Introduction
Tongue cancer is one of the most common oral
malignant tumors, and its pathological type is mainly squamous
epithelial cells. It has high malignancy and develops rapidly
(1). At present, tongue cancer is
still treated by surgical resection combined with radiotherapy and
chemotherapy. However, there are rich blood and lymph node tissues
in the tongue, so tongue cancer has a higher recurrence and
metastasis rate, resulting in a poor prognosis and with less than
50% 5-year survival rate in patients (2). The surgical resection rate of tongue
cancer has been significantly increased in recent years, but high
recurrence rate and metastasis remain. There is related literature
(3) reporting that the highest lymph
node metastasis rate of tongue cancer is 80%. Therefore, it is of
great clinical significance to improve the survival rate of
patients to effectively improve the recurrence and metastasis rate
of tongue cancer by radiotherapy and chemotherapy.
Adriamycin (ADM), a broad-spectrum aminoglycoside
antitumor drug, is widely used in clinical chemotherapy due to its
high anticancer activity (4). As a
drug with non-specific cell cycle, ADM has a unique role in
inhibiting the growth of tumor cells and promoting their apoptosis.
It also increases the sensitivity of cancer cells to radiotherapy,
so as to better exert its anticancer effect (5). ADM is the first choice drug for tongue
cancer chemotherapy, but it also has myelosuppression,
cardiotoxicity and other severe adverse reactions. This causes dose
reduction and even discontinuation in many patients due to the fact
that they are intolerant to those adverse reactions caused by ADM.
As a result, the clinical efficacy is reduced (6). Metformin (MET) is a clinically common
oral hypoglycemic drug for the treatment of type 2 diabetes. It is
widely used in clinical practice because of its low price, good
safety and less adverse reactions (7). There is a previous study (8) reporting that MET has an antitumor
effect. In the investigation of epidemiology (9), it is also found that diabetic patients
taking MET for a long time have a lower risk of cancer and
tumor-related mortality than patients taking other hypoglycemic
drugs. In the study by Wang et al (10), MET was found to inhibit the
proliferation in vitro and in vivo of tongue cancer
HSC-3 and HSC-4 cells. There is a study showing that
chemotherapeutic drugs combined with MET enhance the efficacy in
liver (11), ovarian (12) and breast cancer (13).
Currently, there are few reports on the effect of
MET combined with chemotherapeutic drugs on the proliferation,
apoptosis, invasion and migration of human tongue cancer cells.
Therefore, in this study, the effects of ADM alone, MET alone and
ADM+MET groups (their drug combination) on the proliferation,
apoptosis, invasion and migration ability of human tongue cancer
SCC-15 cells were respectively investigated, to explore the effect
of traditional chemotherapeutic drug ADM combined with new
antitumor drug MET on the biological function of human tongue
cancer cells, so as to provide a more optimized solution for
reducing the postoperative metastasis and recurrence rate of tongue
cancer in clinical practice.
Materials and methods
Experimental instruments and
reagents
The DMIL LED inverted microscope was purchased from
Leica, Microsystems GmbH (Wechsler, Germany), microplate reader
SpectraMax M5 from Meigu (Shanghai, China), flow cytometer CytoFLEX
LX from Beckman Coulter, Inc. (Brea, CA, USA), sterile plus sample
gun head from Axygen Scientific, Inc. (Union City, CA, USA), human
tongue squamous cancer SCC-15 cell line from ATCC (cat. no.
ATCC® CRL-1623; Manassas, VA, USA) and cryopreserved in
this laboratory, DMEM-F12 medium from Gibco; Thermo Fisher
Scientific Inc. (Waltham, MA, USA), fetal bovine serum (FBS),
trypsin and phosphate buffer powder from HyClone; GE Healthcare
(Chicago, IL, USA), sterile gun head from Axygen Scientific, Inc.,
CCK-8 reagent from Tongren Company (Tokyo, Japan), Transwell
chamber from Corning Incorporated (Corning, NY, USA), Annexin
V-FITC/PI apoptosis kit from Kaiji Biological Co., Ltd. (Jiangsu,
China), MET powder and ADM powder from Baole Pharmaceutical Co.,
Ltd. (Shenzhen, China).
The study was approved by the Ethics Committee of
Qianfoshan Hospital of Shandong Province (Jinan, China).
