–3.745) | 0.089 | 2.242
(0.893–5.628) | 0.086 |
| Lymph node
metastasis |
|
|
|
|
| Yes vs.
no | 1.027
(0.487–2.164) | 0.944 | 0.490
(0.190–1.263) | 0.140 |
| Number of
metastatic sites, n |
|
|
|
|
| <3
vs. ≥3 | 1.029
(0.556–1.902) | 0.928 | 0.495
(0.224–1.098) | 0.084 |
| Hypertension |
|
|
|
|
| Yes vs.
no | 1.786
(0.937–3.404) | 0.078 | 1.122
(0.486–2.588) | 0.788 |
| Hand-foot skin
reaction |
|
|
|
|
| Yes vs.
no | 0.655
(0.333–1.289) | 0.221 | 0.492
(0.196–1.238) | 0.132 |
| Proteinuria |
|
|
|
|
| Yes vs.
no | 0.398
(0.173–0.915) | 0.030 | 0.160
(0.031–0.826) | 0.029 |
|
Chemotherapeutic |
|
|
|
|
|
Plant-derived vs.
non-plant-derived agents | 1.055
(0.632–1.761) | 0.837 | 1.459
(0.754–2.823) | 0.262 |
Case presentation
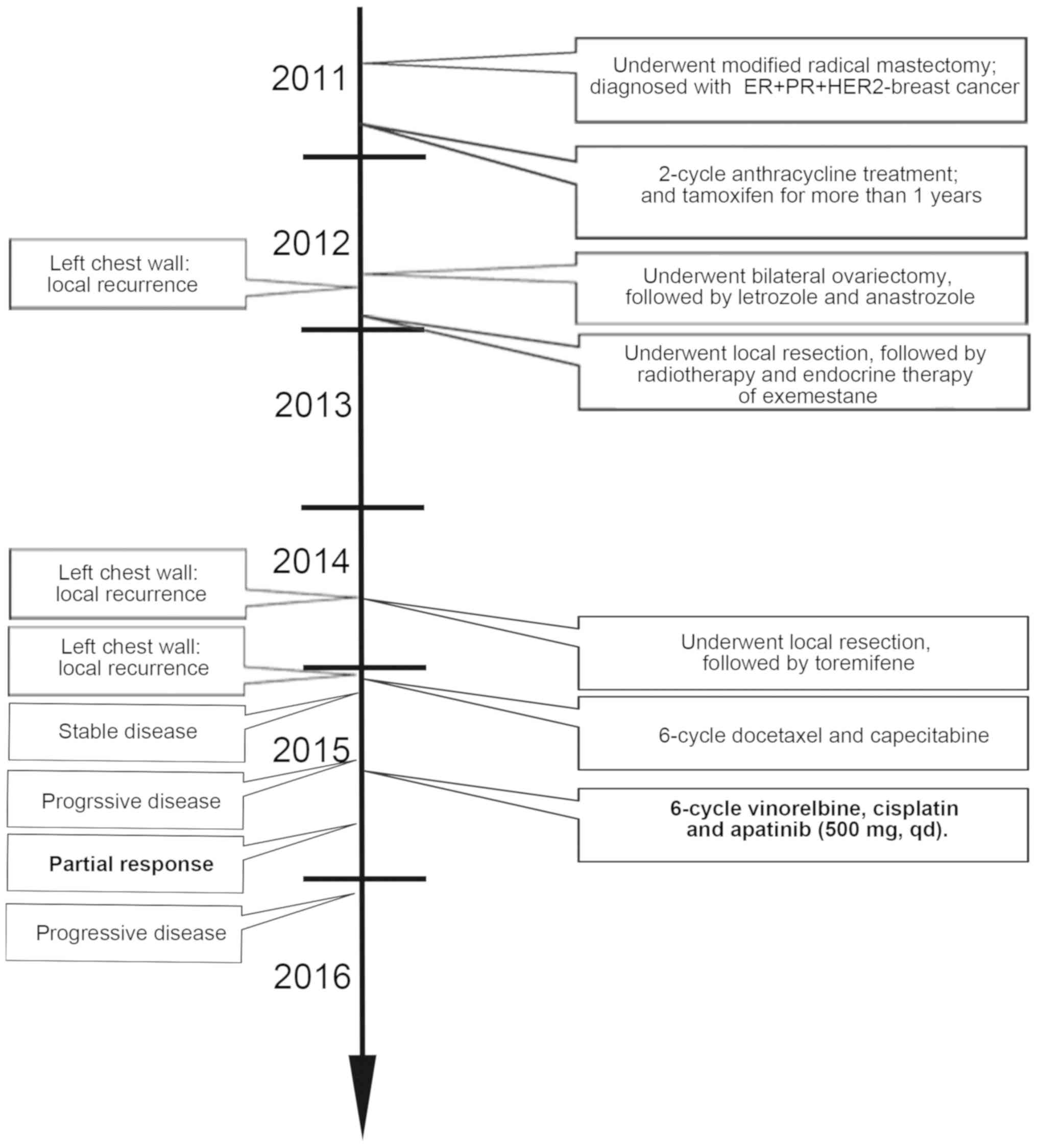
The patient management is presented in Fig. 3. A 54-year-old Chinese female patient
with invasive ductal carcinoma in her left breast underwent a
modified radical mastectomy in January 2011. The pathological stage
of her cancer was T2N0M0. The estrogen receptor (ER) and
progesterone receptor (PR) immunohistochemistry data were scored
according to the Allred scoring system and staining was considered
positive if the Allred score was ≥3. HER2 expression was reported
as positive if >30% of tumor cells demonstrated strong (3+)
membrane staining. Immunohistochemistry revealed positive staining
for ER and PR but negative staining for HER2. The patient was
prepared to receive anthracycline-taxane-based adjuvant
chemotherapy. However, due to intolerable toxicity, adjuvant
chemotherapy was discontinued following two cycles of anthracycline
treatment and tamoxifen was subsequently received.
In September 2012, the patient underwent bilateral
