Introduction
Ovarian cancer (OC) has one of the highest mortality
rates among all gynaecological malignancies (1). Paclitaxel (PTX) combined with platinum
is the standard chemotherapy regimen for OC. However, treatment
failure may occur due to the development of PTX resistance, along
with invasion and metastasis of tumour cells.
In cases of resistance, a subset of the tumour cell
population exhibits inherited or acquired drug resistance and
therefore survives chemotherapy, resulting in tumour recurrence
(2). These drug-resistant cells are
considered to be cancer stem cells (CSCs), which are responsible
for poor prognosis in patients with cancer (3–5). CSCs
have the capacity for unlimited proliferation, self-renewal and
multilineage differentiation, and may also avoid the effects of
chemotherapy, leading to local invasion and distant metastasis.
Therefore, an ideal strategy for preventing tumour recurrence is
one that targets CSCs (6). In
addition, clarifying the mechanisms of action for drug resistance
may reveal novel avenues for treatment.
OC is heterogeneous (7), and the tumours contain subpopulations
of cells with SC characteristics (8). A number of these subpopulations have
been identified, including those positive for CD44 antigen (CD44)
and prominin 1 (CD133) (9–11). Cultured epithelial ovarian
adenocarcinoma ascite cells have been revealed to exhibit
self-renewal and long-term proliferative potential, which is
associated with the overexpression of typical CSC markers,
including CD44 (12). Recurrent
tumours have been demonstrated to exhibit a larger fraction of CSCs
expressing aldehyde dehydrogenase 1 family member A1, CD44 and
CD133 compared with matched primary OC specimens. Furthermore,
several genes involved in SC maintenance, including endoglin
(CD105), are upregulated in residual tumour cells in samples from
relapsed patients at the end of primary therapy (2), suggesting that resistant tumours
overexpress SC genes. Cells expressing the mesenchymal SC marker
CD105 isolated from human renal carcinoma samples and
CD105-positive cells and clones derived from renal carcinoma
samples are enriched in tumour-initiating cells with SC
characteristics (13). Vascular cell
adhesion molecule 1 (CD106) is a surface marker expressed by
mesenchymal and neural SCs (14,15) that
is associated with OC metastasis and recurrence (16). All of these factors are associated
with the self-renewal, chemoresistance and metastasis of cancer
cells and may be CSC surface markers. However, to the best of our
knowledge, the significance of CD105, CD44 and CD106 as CSC markers
in OC has not been investigated previously.
We previously established the PTX-resistant OC
OC3/TAX300 cell line with a resistance index of 6.70 by exposing
OVCAR3 cells to PTX (17). We
hypothesised that resistance to PTX treatment leads to an
enrichment of the CSC population in OC cells, with increased
expression of SC surface markers including CD105. To examine this
hypothesis, the present study analysed the expression of CD105 and
other SC surface markers, including CD44 (2) and CD106 (16), in OC3/TAX300 cells and clinical OC
tissue samples that were graded in terms of the degree of
malignancy in our previous study (7). The invasiveness and metastatic
potential of PTX-resistant OC3/TAX300 cells was also evaluated, and
the association between the clinical features of the tumours and
the expression of SC factors was examined.
Materials and methods
Ethics statement
The present study was approved by the Ethics
Committee of the Beijing Shijitan Hospital of Capital Medical
University (Beijing, China). Written informed consent was obtained
from all participants prior to surgery. All procedures were
performed in accordance with the Declaration of Helsinki.
Cell lines and culture conditions
The OVCAR3 cell line was provided by the Basic
Medical Research Institute (Beijing, China) and has been described
in other studies (18,19). The PTX-resistant OC3/TAX300 cell line
used was previously established (17). Cells were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% bovine calf serum (BCS; Beijing Dingguo
Changsheng Biotechnology Co., Ltd., Beijing, China), 0.1%
penicillin and 0.1% streptomycin at 37°C in an environment
containing 5% CO2.
Reagents and antibodies
TRIzol® reagent and primers were obtained
from Invitrogen (Thermo Fisher Scientific, Inc.). Dimethyl
sulfoxide was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). Microplates were purchased from Beijing Dingguo
Changsheng Biotechnology Co., Ltd. Mouse monoclonal anti-human
CD105 (cat. no. 14606), CD44 (cat. no. 3570) and CD106 (cat. no.
3565) antibodies were purchased from Cell Signalling Technology,
Inc. (Danvers, MA, USA).
Flow cytometry analysis of CD105, CD44
and CD106 expression
OVCAR3 and OC3/TAX300 cells in the logarithmic phase
were collected and washed twice with PBS. The cells were
resuspended in PBS at a concentration of 1×106/ml, and a
100-µl suspension was incubated with 5 µl PerCP-Cy5.5-conjugated
anti-CD105, phycoerythrin-conjugated anti-CD44 or fluorescein
isothiocyanate-conjugated anti-CD106 antibodies (BD Biosciences,
San Jose, CA, USA) for 1 h at 37°C. The cells were washed twice
with PBS and analysed with a FACSCalibur flow cytometer (FlowJo
7.6.1; BD Biosciences).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from cells using TRIzol
reagent, according to the manufacturer's protocol and reverse
transcribed using moloney murine leukemia virus reverse
transcriptase, 5X first strand buffer (dithiothreitol), 10 mM
deoxynucleotide triphosphates, Oligo (dT)18 (all from Tiangen
Biotech Co., Ltd., Beijing, China). The temperature protocol used
for RT was 42°C for 50 min and 95°C for 5 min. Primers targeting
CD105, CD44 and CD106 genes were designed according to sequence
data obtained from GenBank (20),
with β-actin (ACTB) used as an internal control. The primer
sequences were as follows: CD105, 5′-GCCAAGGGCAACTGTGTGA-3′(sense)
and 5′-CCGGTTTTGGGTATGGGTACT-3′ (antisense); CD44,
5′-CCTCTTGGCCTTGGCTTTG-3′ (sense) and 5′-CTCCATTGCCACTGTTGATCAC-3′
(antisense); CD106, 5′-TGGTCAGCCCTTCCTCCAT-3′ (sense) and
5′-AGGATTTTCGGAGCAGGAAAG-3′ (antisense); and ACTB,
5′-AGGTCACCATTGGCAATG-3′ (sense) and 5′-GGTAGTTTCGTGGATGCCACA-3′
(antisense). The cDNA was amplified using SYBR Green PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and the
thermocycling conditions were as follows: 50°C for 2 min, 95°C for
10 min, then 40 cycles of 95°C for 15 sec and 60°C for 1 min for
the amplification curve and 95°C for 15 sec, 60°C for 15 sec and
95°C for 15 sec for the dissociation curve. Data were analysed
using Sequence Detection Software V2.2 (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and exported to an Excel spreadsheet
(Microsoft Corporation, Redmond, WA, USA). Target gene expression
levels were normalised to that of ACTB. The mRNA expression ratio
of CD105, CD44 or CD106 to ACTB was calculated to obtain relative
expression values using the formula: ΔCq=Cq (target gene)-Cq
(ACTB); where q is the number of cycles when the DNA concentration
reached the threshold (21).
