Introduction
Prostate cancer (PCa) is the second most common
cancer type in men worldwide (1). In
Japan, the number of patients diagnosed with PCa has increased
since the use of prostate-specific antigen screening (2). In 2017, the number of individuals
diagnosed with PCa was 86,100, which ranked as the third most
common type of disease in Japanese men. Radiotherapy is effective
for treating localized PCa; however, 20–40% of patients with
high-risk PCa experience tumor recurrence or distant metastases
(3–6).
One of the causes of tumor recurrence and metastasis
is that cancer cells acquire radioresistance (RR) during
fractionated irradiation (7). It has
been reported that RR is acquired in various cancer types,
including head and neck cancer, non-small cell lung cancer, breast
cancer and PCa (8–11). RR cells demonstrate resistance to the
induction of apoptosis, decreased production of reactive oxygen
species (ROS) (12), increased
activation of DNA repair (13), and
exhibit high migratory and invasive abilities (14). RR PCa cells exhibit a number of
features, including an increase in the number of cancer stem cells
(CSCs) (15–17), increased neuroendocrine
differentiation (NED) (18–20) and increased epithelial-mesenchymal
transition (15,16).
The prostatic epithelium is composed of basal cells,
luminal cells and neuroendocrine (NE) cells, the majority of which
are secretory cells. Although the physiological role of NE cells is
unknown (21), NED has been reported
to be caused by androgen deprivation or radiotherapy, which results
in the emergence of NE-like cells (19,22).
NE-like cells do not proliferate and this dormant phenotype renders
them resistant to the cancer treatment (19,20).
However, CSCs cause tumor recurrence and metastasis following
treatment due to their ability to undergo self-renewal and their
differentiation into various cell types to form the tumor bulk
(23–27). PCa cells expressing surface antigens,
including cluster of differentiation (CD)44 (also termed Hermes)
(23,24), CD133 (also termed prominin-1)
(16) and CD138 (also termed
syndecan-1) (26), exhibit CSC-like
properties. Therefore, these surface antigens are used as CSC
markers. Furthermore, it has been reported that
pluripotency-associated genes serve important roles in maintaining
CSC characteristics (28–30) and in regulating NED (31).
Cancer cells acquire RR by fractionated irradiation,
and certain features of RR cells have been described (8–17).
However, to the best of our knowledge, the mechanism underlying RR
acquisition during fractionated irradiation is unclear. An improved
understanding of the mechanism of RR acquisition is required to
facilitate the development of novel and effective treatment
strategies. The present study aimed to identify the factors
associated with the acquisition of RR during fractionated
irradiation and investigate the acquisition mechanism.
Materials and methods
Cell lines and reagents
The human PCa cell lines, PC3 (bone metastatic cell
line), DU145 (brain metastatic cell line) and LNCaP (lymph node
metastatic cell line), were purchased from RIKEN BioResource Center
(Tsukuba, Japan). The cells were cultured at 37°C in a 5%
CO2 environment in RPMI-1640 medium (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Japan Bioserum Co. Ltd.
Hiroshima, Japan) and 1% penicillin/streptomycin (Wako Pure
Chemical Industries, Ltd., Osaka, Japan). Phycoerythrin
(PE)-conjugated monoclonal mouse anti-human CD44 (catalog no.
338808), PE mouse IgG1, κ isotype control (catalog no. 400114),
peridinin chlorophyll protein complex/cyanine5.5
(PerCP/Cy5.5)-conjugated monoclonal mouse anti-human CD138 (catalog
no. 356510) and PerCP/Cy5.5 mouse IgG1, κ isotype control (catalog
no. 400150) were purchased from BioLegend, Inc. (Tokyo, Japan).
Allophycocyanin (APC)-conjugated monoclonal mouse anti-human
CD133/1 (catalog no. 130-090-826) and APC mouse IgG1, κ isotype
control antibody (catalog no. 130-092-214) were purchased from
Miltenyi Biotec GmbH (Bergisch Gladbach, Germany). TB Green Premix
Ex Taq and ROX Reference Dye (catalog no. RR820A) were purchased
from Takara Bio Inc. (Otsu, Japan).
X-ray irradiation of PCa cell
lines
DU145, PC3 and LNCaP cells were irradiated with
X-ray (150 kV, 20 mA, 1.0 Gy/min) through a 0.5-mm aluminum and
0.3-mm copper filter using an X-ray generator (MBR-1520R-3; Hitachi
Ltd., Tokyo, Japan), with a distance of 45 cm between the focus and
target. The dose was monitored with a thimble ionization chamber
placed next to the sample during irradiation. Cells were subjected
to fractionated irradiation according to the following schedule:
Irradiation (IR)1, 2 Gy/day with a total dose of 20 Gy; IR2, 4
Gy/day with a total dose of 20 Gy; and IR3, 4 Gy/day with a total
dose of 56 Gy.
Colony formation assay
The clonogenic potency was estimated by a colony
formation assay. DU145, PC3 and LNCaP cells were seeded in
appropriate numbers as presented in Table I, subjected to X-ray irradiation,
fixed with methanol (Wako Pure Chemical Industries, Ltd.) for 1 min
at room temperature, 10–20 days after irradiation and stained with
Giemsa for 2 h at room temperature (Wako Pure Chemical Industries,
Ltd.). Colonies consisting of >50 cells were counted. The
survival fraction for each cell line was calculated as the plating
efficiency of the irradiated samples compared with that of the
non-irradiated samples.
 | Table I.Cell number seeded for the colony
formation assay. |
Table I.
Cell number seeded for the colony
formation assay.
|
| Cell line |
|---|
|
|
|
|---|
| Absorbed dose
[Gy] | PC3-P | PC3-IR1 | PC3-IR2 | DU145-P | DU145-IR1 | DU145-IR2 | DU145-IR3 | LNCaP-P | LNCaP-IR1 |
|---|
| 0 |
200 |
100 |
100 |
200 |
200 |
200 |
100 |
200 |
200 |
| 1 |
|
300 |
|
|
300 |
|
|
300 |
800 |
| 2 |
500 |
500 |
200 |
500 |
500 |
500 |
200 |
500 |
500 |
| 3 |
800 |
|
|
|
|
|
|
|
|
| 4 |
1,000 |
1,000 |
1,000 |
1,000 |
1,000 |
1,000 |
1,000 |
1,000 |
2,000 |
| 5 |
2,000 |
|
|
|
|
|
|
| 10,000 |
| 6 |
3,000 |
2,000 |
5,000 |
3,000 |
2,000 |
3,000 |
3,000 |
5,000 | 20,000 |
| 8 |
5,000 |
3,000 | 30,000 |
5,000 |
3,000 | 10,000 | 10,000 | 20,000 | 20,000 |
| 10 |
8,000 |
5,000 | 60,000 | 20,000 |
5,000 | 20,000 | 20,000 |
| 40,000 |
Flow cytometric analysis
To analyze the expression of the CSC markers, the
cells were incubated in 100 µl PBS without calcium chloride and
magnesium chloride [PBS(−); Takara Bio Inc.] containing 5% FBS used
as blocking agent and PE anti-human CD44 (3 µl/106
cells), CD133/1-APC (3 µl:106 cells), PerCP/Cy5.5
anti-human CD138 (3 µl/106 cells) or respective mouse
IgG1 isotype control antibodies (3 µl/106 cells) for 15
min at 4°C in the dark. Following staining, the cells were
collected by centrifugation at 300 × g for 5 min at 4°C,
resuspended in PBS and analyzed by direct immunofluorescence flow
cytometry using a BD FACS Aria™ Cell Sorter (BD Biosciences, San
Jose, CA, USA). The fluorescence values of the respective isotype
controls were subtracted from the fluorescence data of CD44, CD133
and CD138. The results were analyzed using the Kaluza software
(version 1.5a; Beckman Coulter, Inc., Brea, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from DU145, PC3 and LNCaP
cells using the RNeasy® Mini kit (Qiagen GmbH, Hilden,
Germany) and diluted to 100 ng/µl in nuclease-free water.