Cell recovery, culture, passage and
cryopreservation
Relevant literature was consulted and the
cryopreserved SCC-15 cell line was taken out from the liquid
nitrogen container, and quickly placed in an incubator at 37°C to
melt the cryoprotectant. The melted cell sap was then transferred
to a centrifuge tube under aseptic conditions, and the DMEM-F12
medium containing 10% FBS was added and cultured in the incubator
at 5% CO2 and 37°C. The inverted microscope was used to
observe the cell growth, and the solution was changed at an
appropriate time. When cells were adherently grown in a culture
flask to more than 80%, the old medium was first aspirated and
discarded, then washed with 3 ml of PBS to remove residual serum
and suspended dead cells. After that, trypsin was added for
digestion. When cells became translucent and their morphology was
round, they were observed under a microscope, then added to the
DMEM complete medium containing FBS in order to terminate the
digestion. The bottom of the flask was strongly tapped until cells
were detached from the bottle wall, and the suspension was then
transferred to the centrifuge tube, centrifuged at 800 × g for 5
min at 20°C. Next, the complete medium was added to resuspend cells
that were distributed evenly into 3 new culture flasks to complete
the passage. Cells continued to be cultured in the incubator at
37°C. The SCC-15 cell line in log phase was used to repeat the
above process of cell digestion, washing and passage. After that,
it was transferred to a 1.5 ml cryogenic vial and stored overnight
in a −80°C refrigerator. The cryogenic vial was transferred to the
liquid nitrogen container the next day.
CCK-8 detection of inhibition in cell
proliferation
The inhibition of ADM alone and MET alone in cell
proliferation was compared. The cryopreserved SCC-15 cells in log
phase were resuscitated according to the above steps, and incubated
in a 96-well plate (four plates in total) for culture. The drug
intervention group, the control group and the blank group were set
up in each plate. The blank group was added only with medium, the
control group and the drug intervention group with 3×105
SCC-15 cells per well. After cells were adherently grown, the drug
was added to the drug intervention group. First, each of 2 ml of
ADM with concentrations of 0.01, 0.05, 0.1/l, 0.5 and 1 mg/l were
added to two plates for ADM, 5 groups of MET with concentrations of
1, 5, 10, 20 and 40 mmol/l to two plates for MET, respectively.
CCK-8 solution (10 µl/well) was respectively added at 24, 48 and 72
h after dosing. Then, cells continued to be cultured for 2 h in the
incubator. The SpectraMax M5 microplate reader was used to measure
the optical density (OD) value of each well at a wavelength of 450
nm in order to detect cell proliferation. The experiment was
repeated 3 times. Next, the inhibition rate of cell growth was
calculated according to the formula: cell survival rate (%) = (OD
value in the experimental group - OD value in the control
group)/(OD value in the control group - OD value in the blank
group) × 100%. It was concluded that the 50% inhibiting
concentration (IC50) of ADM was close to 0.05 mg/l and
the IC50 of MET was close to 10 mmol/l after 48 h of
intervention. Then, the above-mentioned incubation plate method was
repeated, and the cell proliferation was compared with drug
combination. The 96-well plate was grouped into the blank group,
the control group, the ADM group, the MET group and the ADM+MET
group. The blank group was added only with 100 µl of medium, other
groups with 3×105 SCC-15 cells per well, respectively.
After cells were adherently grown, 2 ml of ADM 0.05 mg/l, MET 10
mmol/l and ADM 0.05 mg/l plus MET 10 mmol/l were respectively added
to the ADM group, the MET group and the ADM+MET group. After
cultured for 48 h, CCK-8 solution (10 µl/well) was added. After
cultured for 2 h, the OD value of each group was measured. The
experiment was repeated 3 times. The cell survival rate in
vitro was calculated according to the above given formula.
Flow cytometry detection of
apoptosis
The cryopreserved SCC-15 cells in log phase were
taken out, and 4 flasks of them were cultured in the incubator.
Cells (3×105) were collected from each flask. Flask 1
was used as the control group with only medium added, flask 2 as
the ADM group with 4 ml of ADM 0.05 mg/l added, flask 3 as the MET
group with 4 ml of MET 10 mmol/l, and flask 4 as the ADM+MET group
with 2 ml of ADM 0.05 mg/l plus 2 ml of MET 10 mmol/l. After 48 h
of dosing, a single cell suspension was prepared, washed with PBS
and centrifuged twice at 1,580 × g for 5 min at 4°C. The
supernatant was removed, and cells were resuspended. Annexin V-FITC
(5 µl) and 5 ml of PI were added, and incubated for 20 min at room
temperature in the dark. The CytoFLEX LX flow cytometer was used to
detect apoptosis. The experiment was repeated 3 times.
