Introduction
Ovarian tumor is a common tumor in the female
reproductive organ, and the incidence rate of malignant ovarian
tumor ranks 3rd in gynecological tumors (1). The mortality rate for ovarian cancer is
the highest in a variety of gynecological tumors, seriously
threatening women's health and lives. The ovary is located deeply
in the pelvic cavity, and there are often no typical clinical
manifestations when the tumor occurs, so most patients are in the
late stage at the time of diagnosis. The 5-year survival rate of
patients with advanced ovarian cancer is as low as 30–40%, and the
survival rate of early-stage patients is ~90% (2,3).
Therefore, the early diagnosis of ovarian cancer is the key to
improving the survival rate of patients, which can provide a basis
for treatment and prognosis.
Ultrasound, computed tomography (CT) and magnetic
resonance imaging (MRI) are commonly-used imaging examination
methods in clinic. Among them, MRI is difficult to be the preferred
examination method and screening means for lesions, due to the high
costs of equipment and examination. Ultrasound examination is
currently the most commonly-used and convenient method in clinical
screening of ovarian tumor, which can detect the morphology, size,
capsule and wall thickness of ovarian tumors, the characteristics
of echo in nodules and whether there are ascites, and can also
preliminarily diagnose benign and malignant ovarian tumors
according to the Finkler scoring system criteria (4). Color Doppler ultrasound flow imaging
can also detect the blood flow resistance index (RI) and the
velocity, based on the observation of the distribution
characteristics and morphological features of tumor vessels, thus
improving the diagnostic accuracy rate (5). RI can directly reflect the resistance
against blood flow, and it is higher in benign ovarian tumors than
in malignant ovarian tumors. Therefore, benign and malignant tumors
can be differentially diagnosed by RI, but there is an overlap in
the RI value between benign and malignant tumors (6). Moreover, the 64-slice spiral CT scan
can position and qualitatively determine the tumor and indicate the
lesion scope, because it can clearly display the position of the
tumor and clarify the adjacent relationship between the tumor and
pelvic organs (7–9).
In recent years, the diagnostic value of ultrasound
combined with tumor markers in serum for ovarian tumors has been
reported, but the detection of RI has not received much attention
(10). Reports on the combination of
several examination methods are rare, and no reports on CT
diagnosis of ovarian tumors exist; specifically the application of
64-slice spiral CT in ovarian tumor has been less reported
worldwide (11). Therefore, in this
study, Finkler ultrasound score, color Doppler ultrasound RI and
64-slice spiral CT signs were compared with pathological diagnosis
results, the sensitivity, specificity and accuracy were analyzed,
and the diagnostic value of the combined application of the three
methods for ovarian tumor was evaluated.
Patients and methods
Sample collection
A retrospective analysis was performed on 224
patients pathologically diagnosed with ovarian tumor after
operation in Cangzhou Central Hospital (Cangzhou, China) from
January 2010 to January 2014. There were 120 patients with benign
tumor, aged 42±14.68 years, and 104 patients with malignant tumor
aged 53±12.73 years. Inclusion criteria: patients were confirmed
with ovarian tumor by postoperative pathology with complete
clinical data and imaging. Exclusion criteria: patients with
endometriosis, pelvic inflammation, adenomyosis, liver disease,
ovarian hyperstimulation syndrome, uterine fibroids or diabetes
mellitus were excluded. The study was approved by the Ethics
Committee of Cangzhou Central Hospital (Cangzhou, China). Patients
who participated in this research had complete clinical data.
Signed informed consents were obtained from the patients or the
guardians.
Instruments
Color Doppler ultrasonic apparatus was purchased
from Siemens AG (Munich, Germany), and Philips Brilliance spiral CT
64-slice apparatus was purchased from Philips Medical Systems, Inc.
(Bothell, WA, USA).