Complementary DNA (cDNA) was synthesized from the isolated total
RNA using a High-Capacity cDNA Reverse Transcriptase kit (Thermo
Fisher Scientific Inc.). RT-qPCR was performed in a 20-µl reaction
mixture containing 1X TB Green Premix Ex Taq II, primer pairs
(sequences summarized in Table II)
at a concentration of 0.5 µM and the cDNA template (100 ng/µl total
RNA for cDNA synthesis). GAPDH mRNA was used as an endogenous
control. The conditions for the Real-Time qPCR system (StepOne
Plus; Thermo Fisher Scientific, Inc.) were set at 95°C for 30 sec,
followed by 40 cycles of incubation at 95°C for 5 sec and 54°C for
30 sec. The results were analyzed using the 2−ΔΔCq
method (32).
 | Table II.Primer sequences of the target
genes. |
Table II.
Primer sequences of the target
genes.
| Primer | Sequence
(5′-3′) |
|---|
| NSE |
|
|
Forward |
AGCTGCCCCTGCCTTAC |
|
Reverse |
GAGACAAACAGCGTTACTTAG |
| CgA |
|
|
Forward |
GCGGTGGAAGAGCCATCAT |
|
Reverse |
TCTGTGGCTTCACCACTTTTCTC |
| SOX2 |
|
|
Forward |
ATGCACAACTCGGAGATCAGC |
|
Reverse |
CCTTCTTCATGAGCGTCTTGG |
| c-Myc |
|
|
Forward |
GCCACGTCTCCACACATCAG |
|
Reverse |
TCTTGGCAGCAGGATAGTCCTT |
| NANOG |
|
|
Forward |
TAGCAATGGTGTGACGCAGAAG |
|
Reverse |
TCTGGTTGCTCCACATTGGAAGG |
| OCT4 |
|
|
Forward |
GAGGCAACCTGGAGAATTTGTTCC |
|
Reverse |
ATGTGGCTGATCTGCTGCAGTG |
| KLF4 |
|
|
Forward |
GCGAGTCTGACATGGCTGT |
|
Reverse |
GTCGCTTCATGTGGGAGAG |
| GAPDH |
|
|
Forward |
GTGAAGGTCGGAGTCAACG |
|
Reverse |
TGAGGTCAATGAAGGGGTC |
Statistical analysis
Statistically significant differences between the
control group and the experimental group were determined using
Student's t-test or Welch's t-test for comparisons between two
groups. Multiple comparisons were performed with one-way analysis
of variance followed by the Tukey-Kramer method or Scheffe's F
test. Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA,
USA) with the add-in software Statcel (version 3; OMS Publishing,
Inc., Saitama, Japan) was used to perform the statistical analyses.
Data are presented as the mean ± standard deviation from three
independent experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
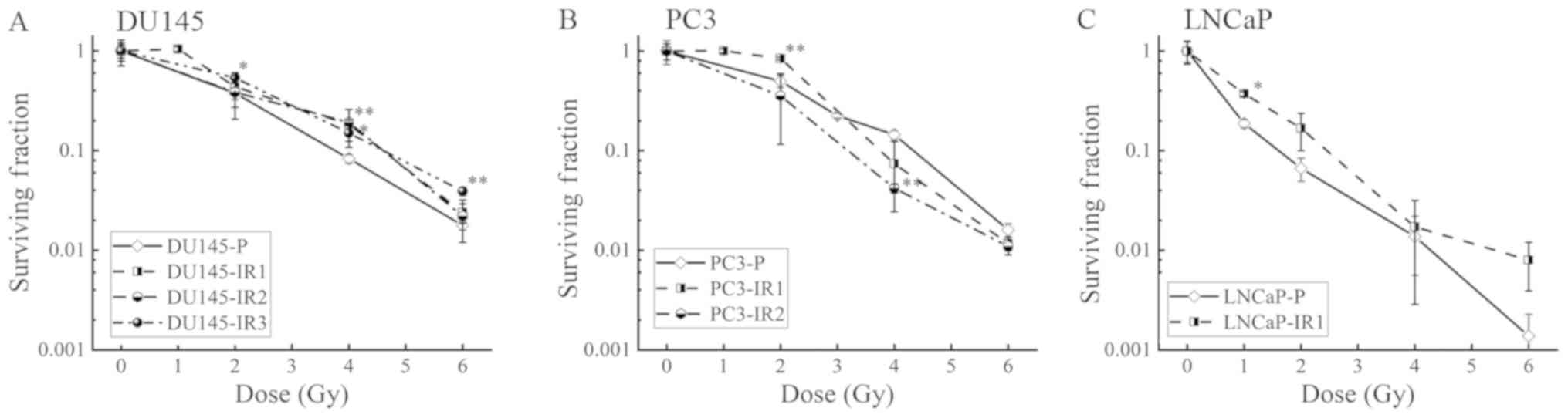
Establishment of RR cells
Fractionated irradiation was performed according to
a previously reported schedule to establish the RR cells. DU145-IR3
cells were obtained by subjecting DU145 cells to 4 Gy/day with a
total irradiation dose of 56 Gy. Since PC3 and LNCaP cells failed
to proliferate following irradiation treatment, IR3 in PC3 cells,
and IR2 and IR3 in LNCaP, these RR cells were not established.
Therefore, the changes in viability of DU145-IR3 cells compared
with DU145-parental (P) cells were analyzed by colony forming
assay. DU145-IR3 cells acquired significant RR between 2–6 Gy
compared with DU145-P cells (Fig.
1A). Since PC3 and LNCaP cells did not proliferate following
irradiation with 56 Gy, these cells were only subjected to
fractionated irradiation with a total dose of 20 Gy. To investigate
whether there was a change in the cell characteristics between 10
fractions and 5 fractions, 2 Gy for 10 fractions (IR1) and 4 Gy for
5 fractions (IR2) were used, respectively. In the DU145 and PC3
cells, IR1 and IR2 cells were established; however, in LNCaP cells
only IR1 cells were established. In the PC3-IR1 and LNCaP-IR1
cells, the cell viability was increased following exposure to 1–2
Gy radiation compared with that in the PC3-P and LNCaP-P cells,
respectively; however, no change was observed in the high dose
range. Additionally, in DU145-IR2 and PC3-IR2 cells, no significant
increase in cell viability was identified compared with that in the
DU145-P and PC3-P cells, respectively (Fig. 1A-C).