Transwell chamber detection of cell
migration ability in vitro
Cells in log phase were incubated at 37°C in a
24-well plate based on the above method, with approximately 3,000
cells per well. They were divided into the control group (only
medium added), the ADM group (2 ml of ADM 0.05 mg/l added), the MET
group (2 ml of MET 10 mmol/l added) and the ADM+MET group (2 ml of
ADM 0.05 mg/l plus MET 10 mmol/l added). After 48 h of drug
intervention, cells were diluted to a density of 3×104
cells/ml with a serum-free DMEM-F12 medium. The diluted cells (200
µl) were added to the upper chamber, 600 µl of DMEM-F12 medium
containing 20% FBS to the lower chamber. After 24 h, the chamber
was rinsed with PBS and fixed with 4% paraformaldehyde solution for
10 min. It was rinsed again with PBS after taken out and stained
with 0.5% crystal violet for 10 min at 20°C. Then, it was taken out
and rinsed with PBS again until clarification. Finally, the cell
invasion of 5 visual fields was randomly calculated with a
microscope, and the average value was calculated. The experiment
was repeated 3 times.
Scratch-healing experiment for
observation of cell migration ability in vitro
Cells in log phase were prepared into a single cell
suspension. Diluted to 3×105 cells/ml based on the above
method, they were added to a 6-well plate, grouped based on the
above method and cultured by adding the medium and the drug. After
48 h, 200 µl of sterile plus sample gun head was used to scratch
the cells in the middle of the plate in order to form a cell-free
area. The cells were rinsed with PBS and a new medium was added for
culture. At 0 h (W0) and 24 h (W24) after the
cells had been scratched, the width of the cell-free area of the
scratches at three different positions was calculated under the
microscope. Cell migration index = (W0 -
W24)/W0 × 100%.
Statistical analysis
In this study, SPSS19.0 software [Boyizhixun
(Beijing) Information Technology Co., Ltd., (Beijing, China)] was
used to statistically analyze the data, and GraphPad Prism 6
software (GraphPad Software, Inc., La Jolla, CA, USA) to plot all
the illustrations in this experiment. Measurement data were
expressed as mean ± standard deviation. Single factor analysis of
variance was used for comparison among multiple groups, and
repeated measurement analysis of variance was used for analysis at
different time-points in the group. LSD test was used for pairwise
comparison after the event and t-test for analysis between two
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of ADM and MET single drug
intervention on proliferation of tongue cancer SCC-15 cells
CCK-8 assay was used to detect the cell
proliferation in each group. It was found that the survival rate of
tongue cancer SCC-15 cells gradually decreased with the increase in
ADM and MET concentrations and in intervention time (P<0.05)
(Tables I and II).
 | Table I.CCK-8 detection of the effect of ADM
with different concentrations on survival rate of SCC-15 cells at
different time-points (%). |
Table I.
CCK-8 detection of the effect of ADM
with different concentrations on survival rate of SCC-15 cells at
different time-points (%).
| Concentration
mg/l | 24 h | 48 h | 72 h | F value | P-value |
|---|
| 0 | 100±0 | 100±0 | 100±0 | – | – |
| 0.01 | 93.1±2.3c | 77.3±2.7a,c | 61.2±3.1a–c | 103.2 | <0.001 |
| 0.05 | 85.6±2.9c | 60.8±3.3a,c | 53.9±3.7a–c | 75.82 | <0.001 |
| 0.1 | 78.7±3.4c | 49.6±2.1a,c | 40.2±3.2a–c | 138.3 | <0.001 |
| 0.5 | 63.5±3.1c | 37.3±3.8a,c | 24.1±3.8a–c | 94.04 | <0.001 |
| 1 | 54.5±5.8c | 21.2±5.3a,c | 13.7±3.5a–c | 57.38 | <0.001 |
| F value | 80.06 | 221.0 | 282.0 | – | – |
| P-value | <0.001 | <0.001 | <0.001 | – | – |
 | Table II.CCK-8 detection of the effect of MET
with different concentrations on survival rate of SCC-15 cells at
different time-points (%). |
Table II.