Methods
Color Doppler ultrasound
examination
After filling of bladder, the probe scanned in
multiple directions above the lower abdominal pubic symphysis of
ovarian tumor patients under a supine position on the bed to
observe the internal echo in tumor in the uterus and bilateral
adnexa areas and whether it was accompanied by ascites, site of
lesion, tumor morphology and boundary, whether there was isolation
in the tumor and isolation thickness. Each tumor was scored based
on the Finkler ultrasound scoring criteria (12), in which the score <7 points
indicates a benign lesion and the score ≥7 points indicates a
malignant lesion. The ultrasound scoring system is shown in
Table I.
 | Table I.Ultrasound scoring system. |
Table I.
Ultrasound scoring system.
| Scoring criteria | Score |
|---|
| No echo in cysts with
clear border, fibroma, and nodular cysts, such as hydrosalpinx | 1 |
| No echo in cysts with
slightly irregular border, smooth cyst capsule with low echo | 2 |
| Low echo in cysts
with slightly irregular border and no nodules (endometrioid tumor),
and postmenopausal non-echo cysts | 3 |
| Iso-echoic and
non-specific ultrasound manifestations: solid ovarian enlargement,
small cysts with irregular border accompanied with internal echo
reflex (hematoma or benign ovarian tumor) | 4–6 |
| Consistent
manifestations with ovarian tumor: multiple isolated or irregular
cystic masses (7 points for a small number of nodules, and 8–9 for
a large number of nodules) | 7–9 |
| Above characteristics
accompanied with ascites | 10 |
RI record
Color Doppler ultrasound examination was performed
to observe the blood flow distribution around and in the tumor and
the morphological characteristics of vessels, and to measure the
blood flow RI around and in the tumor. RI≤0.45 was taken as a
diagnostic criterion for a malignant tumor, and RI>0.45
indicated a benign tumor. The blood flow signal characteristics
around and in the tumor can be divided into 3 types: type 1, there
is no blood flow signal around or in the tumor; type 2, there are
punctiform and short line-like blood flow signals around the tumor
or septum; and type 3, there are punctiform, strip, line or
branched blood flow signals around the tumor and in the parenchymal
area (13).
64-slice spiral CT examination
After fasting for >12 h and filling of bladder
until the need for micturition, scanning was performed for patients
under the supine position from the inferior margin of pubis to the
upper edge of mass. The whole abdominal scanning was performed for
patients with large mass or abdominal metastasis. All patients
received enhanced scanning, while some underwent delayed scanning,
and the tumor was preliminarily positioned and qualitatively
determined (14,15). In the image analysis, benign and
malignant ovarian tumors were identified, combined with AFP
examination, by two imaging physicians with >10 years
experience. Diagnostic criteria: positioning and qualitative
judgment of ovarian tumors. CT diagnosis of ovarian cancer requires
at least two doctors with the corresponding titles of radiology to
evaluate the CT image, including the location, shape, size, cystic
solidity, blood supply, degree of enhancement and peritoneal
implantation (16).
Statistical analysis
Statistical Product and Service Solutions (SPSS)
16.0 software (Shanghai Cabit Information Technology Co., Ltd.,
Shanghai, China) was used for the statistical analysis of the
results. Chi-square test was used for categorical data. The
receiver operating characteristics (ROC) curve analysis was used
for the diagnostic value of ultrasound score, color Doppler
ultrasound RI, spiral CT, and the combined application of the three
methods in ovarian tumors. ANOVA was used for the comparison
between multiple groups and LSD test was the post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
General data
The clinicopathological data of 224 patients with
ovarian cancer are shown in Table
II.
 | Table II.Clinicopathological data of patients
(%). |
Table II.
Clinicopathological data of patients
(%).
