Introduction
Laryngeal cancer is one of the most common
malignancies in the respiratory system globally (1), and the number of new cases of laryngeal
cancer has reached 3.0 per 100,000 people annually between 2011 and
2015 due to excessive tobacco and alcohol consumption (2–4).
Furthermore, exposure to asbestos, dust and polycyclic aromatic
hydrocarbons is associated with an increased risk of laryngeal
squamous cell carcinoma (LSCC) development (3,5,6). Despite the fact that therapeutic
strategies, including surgical techniques, chemotherapeutic drugs
and radiotherapy, have advanced, the 5-year overall survival rate
of patients with LSCC remains as low as 61% (7). The pathogenesis of LSCC remains unclear
and understanding the factors contributing to the development and
progression is crucial for the discovery of novel therapeutic
targets, in addition to the management of patients with LSCC.
Epithelial cell transforming sequence 2 (ECT2) is a
guanine nucleotide exchange factor and can regulate the process of
cell cycling and cytokinesis (8).
ECT2 contains two BRCA1 C Terminus (BRCT) domains within the
N-terminus, including a DBL-homology domain and a pleckstrin
homology domain (9). Previous
studies reported that ECT2 is localized in the actin cortex and
central spindles, as ECT2 is crucial for the cleavage furrow
formation during cytokinesis (10,11). The
upregulated ECT2 expression and its misplacement have been
indicated to be associated with the tumorigenesis and the
development of numerous malignant tumors, including lung cancer,
ovarian cancer and primary brain cancer (12–14), in
addition to poor survival in patients with colorectal cancer or
lung adenocarcinoma (15,16). However, to the best of our knowledge,
there is no information on the levels of ECT2 expression and its
role in the development and progression of LSCC.
In the present study, ECT2 expression was analyzed
in 81 LSCC specimens and adjacent non-tumor tissues, and the
association between the levels of ECT2 expression and clinical
characteristics of patients with LSCC was examined.
Patients and methods
LSCC tissue specimens
A total of 81 patients (79 males and 2 females,
median age of 61.3 years old, ranging from 41 to 76 years old) with
LSCC were recruited at the in-patient service of the Department of
Otolaryngology, Head and Neck Surgery of The Affiliated Hospital of
SouthwestMedical University (Luzhou, China) between September 2012
and March 2015. Individual patients with LSCC were diagnosed and
staged, according to the criteria of the Union for International
Cancer Control and the Tumor-Node-Metastasis (TNM) classification
system (2012) (17). Patients
underwent partial (62 patients) or total laryngectomy (19
patients), and their respective LSCC samples were collected. The
inclusion criteria included that individual patients had complete
clinical data and did not receive antitumor radiotherapy and
chemotherapy. Otherwise, individual patients with incomplete
medical record or received any preoperative antitumor treatment
were excluded. These 81 pairs of human LSCC and their adjacent
non-tumor tissue specimens were used for immunohistochemistry.
Finally, 25 pairs of human LSCC and their adjacent non-tumor
tissues were randomly selected for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR),
based on positive ECT2 expression by immunohistochemistry. Written
informed consent was obtained from individual subjects
participating in the present study. The experimental protocol was
approved by the Ethnic Committee of The Affiliated Hospital of
Southwest Medical University. One part of the tissue samples was
fixed in 10% neutral buffered formalin for 24 h at room temperature
and paraffin-embedded. The remaining tissues were immediately
frozen in liquid nitrogen, and stored at −80°C until RNA and
protein extraction.
The 81 patients with LSCC were followed-up by
telephone communication or out-patient visiting until April 31,
2018. Their overall survival time was defined as the time between
surgery and mortality from LSCC.