CCK-8 detection of the effect of MET
with different concentrations on survival rate of SCC-15 cells at
different time-points (%).
| Concentration
mg/l | 24 h | 48 h | 72 h | F value | P-value |
|---|
| 0 | 100±0 | 100±0 | 100±0 | – | – |
| 1 | 91.1±1.4c |
72.3±12.7a,c |
62.2±2.4a–c | 11.45 | <0.050 |
| 5 |
85.3±2.9c |
52.8±2.3a,c |
42.9±3.1a–c | 190.0 | <0.001 |
| 10 |
74.7±2.4c |
49.6±2.4a,c |
31.2±3.0a–c | 209.1 | <0.001 |
| 20 |
61.7±4.3c |
31.2±2.2a,c |
23.1±3.9a–c | 96.75 | <0.001 |
| 40 |
42.5±2.5c |
25.2±4.3a,c |
11.7±2.5a–c | 69.22 | <0.001 |
| F value | 195.4 | 70.14 | 400.9 | – | – |
| P-value | <0.001 | <0.001 | <0.001 | – | – |
Effect of ADM and MET in combination
on proliferation of tongue cancer SCC-15 cells
According to the above experimental results, it was
calculated that the close concentration of the LD50 of ADM was 0.05
mg/l and the LD50 of MET was 10 mmol/l after 48 h of intervention.
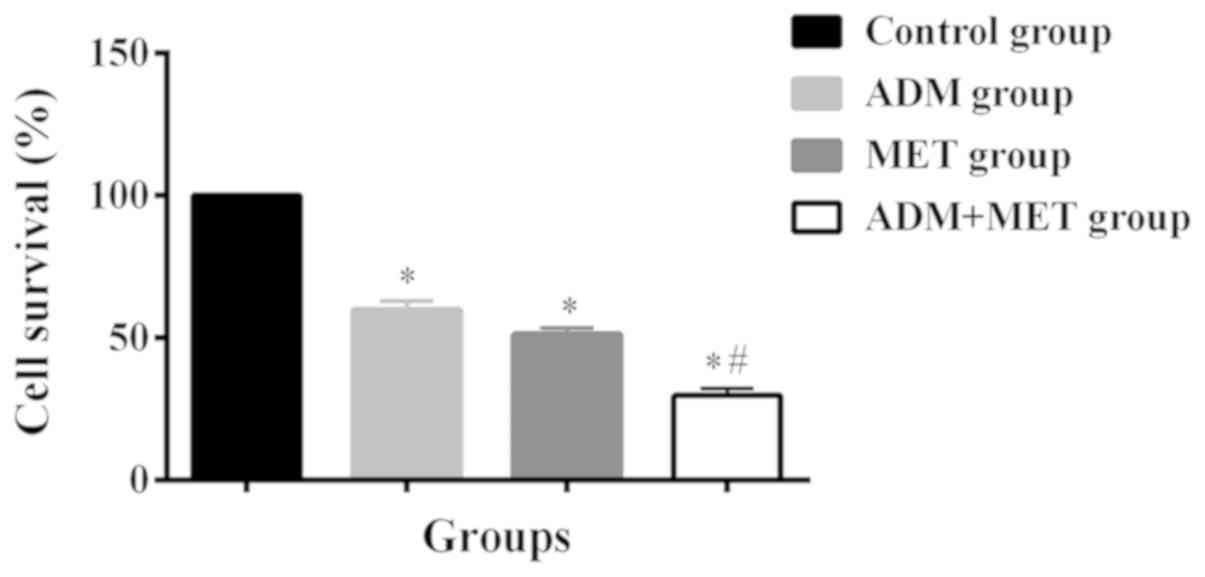
They were used for drug combination experiment. The cell survival
rate at 48 h was 100% in the control group, 59.8±3.1% in the ADM
group, 51.2±2.1% in the MET group, and 29.7±2.3% in the ADM+MET
group. There was no significant difference in the cell survival
rate between the ADM and MET groups (P>0.05). The cell survival
rate in the ADM+MET group was significantly lower than that in the
ADM and MET groups (P<0.05) (Fig.
1).