Invasion assay
The invasive capabilities of OVCAR3 and OC3/TAX300
cells were assessed using a Transwell assay. Cells in the
logarithmic phase were collected and washed with PBS, and then
cultured in serum-free medium for 24 h. In total, 40 µl Matrigel
was coated on the membrane of the upper chamber surface and
incubated at 37°C for 30 min to solidify. The cells were then
collected, and their concentration was adjusted to 1×105
cells/ml; they were then seeded in the upper chamber of a 24-well
Transwell insert coated with a thin layer of Matrigel (BD
Biosciences). The lower chamber was filled with RPMI-1640 medium
supplemented with 20% BCS. The cells were incubated at 37°C and 5%
CO2. After 24 h, cells remaining in the upper chamber
were scraped off along with the Matrigel using a sterile swab, and
cells that had invaded into the insert were fixed with 40 g/l
paraformaldehyde for 20 min at room temperature and stained with
0.01% crystal violet for 20 min at room temperature for observation
by light microscopy (magnification, ×40). Images of cells in at
least five randomly selected microscopic fields were captured and
the number of cells was counted. The average number of cells was
used to assess the invasive capacity of the 2 cell lines.
Clinical OC samples
A total of 80 epithelial OC tissue samples were
collected from the specimen repository of Beijing Shijitan Hospital
between April 2012 and February 2013 (7). The median age of the patients was 56.15
years (range, 23–79 years). There were 52 primary and 28 recurrent
cases; 64 were poorly differentiated and 16 were moderately or
highly differentiated OC tissue; and 63 samples were advanced-stage
(III) whereas 17 were early-stage (I and II) OC. Immediately
following cytoreductive surgery, all specimens were analysed with
the ATP-based tumour chemosensitivity assay (ATP-TCA) as described
previously (7). These specimens were
graded according to the National Comprehensive Cancer Network
guidelines (22). Routine
histopathological analysis was performed for samples obtained from
the same tissues to determine the stage and histological features
of the tumour samples simultaneously with ATP-TCA testing. The
sensitivities of specimens to PTX, carboplatin (CBP), topotecan,
gemcitabine (GEM), docetaxel (TXT), bleomycin, etoposide and
4-hydroperoxycyclophosphamide were examined using an in
vitro ATP-TCA procedure, Cancer recurrence was defined
according to the current clinical criteria as: Return of cancer
following completion of treatment following a period of time during
which the cancer was not detected (23). In OC, patients with
platinum-sensitive cancer were those who achieved complete
remission and experienced relapse at 6 months or later following
initial platinum-based chemotherapy, whereas patients with
platinum-resistant cancer were those who exhibited recurrence
within 6 months (24).
Western blot analysis
Total cell lysates were prepared using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) and the supernatant was collected
via centrifugation at 4°C and 4,024 × g for 10 min. A bicinchoninic
acid assay was used to determine the protein concentration.
Aliquots (30–40 µl) of cell lysates were heated at 100°C for 5 min,
and 10 µg of protein was loaded into each well of a 10% SDS-PAGE
gel for electrophoresis. The proteins on the electrophoresis gel
were then transferred to an Immobilon-P membrane that was incubated
in blocking solution [5% bovine serum albumin (Beijing Dingguo
Changsheng Biotechnology Co., Ltd.) in TBS-Tween 20] for 1–3 h at
25°C followed by overnight incubation at 4°C with mouse monoclonal
anti-human CD105, CD44 and CD106 antibodies at dilutions of
1:1,000. Subsequent to washing 3 times in TBS with 0.1% Tween 20,
the membrane was incubated for 1–2 h at 25°C with a
fluorophore-conjugated secondary antibody (cat. no. 610-132-121;
Rockland Immunochemicals, Inc., Limerick, PA, USA) at a dilution of
1:5,000. The membrane was washed and analysed using an Odyssey
two-colour infrared imaging system (LICOR Odyssey, LI-COR
Biosciences, Lincoln, NE, USA). The signal intensity of protein
bands was calculated using Image J software (v1.8.0; National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
and were analysed using SPSS v.17.0 for Windows software (SPSS
Inc., Chicago, IL, USA). Means were compared using a two-sided
t-test. Linear regression analysis was used to detect the
correlation between sensitivity index (SI) and expression levels of
target genes. All experiments were independently repeated a minimum
of three times. P≤0.05 was considered to indicate a statistically
significant difference.
Results
CD105, CD44 and CD106 are
overexpressed in PTX-resistant OC cells
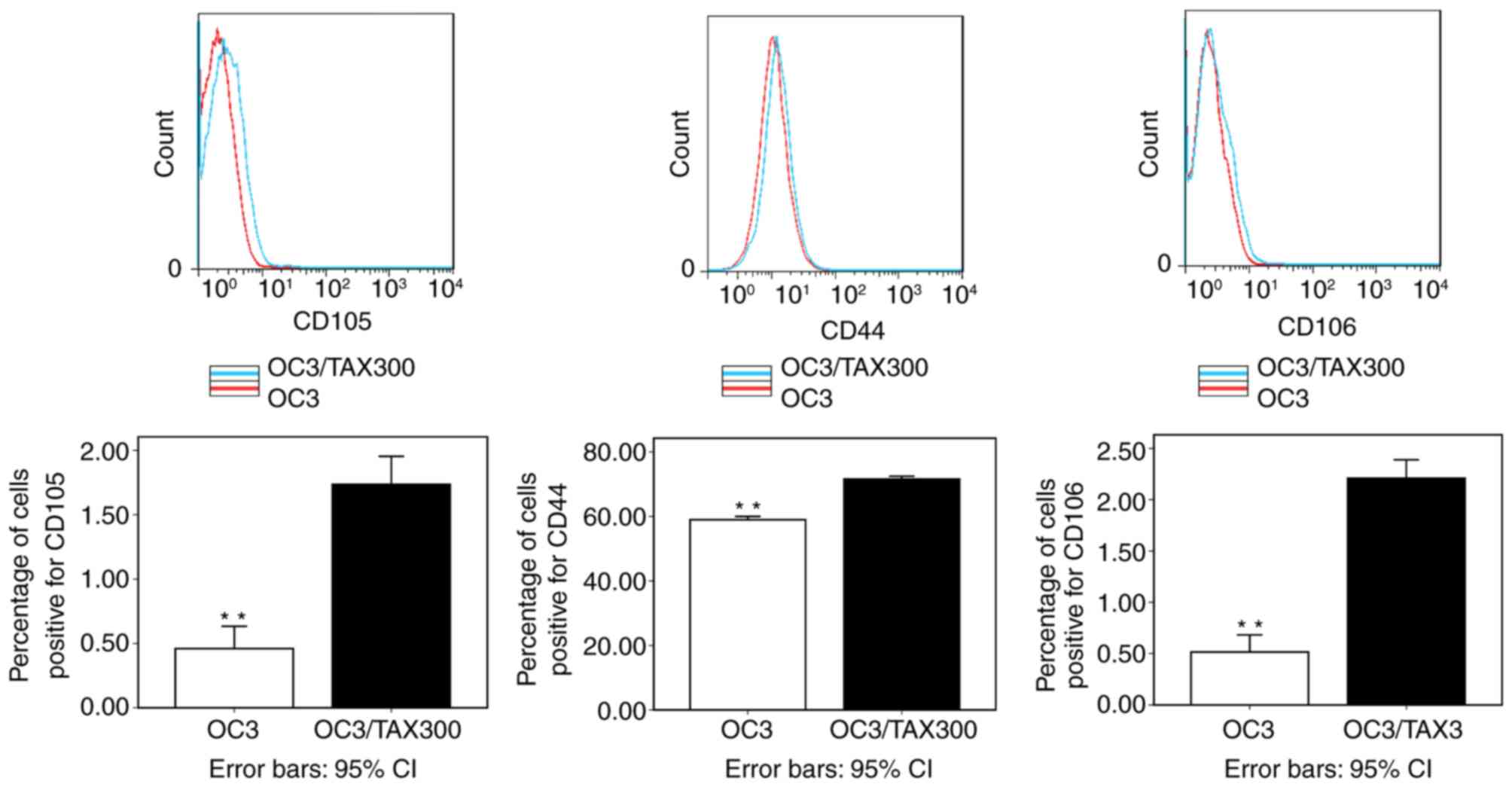
The percentage of cells positive for the 3 proteins
was increased in the PTX-resistant cell line compared with the
PTX-sensitive cell line (P<0.01; Fig.
