Introduction
Hepatocellular carcinoma (HCC) is the fourth most
common cancer and ranks third in digestive system in annual
mortality worldwide (1). The early
stage of the disease is characterized by dysplastic nodules, also
known as preneoplastic lesions, which frequently develop in chronic
inflammatory liver disease or hepatitis, and promote fibrosis,
cirrhosis, and HCC progression (2).
In the past, surgery was the main treatment for HCC. To date, the
prognosis of patients with HCC has improved due to various
combinations of treatment strategies including, surgery,
chemotherapy, and molecular-targeted therapy; however, therapeutic
options remain limited.
Transmembrane 4 L six family member 5 (TM4SF5) is a
transmembrane glycoprotein of the transmembrane 4 L six family, a
branch of the tetraspanin family. The TM4SF5 gene is located on
human chromosome 17q13.3, and encodes a 197 amino acid protein that
has two cysteine residues and two N-glycosylation sites within the
extracellular loops (3–5). TM4SF5 is highly expressed in esophageal
cancer (6), colorectal cancer
(7), and HCC cells (8). In the study of HCC, TM4SF5 has only
been reported to induce HCC metastasis in cell line experiments
(8). Therefore, there has been
limited research on the association between TM4SF5 expression and
clinicopathological factors in HCC.
In the present study, the clinicopathological
significance of TM4SF5 expression was examined using formalin-fixed
paraffin-embedded (FFPE) specimens from patients with HCC; the aim
was to investigate the potential of TM4SF5 as a biomarker and to
analyze the prognostic significance of TM4SF5 status.
Materials and methods
Patient selection
In this retrospective study, the pathology archives
from the Liaoning Cancer Hospital and Institute (Shenyang, China)
were reviewed to identify patients diagnosed with HCC. A spectrum
of carcinoma cases was selected from the period between January
2009 and December 2011. Patients with intrahepatic
cholangiocarcinoma or mixed hepatocholangiocarcinoma were not
included in the present study; as a result, 89 cases of HCC were
retrieved.
Clinical details were obtained from patients'
pathology reports and original electronic medical records. The
morphological features and histopathological variants were
classified according to the National Comprehensive Cancer Network
(NCCN) Clinical Practice Guidelines in Oncology (version 2018). In
addition, other clinicopathological data, including age, sex,
hepatitis B virus (HBV) or hepatitis C virus (HCV) infection and
survival data were obtained from medical records. Time interval
between surgery and final follow-up was identified as a patient's
survival time. All aspects of this study were approved by the
Liaoning Cancer Hospital and Institute Ethics Review Board
(approval no. 20180902).
Tissue sections
Liver tissue sections were obtained from original
FFPE tissue blocks. All tissue blocks were cut into regular 5-µm
sections and subsequently stained with hematoxylin for 3 min and
eosin for 30 sec (H&E) at room temperature to confirm
previously rendered histologic diagnoses based upon current World
Health Organization classifications. In addition, all the
pathologic slides were reviewed independently by two pathologists,
who reevaluated tumor differentiation, lymph node metastasis, and
vascular invasion.
Immunohistochemistry
Tissue samples from FPPE blocks were sectioned (4
µm) and subsequently deparaffinized at 70°C for 120 min, followed
by antigen retrieval in citrate buffer, pH 6. The slides were
incubated with 1:200 rabbit polyclonal anti-TM4SF5 primary antibody
(cat. no. HPA041259; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Subsequently, the sections were incubated by using
UltraSensitiveTM SP (Mouse/Rabbit) IHC kit (cat. no. KIT-9710;
Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China) and expression was
visualized using a DAB Detection kit (cat. no. DAB-0031; Fuzhou
Maixin Biotech Co., Ltd.). High expression TM4SF5 positive control
and untreated with primary antibody negative control were
included.
Evaluations of the pattern of immunoreactivity,
percentage of cell staining, and staining intensities for TM4SF5
were performed by two independent pathologists. The percentage of
cells stained was graded on a scale of 1–4 (1, 1–25%; 2, 26–50%; 3,
51–75%; 4, 76–100%). Staining intensity was scored on a scale of
1–3 (1, weak; 2, intermediate; 3, strong). TM4SF5 expression was
evaluated in each sample by calculating a final
immunohistochemistry score as the sum of the percentage score and
the intensity score. The tissue samples were divided into two
groups, a high-TM4SF5 expression group with a total score ≥6 and a
low-TM4SF5 expression group with a total score <6.