ovariectomy, followed by letrozole therapy. One month later, the
patient changed to anastrozole due to intolerable toxicity during
letrozole treatment.
In December 2012, local recurrence was identified in
the patient's left chest wall. Local resection followed by
radiotherapy was performed and endocrine therapy with exemestane
was administered.
In September 2014, local recurrence was again
identified in the patient's left chest wall. Following local
resection, toremifene was administered as endocrine therapy.
In January 2015, local recurrence was identified in
the patient's left chest wall for a third time and the recurrent
tumor lesions enlarged gradually. Next, six cycles of docetaxel and
capecitabine were administered. SD was achieved following two and
four cycles of treatment but therapy failed following six
cycles.
The patient decided to stop treatment with further
hormone therapy due to the economic burden. Following consultation,
the patient was treated with vinorelbine, cisplatin and apatinib
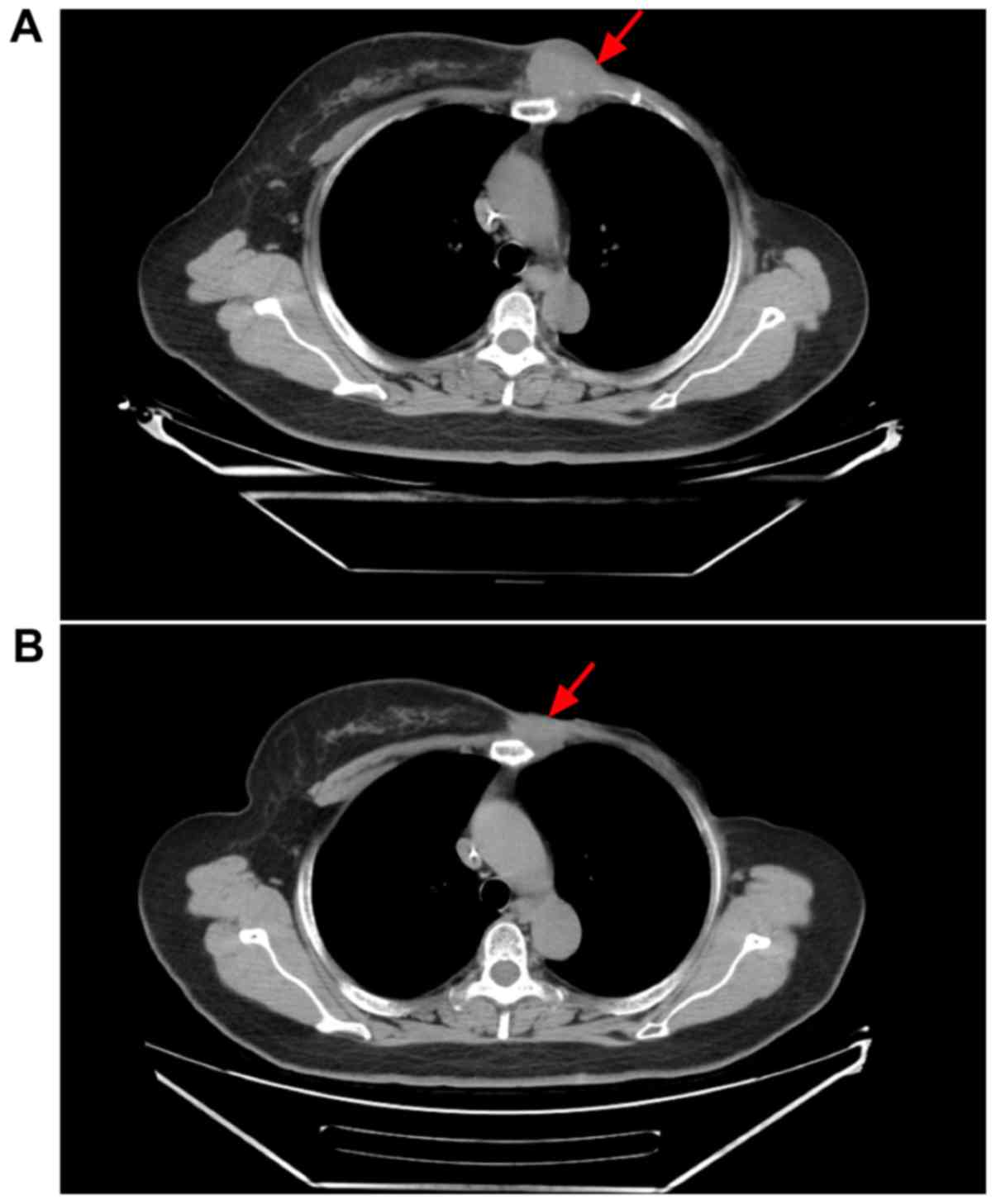
(500 mg per day). Following two cycles, the maximum diameter of the
tumor in the patient's left chest wall reduced in size from 4.5 to
3.1 cm (Fig. 4). PR was subsequently
achieved according to RECIST. In January 2016, the patient
developed PD and achieved a PFS of 6 months.
Discussion
To the best of our knowledge, the current study is
the first to evaluate the efficacy and safety of apatinib combined
with chemotherapeutic agents in patients with advanced breast
cancer who were previously exposed to anthracyclines or taxanes. In
the current study, the median PFS of all 85 patients was 4.4 months
(95% CI=2.8–6.0 months) and the median OS was 11.3 months (95%
CI=8.9–13.8 months). Among the 82 patients eligible for efficacy