Expression of CSC markers following
fractionated irradiation
It has been reported that the expression of CSC
markers increases upon X-ray irradiation and that the markers are
highly expressed in RR cells compared with P cells (16). Therefore, the present study performed
flow cytometry analysis to evaluate the variations in the
expression of CD44, CD133 and CD138 in PC3, DU145, and LNCaP cell
lines following exposure to fractionated irradiation (IR1, 2 Gy/day
with a total dose of 20 Gy). Since the expression of CD133 in PC3
cells, and of CD44 in LNCaP cells were not detectable, the
expression profiles of CD44 and CD138 in PC3 cells, CD44, CD133 and
CD138 in DU145 cells, and CD133 and CD138 markers in LNCaP cells
were confirmed (Fig. 2A and B). The
variations in the ratio of CD44, CD133 and CD138 markers were
analyzed during fractionated irradiation. In addition, the
percentages of CD44+/CD138+ PC3 cells,
CD44+/CD133+/CD138+ DU145 cells
and CD133+/CD138+ LNCaP cells were analyzed
(Fig. 2C-E). CD44 was highly
expressed in PC3-P cells (51.99±12.45%) and DU145-P cells
(74.84±0.79%), and the expression was significantly increased in
IR1 cells (PC3-IR1, 79.32±1.44%; DU145-IR1, 83.71±5.18%) compared
with that in P cells (Fig. 2C and
D). CD133 was increased by 2 Gy × 1 fraction in the DU145 and
LNCaP cells (1.73±1.07 and 5.99±0.39%, respectively) compared with
that in the P cells (0.43±0.44 and 0.120±0.19%, respectively). In
LNCaP cells, CD133 expression decreased by 2 Gy × 2–8 fractions,
but increased again following 2 Gy × 9 and 10 fractions (0.27±0.24
and 0.82±1.05%, respectively; Fig.
2E). The expression of CD138 could not be confirmed in PC3-P
cells, but was increased by 2 Gy × 4 fractions (3.51±0.59%) and
decreased by subsequent fractions. In DU145 cells, CD138 was highly
expressed in P cells (5.15±1.30%), but decreased following
fraction. In LNCaP cells, the expression of CD138 increased by 2 Gy
× 1 fraction compared with that in P cells (15.93±1.72 vs.
12.16±3.05%), decreased with subsequent fraction and began to
increase with 2 Gy × 9 and 10 fractions (1.10±0.47 and 2.99±3.05%,
respectively; Fig. 2E). Expression
of CD44/CD138 in PC3-P cells was increased by 2 Gy × 1 fraction
(0.27±0.34%) and was enhanced the most by 2 Gy × 4 fractions
(3.23±0.51%). In DU145 cells, no marked variation in the frequency
of CD44+/CD133+/CD138+ cells was
identified following fractionated irradiation. In LNCaP cells, the
expression of CD133/CD138 was significantly increased by 2 Gy × 1
fraction (4.62±0.40%) compared with that in P cells (0.68±0.31%),
and the expression was decreased by subsequent fraction. Therefore,
it is clear that the proportion of CD44+ cells increases
following fractionated irradiation. The fractions of
CD133+ and CD138+ cells increased by 2 Gy × 1
fraction or subsequent irradiations; however, their expression was
decreased by subsequent irradiation.
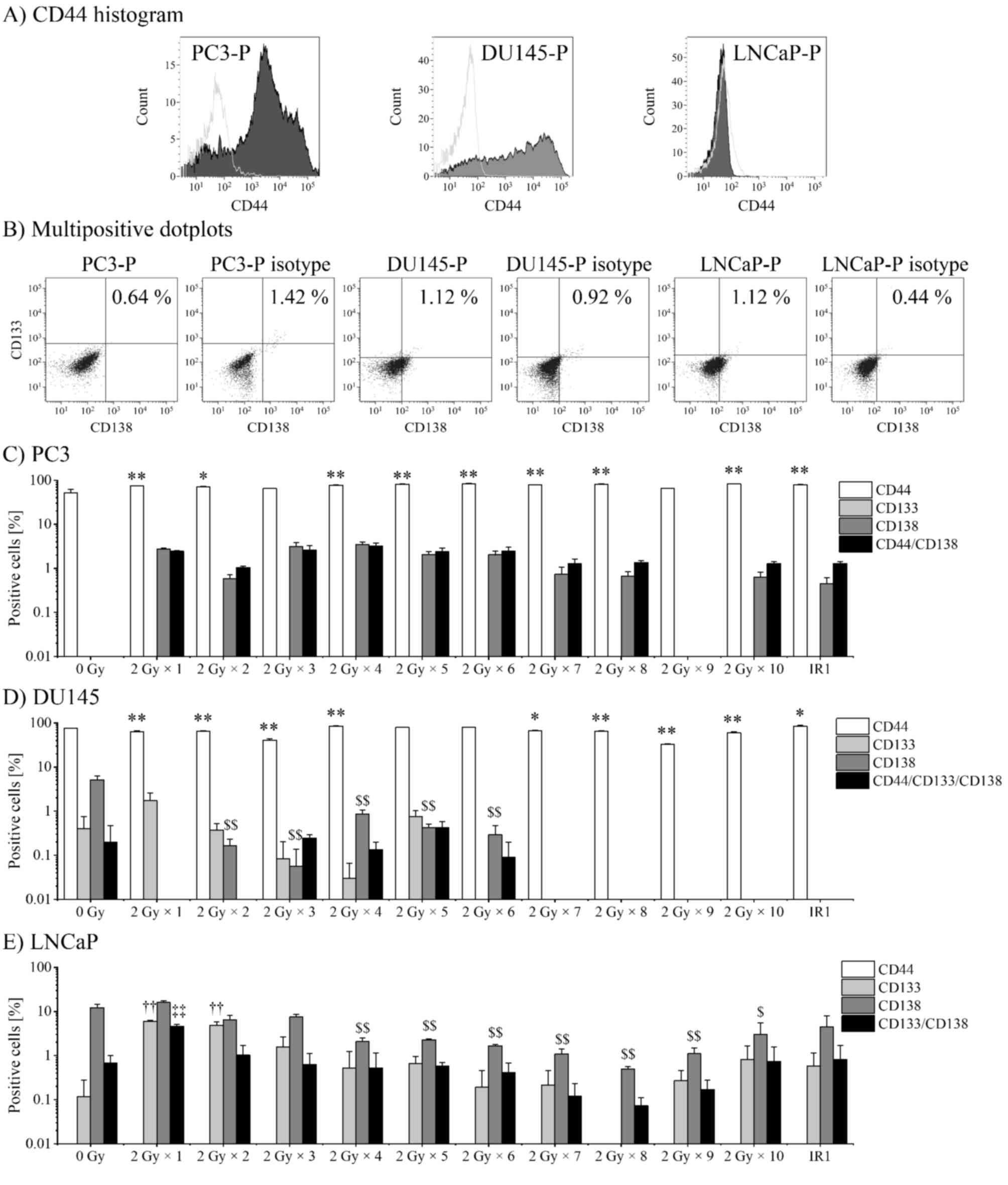 | Figure 2.Expression of cancer stem cell
markers following fractionated irradiation. The fraction of cells