RT-qPCR
Total RNA was extracted from individual tissue
samples with TRIzol®(Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocols, and the RNA (1 µg per sample) was
reversely transcribed into cDNA using a PrimeScript™ RT-PCR kit
(Takara Bio, Inc., Otsu, Japan), according to the manufacturer's
protocols. The relative expression levels of ECT2 to the control
GAPDH mRNA transcripts were determined by RT-qPCR using a SYBR™
Green PCR Master mix (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and specific primers. The sequences of the primers were as
follows: ECT2 (119 bp) forward, 5′-GCCATAATGCTTTTGCCTTGCTTG-3′ and
reverse 5′-TCGACACAGCATCTTTAGCCAGTTT-3′; GAPDH (177 bp) forward,
5′-GGGCCAAAAGGGTCATCATCTC-3′and reverse 5′-GCCCTTCCACGATGCCAAA-3′.
The PCR reactions were performed in triplicate and the
thermocycling conditions were as follows: 95°C for 5 min; and 40
cycles of 95°C for 30 sec, 57°C for 30 sec and 72°C for 30 sec. The
data were analyzed by the 2−ΔΔCq method (18).
Immunohistochemistry
The tissues were fixed in 10% neutral buffered
formalin for 24 h at room temperature and then dehydrated in an
ascending ethanol series and cleared with 3 changes of xylene,
followed by paraffin-embedding. The paraffin-embedded tissue
sections (thickness, 4 µm) were de-paraffinized, rehydrated in a
descending ethanol series and treated with 0.3%
H2O2. The sections were heated in 1% citrate
buffer for 5 min at 90–100°C for antigen retrieval, and blocked
with 10% normal goat serum (Gemini Bio-Products, Sacramento, CA,
USA) for 1 h at room temperature. Subsequent to being washed, the
sections were incubated with rabbit anti-ECT2 antibodies (cat. no.
bs-4102R; 1:50 dilution; BIOSS, Beijing, China) (19,20)
overnight at 4°C. The negative control sections were incubated with
PBS. After being washed three times with PBST, the bound antibodies
were detected with horseradish peroxidase-conjugated anti-IgG (cat.
no. 111-035-144; 1:5,000 dilution; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) and immunoreactive signals
were developed using diaminobenzidine tetrachloride, followed by
counterstaining with haematoxylin for 2 min at room temperature.
The staining was observed in a light microscope (×200,
magnification). The levels of ECT2 expression in 81 LSCC samples
were semi-quantitative analyzed by two pathologists (Department of
Pathology, The Affiliated Hospital of Southwest Medical University,
Luzhou, China) in a blinded manner. Briefly, the staining
intensities were scored as follows: 0, no staining; 1, weak
staining; 2, moderate staining; and 3, strong staining (21). The percentage of positive cells was
recorded as 0, ≤5%; 1, 6–25%; 2, 25–50%; and 3, >50%. The final
scores were calculated by combining the staining intensity score
and the positive percentage score. A score of >3 was regarded as
high expression, while anything ≤3 was considered as low
expression.
Statistical analysis
Data are presented as the mean ± standard deviation.
The experiments were repeated three times. SPSS statistical
software package 23.0 (IBM Corp., Armonk, NY, USA) was applied in
statistical analysis. The difference between groups was analyzed by
paired Student's t-test, χ2 test or Fisher's exact test
when applicable. Survival time was estimated by Kaplan-Meier method
and analyzed by the log-rank test. The potential correlation of
ECT2 mRNA transcripts and anti-ECT2 immunochemistry staining was
analyzed by Pearson's correlation. P<0.05 was considered to
indicated a statistically significant difference.