analysis, the ORR was 23.2% (19/82) and the CBR was 47.6% (39/82).
These results indicated that apatinib combined with chemotherapy
performs efficiently in the treatment of advanced breast
cancer.
Previously, apatinib monotherapy has demonstrated
success in treating advanced breast cancer following standard
treatment. Hu et al (22,23)
performed two prospective, multicenter, phase II trials to evaluate
the efficacy and safety of apatinib as a single agent in patients
with pretreated metastatic TNBC or non-TNBC. Following their
results, among 56 patients with TNBC available for response
evaluation, the median PFS and OS were 3.3 months (95% CI=1.7–5.0
months) and 10.6 months (95% CI=5.6–15.7 months), respectively, and
the ORR and CBR were 10.7% and 25.0%, respectively (22). Among 38 patients with advanced
non-TNBC who were pretreated with anthracycline, taxanes and
capecitabine, apatinib monotherapy achieved a median PFS and OS of
4.0 months (95% CI=2.8–5.2 months) and 10.3 months (95% CI=9.1–11.6
months), respectively, and an ORR of 16.7% and a disease control
rate (DCR) of 66.7% among 36 evaluable patients (23). In the subgroup analysis in the
current study, the patients with TNBC achieved a median PFS of 5.2
months and a median OS of 11.4 months, which was longer than that
of patients with TNBC reported by Hu et al (22). In addition, the median PFS and OS of
patients with non-TNBC were 4.3 and 11.3 months, respectively,
which were longer than those of patients with non-TNBC reported by
Hu et al (23). Although
these findings arise from different study populations and
measurements, they are encouraging since the efficacy of combined
therapy appears to be superior to apatinib monotherapy in advanced
breast cancer.