expressing CD44, CD133 and CD138 markers was analyzed by flow
cytometry. Representative histograms and dot plots of PC3-P,
DU145-P and LNCaP-P cells are presented to illustrate the
identification of multipositive cells. (A) The CD44+
cell population (grey area) was gated and the fluorescence value of
the isotype control population (empty area) was subtracted. (B)
CD133+ and CD138+ cells in the gated
CD44+ population of PC3-P cells and DU145-P cells, and
the ungated LNCaP-P cells. CD133+ and CD138+
cells were subtracted from their respective isotype controls. The
percentage of CD44+, CD133+,
CD138+ and multipositive cells of (C) PC3 cells, (D)
DU145 cells and (E) LNCaP cells. The ratio of
CD44+/CD138+ cells in the PC3 cell
population, the ratio of
CD44+/CD133+/CD138+ cells in DU145
cell population and the ratio of
CD133+/CD138+ cells in the LNCaP cell
population have been depicted for the multipositive cells. P-values
were calculated by the Tukey-Kramer method following one-way
analysis of variance. *P<0.05, **P<0.01 vs. CD44+
population of P cells; ††P<0.01 vs. CD133+
population of LNCaP-P cells; $P<0.05,
$$P<0.01 vs. CD138+ population of P cells;
and ‡‡P<0.01 vs. CD133+/CD138+
population of LNCaP-P cells. IR1, 2 Gy/day (total 20 Gy); P,
parental; CD, cluster of differentiation. |
Analysis of CSC markers, NED markers
and pluripotency- associated genes in RR cells
To investigate how the levels of CSC markers, NED
markers and pluripotency-associated genes change during RR
acquisition by fractionated irradiation, these factors were
analyzed in P cells, cells that did not acquire RR by fractionated
irradiation and RR cells. PC3 cells and LNCaP cells did not exhibit
RR with 4 Gy. Since DU145 cells did acquire RR with 2–6 Gy, CSC
markers, NED markers and pluripotency-associated genes were
examined in DU145-P, DU145-IR1, DU145-IR2 and DU145-IR3 cells.
Representative histograms and dot plots are
presented in Fig. 3A and B.
Expression of the CD44 marker was significantly increased in
DU145-IR1 cells (Fig. 3C) compared
with that in DU145-P cells; however, the expression of CD133 and
CD138 markers was not identified in DU145-IR1 cells. In DU145-IR2
and DU145-IR3, the proportion of CD44+ cells increased
compared with that observed for DU145-P and DU145-IR1 cells
(DU145-IR2, 91.27±0.79%; DU145-IR3, 90.75±0.58%). In addition, the
proportion of CD133+ (DU145-IR2, 0.23±0.27%; DU145-IR3,
0.75±0.28%) and CD138+ cells (DU145-IR2, 2.70±0.53%;
DU145-IR3, 1.93±0.20%) was significantly higher for DU145-IR2 and
DU145-IR3 cells compared with that for DU145-P cells (Fig. 3D and E). Furthermore, the proportion
of CD44+/CD133+/CD138+ cells was
low for the P cells (0.22±0.24%) and was not confirmed in IR1
cells, and was significantly increased in the IR2 (0.31±0.10%) and
IR3 cells (0.51±0.13%) compared with that in the IR1 cells
(Fig. 3F).
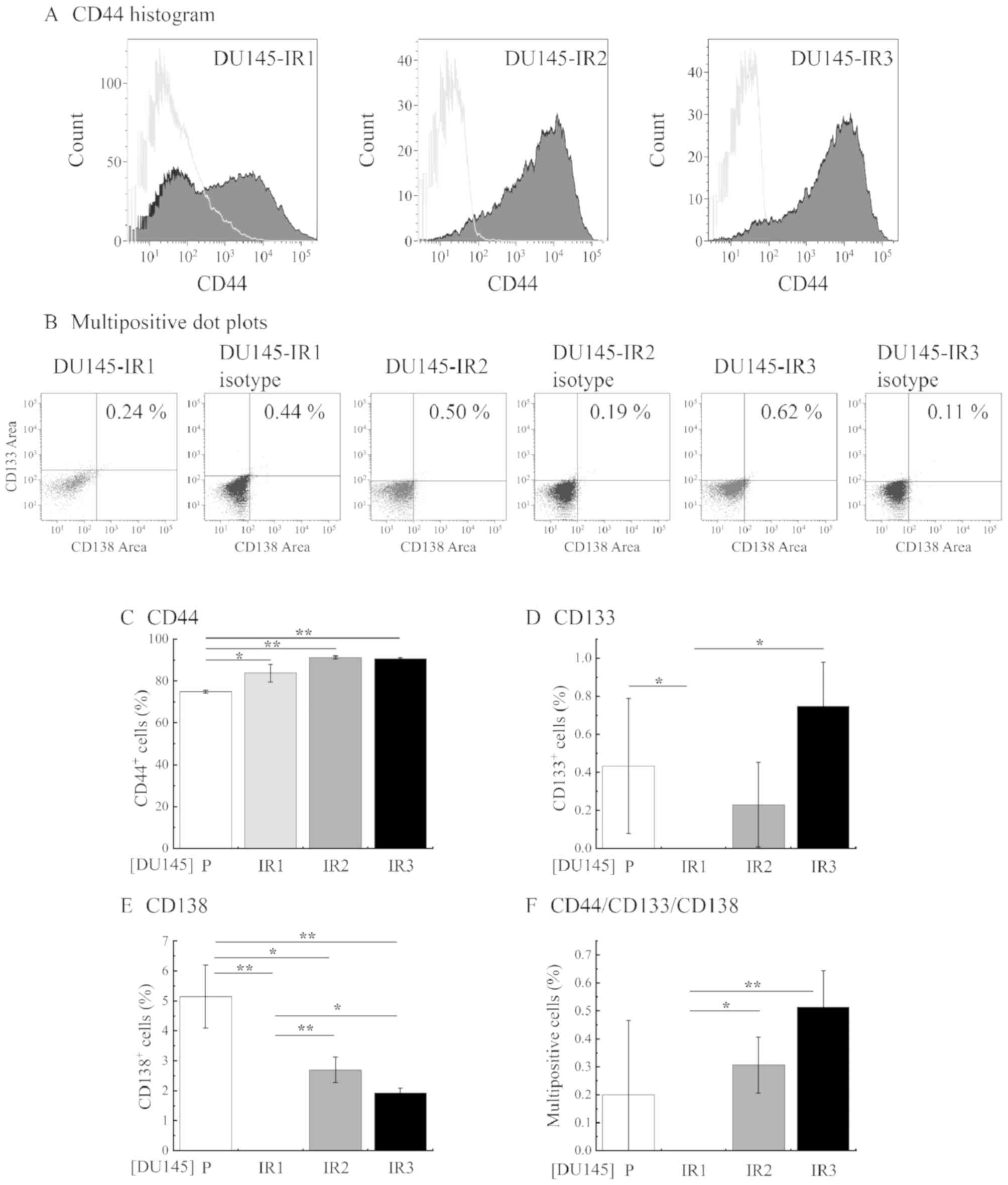 | Figure 3.Changes in the ratio of CSC markers
in cells subjected to fractionated irradiation. The ratio of cells
expressing CD44, CD133 and CD138 was analyzed by flow cytometry.