Results
ECT2 expression was upregulated in
LSCC tissues and correlated with poor differentiation of LSCC
To determine the potential role of ECT2 in the
development and progression of LSCC, 81 patients with LSCC were
recruited and their demographic and clinical characteristics are
presented in Table I. The expression
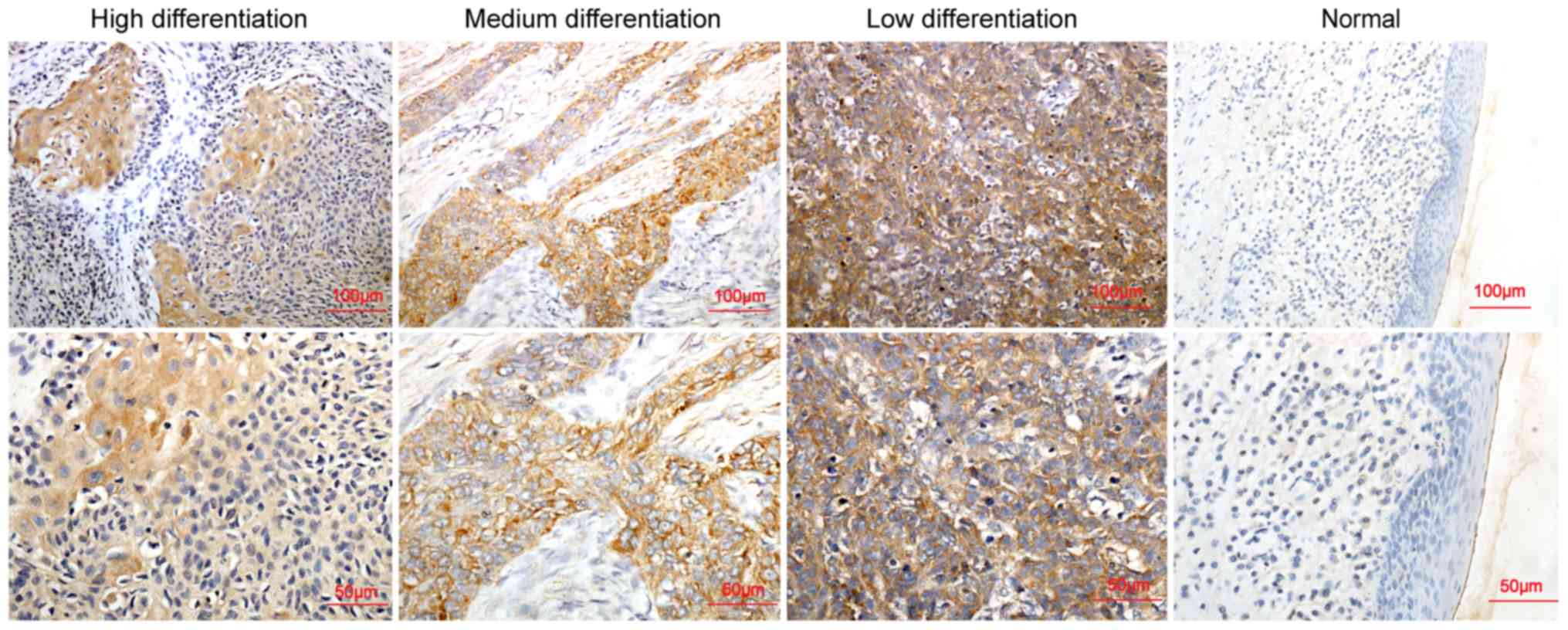
levels of ECT2 expression were examined by immunohistochemistry. As
indicated in Fig. 1, ECT2 protein
was primarily expressed in the cytoplasm of tumor cells. A limited
expression of ECT2 was detected in the matched adjacent non-tumor
tissues, while an upregulated ECT2 protein expression was detected
in LSCC, particularly in the low differentiated tumor tissues
(P=0.039). Stratification analysis indicated that the increased
levels of ECT2 expression were significantly associated with the
medium and low differentiation of LSCC (P=0.039; Table I), increased TNM stages (P=0.016),
increased overall stages (P=0.007) and lymph node metastasis
(P=0.04); however, it was not significantly associated with sex,
age, primary tumor site and smoking status in this population
(Table I).
 | Table I.Association of ECT2 expression with
clinicopathological characteristics of patients with LSCC. |
Table I.