Statistical analysis
Comparison of distribution among different
histologies in this study was performed using a Wilcoxon
signed-ranked test (GraphPad Prism software 7.0; GraphPad, La
Jolla, CA, USA). χ2 test was used to assess the
association between TM4SF5 expression and clinicopathologic
parameters using SPSS 24.0 (SPSS, Inc., Chicago, IL, USA). The
survival curves of the patients were determined using the
Kaplan-Meier method and the log-rank test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Clinicopathological
characteristics
The study included 89 patients with HCC (Table I): 72 male, 17 female; median age at
diagnosis was 54 years (range 25–76 years). The number of patients
infected with HBV was 80 (89.9%), whereas the number of patients
infected with HCV was 13 (14.6%). According to NCCN Clinical
Practice Guidelines in Oncology (version 2018), 14 tumors were
classified as well differentiated, 70 as moderately differentiated,
and 4 as poorly differentiated carcinomas. Twelve patients (13.5%)
were staged as IA, 41 (46.1%) as IB, 11 (12.4%) as II, 10 (11.2%)
as IIIA, 11 (12.4%) as IIIB, and 4 (4.5%) as IVB.
 | Table I.Association between TM4SF5 expression
and clinicopathological factors. |
Table I.
Association between TM4SF5 expression
and clinicopathological factors.
|
|
| TM4SF5
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factor | Number (n=89) | Low | High | P-value |
|---|
| Sex |
|
|
| 0.166 |
| Male | 72 | 29 | 43 |
|
|
Female | 17 | 10 | 7 |
|
| Age |
|
|
| 0.650 |
| ≤60 | 64 | 29 | 35 |
|
|
>60 | 25 | 10 | 15 |
|
| HBV infection |
|
|
| 0.454 |
|
Negative | 9 | 5 | 4 |
|
|
Positive | 80 | 34 | 46 |
|
| HCV infection |
|
|
| 0.305 |
|
Negative | 76 | 35 | 41 |
|
|
Positive | 13 | 4 | 9 |
|
| Tumor size (cm) |
|
|
| <0.001 |
| ≤5 | 59 | 11 | 48 |
|
|
>5 | 30 | 28 | 2 |
|
| Vascular
invasion |
|
|
| <0.001 |
| No | 75 | 26 | 49 |
|
| Yes | 14 | 13 | 1 |
|
| Tumor
differentiation |
|
|
| 0.006 |
| G1 | 14 | 2 | 12 |
|
| G2 | 70 | 33 | 37 |
|
| G3 | 4 | 4 | 0 |
|
| TNM stage |
|
|
| 0.005 |
| IA | 12 | 1 | 11 |
|
| IB | 41 | 17 | 24 |
|
| II | 11 | 4 | 7 |
|
| IIIA | 10 | 5 | 5 |
|
| IIIB | 11 | 10 | 1 |
|
| IVB | 4 | 2 | 2 |
|
Association between TM4SF5 expression
and clinicopathological factors
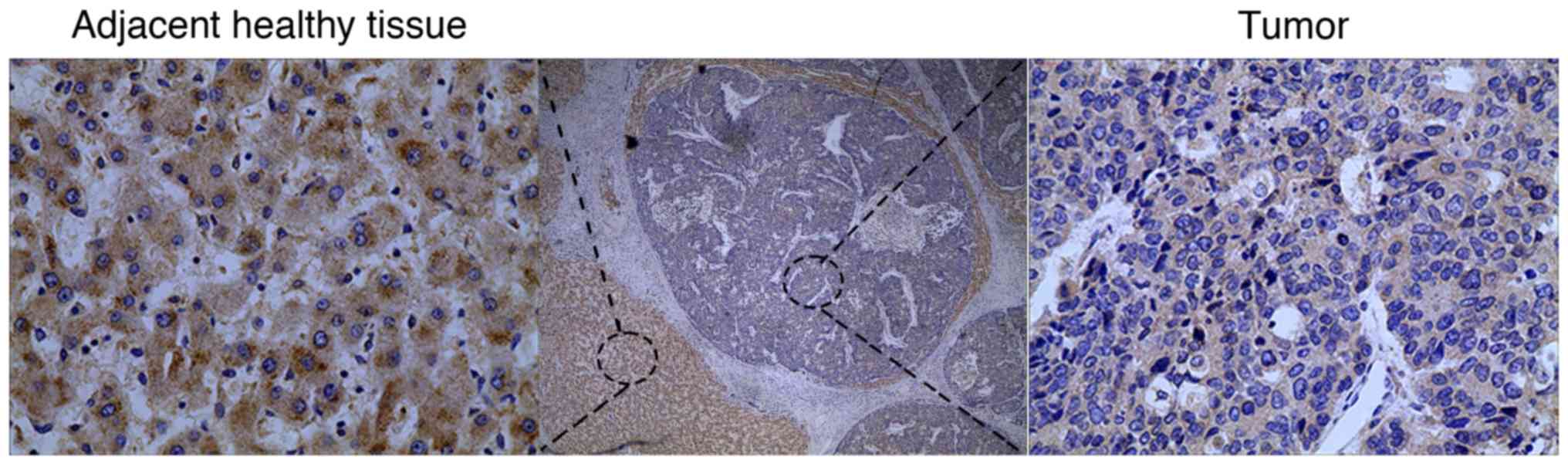
TM4SF5 expression was investigated in 89 HCC tissues