| Factor | n | Ratio |
|---|
| Age (years) |
|
|
| ≥53 | 118 | 52.68 |
|
<53 | 106 | 47.32 |
| Clinical stage |
|
|
|
T1+T2 | 68 | 65.38 |
|
T3+T4 | 36 | 34.62 |
| Depth of
infiltration |
|
|
| Muscular
layer | 57 | 54.81 |
|
Serosa | 47 | 45.19 |
| Degree of
differentiation |
|
|
| High
differentiation | 79 | 35.27 |
|
Moderate-low
differentiation | 145 | 64.73 |
| Lymph node
metastasis |
|
|
| Yes | 40 | 38.46 |
| No | 64 | 61.54 |
Ultrasound manifestations of ovarian
tumor in both groups
The cystic echo mostly appeared in benign ovarian
tumors, while the cystic-solid echo mostly appeared in malignant
ovarian tumors, displaying a statistically significant difference
(P<0.05). The vascular resistance of benign ovarian tumors was
larger than that of malignant tumors. It was easier to measure
blood flow signals in malignant ovarian tumors. The difference
between the two groups was statistically significant (P<0.001)
(Table III). Compared with cystic
echo and blood flow signals, the cystic echo, used to judge the
benign and malignant ovarian cancer tumors, had the highest
specificity and accuracy. Compared with cystic echo and cystic
mixed echo, blood flow signal had the highest sensitivity in the
assessment of the benign and malignant ovarian tumors (Table IV).
 | Table III.Ultrasound imaging of benign and
malignant ovarian tumors [n (%)]. |
Table III.
Ultrasound imaging of benign and
malignant ovarian tumors [n (%)].
| Item | Benign (n=120) | Malignant
(n=104) | χ2 | P-value |
|---|
| Ultrasound
characteristics |
|
| 29.680 | <0.001 |
| Cystic
echo | 67 (55.83) | 21 (20.19) | 29.670 | <0.001 |
|
Cystic-solid echo | 35 (29.17) | 54 (51.92) | 12.050 | <0.001 |
| Solid
echo | 18 (15.00) | 29 (27.88) | 5.579 | 0.018 |
| Blood flow
signal | 82 (68.33) | 91 (87.50) | 11.64 | <0.001 |
 | Table IV.Diagnostic value of different imaging
features in benign and malignant ovarian tumors. |
Table IV.
Diagnostic value of different imaging