Association of ECT2 expression with
clinicopathological characteristics of patients with LSCC.
|
|
| ECT2 expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | n | High (%) | Low (%) | P-value |
|---|
| Type of
organization |
|
|
| <0.001 |
| LSCC
tissue | 81 | 56 (69.37) | 25 (31.63) |
|
| Adjacent
non-tumor tissue | 81 | 21 (27.16) | 60 (72.84) |
|
| Sex |
|
|
|
0.553 |
| Male | 79 | 55 (69.62) | 24 (30.38) |
|
|
Female | 2 | 1 (50.00) | 1 (50.00) |
|
| Age (years) |
|
|
|
0.109 |
|
<60 | 38 | 25 (65.78) | 13 (33.22) |
|
|
60–70 | 35 | 25 (71.43) | 7 (28.57) |
|
|
>70 | 8 | 6 (75.00) | 2 (25.00) |
|
| Degree of
differentiation |
|
|
|
0.039 |
| High | 42 | 23 (54.76) | 19 (45.24) |
|
|
Medium | 35 | 29 (82.86) | 6 (17.14) |
|
| Low | 4 | 4 (100.00) | 0 (0.00) |
|
| TNM staging (17) |
|
|
|
|
|
Tis+T1 | 17 | 8 (47.71) | 9 (52.29) |
0.002 |
| T2 | 32 | 21 (65.63) | 11 (34.47) |
|
| T3 | 21 | 18 (86.87) | 3 (13.33) |
|
| T4 | 11 | 9 (81.82) | 2 (18.18) |
|
|
Tis+T1+T2 | 49 | 29 (59.18) | 20 (40.82) |
0.016 |
|
T3+T4 | 32 | 27 (84.38) | 5 (15.62) |
|
| Lymph node
metastasis |
|
|
|
0.040 |
|
Yes | 22 | 19 (86.36) | 3 (13.64) |
|
| No | 59 | 37 (62.17) | 22 (37.29) |
|
| Smoking |
|
|
|
0.492 |
|
Yes | 53 | 38 (71.71) | 3 (13.64) |
|
| No | 28 | 18 (64.28) | 22 (37.29) |
|
| Overall staging
(17) |
|
|
|
0.003 |
| I | 17 | 8 (44.71) | 9 (55.29) |
|
| II | 30 | 19 (63.33) | 20 (37.67) |
|
|
III | 23 | 20 (86.95) | 2 (23.05) |
|
| IV | 11 | 9 (81.82) | 2 (18.18) |
|
|
I+II | 47 | 27 (59.79) | 20 (40.21) |
0.007 |
|
III+IV | 34 | 29 (85.29) | 5 (14.71) |
|
| Glottic | 58 | 40 (68.97) | 18 (31.03) |
0.958 |
| Supraglottic | 23 | 16 (69.09) | 7 (30.91) |
|
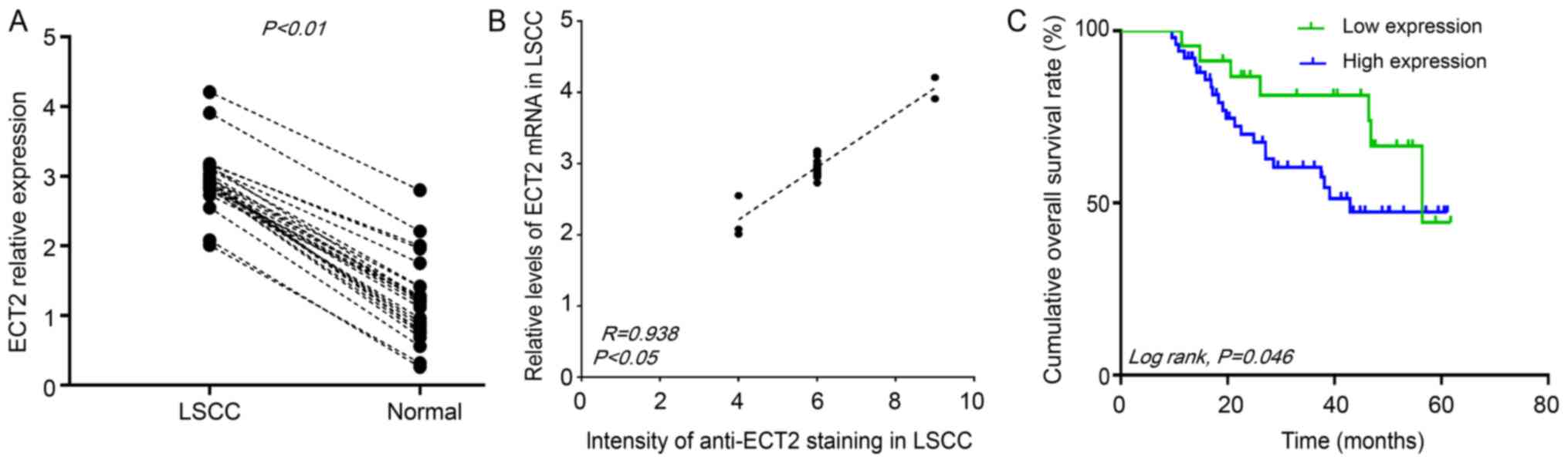
To further validate the data, the expression levels
of ECT2 mRNA transcripts were determined in 25 pairs of LSCC and
matched non-tumor tissue samples by RT-qPCR. The relative
expression levels of ECT2 mRNA transcripts in the LSCC tissues were
significantly increased, compared with the matched adjacent
non-tumor tissues (P<0.01; Fig.