1). Accordingly, the median fluorescence intensities of CD105,
CD44 and CD106 were increased in OC3/TAX300 cells compared with the
OVCAR3 cells (P<0.01; Fig. 2A-C).
The results from the RT-qPCR analysis demonstrated a similar trend
to those obtained by flow cytometry, with increased relative
expression levels of CD105, CD44 and CD106 mRNA in the OC3/TAX300
cells compared with the OVCAR3 cells (P<0.05; Fig. 2D).
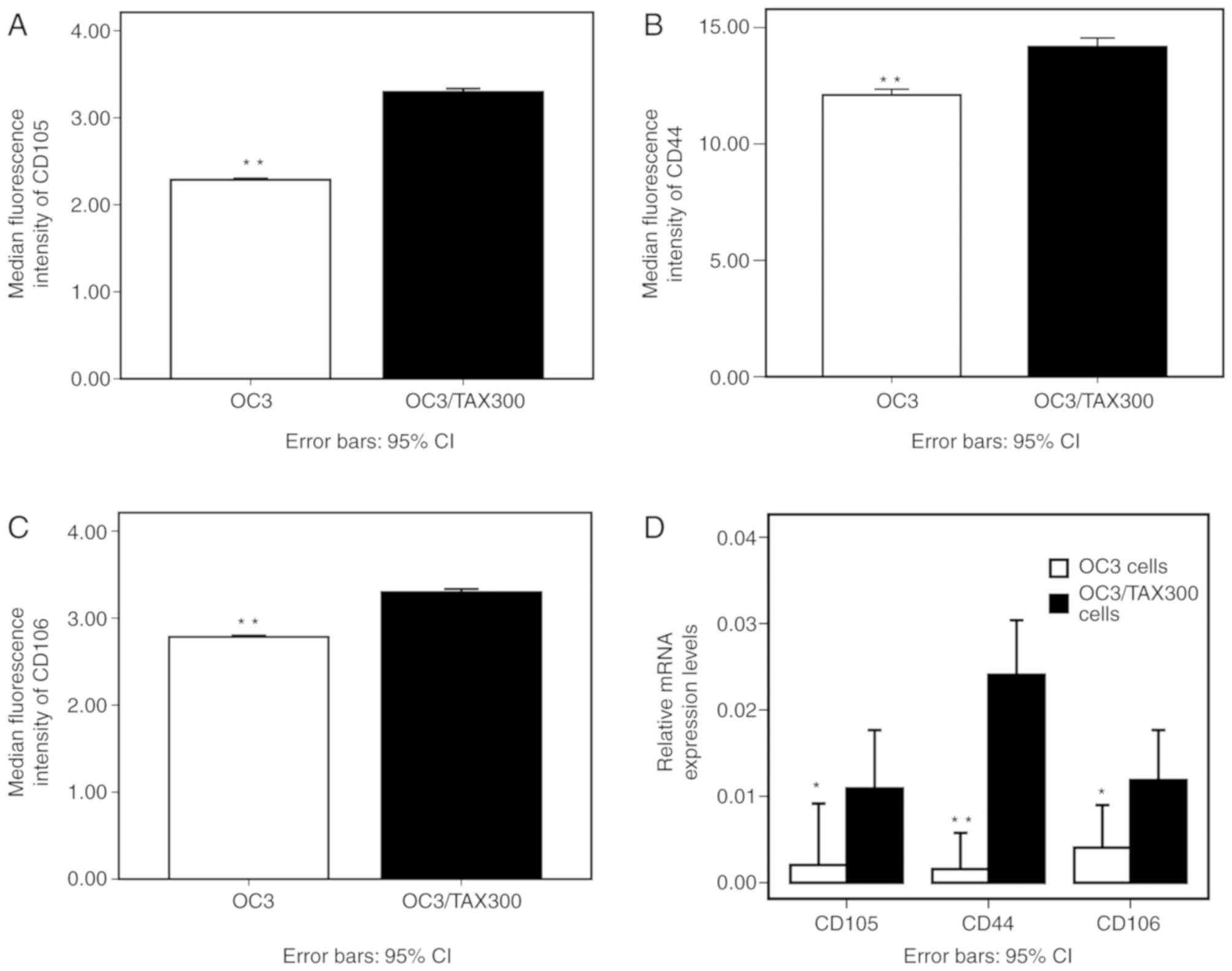 | Figure 2.Median fluorescence intensity of
CD105, CD44 and CD106 in PTX-resistant OC3/TAX300 and PTX-sensitive
OVCAR3 cells, as determined by flow cytometry. (A-C) (A) CD105, (B)
CD44 and (C) CD106 overexpression in OC3/TAX300 cells. (D) Relative
mRNA expression levels of CD105, CD44 and CD106 in OC3/TAX300
cells. *P<0.05 and **P<0.01. PTX, paclitaxel; CD44, CD44
antigen; CD105, endoglin; CD106, vascular cell adhesion molecule 1;
PTX, paclitaxel; CI, confidence interval. |
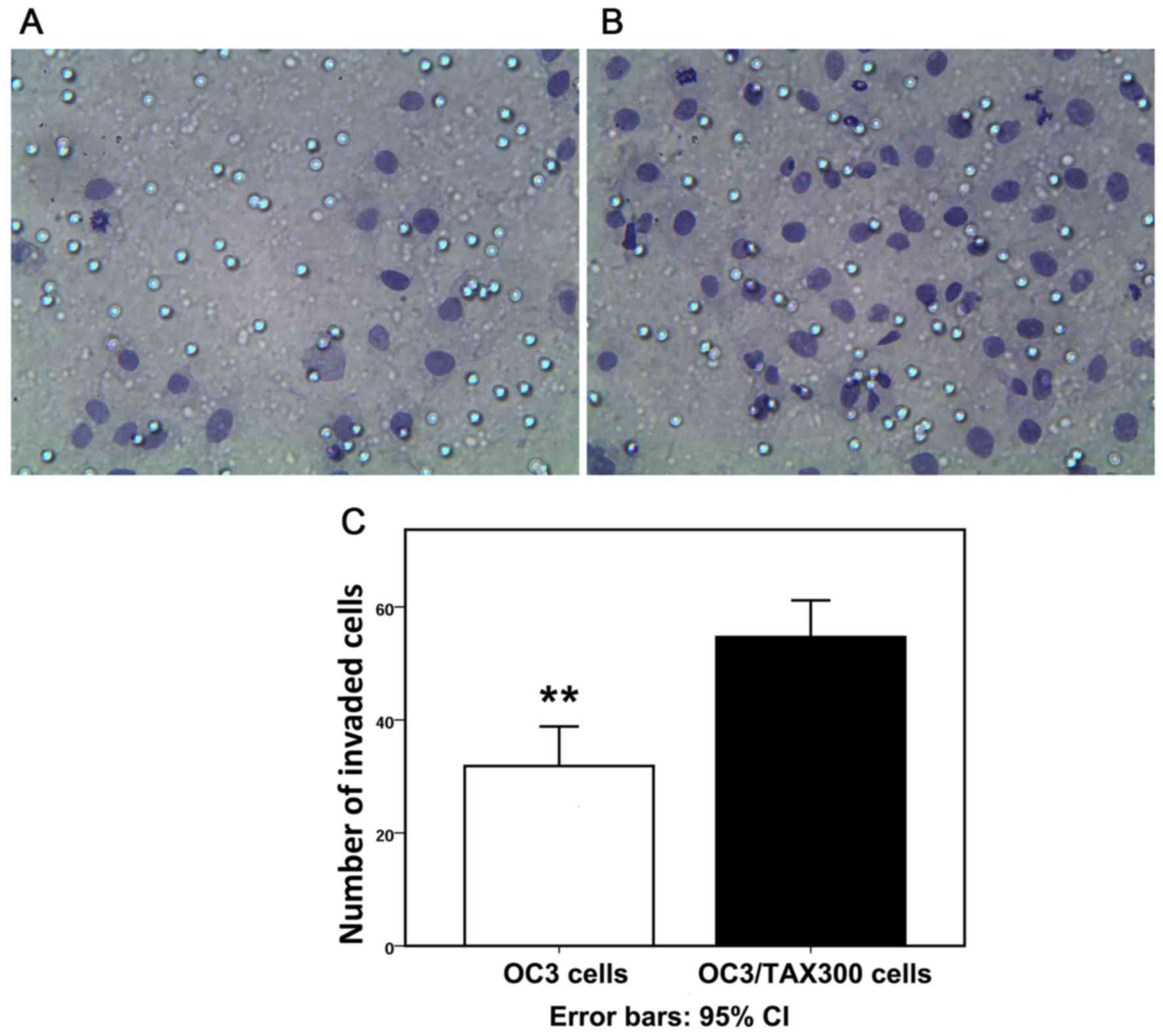
PTX-resistant OC cells exhibit
increased invasive capabilities
The Transwell assay revealed that numerous OC cells
had invaded the membrane filter (Fig. 3A
and B). The numbers of invaded cells in 12 different fields of
vision were counted after 24 h culture, and the quantitative
analysis indicated that the number of invaded cells was increased
in the OC3/TAX300 group compared with the OVCAR3 group (54.7±6.65
vs. 31.8±6.55; P<0.01; Fig.
3C).
CD105, CD44 and CD106 are highly
expressed in drug-resistant epithelial OC tissue samples
In the western blot analysis, the protein expression
levels of CD105, CD44 and CD106 in 80 epithelial ovarian cancer
tissues were markedly different. This may be associated with the
heterogeneity of chemotherapy treatments in patients (7). It was identified that 66/80 were
PTX-sensitive and 14/80 were PTX-resistant; and 47/80 were
CBP-sensitive and 33/80 were CBP-resistant (Table I). The difference of protein
expression in chemoresistant or -sensitive samples was demonstrated
by the following: CD105, CD44 and CD106 were expressed at high and
low levels in PTX-resistant and PTX-sensitive tissue samples,
respectively (Fig. 4A). Statistical
analysis of the data demonstrated the significant difference:
Protein expression of CD105 was different in 80 specimens with
different sensitivity to 8 drugs, and there were increased protein
expression levels of CD105 in PTX/CBP/TXT resistant samples
compared with sensitive specimens (Fig.
4B). There was an increased protein expression level of CD44 in
PTX resistant samples compared with sensitive specimens (Fig. 4C). Among patients exhibiting
chemoresistance or sensitivity to the commonly used chemotherapy
drugs including PTX, CBP or TXT, CD106 levels were increased in the
chemoresistance group compared with the chemosensitive group
(Fig. 4D).
 | Figure 4.CD105, CD44 and CD106 are highly
expressed in drug-resistant epithelial OC tissue samples. (A)
Representative blots of 3 experiments of PTX-resistant and
PTX-sensitive samples were subjected to western blot analysis for
CD105, CD44 and CD106 protein levels. ACTB was used as a loading
control. Each number corresponds to a different patient. CD105,
CD44 and CD106 were expressed at high and low levels in
PTX-resistant and PTX-sensitive tissue samples, respectively. (B)
Difference of expression of CD105 protein in all specimens with
different sensitivities to 8 drugs. There were increased protein
expression levels of CD105 in PTX/CBP/TXT resistant samples. (C)
Difference of expression of CD44 protein in all specimens with
different sensitivity to 8 drugs. There was an increased protein
expression level of CD44 in PTX resistant samples compared with the
sensitive specimens. (D) Difference of expression of CD106 protein
in all specimens with different sensitivity to 8 drugs. There were
increased protein expression levels of CD106 in PTX/CBP/TXT
resistant samples compared with the sensitive specimens. *P<0.05
and **P<0.01. CD44, CD44 antigen; CD105, endoglin; CD106,
vascular cell adhesion molecule 1; BLM, bleomycin; CBP,
carboplatin; GEM, gemcitabine; PTX, paclitaxel; TPT, topotecan;
TXT, docetaxel; VP-16, etoposide; 4-HC,
4-hydroperoxycyclophosphamide; R, PTX-resistant samples; S,
PTX-sensitive samples; ACTB, β-actin; CI, confidence interval; OC,
ovarian cancer. |
 | Table I.Results of chemosensitivity assay in
OC samples. |
Table I.