features in benign and malignant ovarian tumors.
| Feature | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|
| Cystic echo | 55.83 | 79.81 | 66.96 |
| Cystic-solid
echo | 29.17 | 48.08 | 37.95 |
| Blood flow
signal | 68.33 | 12.50 | 42.41 |
Diagnostic evaluation of different
examination methods for ovarian tumors
The diagnostic results of the four methods in
ovarian tumors were compared. With the pathological results as the
gold standard, it was found that the sensitivity, specificity and
accuracy of the combined application of ultrasound scoring, RI and
64-slice spiral CT in the diagnosis of benign ovarian tumor were
higher than those of the single application. The diagnosis
coincidence rate of RI for ovarian malignant tumors was higher than
that of benign tumors (P<0.05). There was no significant
difference in the diagnosis coincidence rate of the other three
methods for benign and malignant ovarian tumors (P>0.05)
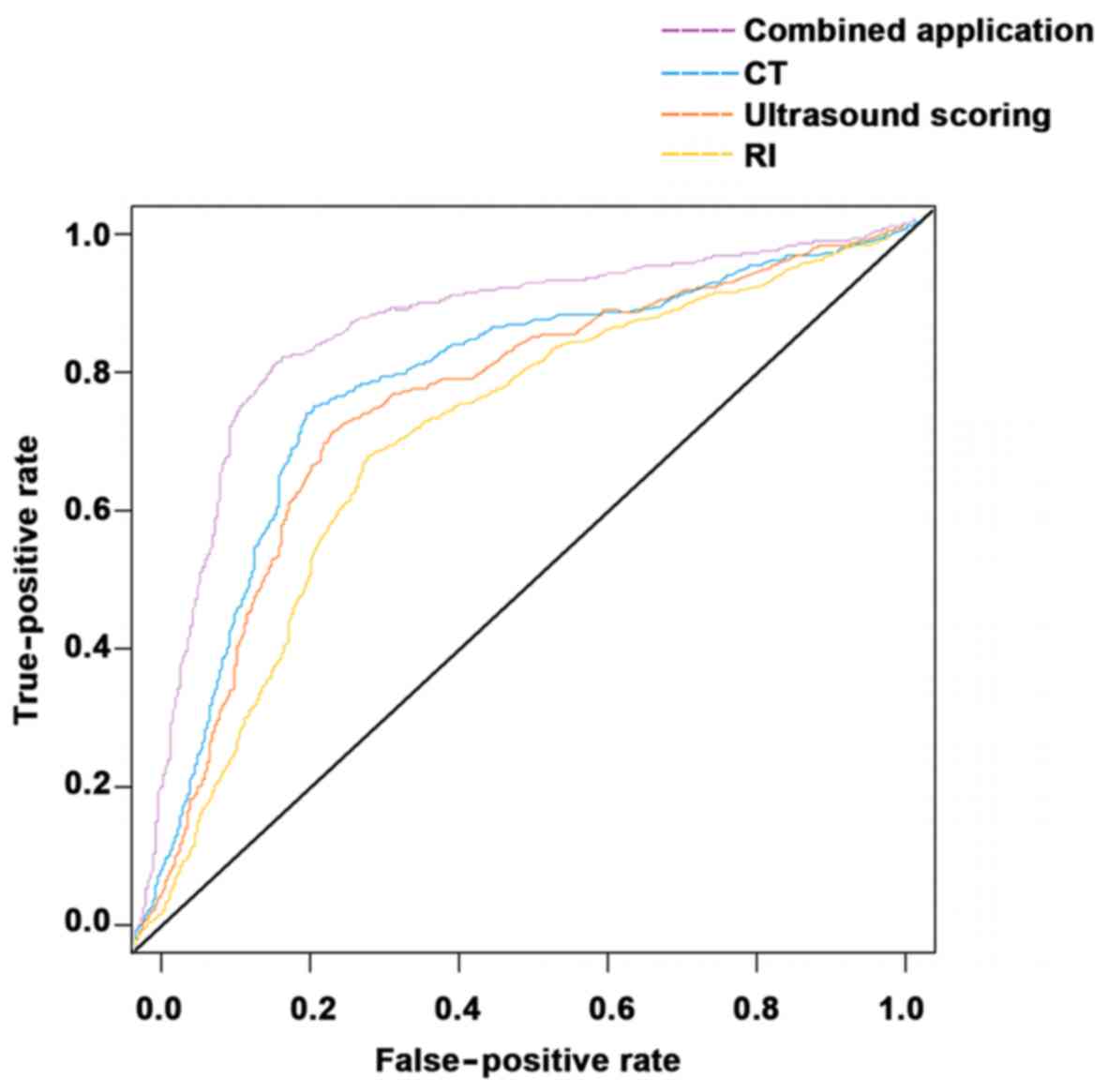
(Tables V–VII). The area under the ROC curve of the
RI was smaller than that of the ultrasound score, which was smaller
than that of the 64-slice spiral CT, and the area under the ROC
curve of the 64-slice spiral CT was smaller than that of the
combination of the three methods (Fig.
1).
 | Table V.Diagnostic results of each examination
method for ovarian tumors. |
Table V.
Diagnostic results of each examination
method for ovarian tumors.
| Pathological
result | Benign (n=120) | Malignant
(n=104) | Total |
|---|
| Ultrasound score |
|
|
|
|
Benign | 102 | 12 | 114 |
|
Malignant | 18 | 92 | 110 |
| RI |
|
|
|
|
Benign | 82 | 13 | 95 |
|
Malignant | 38 | 91 | 129 |
| 64-slice spiral
CT |
|
|
|
|
Benign | 106 | 13 | 119 |
|
Malignant | 14 | 91 | 105 |
| Combined
application |
|
|
|
|
Benign | 110 | 4 | 114 |
|
Malignant | 10 | 100 | 110 |
 | Table VII.Comparison of the diagnosis
coincidence rates of benign and malignant ovarian tumors for each
examination method. |
Table VII.