and corresponding adjacent normal tissues. Higher TM4SF5 protein
expression was observed in normal tissues compared with carcinoma
tissues (Fig. 1). To demonstrate the
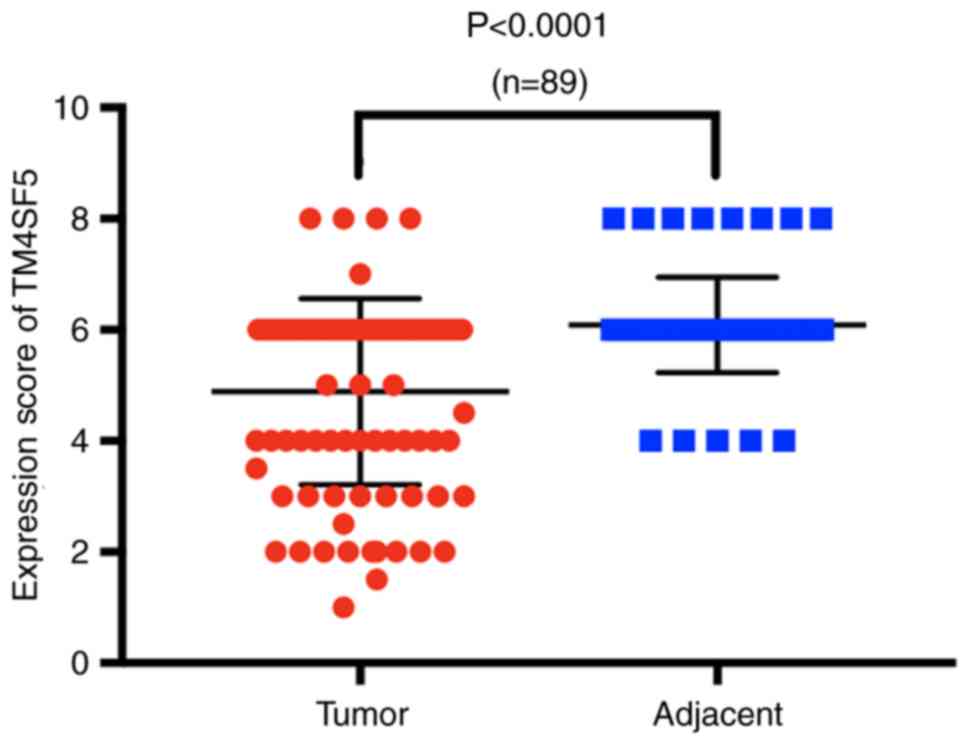
difference in immunohistochemistry scores between the two groups, a
Wilcoxon signed-rank test was applied, and the result revealed that
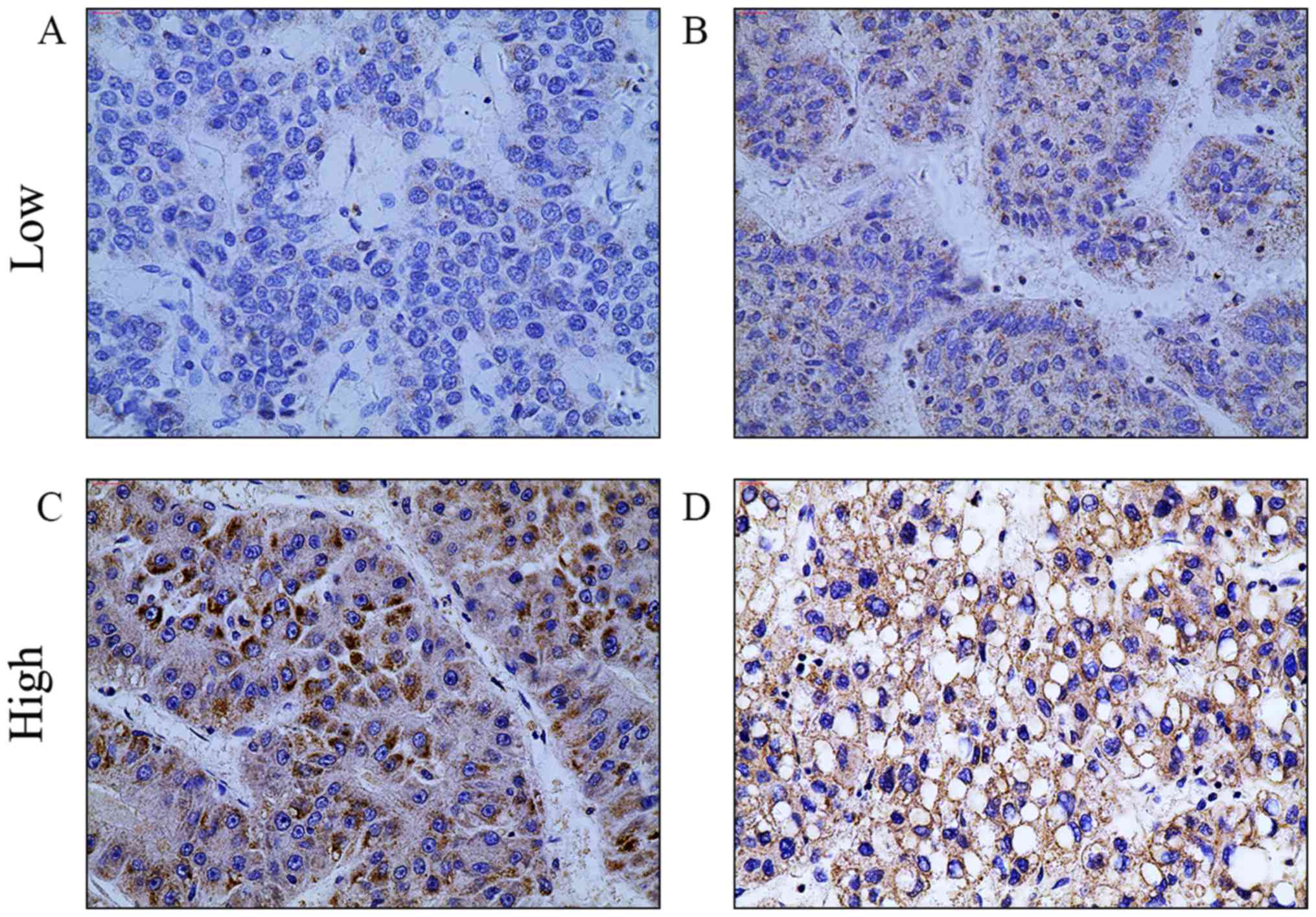
the difference was statistically significant (Fig. 2). The carcinoma tissues were further
divided into two groups; high-TM4SF5 expression and low-TM4SF5
expression (Fig. 3), to assess the
association between TM4SF5 expression and clinicopathological
factors. Tumor size, vascular invasion, tumor differentiation, and
tumor-node-metastasis (TNM) stage were associated with low TM4SF5
expression (Table I). No significant
associations were identified between TM4SF5 expression and the
remaining factors.
Survival analysis
A total of 86 patients completed the follow-up
period and the median period was 40 months (range 1–113 months).
Among these, the number of patients in the high-TM4SF5 expression
group was 47 and in the low-TM4SF5 expression group was 39.