During combined treatment, no treatment-associated
cases of mortality occurred. Five patients (6%) discontinued
apatinib due to severe AEs. Most of the AEs were manageable
following symptomatic treatment, dose adjustment or dose
interruption. As reported previously, myelosuppression is the most
common apatinib-associated haematologic toxicity (22,23,28) and
is characterized by thrombocytopenia, leukopenia, neutropenia and
anemia. In the current study, 42/85 (49.4%) patients exhibited
myelosuppression during combined treatment. In addition,
hypertension, hand-foot skin reaction and proteinuria are the most
common non-hematologic toxicities during apatinib treatment
(22,23,28);
this accounted for the fourth (32/85), fifth (22/85) and seventh
(14/85) most common AEs of all grades in the current study. Fatigue
is one of the most commonly reported AEs among patients with
advanced solid tumors, including lung cancer and thyroid tumors,
during or following apatinib treatment (29,30). In
the current study, 37/85 (43.5%) patients developed fatigue. The
toxicity profile in our study was similar with that reported
previously (22,23,28).
In apatinib-treated advanced gastric cancer, the PFS
was previously identified to be strongly associated with OS
(31). Recently, Huang et al
(32) applied mediation analysis to
apatinib phase III clinical trial data. In their study, the
mediating effect of apatinib on patient OS was systematically
quantified through PFS, post-progression survival and the DCR
(32). Following the results, Huang
et al demonstrated that PFS and DCR were the significant
mediators of the association between apatinib treatment and OS,
indicating that the tumor response to apatinib treatment may be
associated with patient OS. The current study investigated the
association between the response to apatinib treatment and patient
survival outcome, including PFS and OS, in apatinib-treated
advanced breast cancer. Based on the current results, no
significant difference was identified in either PFS or OS between
patients who achieved remission and those who did not. However, PFS
and OS were significantly prolonged in patients who achieved a
clinical benefit following apatinib treatment. These results
suggested that not only the ORR but also the CBR may be used to
predict patient survival outcome.
Most patients (52.9%) received apatinib combined
with chemotherapy as >3 lines of therapy in the current study.
For heavily pretreated advanced breast cancer (≥3 lines), the
patients were difficult to treat in the majority of cases because
they had received several lines of potent cytotoxic and hormonal
therapies. Non-responsiveness or refractoriness to cytotoxic agents
generally leads to poor efficacy of third and subsequent lines of
chemotherapy, with response rates ranging between 10 and 20%
(33,34). Additionally, due to the heterogeneous
nature of breast cancer, patients substantially vary in symptoms,
growth rate and responsiveness to therapy, and numerous patients
receive several lines of chemotherapy, occasionally provided until
mortality (35). In addition to
anti-angiogenic activity, apatinib has been identified to reverse
ATP-binding cassette sub-family B member 1 (ABCB1)- and ATP-binding
cassette sub-family G member 2 (ABCG2)-mediated multidrug
resistance (MDR) by directly inhibiting ABCB1 and ABCG2 transport
function, resulting in an elevated intracellular concentration of
the substrate chemotherapeutic drug (36,37).
Therefore, apatinib has been selected for reversal of MDR in
gastric cancer cells (38,39). In the current study, patients who had
received at least two lines of prior therapy achieved a median PFS
of 4.2 months and a median OS of 10.3 months following apatinib
combined with chemotherapy treatment, durations that were longer
than those in previous studies of chemotherapy alone (35). These promising results indicated that
the combined therapy may be effective for heavily pretreated
advanced breast cancer.
Predictive biomarkers are urgently required to
identify specific patients who are more sensitive to therapies and
to avoid exposure to useless toxic agents. Demographic
characteristics, baseline clinical information and AEs attributed
to therapies may be used as predictive biomarkers. Hypertension,
proteinuria and hand-foot skin reaction are common AEs associated
with angiogenesis inhibitors that target the VEGF pathway. A recent
study indicated that the presence of AEs, including hypertension,
proteinuria and hand-foot skin reaction, was a viable biomarker for
apatinib monotherapy in treating gastric cancer (40). In addition, Fan et al
(41) revealed that hypertension was
an independent predictive factor for PFS and CBR in patients with
metastatic breast cancer following apatinib monotherapy. In
contrast to the results for apatinib treatment alone, only
proteinuria was a predictive factor for apatinib combined with
chemotherapeutic agents in prolonging PFS and OS in advanced breast
cancer in the current study. Although a significant difference was
identified in OS when patients were stratified based on the
presence of hypertension or hand-foot skin reaction, when other
variables were considered, hypertension or hand-foot skin reaction
were not independent factors associated with patient OS in
multivariate analysis. In addition to proteinuria, the lines of
combined treatment may be used to predict patient survival outcome
in the current study. Certainly, these findings should be confirmed
in a further prospective clinical trial.