Results of chemosensitivity assay in
OC samples.
| Drug | Sensitivity, n
(%) | Weak sensitivity, n
(%) | Resistance, n
(%) | Sensitivity
(%) |
|---|
| PTX | 44 (55) | 22 (27.5) | 14 (17.5) | 82.5 |
| CBP | 21 (26.4) | 26 (32.4) | 33 (41.2) | 58.8 |
| TPT | 14 (17.5) | 23 (28.7) | 43 (53.8) | 46.2 |
| TXT | 18 (22.5) | 18 (22.5) | 44 (55) | 45 |
| GEM | 13 (16.3) | 13 (16.3) | 54 (67.5) | 32.5 |
| 4-HC | 3 (3.7) | 18 (22.5) | 59 (73.8) | 26.2 |
| VP-16 | 4 (6.3) | 10 (11.2) | 66 (82.5) | 17.5 |
| BLM | 0 | 0 | 80 (100) | 0 |
In our previous study, the SI was used to evaluate
drug sensitivity rates and was calculated using the formula:
SI=500-% tumour growth inhibition at 200, 100, 50, 25 and 12.5% +
test drug concentration (7). The
expression levels of these target genes in the tissue samples were
associated with the SI of a number of the drugs examined; for
example, the CD105 level was correlated with the SI value of PTX
and TXT (Table II).
 | Table II.Results of correlation analysis
between expression levels of target genes and sensitivity index of
tested chemotherapy drugs. |
Table II.
Results of correlation analysis
between expression levels of target genes and sensitivity index of
tested chemotherapy drugs.
| Drug
combination | Correlation
coefficient, r | P-value |
|---|
| CD105 and PTX | 0.327 | 0.003 |
| CD105 and TXT | 0.285 | 0.010 |
| CD44 and PTX | 0.353 | 0.001 |
| CD106 and PTX | 0.344 | 0.002 |
| CD106 and TXT | 0.321 | 0.004 |
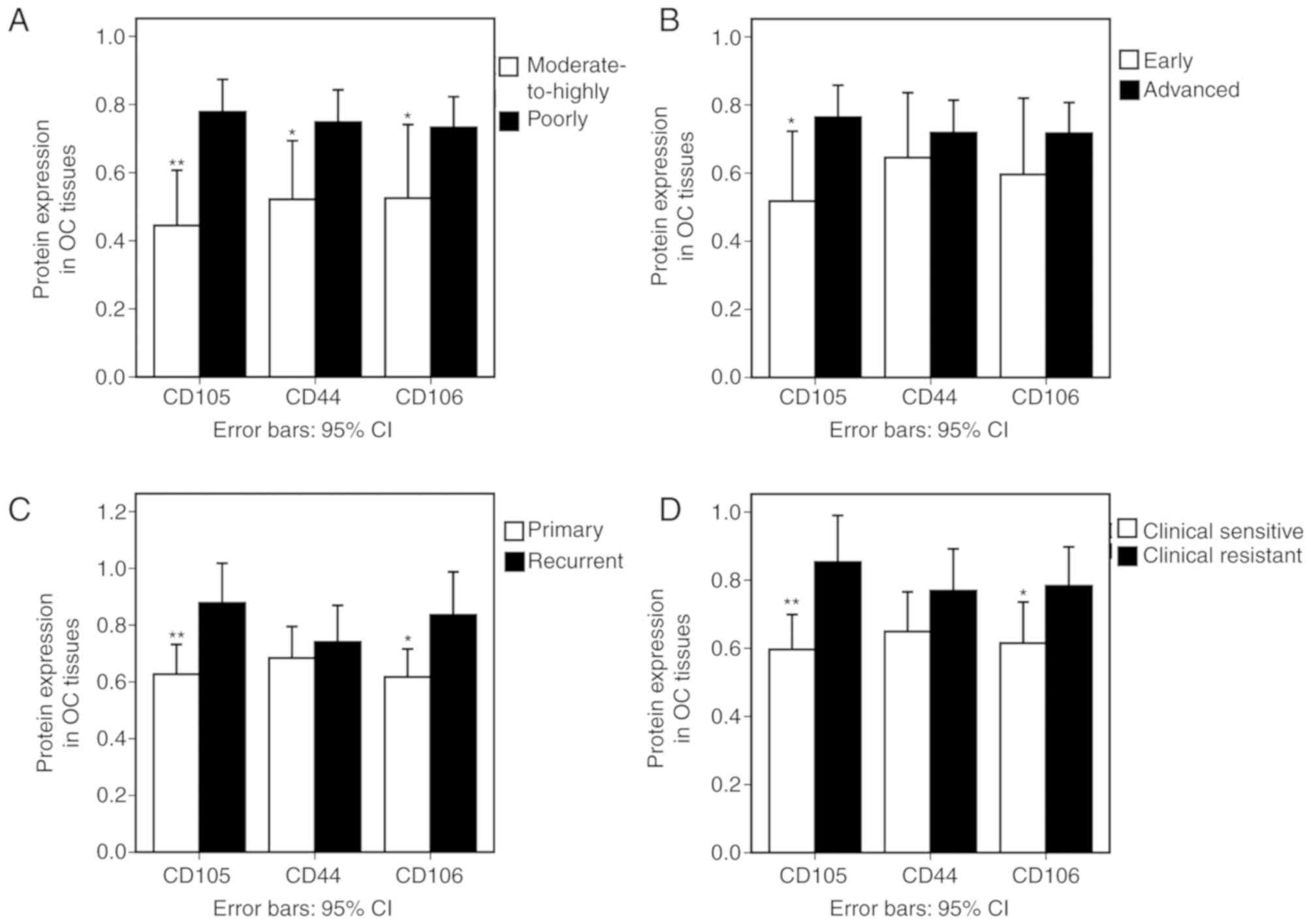
CD105, CD44 and CD106 expression
levels are associated with clinical parameters of epithelial
OC
Moderately and highly differentiated OC tissue
samples exhibited decreased CD105 protein expression compared with
those that were poorly differentiated (P=0.002). Furthermore, CD105
expression was decreased at the early stage (I and II) compared
with the advanced stage (III) tissues (P=0.019) and decreased in
the primary tumour samples compared with the recurrent ovarian
epithelial cancer specimens (P=0.006). Similar trends were observed
for CD44 and CD106 expression (Fig.
5A-C).
All of the cases in the present study were followed
up for at least 2 years after initial chemotherapy. A total of 44
patients (55%) were classified as clinically CBP-sensitive and 36
(45%) were clinically CBP-resistant. Clinically CBP-sensitive OC
tissue samples exhibited decreased CD105 and CD106 expression
compared with the resistant cases (Fig.
5D).
Discussion
The present study investigated the association
between the expression of the SC markers CD105, CD44 and CD106 and
the invasive capabilities and chemotherapeutic resistance of OC
cell lines. It was identified that all 3 proteins were
overexpressed in PTX-resistant OC3/TAX300 cells and in
chemoresistant and poorly differentiated or advanced-stage
epithelial OC tissues, and that this was associated with enhanced
invasive capacity. These data suggest that exposure to high doses
of PTX enhances the SC properties of ovarian tumour cells
(including CD105, CD44 and CD106 overexpression), leading to the
development of PTX resistance, increased invasion and long-distance
metastasis, and poor prognosis in OC.
CD105 is a co-receptor for transforming growth
factor (TGF)-β family proteins including TGF-β1 and -β3, and serves
a key role in development, cell proliferation, extracellular matrix
synthesis, angiogenesis and the immune response (25). CD105 exhibits SC characteristics and
may stimulate endothelial cell growth; its high level of expression
on peri- and intratumoural vessels is associated with poor
prognosis following cancer treatment (26–28).