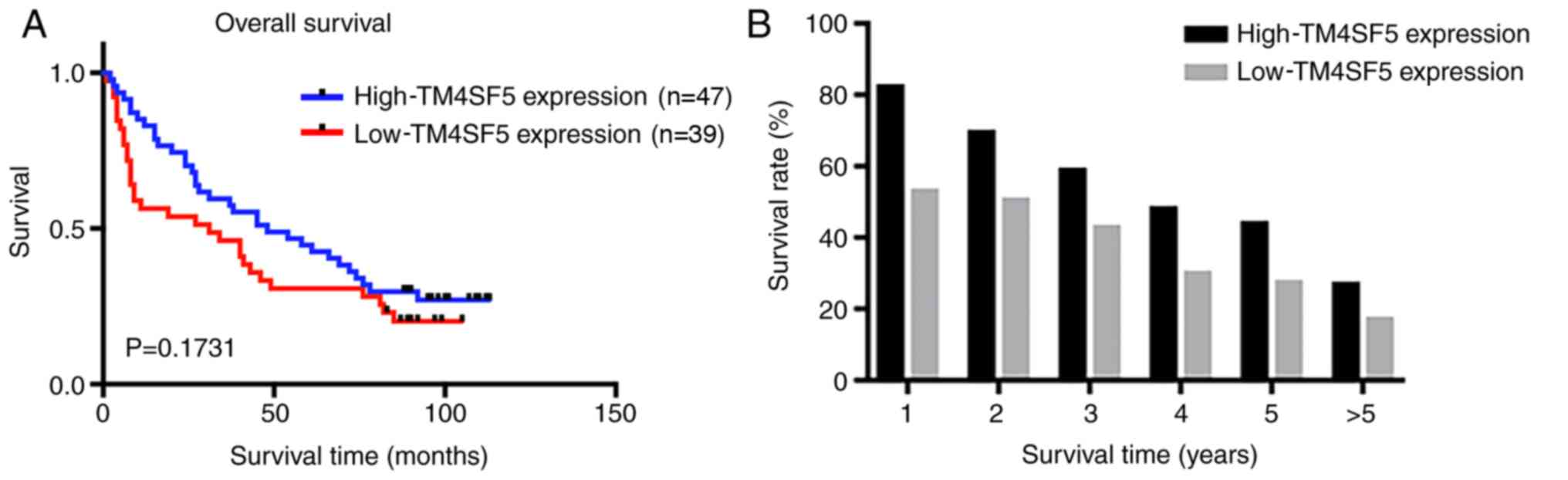
Kaplan-Meier survival analysis demonstrated that patients with
low-TM4SF5 expression exhibited shorter overall survival (OS)
compared with those with high-TM4SF5 expression, although the
difference was not significant (Fig.
4A). In addition, the 5-year survival rate of the low-TM4SF5
expression group (30.8%) was lower compared with the high-TM4SF5
expression group (44.7%) (Fig.
4B).
Discussion
Human malignant tumors develop by genetic
alterations and are made up of a heterogeneous population of cells.
HCC develops through a multistep process, including cell mutation
and regeneration; it is one of the most common cancers, and is
characterized by a high mortality rate (9). Previous studies have demonstrated that
the risk factors for the occurrence of HCC primarily include
chronic HBV and HCV infections, chronic alcohol consumption, and
non-alcoholic fatty liver disease (10–12).
Despite the numerous therapeutic regimens for HCC that have been
proposed, including etiology and oncology treatment, and currently
approved standard therapies, the disease is still progressive for
the majority of patients.
TM4SF5 is highly expressed in numerous types of
cancers and serves a crucial role in tumorigenesis (6–8). In
human hepatocytes, ectopic expression of TM4SF5 enhances focal
adhesion kinase (FAK) Tyr577 phosphorylation as well as
the association between FAK, Rho GTPase-activating protein 35 and
Rho GTPase-activating protein 26, leading to transforming protein
RhoA inactivation (13).
Additionally, TM4SF5 induces stabilization of cytosolic cyclin
dependent kinase inhibitor 1B (p27kip1), which may
function as an inhibitor of the RhoA signaling pathway (14). Consequently, TM4SF5-mediated RhoA
inactivation results in the process of epithelial-mesenchymal
transition, leading to tumor cell migration, invasion, and
proliferation due to the loss of contact inhibition (15). Tumor initiation and progression also
involve complex communications between tumor cells and their
microenvironments, including cytokines, extracellular matrix, and
growth factors (16). In this
complicated network, TM4SF5 protein not only cooperates with
integrins to communicate with the microenvironment (17), but also mediates the activation of
vascular endothelial growth factor transcription and secretion
(18). This study suggests these
factors contribute to the communication between cancer cells and
extracellular environments, as well as angiogenesis, which provides
beneficial conditions for tumor growth.
Advances in our knowledge about tumor initiation and
progression have enriched the way the role of TM4SF5 is perceived.