Furthermore, no statistically significant difference
was identified in either PFS or OS between patients with different
molecular types (TNBC vs. non-TNBC; HER2-positive vs.
HER2-negative; and hormone receptor-positive vs. negative),
indicating that apatinib combined with chemotherapy may be used for
the treatment of pretreated advanced breast cancer regardless of
molecular type. For patients with HER2-positive breast cancer,
HER2-targeted therapy is first recommended. However, due to the
heavy economic burden, a number of patients refuse to receive
HER2-targeted therapy. Based on the results in the current study,
apatinib combined with chemotherapy may be considered a treatment
choice for such patients who are unwilling to receive HER2-targeted
therapy.
The current study is a real-world observational
study. Several limitations, including using a retrospective design
and being a single-center study may inevitably lead to bias.
Additionally, the difference in chemotherapy regimens may increase
the occurrence of AEs. However, based on the promising outcome of
apatinib monotherapy in breast cancer, the results of the current
study further demonstrated that apatinib combined with
chemotherapeutic agents may bring clinical benefits for patients
with pretreated advanced breast cancer. Furthermore, the presence
of proteinuria may be a predictive factor for the efficacy of the
combined treatment. Considering the manageable toxicity and lack of
treatment-associated cases of mortality, this combined treatment
presents a new alternative therapy for patients with pretreated
advanced breast cancer.
Acknowledgements
Not applicable.
Funding
The current study was funded by the Clinical
Oncology Research Foundation, Chinese Society of Clinical Oncology
(grant no. Y-HR2015-105).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AZ, PY and BX participated in the conception and
design of the study. All authors collected and interpreted the
data. AZ, JW, YF, YL, RC, PZ, QL and FM performed the statistical
analysis. AZ drafted the manuscript, and PY and BX edited it
critically. All authors gave final approval of the version to be
published.
Ethics approval and consent to
participate
This study was approved by the National Cancer
Center/National Clinical Research Center for Cancer/Cancer
Hospital, Chinese Academy of Medical Sciences and Peking Union
Medical College (Beijing, China). Due to the retrospective design
of the current study and patient anonymization, the review board
determined that informed consent was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mayer EL and Burstein HJ: Chemotherapy for
metastatic breast cancer. Hematol Oncol Clin North Am. 21:257–272.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: Breast cancer, version 4.2017, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
16:310–320. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schneider BP and Miller KD: Angiogenesis
of breast cancer. J Clin Oncol. 23:1782–1790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fox SB, Generali DG and Harris AL: Breast
tumour angiogenesis. Breast Cancer Res. 9:2162007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Banerjee S, Dowsett M, Ashworth A and
Martin LA: Mechanisms of disease: Angiogenesis and the management
of breast cancer. Nat Clin Pract Oncol. 4:536–550. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Willett CG, Boucher Y, di Tomaso E, Duda
DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, et
al: Direct evidence that the VEGF-specific antibody bevacizumab has
antivascular effects in human rectal cancer. Nat Med. 10:145–147.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miller K, Wang M, Gralow J, Dickler M,
Cobleigh M, Perez EA, Shenkier T, Cella D and Davidson NE:
Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic
breast cancer. N Engl J Med. 357:2666–2676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bianchi G, Loibl S, Zamagni C, Salvagni S,
Raab G, Siena S, Laferriere N, Peña C, Lathia C, Bergamini L and
Gianni L: Phase II multicenter, uncontrolled trial of sorafenib in
patients with metastatic breast cancer. Anticancer Drugs.