Comparison of the diagnosis
coincidence rates of benign and malignant ovarian tumors for each
examination method.
| Method | Benign tumor
(n=120) | Malignant tumor
(n=104) | χ2 | P-value |
|---|
| Ultrasound
score | 102 (85.00) | 92 (88.46) | 0.576 | 0.448 |
| RI | 82 (68.33) | 91 (87.5) | 11.640 | 0.001 |
| 64-slice spiral
CT | 106 (88.33) | 91 (87.5) | 0.036 | 0.849 |
| Combined
application | 110 (91.67) | 100 (96.15) | 1.915 | 0.167 |
Discussion
Patients with ovarian cancer mostly have no or mild
symptoms in the early stage, and metastasis has often occurred with
poor prognosis when treated. If patients can be diagnosed in the
early stage, the therapeutic effect on ovarian tumor will be
improved. Modern medical imaging techniques have provided
examination means for the diagnosis of ovarian tumor, which are
characterized by non-invasiveness and no pain and play important
roles in the diagnosis of gynecological tumors (17). Although a variety of imaging methods
have been widely used in the examination and diagnosis of ovarian
tumor, the imaging application in ovarian tumor is diversified and
variable due to the diverse sources of ovarian tumor cells and
various tissue types. None of the imaging methods is able to
diagnose accurately and qualitatively the ovarian mass in patients.
In particular, the surrounding organs are squeezed and displaced
when the ovarian tumor is >50 mm, so it is difficult to
determine the source of the tumor and easy to confuse it with
tumors from other sources (18).
In recent years, the measurement of RI has not
attracted attention, and the combined application of ultrasound
scoring, color Doppler ultrasound RI and spiral CT in ovarian
tumors is less reported. CT diagnosis of ovarian tumors has been
extensively reported worldwide, but not the application of 64-slice
spiral CT (11). Therefore, in this
study, the ultrasound score, RI and CT signs were compared with
pathological diagnosis results, the differences in sensitivity and
specificity were analyzed, and the value of each method in the
differential diagnosis of ovarian tumor was evaluated, so as to
provide insights for the selection and development of a clinical
therapeutic regimen.
At present, ultrasound examination is the most
common and convenient method in the clinical diagnosis of ovarian
tumors. Observing the morphology and echo of an ovarian tumor and
whether ascites are presented in ultrasound images can
preliminarily determine the benign and malignant tumors according
to the ultrasound scoring criteria. In this study, the ultrasound
manifestation was mainly cystic echo in the benign tumor and
cystic-solid echo in the malignant tumor. In some malignant tumors,
the early morphology is regular and there is a lack of typical
changes in the ultrasonogram, so it is difficult to accurately
determine the benign and malignant ovarian tumors in the
ultrasonogram of complex or early-stage malignant tumors only by
using ultrasound examination. The sensitivity, specificity and
accuracy of Finkler ultrasound scoring in the analysis of ovarian
tumor were 89.47, 83.64 and 86.61%, respectively. Alanbay et
al (19) obtained research
results that are basically consistent with ours. Fleischer and
Brader (20) have shown that the
pathological parameters of patients can be obtained through
observing the characteristics of vascular distribution in tumor
tissues via ultrasound examination. In the present study, blood
flow signals could be detected around the benign tumor in 82 cases,
in which the blood vessels were sparse with the type 1
characteristics of blood flow. Besides, blood flow signals could be
detected in malignant ovarian tumors in 91 cases, in which there
were abundant blood vessels and complex branches mainly distributed
in and around the tumor with the type 3 characteristics of blood
flow. The sensitivity, specificity and accuracy were 86.32, 70.54
and 77.23%, respectively. The RI value of malignant ovarian tumor
was lower than that of benign ovarian tumor, and the difference was
statistically significant (P<0.001). According to the study of
Kurjak et al (21), RI≤0.4 is
the diagnostic criterion for malignant ovarian tumor. Waltmire
et al (22) considered that
RI<0.5 can be taken as the criterion for positive prediction.
The above conclusion is different from the results in this study,
indicating that RI has no unified criteria for benign and malignant
tumors, and the possible reason is that there are a variety of
ovarian tumors with complex tissue components. Therefore, there is
a partial overlap of RI value in benign and malignant ovarian
tumors, and blood flow signals cannot be detected. In this study,
120 patients were pathologically diagnosed with benign ovarian
tumor and 104 patients were diagnosed with malignant ovarian tumor.