A study by Lee et al indicated that the interaction between
TM4SF5 and CD44 was essential for the self-renewal and circulating
capacities of HCC cells, leading to metastasis (19). In addition, mitogen-activated protein
kinase 8 signaling activity has been demonstrated to regulate
cell-cell adhesions through TM4SF5-mediated p27kip1
phosphorylation (20). In the
process of tumor immune escape, Ryu et al revealed that
crosstalk between the TM4SF5/FAK pathway and the interleukin 6
(IL-6)/signal transducer and activator of transcription 3 pathway
promoted metastatic potential by lowering IL-6 expression levels
and avoiding its immunological action (21). However, studies using human HCC
tissues to evaluate the association between TM4SF5 expression and
clinicopathological factors have been very limited.
In contrast with results of Lee et al
(19), the present study
demonstrated a cytoplasmic staining pattern for TM4SF5 in both
tumors and corresponding adjacent normal cells. Notably, TM4SF5
expression was demonstrated to be significantly higher in adjacent
normal cells compared with tumors. The data were separated into two
groups, a low-TM4SF5 expression group and a high-expression group,
to estimate the association between TM4SF5 expression and
clinicopathological data. Low TM4SF5 expression was associated with
increased tumor size, vascular invasion, tumor differentiation and
TNM stage. In addition, survival analysis indicated that patients
with low-TM4SF5 expression exhibited shorter OS, although no
significance was identified. These results are contrary to previous
findings, which may have resulted from the following: Experiments
using cell lines and mice merely reflect the biological features of
human bodies, whereas the initiation of human tumors is a complex,
multistep process. The multistep development of human tumors
involves several biological capabilities; for example, sustaining
proliferative signaling, evading growth suppressors, resisting cell
death, enabling replicative immortality, inducing angiogenesis,
reprogramming of energy metabolism, evading immune destruction, and
activating invasion and metastasis (22). By comparing the data of HCC
microarray in GEO database (https://www.ncbi.nlm.nih.gov/gds/), it was found that
the gene expression profiles and tumor biological functions were
notably different due to individual discrepancy in the study of a
series of patients with the same type of cancer. In the present
study, the data of a number of patients with high-TM4SF5-expressing
tumors were consistent with findings of a previous study (21). When all patient data were summarized,
the results revealed distinct and opposing differences, which
contributed to the conclusion that there are large differences in
biological processes between cell lines, mice, and human
bodies.
In the majority of previous studies (5,18,19), a
monoclonal antibody was applied to detect TM4SF5. By contrast, in
the present study, only the commercial polyclonal antibody
(HPA041259 from Sigma) was utilized; no comparison of
immunohistochemistry specificity between two antibodies was
performed, which may be a limitation to the present study.
In summary, associations between TM4SF5 expression
and clinicopathological factors were identified, and the prognostic
significance of TM4SF5 as a potential biomarker was further
evaluated using a number of human HCC FFPE samples. To the best of
our knowledge, this was the first study to evaluate TM4SF5
expression and clinicopathological factors using follow-up records
and surgically resected specimens. The conclusion that high-TM4SF5
expression may be associated with OS was different from a previous
study. These results indicated that TM4SF5 expression may not only
be a prognostic factor, but also may be a predictive factor for
HCC. However, a large-scale investigation is required to confirm
these results.
Acknowledgements
The authors would like to thank Liaoning Cancer
Hospital and Institute (Shenyang, China) for providing the tissue
samples.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JL conceived and designed the study. BX and WL
completed data extraction and analysis. XL and LZ performed the
pathological evaluations. BX, XL and LZ drafted the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by Liaoning Cancer Hospital
and Institute Ethics Review Board (Shenyang, China) (approval no.
20180902). Written informed consent was obtained from the patients
for use of their information and materials for research
purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tummala KS, Gomes AL, Yilmaz M, Graña O,
Bakiri L, Ruppen I, Ximénez-Embún P, Sheshappanavar V,
Rodriguez-Justo M, Pisano DG, et al: Inhibition of de novo NAD(+)
synthesis by oncogenic URI causes liver tumorigenesis through DNA
damage. Cancer Cell. 26:826–839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee SA, Park KH and Lee JW: Modulation of
signaling between TM4SF5 and integrins in tumor microenvironment.