20:616–624. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moreno-Aspitia A, Morton RF, Hillman DW,
Lingle WL, Rowland KM Jr, Wiesenfeld M, Flynn PJ, Fitch TR and
Perez EA: Phase II trial of sorafenib in patients with metastatic
breast cancer previously exposed to anthracyclines or taxanes:
North central cancer treatment group and mayo clinic trial N0336. J
Clin Oncol. 27:11–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burstein HJ, Elias AD, Rugo HS, Cobleigh
MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, DePrimo
SE, et al: Phase II study of sunitinib malate, an oral
multitargeted tyrosine kinase inhibitor, in patients with
metastatic breast cancer previously treated with an anthracycline
and a taxane. J Clin Oncol. 26:1810–1816. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yardley DA, Dees EC, Myers SD, Li S,
Healey P, Wang Z, Brickman MJ, Paolini J, Kern KA and Citrin DL:
Phase II open-label study of sunitinib in patients with advanced
breast cancer. Breast Cancer Res Treat. 136:759–767. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wildiers H, Fontaine C, Vuylsteke P,
Martens M, Canon JL, Wynendaele W, Focan C, De Greve J, Squifflet P
and Paridaens R: Multicenter phase II randomized trial evaluating
antiangiogenic therapy with sunitinib as consolidation after
objective response to taxane chemotherapy in women with
HER2-negative metastatic breast cancer. Breast Cancer Res Treat.
123:463–469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barrios CH, Liu MC, Lee SC, Vanlemmens L,
Ferrero JM, Tabei T, Pivot X, Iwata H, Aogi K, Lugo-Quintana R, et
al: Phase III randomized trial of sunitinib versus capecitabine in
patients with previously treated HER2-negative advanced breast
cancer. Breast Cancer Res Treat. 121:121–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mariani G, Burdaeva O, Roman L,
Staroslawska E, Udovitsa D, Driol P, Goisis G, Zamagni C,
Semiglazov V and Gianni L: A double-blind, randomized phase lib
study evaluating the efficacy and safety of sorafenib (SOR)
compared to placebo (PL) when administered in combination with
docetaxel and/or letrozole in patients with metastatic breast
cancer (MBC): FM-B07-01 Trial. Eur J Cancer. 47:102011. View Article : Google Scholar
|
|
17
|
Hudis C, Tauer KW, Hermann RC,
Makari-Judson G, Isaacs C, Beck JT, Kaklamani VG, Stepanski EJ,
Rugo HS, Wang W, et al: Sorafenib (SOR) plus chemotherapy (CRx) for
patients (pts) with advanced (adv) breast cancer (BC) previously
treated with bevacizumab (BEV). J Clin Oncol. 29:1009. 2011.
View Article : Google Scholar
|
|
18
|
Gradishar WJ, Kaklamani V, Sahoo TP,
Lokanatha D, Raina V, Bondarde S, Jain M, Ro SK, Lokker NA and
Schwartzberg L: A double-blind, randomised, placebo-controlled,
phase 2b study evaluating sorafenib in combination with paclitaxel
as a first-line therapy in patients with HER2-negative advanced
breast cancer. Eur J Cancer. 49:312–322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baselga J, Segalla JG, Roché H, Del Giglio
A, Pinczowski H, Ciruelos EM, Filho SC, Gómez P, Van Eyll B,
Bermejo B, et al: Sorafenib in combination with capecitabine: An
oral regimen for patients with HER2-negative locally advanced or
metastatic breast cancer. J Clin Oncol. 30:1484–1491. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aalders KC, Tryfonidis K, Senkus E and
Cardoso F: Anti-angiogenic treatment in breast cancer: Facts,
successes, failures and future perspectives. Cancer Treat Rev.
53:98–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Zhao X, Chen L, Guo H, Lv F, Jia K,
Yv K, Wang F, Li C, Qian J, et al: Safety and pharmacokinetics of
novel selective vascular endothelial growth factor receptor-2
inhibitor YN968D1 in patients with advanced malignancies. BMC
Cancer. 10:5292010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu X, Zhang J, Xu B, Jiang Z, Ragaz J,
Tong Z, Zhang Q, Wang X, Feng J, Pang D, et al: Multicenter phase
II study of apatinib, a novel VEGFR inhibitor in heavily pretreated
patients with metastatic triple-negative breast cancer. Int J
Cancer. 135:1961–1969. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu X, Cao J, Hu W, Wu C, Pan Y, Cai L,
Tong Z, Wang S, Li J, Wang Z, et al: Multicenter phase II study of
apatinib in non-triple-negative metastatic breast cancer. BMC
Cancer. 14:8202014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
U.S.Department of Health, Human Services,
National Institutes of Health and National Cancer Institute: Common
Terminology Criteria for Adverse Events (CTCAE). Version 4.03.
https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdJune
14–2010
|
|
25
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carninci P, Kasukawa T, Katayama S, Gough
J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, et al:
The transcriptional landscape of the mammalian genome. Science.