Effects of ADM alone, MET alone and
their drug combination on apoptosis, invasion and migration ability
of tongue cancer SCC-15 cells
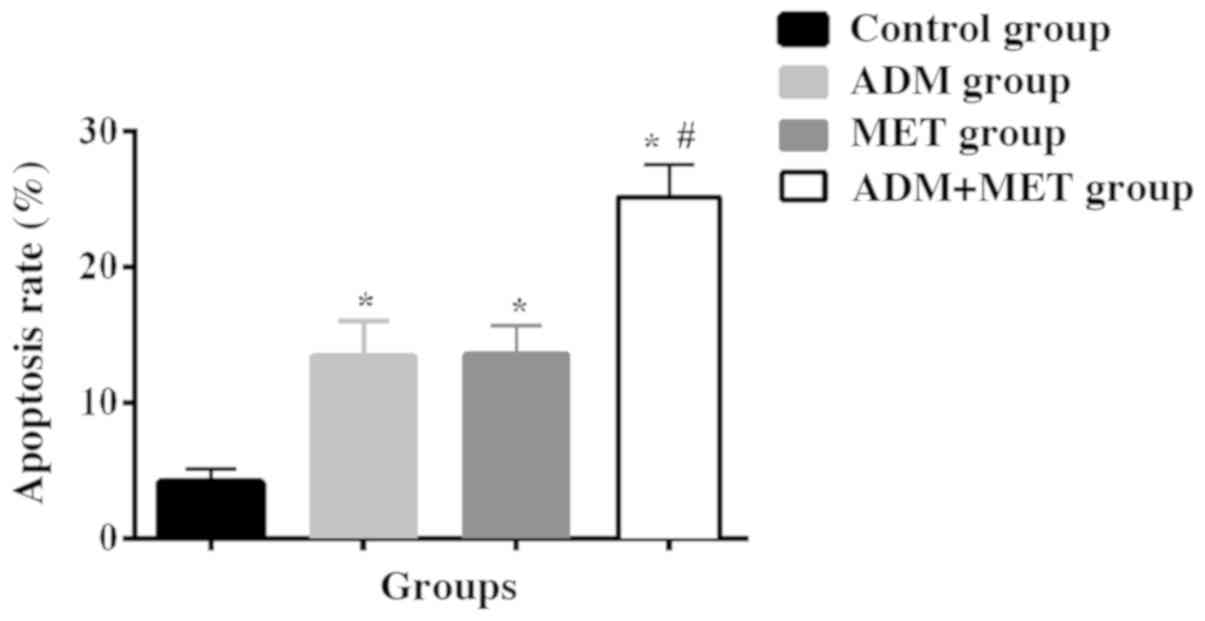
Apoptosis rate in the ADM, MET and ADM+MET group was
significantly higher than that in the control group (P<0.05).
There was no significant difference in the apoptosis rate between
the ADM and MET groups (P>0.05). The apoptosis rate in the
ADM+MET group was higher than that in the ADM and MET groups
(P<0.05). Transwell chamber was used to detect the cell invasion
ability in each group after 24 h of routine culture. It was found
that the cell membrane number was (65.7±5.6) in the control group,
(31.5±6.7) in the ADM group, (29.2±7.1) in the MET group and
(8.2±1.3) in the ADM+MET group. There was a difference in the cell
invasion ability between the ADM, MET, ADM+MET groups and the
control group (P<0.05), but there was no significant difference
in the cell membrane number between the ADM and MET groups
(P>0.05). The cell membrane number in the ADM+MET group was
significantly less than that in the ADM and MET groups (P<0.05).
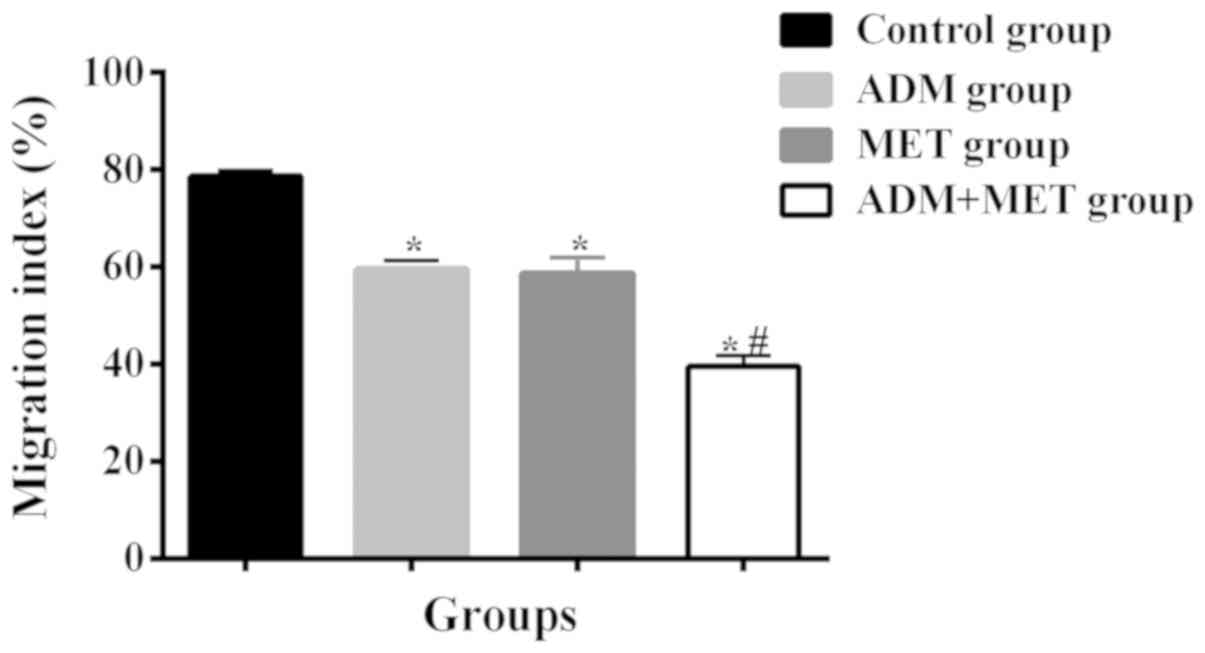
After 24 h of cell scratching, the migration of cells was
78.6±1.24% in the control group, 59.5±1.78% in the ADM group,
58.7±3.22% in the MET group and 39.5±2.31% in the ADM+MET group. In
addition, there was a difference in the cell migration ability
between the ADM, MET, ADM+MET groups and the control group
(P<0.05), but there was no significant difference in the
migration between the ADM and MET groups (P>0.05). The migration
in the ADM+MET group was significantly lower than that in the ADM
and MET groups (P<0.05) (Tables
III–V and Figs. 2–4).
 | Table III.Apoptosis rates of SSC-15 cells after
48 h of ADM alone, MET alone and their drug combination (%). |
Table III.
Apoptosis rates of SSC-15 cells after
48 h of ADM alone, MET alone and their drug combination (%).
| Groups | Control group | ADM group | MET group | ADM+MET group |
|---|
| Apoptosis rate | 4.12±1.02 |
13.47±2.57a |
13.56±2.13a |
25.17±2.33a,b |
| Cell membrane
number | 65.7±5.6 |
31.5±6.7a |
29.2±7.1a |
8.2±1.3a,b |
| Cell migration | 78.6±1.24 |
59.5±1.78a |
58.7±3.22a |
39.5±2.31a,b |
 | Table V.Effects of ADM and MET alone and in