(A) The CD44+ cell population (grey area) was gated and
the fluorescence value of the isotype control population (empty
area) was subtracted. (B) CD133+ and CD138+
cells in the gated CD44+ population of DU145-P, -IR1,
-IR2, and -IR3 cells. CD133+ and CD138+ cells
were subtracted from their respective isotype controls. The ratio
of (C) CD44+, (D) CD133+, (E)
CD138+ and (F)
CD44+/CD133+/CD138+ cells.
P-values were calculated by the Tukey-Kramer method following
one-way analysis of variance. *P<0.05 and **P<0.01. IR1, 2
Gy/day (total 20 Gy); IR2, 4 Gy/day (total 20 Gy); IR3, 4 Gy/day
(total 56 Gy); P, parental; CD, cluster of differentiation. |
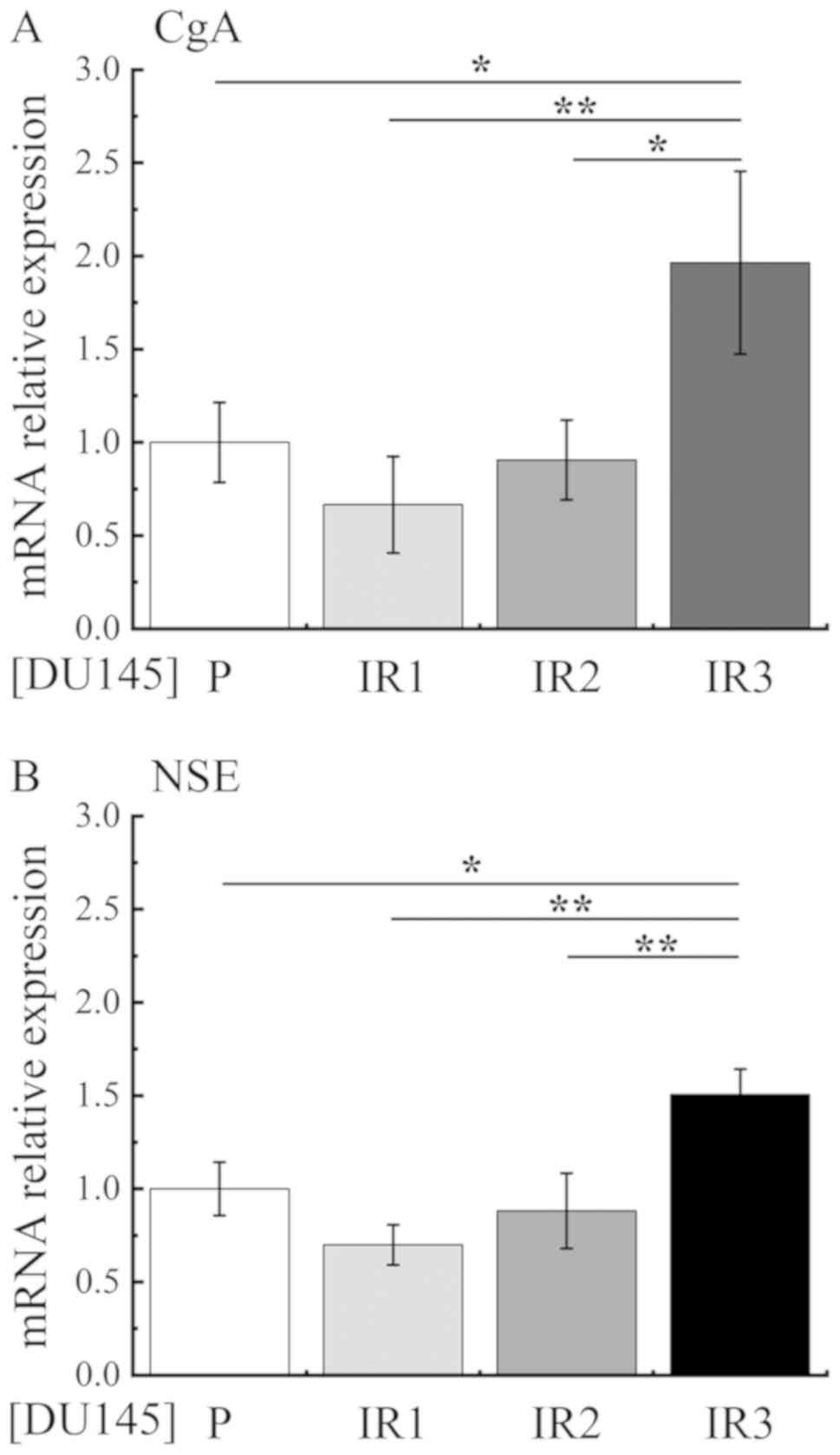
Secondly, to investigate NED, mRNA expression levels
of the NED markers chromogranin A (CgA) and neuron-specific enolase
(NSE) were measured by RT-qPCR. The expression levels of CgA and
NSE were decreased in DU145-IR1 cells compared with those in
DU145-P cells, whereas the expression levels were significantly
increased in DU145-IR3 cells compared with those in DU145-P,
DU145-IR1 and DU145-IR2 cells (Fig.
4).
Thirdly, the relative mRNA expression levels of the
pluripotency-associated genes c-Myc, octamer-binding transcription
factor 4 (OCT4), Nanog homeobox (NANOG), sex determining region
Y-box 2 (SOX2) and Kruppel-like factor 4 (KLF4) were analyzed by
RT-qPCR. The relative mRNA expression levels of OCT4 and NANOG were
significantly increased in DU145-IR3 cells compared with those in
DU145-IR2 cells (Fig. 5A and B).
However, no significant increases in the relative mRNA expression
levels of c-Myc, SOX and KLF4 were identified in DU145-IR3 cells
(Fig. 5C-E).