309:1559–1563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gerard T: Eastern Cooperative Oncology
Group Performance Status. Chemotherapy. 5:102012.
|
|
28
|
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y,
Liu W, Tong J, Liu Y, Xu R, et al: Randomized, double-blind,
placebo-controlled phase III trial of apatinib in patients with
chemotherapy-refractory advanced or metastatic adenocarcinoma of
the stomach or gastroesophageal junction. J Clin Oncol.
34:1448–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu J, Liu X, Yang S, Zhang X and Shi Y:
Apatinib plus icotinib in treating advanced non-small cell lung
cancer after icotinib treatment failure: A retrospective study.
Onco Targets Ther. 10:4989–4995. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin Y, Wang C, Gao W, Cui R and Liang J:
Overwhelming rapid metabolic and structural response to apatinib in
radioiodine refractory differentiated thyroid cancer. Oncotarget.
8:42252–42261. 2017.PubMed/NCBI
|
|
31
|
Liu L, Yu H, Huang L, Shao F, Bai J, Lou D
and Chen F: Progression-free survival as a surrogate endpoint for
overall survival in patients with third-line or later-line
chemotherapy for advanced gastric cancer. Onco Targets Ther.
8:921–928. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang L, Wei Y, Shen S, Shi Q, Bai J, Li
J, Qin S, Yu H and Chen F: Therapeutic effect of apatinib on
overall survival is mediated by prolonged progression-free survival
in advanced gastric cancer patients. Oncotarget. 8:29346–29354.
2017.PubMed/NCBI
|
|
33
|
Cortes J, O'Shaughnessy J, Loesch D, Blum
JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Diéras V,
Delozier T, et al: Eribulin monotherapy versus treatment of
physician's choice in patients with metastatic breast cancer
(EMBRACE): A phase 3 open-label randomised study. Lancet.
377:914–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Blum JL, Jones SE, Buzdar AU, LoRusso PM,
Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS and Griffin T:
Multicenter phase II study of capecitabine in paclitaxel-refractory
metastatic breast cancer. J Clin Oncol. 17:485–493. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jehn CF, Hemmati P, Lehenbauer-Dehm S,
Kümmel S, Flath B and Schmid P: Biweekly pegylated liposomal
doxorubicin (caelyx) in heavily pretreated metastatic breast
cancer: A phase 2 study. Clin Breast Cancer. 16:514–519. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tong XZ, Wang F, Liang S, Zhang X, He JH,
Chen XG, Liang YJ, Mi YJ, To KK and Fu LW: Apatinib (YN968D1)
enhances the efficacy of conventional chemotherapeutical drugs in
side population cells and ABCB1-overexpressing leukemia cells.
Biochem Pharmacol. 83:586–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu J, Li X, Xie C, Li Y and Zhong G:
Apatinib, a new small molecular VEGFR2 inhibitor, suppresses the
activity of lung cancer stem cells. J Thorac Oncol. 12 (Suppl
1):S12792017. View Article : Google Scholar
|
|
39
|
Wu Q, Yang Z, Nie Y, Shi Y and Fan D:
Multi-drug resistance in cancer chemotherapeutics: Mechanisms and
lab approaches. Cancer Lett. 347:159–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu X, Qin S, Wang Z, Xu J, Xiong J, Bai
Y, Wang Z, Yang Y, Sun G, Wang L, et al: Early presence of
anti-angiogenesis-related adverse events as a potential biomarker
of antitumor efficacy in metastatic gastric cancer patients treated
with apatinib: A cohort study. J Hematol Oncol. 10:1532017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fan M, Zhang J, Wang Z, Wang B, Zhang Q,
Zheng C, Li T, Ni C, Wu Z, Shao Z and Hu X: Phosphorylated VEGFR2
and hypertension: Potential biomarkers to indicate VEGF-dependency
of advanced breast cancer in anti-angiogenic therapy. Breast Cancer
Res Treat. 143:141–151. 2014. View Article : Google Scholar : PubMed/NCBI
|