CD105 overexpression has also been identified to be associated with
decreased patient survival rates and distant metastasis (27–30);
this is likely due to the angiogenesis-promoting function of CD105,
which has been demonstrated to increase tumour vasculature and
ultimately lead to poor prognosis (31–33).
CD105 is expressed not only in vascular endothelial
cells, but it is also detected in several malignancies including
gastrointestinal stromal tumours, hepatocellular carcinoma, and
breast cancer (34–36), head and neck paragangliomas (37), and OC ascites (38). Furthermore, CD105-expressing cells
have multi-differentiation potential: CD105-positive rhabdoid
meningioma cells exhibit SC-like features and have the capacity to
differentiate into adipocytes and osteocytes (39).
Various studies have demonstrated that CD105
overexpression is associated with chemoresistance. CD105 is rarely
detected in primary OC cells, but is expressed at an increased
level in platinum-resistant cells compared with primary untreated
tumour cells (2). Notably, the
protein is predominantly localised in the cytoplasm, which is
consistent with the features of a CSC-like population. CD105
inhibition increases cisplatin sensitivity and decreases OC cell
viability while enhancing apoptosis via induction of
double-stranded DNA damage (40). It
has also been suggested that chemotherapy stimulates CD90 and CD105
expression in hepatocellular carcinoma cells (41). These studies suggest that poor
prognosis in cancer is not solely due to the induction of tumour
angiogenesis by CD105 (42), but
that it is also caused by CD105 overexpression in tumour cells.
Accordingly, CD105 has been investigated as a potential therapeutic
target: One study identified that downregulation of CD105 decreased
tumourigenicity and GEM resistance, suggesting that CD105
expression not only distinguishes a CSC subpopulation but also
confers self-renewal capacity and contributes to chemoresistance in
renal cell carcinoma (43).
Additionally, the anti-CD105 antibody TRC105 inhibited tumour
growth and improved survival without off-target toxicity in a mouse
model of mammary carcinoma (44),
with similar results demonstrated in acute leukaemia (45). A previous clinical study that
enrolled 26 patients with hepatocellular carcinoma showed that
TRC105 combined with sorafenib was well tolerated at the
recommended single agent doses of both drugs (46), and another clinical trial that
enrolled 13 patients with urothelial carcinoma also found TRC105
was well tolerated, although the benefits of extended survival of
patients need further examination (47).
CD106, also known as vascular cell adhesion molecule
1, is a member of the immunoglobulin superfamily of transmembrane
proteins that bind integrin (48).
CD106 mediates leukocyte adhesion to endothelial cells and
downstream signalling cascades (49), and serves an important role in the
oncogenesis, tumour angiogenesis, tumour progression, and
metastasis of human cancer (50)
including colorectal carcinoma (51), non-Hodgkin lymphoma (52) and gastric carcinoma (53). CD106 is highly expressed in OC
(54), has been associated with
ovarian tumour growth, and may be a prognostic indicator and
potential therapeutic target (16).
CD106 was also demonstrated to be overexpressed in breast cancer
(55) and enhances breast cancer
cell metastasis to the lungs (56).
It has previously been demonstrated that CD106 is highly
overexpressed in lung cancer compared with normal lung tissue, and
that it is associated with poor survival. Additionally, the
invasive potential of lung cancer cells is significantly weakened
by CD106 silencing (57).
CD44 is a classic surface marker of SCs, which
promotes oncogenesis and tumour progression (58). Cells with this phenotype are more
likely to form tumours compared with those with alternate
phenotypes. A number of studies have suggested that CD44 is a
reliable cell surface marker for CSCs in gastric (59) and breast cancer (60), glioma (61), colon cancer (62) and OC (11). High CD44 expression is associated
with metastasis, recurrence, chemoresistance and survival rate in
OC, whereas its downregulation suppresses tumour cell proliferation
and metastasis and reverses chemoresistance (63). The present study demonstrated that OC
cells expressing CD105, CD44 and CD106 on their surface exhibited
greater invasive capabilities and drug resistance. Although the
results are consistent with the earlier studies, additional studies
are required to determine whether targeting these factors would be
effective for the treatment of OC.
In conclusion, the results from the present study
demonstrated that CD105, CD44 and CD106 were upregulated in
PTX-resistant OC cell lines and chemoresistant epithelial OC
tissues. This was associated with poor prognosis, distant
metastasis and early recurrence. At present, these results have
certain limitations, as they are only based on a PTX-resistant OC
cell line and its primary parent cell line, and also require
validation by knocking down CD105, CD44 or CD106 genes. However,
these positive results suggested future avenues of study, and other
PTX or platinum resistant cell lines will be examined in subsequent
experiments, and CD105 gene knockdown will be performed to study
the changes of invasiveness of OC3/TAX300 cells following
inhibition of the expression of CD105 gene and the tumourigenicity
in nude mice. Therefore, inhibiting CD105, CD44 or CD106 expression
has potential as an adjuvant therapy for OC. Additionally, as these
factors confer SC characteristics, investigating other CSCs markers
may provide a basis for targeted therapy and for predicting patient
prognosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Hospital
Subject of Beijing Shijitan Hospital, Capital Medical University
(Beijing, China: Grant no. 2014-C16).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ, BY and HZ performed the experiments. JZ and HL
performed the statistical analysis and wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Beijing Shijitan Hospital of Capital Medical
University (Beijing, China). Written informed consent was obtained
from all participants prior to surgery. All procedures were
performed in accordance with the Declaration of Helsinki.
Patient consent for publication
Written informed consent was obtained from all
participants prior to surgery.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steg AD, Bevis KS, Katre AA, Ziebarth A,
Dobbin ZC, Alvarez RD, Zhang K, Conner M and Landen CN: Stem cell
pathways contribute to clinical chemoresistance in ovarian cancer.
Clin Cancer Res. 18:869–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosen JM and Jordan CT: The increasing
complexity of the cancer stem cell paradigm. Science.
324:1670–1673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: Models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsieh CH, Hsiung SC, Yeh CT, Yen CF, Chou
YW, Lei WY, Pang ST, Chuang CK and Liao SK: Differential expression
of CD44 and CD24 markers discriminates the epithelioid from the
fibroblastoid subset in a sarcomatoid renal carcinoma cell line:
Evidence suggesting the existence of cancer stem cells in both
subsets as studied with sorted cells. Oncotarget. 8:15593–15609.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang XF, Weng DS, Pan K, Zhou ZQ, Pan QZ,
Zhao JJ, Tang Y, Jiang SS, Chen CL, Li YQ, et al:
Dendritic-cell-based immunotherapy evokes potent anti-tumor immune
responses in CD105+ human renal cancer stem cells. Mol Carcinog.
56:2499–2511. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J and Li H: Heterogeneity of tumor
chemosensitivity in ovarian epithelial cancer revealed using the
adenosine triphosphate-tumor chemosensitivity assay. Oncol Lett.