2A). The relative expression levels of ECT2 mRNA transcripts in
25 LSCC were positively correlated with the intensity of anti-ECT2
staining (R=0.938; P<0.05; Fig.
2B). Therefore, increased levels of ECT2 expression are
associated with lower differentiation of LSCC and may contribute to
the development and progression of poorly differentiated LSCC.
ECT2 expression was negatively
correlated with overall survival time of LSCC patients
A total of 81 patients with LSCC were followed-up.
Among them, seven cases (8.4%) were lost for follow-up, due to
personal reasons. Stratification analysis indicated that the
overall survival time period of patients with increased ECT2
expressing LSCC was significantly reduced, compared with those with
reduced ECT2 expressing LSCC (P=0.046; Fig. 2C). Therefore, increased levels of
ECT2 are associated with poor prognosis of LSCC.
Discussion
LSCC invasion and metastasis are leading causes of
mortality (22). However, to the
best of our knowledge, no reliable prognostic biomarkers for LSCC
have been identified. ECT2 is physiologically expressed primarily
in the nucleus and spreads into the cytoplasm of cells during
mitosis (13). ECT2 can be misplaced
in the cytoplasm, where ECT2 binds to the protein kinase
Cι/polarity protein 6α (PKCι/Par6α) complex and is phosphorylated
by PKCι to activate Rac family small GTPase 1 (Rac1), and promote
the proliferation and invasion of non-small cell lung carcinoma
(NSCLC) cells (23). Furthermore,
PKCι or Par6α silencing also promotes the redistribution of ECT2
from the cytoplasm to nucleus (24).
Therefore, PKCι/Par6α complex in NSCLC regulates ECT2 cytoplasmic
retention to induce phosphorylation and activation of downstream
Rac1, conferring its oncogenic activity (24). In the present study, it was indicated
that the upregulated ECT2 expression was primarily located in the
cytoplasm of LSCC. This indicated that upregulated ECT2 expression
in the cytoplasm may contribute to the development and progression
of LSCC through similar mechanisms.
In the present study, it was indicated that
upregulated ECT2 expression was associated with reduced
differentiation, increased TNM stage, and lymph node metastasis of
LSCC in the sample population. It has been reported that lowly
differentiated tumors are associated with aggressive progression
and poor prognosis in numerous malignant tumor types, including
epithelial ovarian carcinoma, bladder squamous cell carcinoma and
thyroid carcinoma (25–28). Because up-regulation of ECT2 and its
misplacement are demonstrated in lowly differentiated LSCC, ECT2
may serve as a potential biomarker for the prognosis of LSCC.
Increased levels of ECT2 expression were associated
significantly with poor overall survival time period of patients
with LSCC. The data of the present study is in accordance to
previous reports on other malignant tumors including lung cancer,
colorectal cancer, and ovarian cancer (15,16,29,30) and
indicate that ECT2 may serve as an oncogenic factor to promote the
progression of LSCC. Therefore, an increased ECT2 expression is a
valuable biomarker for differentially diagnosis and prognosis of
LSCC.