The sensitivity, specificity and accuracy of 64-slice spiral CT in
the detection of ovarian tumor were 89.08, 86.67 and 87.95%,
respectively, which are consistent with the results of Taïeb et
al (23) on the multi-slice CT
in the diagnosis of ovarian tumor (24,25). The
sensitivity, specificity and accuracy of the combined application
were 96.49, 90.91 and 93.75%, respectively. The sensitivity,
specificity and accuracy of the combined application of ultrasound
scoring, RI and 64-slice spiral CT in the diagnosis of benign
ovarian tumor were higher than those of the single application.
However, there were also some limitations in this
study. Comparisons were not performed between 64-slice spiral CT
and conventional CT, and between multi-planar reconstruction and
curved planar reconstruction. Therefore, these issues will be
further explored in a future study.
In conclusion, the cystic echo mostly appeared in
benign ovarian tumor, while the cystic-solid echo mostly appeared
in malignant ovarian tumor. The RI of benign ovarian tumor was
higher than that of malignant ovarian tumor (P<0.001), so blood
flow signals could be detected more easily in malignant ovarian
tumor. The sensitivity and specificity in the diagnosis of ovarian
tumor can be increased in the combined application of Finkler
ultrasound scoring, RI value and 64-slice spiral CT scan, which can
provide an effective basis for the early therapeutic regimen of
ovarian tumor, thus improving the survival rate of patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ designed the study and drafted the manuscript. LZ
and ZX collected and analyzed the general data. LZ and YW
interpreted the results of the examinations. All the authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Cangzhou Central Hospital (Cangzhou, China). Patients who
participated in this research had complete clinical data. Signed
informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Curtin JP: Management of the adnexal mass.
Gynecol Oncol. 55:S42–S46. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Munkarah A, Chatterjee M and Tainsky MA:
Update on ovarian cancer screening. Curr Opin Obstet Gynecol.
19:22–26. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gansler T, Ganz PA, Grant M, Greene FL,
Johnstone P, Mahoney M, Newman LA, Oh WK, Thomas CR Jr, Thun MJ, et
al: Sixty years of CA. CA Cancer J Clin. 60:345–350. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Timmerman D, Testa AC, Bourne T, Ameye L,
Jurkovic D, VanHolsbeke C, Paladini D, Van Calster B, Vergote I,
Van Huffel S, et al: Simple ultrasound-based rules for the
diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 31:681–690.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hale SA, Schonberg A, Badger GJ and
Bernstein IM: Relationship between prepregnancy and early pregnancy
uterine blood flow and resistance index. Reprod Sci. 16:1091–1096.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hetland TE, Kærn J, Skrede M, Sandstad B,
Tropé C, Davidson B and Flørenes VA: Predicting platinum resistance
in primary advanced ovarian cancer patients with an in vitro
resistance index. Cancer Chemother Pharmacol. 69:1307–1314. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang X, Jacobs R, Hassan B, Li L, Pauwels
R, Corpas L, Souza PC, Martens W, Shahbazian M, Alonso A, et al: A
comparative evaluation of cone beam computed tomography (CBCT) and
multi-slice CT (MSCT) Part I. On subjective image quality. Eur J
Radiol. 75:265–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang G, Bousse A, Toumoulin C and Shu H: A
multiscale tracking algorithm for the coronary extraction in MSCT
angiography. Conf Proc IEEE Eng Med Biol Soc. 1:3066–3069. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Becker N, Motsch E, Gross ML, Eigentopf A,
Heussel CP, Dienemann H, Schnabel PA, Eichinger M, Optazaite DE,
Puderbach M, et al: Randomized study on early detection of lung
cancer with MSCT in Germany: Results of the first 3 years of
follow-up after randomization. J Thorac Oncol. 10:890–896. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deligeoroglou E, Eleftheriades M, Shiadoes
V, Botsis D, Hasiakos D, Kontoravdis A and Creatsas G: Ovarian