9:2374–2380. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muinao T, Deka Boruah HP and Pal M:
Diagnostic and prognostic biomarkers in ovarian cancer and the
potential roles of cancer stem cells-An updated review. Exp Cell
Res. 362:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alvero AB, Montagna MK, Holmberg JC,
Craveiro V, Brown DA and Mor G: Targeting the mitochondria
activates two independent cell death pathways in the ovarian cancer
stem cells. Mol Cancer Ther. 10:1385–1393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Slomiany MG, Dai L, Tolliver LB, Grass GD,
Zeng Y and Toole BP: Inhibition of functional hyaluronan-CD44
interactions in CD133-positive primary human ovarian carcinoma
cells by small hyaluronan oligosaccharides. Clin Cancer Res.
15:7593–7601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartakova A, Michalova K, Presl J, Vlasak
P, Kostun J and Bouda J: CD44 as a cancer stem cell marker and its
prognostic value in patients with ovarian carcinoma. J Obstet
Gynaecol. 38:110–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ho CM, Chang SF, Hsiao CC, Chien TY and
Shih DT: Isolation and characterization of stromal progenitor cells
from ascites of patients with epithelial ovarian adenocarcinoma. J
Biomed Sci. 19:232012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bussolati B, Bruno S, Grange C, Ferrando U
and Camussi G: Identification of a tumor-initiating stem cell
population in human renal carcinomas. FASEB J. 22:3696–3705. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang ZX, Han ZB, Ji YR, Wang YW, Liang L,
Chi Y, Yang SG, Li LN, Luo WF, Li JP, et al: CD106 identifies a
subpopulation of mesenchymal stem cells with unique
immunomodulatory properties. PLoS One. 8:e593542013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kokovay E, Wang Y, Kusek G, Wurster R,
Lederman P, Lowry N, Shen Q and Temple S: VCAM1 is essential to
maintain the structure of the SVZ niche and acts as an
environmental sensor to regulate SVZ lineage progression. Cell Stem
Cell. 11:220–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang J, Zhang J, Li H, Lu Z, Shan W,
Mercado-Uribe I and Liu J: VCAM1 expression correlated with
tumorigenesis and poor prognosis in high grade serous ovarian
cancer. Am J Transl Res. 5:336–346. 2013.PubMed/NCBI
|
|
17
|
Zhang J, Zhao J, Zhang W, Liu G, Yin D, Li
J, Zhang S and Li H: Establishment of paclitaxel-resistant cell
line and the underlying mechanism on drug resistance. Int J Gynecol
Cancer. 22:1450–1456. 2012.PubMed/NCBI
|
|
18
|
Zhang L, Liu P, Li H and Xue F: Effect of
histone deacetylase inhibitors on cell apoptosis and expression of
the tumor suppressor genes RUNX3 and ARHI in ovarian tumors. Mol
Med Rep. 7:1705–1709. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Yin D and Li H: hMSH2 expression
is associated with paclitaxel resistance in ovarian carcinoma, and
inhibition of hMSH2 expression in vitro restores paclitaxel
sensitivity. Oncol Rep. 32:2199–2206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Benson DA, Cavanaugh M, Clark K,
Karsch-Mizrachi I, Lipman DJ, Ostell J and Sayers EW: GenBank.
Nucleic Acids Res 41 (Database Issue). D36–D42. 2013.
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Daly MB, Pilarski R, Berry M, Buys SS,
Farmer M, Friedman S, Garber JE, Kauff ND, Khan S, Klein C, et al:
NCCN Guidelines insights. Genetic/Familial High-risk assessment:
Breast and ovarian. version 2.2017. J Natl Compr Canc Netw.
15:9–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ozga M, Aghajanian C, Myers-Virtue S,
McDonnell G, Jhanwar S, Hichenberg S and Sulimanoff I: A systematic
review of ovarian cancer and fear of recurrence. Palliat Support
Care. 13:1771–1780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fung-Kee-Fung M, Oliver T, Elit L, Oza A,
Hirte HW and Bryson P: Optimal chemotherapy treatment for women
with recurrent ovarian cancer. Curr Oncol. 14:195–208. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barbara NP, Wrana JL and Letarte M:
Endoglin is an accessory protein that interacts with the signaling
receptor complex of multiple members of the transforming growth
factor-beta superfamily. J Biol Chem. 274:584–594. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nassiri F, Cusimano MD, Scheithauer BW,
Rotondo F, Fazio A, Yousef GM, Syro LV, Kovacs K and Lloyd RV:
Endoglin (CD105): A review of its role in angiogenesis and tumor
diagnosis, progression and therapy. Anticancer Res. 31:2283–2290.
2011.PubMed/NCBI
|
|
27
|
Dallas NA, Samuel S, Xia L, Fan F, Gray
MJ, Lim SJ and Ellis LM: Endoglin (CD105): A marker of tumor
vasculature and potential target for therapy. Clin Cancer Res.
14:1931–1937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taskiran C, Erdem O, Onan A, Arisoy O,
Acar A, Vural C, Erdem M, Ataoglu O and Guner H: The prognostic
value of endoglin (CD105) expression in ovarian carcinoma. Int J
Gynecol Cancer. 16:1789–1793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saad RS, El-Gohary Y, Memari E, Liu YL and
Silverman JF: Endoglin (CD105) and vascular endothelial growth
factor as prognostic markers in esophageal adenocarcinoma. Hum
Pathol. 36:955–961. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chien CY, Su CY, Hwang CF, Chuang HC, Chen
CM and Huang CC: High expressions of CD105 and VEGF in early oral
cancer predict potential cervical metastasis. J Surg Oncol.
94:413–417. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cho T, Shiozawa E, Urushibara F, Arai N,
Funaki T, Takehara Y, Tazawa S, Misawa M, Homma M, Norose T, et al:
The role of microvessel density, lymph node metastasis, and tumor
size as prognostic factors of distant metastasis in colorectal
cancer. Oncol Lett. 13:4327–4333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fonsatti E and Maio M: Highlights on
endoglin (CD105): From basic findings towards clinical applications
in human cancer. J Transl Med. 2:182004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ding S, Li C, Lin S, Yang Y, Liu D, Han Y,
Zhang Y, Li L, Zhou L and Kumar S: Comparative evaluation of
microvessel density determined by CD34 or CD105 in benign and
malignant gastric lesions. Hum Pathol. 37:861–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gromova P, Rubin BP, Thys A, Cullus P,
Erneux C and Vanderwinden JM: ENDOGLIN/CD105 is expressed in KIT
positive cells in the gut and in gastrointestinal stromal tumors. J
Cell Mol Med. 16:306–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ribeiro OD, Canedo NH and Pannain VL:
Immunohistochemical angiogenic biomarkers in hepatocellular
carcinoma and cirrhosis: Correlation with pathological features.
Clinics (Sao Paulo). 71:639–643. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Davidson B, Stavnes HT, Førsund M, Berner
A and Staff AC: CD105 (Endoglin) expression in breast carcinoma
effusions is a marker of poor survival. Breast. 19:493–498. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Litwiniuk M, Niemczyk K, Niderla-Bielińska
J, Łukawska-Popieluch I and Grzela T: Soluble endoglin (CD105)
serum level as a potential marker in the management of head and
neck paragangliomas. Ann Otol Rhinol Laryngol. 126:717–721. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bock AJ, Tuft Stavnes H, Kærn J, Berner A,
Staff AC and Davidson B: Endoglin (CD105) expression in ovarian
serous carcinoma effusions is related to chemotherapy status.