There are a number of limitations in the present
study, including small sample size. Therefore, larger sample sizes
are required in order to examine the potential molecular mechanisms
underlying the action of ECT2 in promoting the progression of
LSCC.
Acknowledgements
The authors thank their colleagues in Chongqing
Medical University (Chongqing, China) and Southwest Medical
University (Luzhou, China) for their suggestions and critical
reading of the manuscript.
Funding
No funding was received.
Availability of data and materials
All data from this study are included in the
article.
Authors' contributions
HYC, LY and LZJ were responsible for the conception
and design of the present study. LZ, BL, TYW, YLL and TJZ collected
and analyzed the data. LZ, GQ, BL and TJZ analyzed and interpreted
the data. LZ wrote the manuscript. HYC, LY, GQ, LZJ, TJZ, BL, TYW
and YLL critically revised the manuscript for important
intellectual content. All authors gave intellectual input to the
study and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Affiliated Hospital of Southwest Medical University. All
procedures performed in studies involving human participants were
in accordance with the ethical standards of the institutional and
national research committee and with the 1964 Helsinki declaration
and its later amendments or comparable ethical standards. Written
informed consent was obtained from all individual participants in
the study.
Patient consent for publication
Patients provided written informed consent for the
publication of the data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Bray F, Pisani P and Parkin DM:
GLOBOCAN 2000: Cancer incidence, mortality and prevalence
worldwide, version 1.0. IARC CancerBase No. 5. Lyon. (IARCPress).
2001.
|
|
2
|
Ramroth H, Dietz A and Becher H:
Interaction effects and population-attributable risks for smoking
and alcohol on laryngeal cancer and its subsites. A case-control
study from Germany. Methods Inf Med. 43:499–504. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shangina O, Brennan P, Szeszenia-Dabrowska
N, Mates D, Fabiánová E, Fletcher T, t'Mannetje A, Boffetta P and
Zaridze D: Occupational exposure and laryngeal and hypopharyngeal
cancer risk in central and eastern Europe. Am J Epidemiol.
164:367–375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Talamini R, Bosetti C, La Vecchia C, Dal
Maso L, Levi F, Bidoli E, Negri E, Pasche C, Vaccarella S, Barzan
L, et al: Combined effect of tobacco and alcohol on laryngeal
cancer risk: a case-control study. Cancer Causes Control.
13:957–964. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Becher H, Ramroth H, Ahrens W, Risch A,
Schmezer P and Dietz A: Occupation, exposure to polycyclic aromatic
hydrocarbons and laryngeal cancer risk. Int J Cancer. 116:451–457.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramroth H, Dietz A, Ahrens W and Becher H:
Occupational wood dust exposure and the risk of laryngeal cancer: a
population based case-control study in Germany. Am J Ind Med.
51:648–655. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nachalon Y, Cohen O, Alkan U, Shvero J and
Popovtzer A: Characteristics and outcome of laryngeal squamous cell
carcinoma in young adults. Oncol Lett. 13:1393–1397. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scoumanne A and Chen X: The epithelial
cell transforming sequence 2, a guanine nucleotide exchange factor
for Rho GTPases, is repressed by p53 via protein methyltransferases
and is required for G1-S transition. Cancer Res. 66:6271–6279.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miki T, Smith CL, Long JE, Eva A and
Fleming TP: Oncogene ect2 is related to regulators of small
GTP-binding proteins. Nature. 362:462–465. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yüce O, Piekny A and Glotzer M: An
ECT2-centralspindlin complex regulates the localization and
function of RhoA. J Cell Biol. 170:571–582. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tatsumoto T, Xie X, Blumenthal R, Okamoto
I and Miki T: Human ECT2 is an exchange factor for Rho GTPases,
phosphorylated in G2/M phases, and involved in cytokinesis. J Cell
Biol. 147:921–928. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sano M, Genkai N, Yajima N, Tsuchiya N,
Homma J, Tanaka R, Miki T and Yamanaka R: Expression level of ECT2
proto-oncogene correlates with prognosis in glioma patients. Oncol
Rep. 16:1093–1098. 2006.PubMed/NCBI
|
|
13
|
Saito S, Liu XF, Kamijo K, Raziuddin R,
Tatsumoto T, Okamoto I, Chen X, Lee CC, Lorenzi MV, Ohara N, et al:
Deregulation and mislocalization of the cytokinesis regulator ECT2
activate the Rho signaling pathways leading to malignant
transformation. J Biol Chem. 279:7169–7179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matthews HK, Delabre U, Rohn JL, Guck J,
Kunda P and Baum B: Changes in Ect2 localization couple
actomyosin-dependent cell shape changes to mitotic progression. Dev
Cell. 23:371–383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo Y, Qin SL, Mu YF, Wang ZS, Zhong M and
Bian ZQ: Elevated expression of ECT2 predicts unfavorable prognosis
in patients with colorectal cancer. Biomed Pharmacother.