masses during adolescence: clinical, ultrasonographic and
pathologic findings, serum tumor markers and endocrinological
profile. Gynecol Endocrinol. 19:1–8. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brodoefel H, Klumpp B, Reimann A, Fenchel
M, Heuschmid M, Miller S, Schroeder S, Claussen C, Scheule AM and
Kopp AF: Sixty-four-MSCT in the characterization of porcine acute
and subacute myocardial infarction: Determination of transmurality
in comparison to magnetic resonance imaging and histopathology. Eur
J Radiol. 62:235–246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sankaranarayanan R and Ferlay J: Worldwide
burden of gynaecological cancer: The size of the problem. Best
Pract Res Clin Obstet Gynaecol. 20:207–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurjak A, Kupesic S, Anic T and Kosuta D:
Three-dimensional ultrasound and power Doppler improve the
diagnosis of ovarian lesions. Gynecol Oncol. 76:28–32. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nolz R, Wibmer A, Beitzke D, Gentzsch S,
Willfort-Ehringer A, Lammer J, Thurnher M and Schoder M: Carotid
artery stenting and follow-up: Value of 64-MSCT angiography as
complementary imaging method to color-coded duplex sonography. Eur
J Radiol. 81:89–94. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brodoefel H, Klumpp B, Reimann A, Ohmer M,
Fenchel M, Schroeder S, Miller S, Claussen C, Kopp AF and Scheule
AM: Late myocardial enhancement assessed by 64-MSCT in reperfused
porcine myocardial infarction: Diagnostic accuracy of low-dose CT
protocols in comparison with magnetic resonance imaging. Eur
Radiol. 17:475–483. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khiewvan B, Torigian DA, Emamzadehfard S,
Paydary K, Salavati A, Houshmand S, Werner TJ and Alavi A: An
update on the role of PET/CT and PET/MRI in ovarian cancer. Eur J
Nucl Med Mol Imaging. 44:1079–1091. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahamad A and Jhingran A: New radiation
techniques in gynecological cancer. Int J Gynecol Cancer.
14:569–579. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McLaughlin JR, Risch HA, Lubinski J,
Moller P, Ghadirian P, Lynch H, Karlan B, Fishman D, Rosen B,
Neuhausen SL, et al Hereditary Ovarian Cancer Clinical Study Group,
: Reproductive risk factors for ovarian cancer in carriers of BRCA1
or BRCA2 mutations: A case-control study. Lancet Oncol. 8:26–34.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alanbay I, Akturk E, Coksuer H, Ercan M,
Karaşahin E, Dede M, Yenen MC, Ozan H and Baser I: Comparison of
risk of malignancy index (RMI), CA125, CA 19-9, ultrasound score,
and menopausal status in borderline ovarian tumor. Gynecol
Endocrinol. 28:478–482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fleischer AC and Brader KR: Sonographic
depiction of ovarian vascularity and flow: Current improvements and
future applications. J Ultrasound Med. 20:241–250. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kurjak A, Kupesic S, Sparac V, Prka M and
Bekavac I: The detection of stage I ovarian cancer by
three-dimensional sonography and power Doppler. Gynecol Oncol.
90:258–264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waltmire CN, Alberts DS and Dorr RT:
Sequence-dependent cytotoxicity of combination chemotherapy using
paclitaxel, carboplatin and bleomycin in human lung and ovarian
cancer. Anticancer Drugs. 12:595–602. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taïeb S, Bonodeau F, Leblanc é, Vennin P,
Fournier C and Besson P: Predictive value of preoperative
abdominopelvic CT for optimal cytoreduction surgery in ovarian
carcinoma. Bull Cancer. 87:265–272. 2000.(In French). PubMed/NCBI
|
|
24
|
He B, Gong S, Hu C, Fan J, Qian J, Huang
S, Cui L and Ji Y: Obscure gastrointestinal bleeding: Diagnostic
performance of 64-section multiphase CT enterography and CT
angiography compared with capsule endoscopy. Br J Radiol.
87:201402292014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mathewson JW: Three dimensional imaging
using 64 detector row multi-slice CT should be used more widely for
the diagnosis and management of congenital heart disease. J Saudi
Heart Assoc. 22:179–185. 2010. View Article : Google Scholar : PubMed/NCBI
|