Tumour Biol. 32:589–596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu D, Wang X, Mao Y and Zhou L:
Identification of CD105 (endoglin)-positive stem-like cells in
rhabdoid meningioma. J Neurooncol. 106:505–517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ziebarth AJ, Nowsheen S, Steg AD, Shah MM,
Katre AA, Dobbin ZC, Han HD, Lopez-Berestein G, Sood AK, Conner M,
et al: Endoglin (CD105) contributes to platinum resistance and is a
target for tumor-specific therapy in epithelial ovarian cancer.
Clin Cancer Res. 19:170–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nomura Y, Yamashita T, Oishi N, Nio K,
Hayashi T, Yoshida M, Hayashi T, Hashiba T, Asahina Y, Okada H, et
al: De novo emergence of mesenchymal stem-like CD105+ cancer cells
by cytotoxic agents in human hepatocellular carcinoma. Transl
Oncol. 10:184–189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Minhajat R, Mori D, Yamasaki F, Sugita Y,
Satoh T and Tokunaga O: Organ-specific endoglin (CD105) expression
in the angiogenesis of human cancers. Pathol Int. 56:717–723. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu J, Guan W, Liu P, Dai J, Tang K, Xiao
H, Qian Y, Sharrow AC, Ye Z, Wu L and Xu H: Endoglin is essential
for the maintenance of self-renewal and chemoresistance in renal
cancer stem cells. Stem Cell Rep. 9:464–477. 2017. View Article : Google Scholar
|
|
44
|
Ehlerding EB, Lacognata S, Jiang D,
Ferreira CA, Goel S, Hernandez R, Jeffery JJ, Theuer CP and Cai W:
Targeting angiogenesis for radioimmunotherapy with a
177Lu-labeled antibody. Eur J Nucl Med Mol Imaging.
45:123–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dourado KMC, Baik J, Oliveira VKP,
Beltrame M, Yamamoto A, Theuer CP, Figueiredo CAV, Verneris MR and
Perlingeiro RCR: Endoglin: A novel target for therapeutic
intervention in acute leukemias revealed in xenograft mouse models.
Blood. 129:2526–2536. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Duffy AG, Ma C, Ulahannan SV, Rahma OE,
Makarova-Rusher O, Cao L, Yu Y, Kleiner DE, Trepel J, Lee MJ, et
al: Phase I and preliminary phase II study of TRC105 in combination
with sorafenib in hepatocellular carcinoma. Clin Cancer Res.
23:4633–4641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Apolo AB, Karzai FH, Trepel JB, Alarcon S,
Lee S, Lee MJ, Tomita Y, Cao L, Yu Y, Merino MJ, et al: A phase II
clinical trial of TRC105 (anti-endoglin antibody) in adults with
advanced/metastatic urothelial carcinoma. Clin Genitourin Cancer.
15:77–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Malhotra S and Kincade PW: Canonical Wnt
pathway signaling suppresses VCAM-1 expression by marrow stromal
and hematopoietic cells. Exp Hematol. 37:19–30. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yamada Y, Arao T, Matsumoto K, Gupta V,
Tan W, Fedynyshyn J, Nakajima TE, Shimada Y, Hamaguchi T, Kato K,
et al: Plasma concentrations of VCAM-1 and PAI-1: A predictive
biomarker for post-operative recurrence in colorectal cancer.
Cancer Sci. 101:1886–1890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yurkovetsky Z, Skates S, Lomakin A, Nolen
B, Pulsipher T, Modugno F, Marks J, Godwin A, Gorelik E, Jacobs I,
et al: Development of a multimarker assay for early detection of
ovarian cancer. J Clin Oncol. 28:2159–2166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dymicka-Piekarska V, Guzinska-Ustymowicz
K, Kuklinski A and Kemona H: Prognostic significance of adhesion
molecules (sICAM-1, sVCAM-1) and VEGF in colorectal cancer
patients. Thromb Res. 129:e47–e50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shah N, Cabanillas F, McIntyre B, Feng L,
McLaughlin P, Rodriguez MA, Romaguera J, Younes A, Hagemeister FB,
Kwak L and Fayad L: Prognostic value of serum CD44, intercellular
adhesion molecule-1 and vascular cell adhesion molecule-1 levels in
patients with indolent non-Hodgkin lymphomas. Leuk Lymphoma.
53:50–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ding YB, Chen GY, Xia JG, Zang XW, Yang HY
and Yang L: Association of VCAM-1 overexpression with oncogenesis,
tumor angiogenesis and metastasis of gastric carcinoma. World J
Gastroenterol. 9:1409–1414. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Slack-Davis JK, Atkins KA, Harrer C,
Hershey ED and Conaway M: Vascular cell adhesion molecule-1 is a
regulator of ovarian cancer peritoneal metastasis. Cancer Res.
69:1469–1476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang PC, Weng CC, Hou YS, Jian SF, Fang
KT, Hou MF and Cheng KH: Activation of VCAM-1 and its associated
molecule CD44 leads to increased malignant potential of breast
cancer cells. Int J Mol Sci. 15:3560–3579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen Q, Zhang XH and Massagué J:
Macrophage binding to receptor VCAM-1 transmits survival signals in
breast cancer cells that invade the lungs. Cancer Cell. 20:538–549.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kim MR, Jang JH, Park CS, Kim TK, Kim YJ,
Chung J, Shim H, Nam IH, Han J and Lee S: A human antibody that
binds to the sixth Ig-like domain of VCAM-1 blocks lung cancer cell
migration in vitro. Int J Mol Sci. 18(pii): E5662017.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yan Y, Zuo X and Wei D: Concise review:
Emerging role of CD44 in cancer stem cells: A Promising biomarker
and therapeutic target. Stem Cells Transl Med. 4:1033–1043. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hu J, Li G, Zhang P, Zhuang X and Hu G: A
CD44v+ subpopulation of breast cancer stem-like cells
with enhanced lung metastasis capacity. Cell Death Dis.
8:e26792017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pietras A, Katz AM, Ekström EJ, Wee B,
Halliday JJ, Pitter KL, Werbeck JL, Amankulor NM, Huse JT and
Holland EC: Osteopontin-CD44 signaling in the glioma perivascular
niche enhances cancer stem cell phenotypes and promotes aggressive
tumor growth. Cell Stem Cell. 14:357–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Su YJ, Lai HM, Chang YW, Chen GY and Lee
JL: Direct reprogramming of stem cell properties in colon cancer
cells by CD44. EMBO J. 30:3186–3199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gao Y, Foster R, Yang X, Feng Y, Shen JK,
Mankin HJ, Hornicek FJ, Amiji MM and Duan Z: Up-regulation of CD44
in the development of metastasis, recurrence and drug resistance of
ovarian cancer. Oncotarget. 6:9313–9326. 2015. View Article : Google Scholar : PubMed/NCBI
|