Front Biosci (Landmark Ed). 16:1752–1758. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wright MD, Ni J and Rudy GB: The L6
membrane proteins-a new four-transmembrane superfamily. Protein
Sci. 9:1594–1600. 2009. View Article : Google Scholar
|
|
5
|
Lee SA, Ryu HW, Kim YM, Choi S, Lee MJ,
Kwak TK, Kim HJ, Cho M, Park KH and Lee JW: Blockade of
four-transmembrane L6 family member 5 (TM4SF5)-mediated
tumorigenicity in hepatocytes by a synthetic chalcone derivative.
Hepatology. 49:1316–1325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu YB, Huang YS, Xu YP, Sun YF, Yu DL,
Zhang XQ, Long X, Zhu SQ, Zhou JL and Xu JJ: A high level of TM4SF5
is associated with human esophageal cancer progression and poor
patient survival. Dig Dis Sci. 58:2623–2633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park BK, Park JY, Kim TH, Kim D, Wu G,
Gautam A, Maharjan S, Lee SI, Lee Y, Kwon HJ and Choi KC:
Production of an anti-TM4SF5 monoclonal antibody and its
application in the detection of TM4SF5 as a possible marker of a
poor prognosis in colorectal cancer. Int J Oncol. 53:275–285.
2018.PubMed/NCBI
|
|
8
|
Lee D and Lee JW: Self-renewal and
circulating capacities of metastatic hepatocarcinoma cells required
for collaboration between TM4SF5 and CD44. BMB Rep. 48:127–128.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amicone L and Marchetti A:
Microenvironment and tumor cells: Two targets for new molecular
therapies of hepatocellular carcinoma. Transl Gastroenterol
Hepatol. 3:242018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dutta R and Mahato RI: Recent advances in
hepatocellular carcinoma therapy. Pharmacol Ther. 173:106–117.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reeves HL, Zaki MY and Day CP:
Hepatocellular carcinoma in obesity, type 2 diabetes, and NAFLD.
Dig Dis Sci. 61:1234–1245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trojan J, Zangos S and Schnitzbauer AA:
Diagnositics and treatment of hepatocellular carcinoma in 2016:
Standrads and developments. Visc Med. 32:116–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SA, Lee SY, Cho IH, Oh MA, Kang ES,
Kim YB, Seo WD, Choi S, Nam JO, Tamamori-Adachi M, et al:
Tetraspanin TM4SF5 mediates loss of contact inhibition through
epithelial- mesenchymal transition in human hepatocarcinoma. J Clin
Invest. 118:1354–1366. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Besson A, Gurian-West M, Schmidt A, Hall A
and Roberts JM: p27Kip1 modulates cell migration through the
regulation of RhoA activation. Genes Dev. 18:862–876. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim H, Kang M, Lee SA, Kwak TK, Jung O,
Lee HJ, Kim SH and Lee JW: TM4SF5 accelerates G1/S phase
progression via cytosolic p27Kip1 expression and RhoA activity.
Biochim Biophys Acta. 1803:975–982. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sadej R, Romanska H, Baldwin G,
Gkirtzimanaki K, Novitskaya V, Filer AD, Krcova Z, Kusinska R,
Ehrmann J, Buckley CD, et al: CD151 regulates tumorigenesis by
modulating the communication between tumor cells and endothelium.
Mol Cancer Res. 7:787–798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi S, Lee S-A, Kwak TK, Kim HJ, Lee MJ,
Ye SK, Kim SH, Kim S and Lee JW: Cooperation between integrin
alpha5 and teraspan TM4SF5 regulates VEGF-mediated angiogenic
activity. Blood. 113:1845–1855. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee D, Na J, Ryu J, Kim HJ, Nam SH, Kang
M, Jung JW, Lee MS, Song HE, Choi J, et al: Interaction of
tetraspan(in) TM4SF5 with CD44 promotes self-renewal and
circulating capacities of hepatocarcinoma cells. Hepatology.
61:1978–1997. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim H, Jung O, Kang M, Lee MS, Jeong D,
Ryu J, Ko Y, Choi YJ and Lee JW: JNK signaling activity regulates
cell-cell adhesions via TM4SF5-mediated p27(Kip1) phosphorylation.
Cancer Lett. 314:198–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ryu J, Kang M, Lee MS, Kim HJ, Nam SH,
Song HE, Lee D and Lee JW: Cross talk between the TM4SF5/focal
adhesion kinase and the interleukin-6/STAT3 pathways promotes
immune escape of human liver cancer cells. Mol Cell Biol.
34:2946–2960. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|