73:135–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murata Y, Minami Y, Iwakawa R, Yokota J,
Usui S, Tsuta K, Shiraishi K, Sakashita S, Satomi K, Iijima T, et
al: ECT2 amplification and overexpression as a new prognostic
biomarker for early-stage lung adenocarcinoma. Cancer Sci.
105:490–497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patel SG and Shah JP: TNM staging of
cancers of the head and neck: Striving for uniformity among
diversity. CA Cancer J Clin. 55:242–258; quiz 261–262, 264. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang HB, Yan HC and Liu Y: Clinical
significance of ECT2 expression in tissue and serum of gastric
cancer patients. Clin Transl Oncol. 18:735–742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo Z, Chen X, Du T, Zhu D, Lai Y, Dong W,
Wu W, Lin C, Liu L and Huang H: Elevated levels of epithelial cell
transforming sequence 2 predicts poor prognosis for prostate
cancer. Med Oncol. 34:132017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rizzardi AE, Johnson AT, Vogel RI,
Pambuccian SE, Henriksen J, Skubitz AP, Metzger GJ and Schmechel
SC: Quantitative comparison of immunohistochemical staining
measured by digital image analysis versus pathologist visual
scoring. Diagn Pathol. 7:422012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.0, Cancer incidence and mortality
worldwide: IARC CancerBase No. 11. International Agency for
Research on Cancer. (Lyon, France). 2013.
|
|
23
|
Justilien V, Jameison L, Der CJ, Rossman
KL and Fields AP: Oncogenic activity of Ect2 is regulated through
protein kinase C iota-mediated phosphorylation. J Biol Chem.
286:8149–8157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Justilien V and Fields AP: Ect2 links the
PKCiota-Par6alpha complex to Rac1 activation and cellular
transformation. Oncogene. 28:3597–3607. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vergote I, De Brabanter J, Fyles A,
Bertelsen K, Einhorn N, Sevelda P, Gore ME, Kaern J, Verrelst H,
Sjövall K, et al: Prognostic importance of degree of
differentiation and cyst rupture in stage I invasive epithelial
ovarian carcinoma. Lancet. 357:176–182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ostergaard M, Rasmussen HH, Nielsen HV,
Vorum H, Orntoft TF, Wolf H and Celis JE: Proteome profiling of
bladder squamous cell carcinomas: Identification of markers that
define their degree of differentiation. Cancer Res. 57:4111–4117.
1997.PubMed/NCBI
|
|
27
|
Pollina L, Pacini F, Fontanini G, Vignati
S, Bevilacqua G and Basolo F: bcl-2, p53 and proliferating cell
nuclear antigen expression is related to the degree of
differentiation in thyroid carcinomas. Br J Cancer. 73:139–143.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cook DR, Rossman KL and Der CJ: Rho
guanine nucleotide exchange factors: Regulators of Rho GTPase
activity in development and disease. Oncogene. 33:4021–4035. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mack NA and Georgiou M: The
interdependence of the Rho GTPases and apicobasal cell polarity.
Small GTPases. 5:102014. View Article : Google Scholar : PubMed/NCBI
|