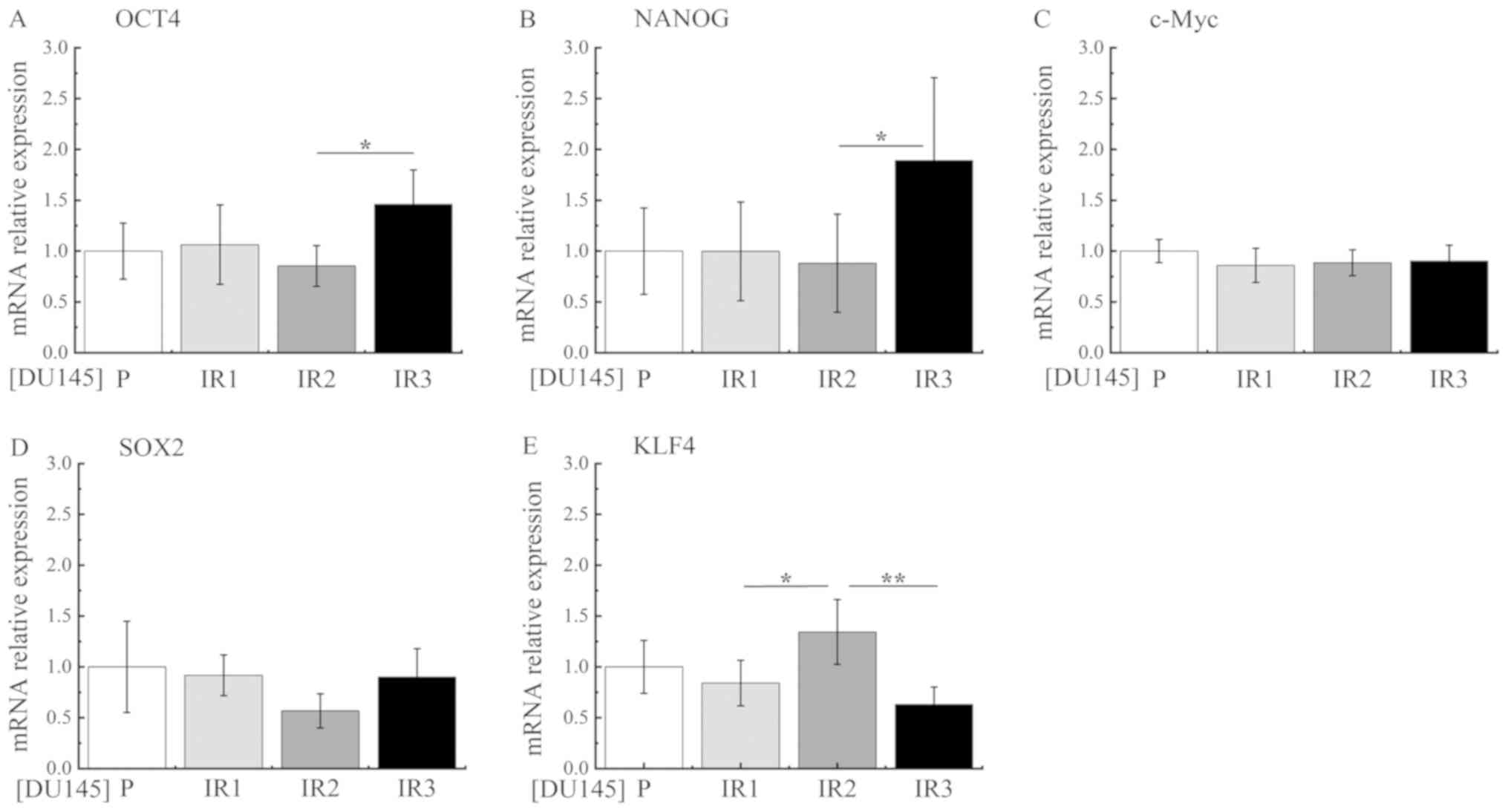 | Figure 5.Expression of pluripotency-associated
genes following fractionated irradiation. The relative mRNA
expression levels of (A) OCT4, (B) NANOG, (C) c-Myc, (D) SOX2 and
(E) KLF4 were analyzed by reverse transcription-quantitative
polymerase chain reaction. P cells were used as the control.
P-values were calculated by the Tukey-Kramer method following
one-way analysis of variance. *P<0.05 and **P<0.01. IR1, 2
Gy/day (total 20 Gy); IR2, 4 Gy/day (total 20 Gy); IR3, 4 Gy/day
(total 56 Gy); P, parental; OCT4, octamer-binding transcription
factor 4; NANOG, Nanog homeobox; SOX2, sex determining region Y-box
2; KLF4, Kruppel-like factor 4. |
Analysis of CSC markers, NED markers and
pluripotency-associated genes in DU145-P, DU145-IR1, DU145-IR2 and
DU145-IR3 cells revealed that the CSC markers were re-expressed,
the levels of NED markers were increased and the mRNA expression
levels of the pluripotency-associated genes OCT4 and NANOG were
increased during fractionated irradiation.
Discussion
The present study examined factors associated with
RR acquisition, including CSC markers, NED markers and
pluripotency-associated genes, to understand the acquisition
mechanism of RR, which remains a predictive factor for a poor
prognosis following radiotherapy (16,19,31,33). The
results of the current study revealed that the proportion of
CD44+ cells increased following fractionated
irradiation. The percentages of CD133+ and
CD138+ cells were identified to increase by single
irradiation or multiple irradiations compared with the percentages
of P cells; however, the expression levels of these markers
decreased with subsequent irradiation. Furthermore, when RR was
acquired by 56 Gy irradiation, the CSC markers CD133 and CD138 were
re-expressed, the levels of NED markers increased and the mRNA
expression levels of the pluripotency-associated genes OCT4 and
NANOG increased.
Radiotherapy has been reported to eliminate the
majority of non-CSCs (6). However,
few CSCs are identified in cancer types that demonstrate relative
RR by lowered ROS production and efficient DNA repair (34,35),
which results in a poor prognosis. The present results revealed
increased expression of CD44 triggered by fractionated irradiation
and increased expression levels of CD133 and CD138 by single
irradiation or multiple irradiations, which is consistent with the
concept of CSCs (16,23). While the expression levels of CD133
and CD138 were decreased by continued fractionated irradiation,
they subsequently increased in RR cells. Consistent with this
result, Lagadec et al (36)
reported that the proportion of
CD24−/low/CD44high cells was increased by
multiple irradiations; however, with 2 Gy × 8 irradiations the
proportion returned to the same level compared with that in
non-irradiated cells, in breast cancer cell lines. These results
suggest that the number of hypothetical CSCs decreases relative to
the non-CSCs if the irradiation exceeds the tolerable dose by
fractionated irradiation. To the best of our knowledge, the present
study provides the first evidence that the frequency of CSCs
decreases once during fractionated irradiation and then increases
again.
Since NE-like cells do not proliferate and express
survival genes, including survivin and B-cell lymphoma 2 (37,38),
they may be a cause of treatment failure. In addition, NE-like
cells secrete several peptide hormones and eutopic bioactive
hormones, including serotonin, and promote the growth of
surrounding tumor cells (18,29,40).