combination on migration ability of SCC-15 cells (%). |
Table V.
Effects of ADM and MET alone and in
combination on migration ability of SCC-15 cells (%).
| Groups | Control group | ADM group | MET group | ADM+MET group |
|---|
| Cell migration
index | 78.6±1.24 |
59.5±1.78a |
58.7±3.22a |
39.5±2.31a,b |
Discussion
Tongue cancer, a mouth cancer that is one of the top
ten common cancers in the world, has shown constantly rising
incidence in recent years (14). The
rich blood circulation and lymph nodes in the tongue are prone to
cause tumor cell invasion in tongue cancer. The most common is the
metastasis of tumor cells to lymph nodes (15). At present, low attention is paid by
patients to tongue cancer that has high malignancy and rapid
development. Therefore, many patients are in the advanced stage
when diagnosed. Tongue cancer is mainly treated by operation
combined with radiotherapy and chemotherapy (16). ADM is a widely used chemotherapeutic
drug for the treatment of tongue cancer in clinical practice, its
main function is to inhibit the spread and proliferation of tongue
cancer cells (17). However, its
long-term use causes severe toxic and side effects, such as kidney
and liver damage and myocardial lesion. It also causes drug
resistance in patients, thereby reducing the efficacy. Therefore,
ADM has certain limitations in clinical application (18). MET is mainly used in the treatment of
type 2 diabetes in clinical practice. Compared to traditional
hypoglycemic drugs, it has the advantages of obvious efficacy,
safety and low price (19). In
recent years, an increasing number of studies have reported the
antitumor effect of MET. Related experiments have also confirmed
that it inhibits the proliferation of gastric cancer cells
(20) and esophageal cancer cells
(21). However, there are currently
few studies on the effect of MET combined with chemotherapeutic
drugs on human tongue cancer cells, especially on the effect of MET
combined with chemotherapeutic drugs on the proliferation,
apoptosis, invasion and migration ability of human tongue cancer
cells. Therefore, in this study, the effect of ADM combined with
MET on the biological function of human tongue cancer cells was
investigated, in order to provide a more optimal treatment for
tongue cancer patients.
First, the survival rates of tumor cells when ADM
and MET were used alone and in combination were compared. It was
found that the survival rate of tongue cancer SCC-15 cells
gradually decreased with the increase in ADM and MET concentrations
used alone and in intervention time (P<0.05). The cell survival
rate in the ADM+MET group was significantly lower than that in the
ADM and MET groups (P<0.05). This indicates that ADM and MET in
combination is more effective than ADM and MET alone in inhibiting
the proliferation of tumor cells. It was also found that the
combination of MET and ADM has a more significant effect on Ehrlich
tumors in mice than ADM or MET alone (22). It was found that the apoptosis rate
in the ADM, MET and ADM+MET groups was significantly higher than
that in the control group (P<0.05). There was no significant
difference in the apoptosis rate between the ADM and MET groups
(P>0.05). The apoptosis rate in the ADM+MET group was higher
than that in the ADM and MET groups (P<0.05). It shows that both
ADM and MET induce apoptosis, and the combination of the two
strengthens the apoptosis effect. The effects of ADM alone, MET
alone and their drug combination on the invasion and migration
ability of tongue cancer cells were investigated. It was found that
both ADM alone and MET alone inhibited the invasion and migration
ability of tumor cells, but the combination of the two was more
effective in the inhibitory effect. In the study by Bao et
al (23), it was confirmed that
MET inhibits the invasion and migration of tumor cells. There is
also a study (24) showing that
conventional chemotherapeutic drugs assisted with MET are helpful
to improve the efficacy, which is consistent with our findings.
In summary, both MET and ADM inhibit the growth,
invasion and migration of tongue cancer SSC-15 cells, and induce
their apoptosis. ADM and MET in combination is more effective than
ADM alone and MET alone in inhibiting the growth, invasion and
migration of tongue cancer cells, and in inducing their apoptosis.
However, in this study, the effect of ADM combined with MET in
vivo on the biological function of tongue cancer cells was not
explored, and the effects of AMD and MET on multiple cell lines
were not compared. These problems will be improved in future
studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Author's contributions
JZ drafted this manuscript, collected and
interpreted the data, revised the manuscript critically for
important intellectual content and was responsible for the
conception and design of the study. JZ read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Qianfoshan Hospital of Shandong Province (Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The author declares no competing interests.
References
|
1
|
He Q, Chen Z, Cabay RJ, Zhang L, Luan X,
Chen D, Yu T, Wang A and Zhou X: microRNA-21 and microRNA-375 from
oral cytology as biomarkers for oral tongue cancer detection. Oral
Oncol. 57:15–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu W, Zhao X, Zhang YJ, Fang GW and Xue
Y: MicroRNA-375 as a potential serum biomarker for the diagnosis,
prognosis, and chemosensitivity prediction of osteosarcoma. J Int
Med Res. 46:975–983. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mao L, Li J, Chen WX, Cai YQ, Yu DD, Zhong
SL, Zhao JH, Zhou JW and Tang JH: Exosomes decrease sensitivity of
breast cancer cells to adriamycin by delivering microRNAs. Tumour
Biol. 37:5247–5256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu DD, Wu Y, Zhang XH, Lv MM, Chen WX,
Chen X, Yang SJ, Shen H, Zhong SL, Tang JH, et al: Exosomes from
adriamycin-resistant breast cancer cells transmit drug resistance
partly by delivering miR-222. Tumour Biol. 37:3227–3235. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dundar HA, Kiray M, Kir M, Kolatan E,
Bagriyanik A, Altun Z, Aktas S, Ellidokuz H, Yilmaz O, Mutafoglu K,
et al: Protective effect of acetyl-L-carnitine against
doxorubicin-induced cardiotoxicity in wistar albino rats. Arch Med
Res. 47:506–514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McCreight LJ, Bailey CJ and Pearson ER:
Metformin and the gastrointestinal tract. Diabetologia. 59:426–435.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang J, Wei D, Wang W, Shen B, Xu S and
Cao Y: TRAF4 enhances oral squamous cell carcinoma cell growth,
invasion and migration by Wnt-β-catenin signaling pathway. Int J
Clin Exp Pathol. 8:11837–11846. 2015.PubMed/NCBI
|
|
9
|
Fan C, Wang Y, Liu Z, Sun Y, Wang X, Wei G
and Wei J: Metformin exerts anticancer effects through the
inhibition of the Sonic hedgehog signaling pathway in breast
cancer. Int J Mol Med. 36:204–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Wang Z, Zhao X, Ji N, Zhou Y and
Li J: The inhibitory effect of metformin on oral squamous cell
carcinoma. Zhonghua Kou Qiang Yi Xue Za Zhi. 50:360–365. 2015.(In
Chinese). PubMed/NCBI
|
|
11
|
Qu Z, Zhang Y, Liao M, Chen Y, Zhao J and
Pan Y: In vitro and in vivo antitumoral action of metformin on
hepatocellular carcinoma. Hepatol Res. 42:922–933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui R, Yue W, Lattime EC, Stein MN, Xu Q
and Tan XL: Targeting tumor-associated macrophages to combat
pancreatic cancer. Oncotarget. 7:50735–50754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alimova IN, Liu B, Fan Z, Edgerton SM,
Dillon T, Lind SE and Thor AD: Metformin inhibits breast cancer
cell growth, colony formation and induces cell cycle arrest in
vitro. Cell Cycle. 8:909–915. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu JC, Sopka DS, Mehra R, Lango MN,
Fundakowski C, Ridge JA and Galloway TJ: Early oral tongue cancer
initially managed with surgery alone: Treatment of recurrence.
World J Otorhinolaryngol Head Neck Surg. 2:193–197. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu GT, Bu LL, Huang CF, Zhang WF, Chen WJ,
Gutkind JS, Kulkarni AB and Sun ZJ: PD-1 blockade attenuates
immunosuppressive myeloid cells due to inhibition of CD47/SIRPα
axis in HPV negative head and neck squamous cell carcinoma.
Oncotarget. 6:42067–42080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai WX, Yu RQ, Ma L, Huang HZ, Zheng LW
and Zwahlen R: Differences between epithelial and mesenchymal human
tongue cancer cell lines in experimental metastasis. Oncol Lett.
15:9959–9964. 2018.PubMed/NCBI
|
|
17
|
Jung HM, Benarroch Y and Chan EK:
Anti-cancer drugs reactivate tumor suppressor miR-375 expression in
tongue cancer cells. J Cell Biochem. 116:836–843. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rogulj D, El Aklouk I, Konjevoda P, Ljubić
S, Pibernik Okanović M, Barbir A, Luburić M, Radman M, Budinski N
and Vučić Lovrenčić M: Age-dependent systemic DNA damage in early
Type 2 Diabetes mellitus. Acta Biochim Pol. 64:233–238. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Merino J, Leong A, Liu CT, Porneala B,
Walford GA, von Grotthuss M, Wang TJ, Flannick J, Dupuis J, Levy D,
et al: Metabolomics insights into early type 2 diabetes
pathogenesis and detection in individuals with normal fasting
glucose. Diabetologia. 61:1315–1324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kato K, Gong J, Iwama H, Kitanaka A, Tani
J, Miyoshi H, Nomura K, Mimura S, Kobayashi M, Aritomo Y, et al:
The antidiabetic drug metformin inhibits gastric cancer cell
proliferation in vitro and in vivo. Mol Cancer Ther. 11:549–560.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J and Zhang Y, Tan X, Zhang Q, Liu C
and Zhang Y: MiR-23b-3p induces the proliferation and metastasis of
esophageal squamous cell carcinomas cells through the inhibition of
EBF3. Acta Biochim Biophys Sin (Shanghai). 50:605–614. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kabel AM, Omar MS, Balaha MF and Borg HM:
Effect of metformin and adriamycin on transplantable tumor model.
Tissue Cell. 47:498–505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bao B, Wang Z, Ali S, Ahmad A, Azmi AS,
Sarkar SH, Banerjee S, Kong D, Li Y, Thakur S, et al: Metformin
inhibits cell proliferation, migration and invasion by attenuating
CSC function mediated by deregulating miRNAs in pancreatic cancer
cells. Cancer Prev Res (Phila). 5:355–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miao L, Guo S, Lin CM, Liu Q and Huang L:
Nanoformulations for combination or cascade anticancer therapy. Adv
Drug Deliv Rev. 115:3–22. 2017. View Article : Google Scholar : PubMed/NCBI
|