Furthermore, NED is a reversible process and the dedifferentiated
cells can resume proliferation (41). Therefore, NE-like cells exhibit a
dormancy phenotype and are a predictive factor for a poor
prognosis. Deng et al (19)
established RR cells by fractionated irradiation and demonstrated
that NED was induced by the increasing nuclear content of cyclic
AMP response element binding protein of the basic leucine zipper
family and cytoplasmic accumulation of activating transcription
factor 2. The same study reported that NE-like cells that emerge by
fractionated irradiation maintain RR even if they are
dedifferentiated and resume proliferation. The current study
confirmed that the NED markers CgA and NSE did not increase by 20
Gy radiation exposure, but increased only in the RR cells.
The OCT4, SOX2 and NANOG proteins co-occupy the
promoters of various target genes and contribute to pluripotency
and the self-renewal of embryonic stem cells (42). In addition, c-Myc, OCT4, SOX2 and
KLF4 are essential for the generation of the pluripotent phenotype
from differentiated cells (43), and
their expression is associated with tumor progression (28–31,44).
OCT4 and NANOG modulate the expression of various CSC-associated
molecules, including CD44 and CD133 (31,33), and
the overexpression of NANOG upregulates CSC markers, including CD44
and CD133 (31). Furthermore, OCT4
is highly expressed in CD133+ cells and OCT4-knockdown
inhibits the expression of CD133 and sensitizes the cells to
radiation and chemotherapy (44). In
addition, OCT4-positive cells have been reported to co-express the
NED markers CgA and synaptophysin in PCa samples (45).
In the present study, CD44 expression increased,
while the CD133 and CD138 markers were re-expressed by fractionated
irradiation, and OCT4 and NANOG mRNA expression levels increased
only in RR cells. These results suggest that
pluripotency-associated genes were upregulated by fractionated
irradiation, resulting in the acquisition of CSC properties by the
cancer cells. These findings strongly suggest that
radiation-induced CSCs emerge by fractionated irradiation. Whether
or not OCT4 can regulate the progression of NED remains unclear;
however, these findings suggest that OCT4 may contribute to
pluripotency and the maintenance of NED.
In summary, the current data demonstrate that the
expression levels of CD133 and CD138 are increased by single
irradiation or multiple irradiations but are decreased by
fractionated irradiation. However, CD133 and CD138 are re-expressed
by repeating fractionated irradiation. It can be suggested that the
increased expression of pluripotency-associated genes caused by
fractionated irradiation may result in the emergence of induced
CSCs and the progression of NED, which triggers RR acquisition in
PCa.
Acknowledgements
Not applicable.
Funding
This study was supported by Grant-in-Aid for
Scientific Research (KAKENHI) (grant no. 16K10339) and Young
Scientists (grant no. 17K16413).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KM, RS, SM, ET and YH conceived the study and
participated in its design and coordination. KM, RS and KH drafted
the manuscript. KM, RS and KH performed the experiments, and
analyzed and interpreted the data. SM, ET and YH critically revised
the manuscript for important intellectual content. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katanoda K, Sobue T, Tanaka H and
Miyashiro I: 2016.JACR Monograph Supplement No. 2. Tokyo:
Japanese Association of Cancer Registries.
|
|
3
|
Takeda K, Takai Y, Narazaki K, Mitsuya M,
Umezawa R, Kadoya N, Fujita Y, Sugawara T, Kubozono M, Shimizu E,
et al: Treatment outcome of high-dose image-guided
intensity-modulated radiotherapy using intra-prostate fiducial
markers for localized prostate cancer at a single institute in
Japan. Radiat Oncol. 7:1052012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zelefsky MJ, Chan H, Hunt M, Yamada Y,
Shippy AM and Amols H: Long-term outcome of high dose intensity
modulated radiation therapy for patients with clinically localized
prostate cancer. J Urol. 176:1415–1419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grimm P, Billiet I, Bostwick D, Dicker AP,
Frank S, Immerzeel J, Keyes M, Kupelian P, Lee WR, Machtens S, et
al: Comparative analysis of prostate-specific antigen free survival
outcomes for patients with low, intermediate and high risk prostate
cancer treatment by radical therapy. Results from the Prostate
Cancer Results Study Group. BJU Int. 109 (Suppl 1):S22–S29. 2012.
View Article : Google Scholar
|
|
6
|
Pahlajani N, Ruth KJ, Buyyounouski MK,
Chen DY, Horwitz EM, Hanks GE, Price RA and Pollack A: Radiotherapy
doses of 80 Gy and higher are associated with lower mortality in
men with gleason score 8 to 10 prostate cancer. Int J Radiat Oncol
Biol Phys. 82:1–16. 2012. View Article : Google Scholar
|
|
7
|
Li F, Zhou K, Gao L, Zhang B, Li W, Yan W,
Song X, Yu H, Wang S, Yu N and Jiang Q: Radiation induces the
generation of cancer stem cells: A novel mechanism for cancer
radioresistance. Oncol Lett. 12:3059–3065. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurth I, Hein L, Mäbert K, Peitzsch C, Koi
L, Cojoc M, Kunz-Schughart L, Baumann M and Dubrovska A: Cancer
stem cell related markers of radioresistance in head and neck
squamous cell carcinoma. Oncotarget. 6:34494–34509. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arechaga-Ocampo E, Lopez-Camarillo C,
Villegas-Sepulveda N, Gonzalez-De la Rosa CH, Perez-Añorve IX,
Roldan-Perez R, Flores-Perez A, Peña-Curiel O, Angeles-Zaragoza O,
Rangel Corona R, et al: Tumor suppressor miR-29c regulates
radioresistance in lung cancer cells. Tumour Biol.
39:10104283176950102017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahmed KM, Dong S, Fan M and Li JJ: Nuclear
factor-kappaB p65 inhibits mitogen-activated protein kinase
signaling pathway in radioresistant breast cancer cells. Mol Cancer
Res. 4:945–955. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hazawa M, Hosokawa Y, Monzen S, Yoshino H
and Kashiwakura I: Regulation of DNA damage response and cell cycle
in radiation-resistant HL60 myeloid leukemia cells. Oncol Rep.
28:55–61. 2012.PubMed/NCBI
|
|
12
|
Kim JS, Chang JW, Yun HS, Yang KM, Hong
EH, Kim DH, Um HD, Lee KH, Lee SJ and Hwang SG: Chloride
intracellular channel 1 identified using proteomic analysis plays
an important role in the radiosensitivity of HEp-2 cells via
reactive oxygen species production. Proteomics. 10:2589–2604. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chin C, Bae JH, Kim MJ, Hwang JY, Kim SJ,
Yoon MS, Lee MK, Kim DW, Chung BS, Kang CD and Kim SH:
Radiosensitization by targeting radioresistance-related genes with
protein kinase A inhibitor in radioresistant cancer cells. Exp Mol
Med. 37:608–618. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin Y, Xu K, Chen Q, Wang B, Pan J, Huang
S, Wei Y and Ma H: Simvastatin inhibits the development of
radioresistant esophageal cancer cells by increasing the
radiosensitivity and reversing EMT process via the PTEN-PI3K/AKT
pathway. Exp Cell Res. 362:1–8. 2017.PubMed/NCBI
|
|
15
|
Chang L, Graham PH, Hao J, Ni J, Bucci J,
Cozzi PJ, Kearsley JH and Li Y: Acquisition of
epithelial-mesenchymal transition and cancer stem cell phenotypes
is associated with activation of the PI3K/Akt/mTOR pathway in
prostate cancer radioresistance. Cell Death Dis. 4:1–13. 2013.
View Article : Google Scholar
|
|
16
|
Cojoc M, Peitzsch C, Kurth I, Trautmann F,
Kunz-Schughart LA, Telegeev GD, Stakhovsky EA, Walker JR, Simin K,
Lyle S, et al: Aldehyde Dehydrogenase is regulated by b-Catenin/TCF
and promotes radioresistance in prostate cancer progenitor cells.
Cancer Res. 75:1482–1494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peitzsch C, Cojoc M, Hein L, Kurth I,
Mäbert K, Trautmann F, Klink B, Schröck E, Wirth MP, Krause M, et
al: An Epigenetic reprogramming strategy to resensitize
radioresistant prostate cancer cells. Cancer Res. 76:2637–2651.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu CD, Choo R and Huang J: Neuroendocrine
differentiation in prostate cancer: A mechanism of radioresistance
and treatment failure. Front Oncol. 5:902015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng X, Liu H, Huang J, Cheng L, Keller
ET, Parsons SJ and Hu CD: Ionizing radiation induces prostate
cancer neuroendocrine differentiation through interplay of CREB and
ATF2: Implications for disease progression. Cancer Res.
68:9663–9670. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suarez CD, Deng X and Hu CD: Targeting
CREB inhibits radiation-induced neuroendocrine differentiation and
increases radiation-induced cell death in prostate cancer cells. Am
J Cancer Res. 4:850–861. 2014.PubMed/NCBI
|
|
21
|
Vashchenko N and Abrahamsson PA:
Neuroendocrine differentiation in prostate cancer: Implications for
new treatment modalities. Eur Urol. 47:147–155. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu C and Huang J: Phosphatidylinositol
3-Kinase-AKT- mammalian target of rapamycin pathway is essential
for neuroendocrine differentiation of prostate cancer. J Biol Chem.
282:3571–3583. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang L, Huang X, Zheng X, Wang X, Li S,
Zhang L, Yang Z and Xia Z: Enrichment of prostate cancer stem-like
cells from human prostate cancer cell lines by culture in
serum-free medium and chemoradiotherapy. Int J Biol Sci. 9:472–479.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ni J, Cozzi PJ, Hao JL, Beretov J, Chang
L, Duan W, Shigdar S, Delprado WJ, Graham PH, Bucci J, et al: CD44
Variant 6 is associated with prostate cancer metastasis and
chemo-/radioresistance. Prostate. 74:602–617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimada K, Anai S, Fujii T, Tanaka N,
Fujimoto K and Konishi N: Syndecan-1 (CD138) contributes to
prostate cancer progression by stabilizing tumour-initiating cells.
J Pathol. 231:495–504. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shimada K, Nakamura M, De Velasco MA,
Tanaka M, Ouji Y and Konishi N: Syndecan-1, a new target molecule
involved in progression of androgen-independent prostate cancer.
Cancer Sci. 100:1248–1254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hiraga T, Ito S and Nakamura H: Cancer
stem-like cell marker CD44 promotes bone metastases by enhancing
tumorigenicity, cell motility, and hyaluronan production. Cancer
Res. 73:4112–4122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rybak AP and Tang D: SOX2 plays a critical
role in EGFR-mediated self-renewal of human prostate cancer
stem-like cells. Cell Signal. 25:2734–2742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang YL, Zhou PJ, Wei L, Li W, Ji Z, Fang
YX and Gao WQ: MicroRNA-7 inhibits the stemness of prostate cancer
stem-like cells and tumorigenesis by repressing KLF4/PI3K/Akt/p21
pathway. Oncotarget. 6:24017–24031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeter CR, Liu B, Liu X, Chen X, Liu C,
Calhoun-Davis T, Repass J, Zaehres H, Shen JJ and Tang DG: NANOG
promotes cancer stem cell characteristics and prostate cancer
resistance to androgen deprivation. Oncogene. 30:3833–3845. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Russo MV, Esposito S, Tupone MG, Manzoli
L, Airoldi I, Pompa P, Cindolo L, Schips L, Sorrentino C and Di
Carlo E: SOX2 boosts major tumor progression genes in prostate
cancer and is a functional biomarker of lymph node metastasis.
Oncotarget. 7:12372–12385. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen YC, Hsu HS, Chen YW, Tsai TH, How CK,
Wang CY, Hung SC, Chang YL, Tsai ML, Lee YY, et al: Oct-4
expression maintained cancer stem-like properties in lung
cancer-derived CD133-positive Cells. PLoS One. 3:e26372008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiao W, Graham PH, Power CA, Hao J,
Kearsley JH and Li Y: CD44 is a biomarker associated with human
prostate cancer radiation sensitivity. Clin Exp Metastasis. 29:1–9.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lagadec C, Vlashi E, Della Donna L, Meng
Y, Dekmezian C, Kim K and Pajonk F: Survival and self-renewing
capacity of breast cancer initiating cells during fractionated
radiation treatment. Breast Cancer Res. 12:R132010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xing N, Qian J, Bostwick D, Bergstralh E
and Young CY: Neuroendocrine cells in human prostate over-express
the anti-apoptosis protein survivin. Prostate. 48:7–15. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gong J, Lee J, Akio H, Schlegel PN and
Shen R: Attenuation of apoptosis by chromogranin a-induced akt and
survivin pathways in prostate cancer Cells. Endocrinology.
148:4489–4499. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dayon A, Brizuela L, Martin C, Mazerolles
C, Pirot N, Doumerc N, Nogueira L, Golzio M, Teissié J, Serre G, et
al: Sphingosine kinase-1 Is central to androgen-regulated prostate
cancer growth and survival. PLoS One. 4:e80482009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Abrahamsson PA: Neuroendocrine cells in
tumour growth of the prostate. Endocr Relat Cancer. 6:503–519.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cox ME, Deeble PD, Lakhani S and Parsons
SJ: Acquisition of neuroendocrine characteristics by prostate tumor
cells is reversible: Implications for prostate cancer progression.
Cancer Res. 59:3821–3830. 1999.PubMed/NCBI
|
|
42
|
Boyer LA, Lee TI, Cole MF, Johnstone SE,
Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG,
et al: Core transcriptional regulatory circuitry in human embryonic
stem cells. Cell. 122:947–956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maina PK, Shao P, Liu Q, Fazli L, Tyler S,
Nasir M, Dong X and Qi HH: c-MYC drives histone demethylase PHF8
during neuroendocrine differentiation and in castration-resistant
prostate cancer. Oncotarget. 7:75585–75602. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sotomayor P, Godoy A, Smith GJ and Huss
WJ: Oct4A is expressed by a subpopulation of prostate
neuroendocrine cells. Prostate. 69:401–410. 2009. View Article : Google Scholar : PubMed/NCBI
|