Introduction
Prostate carcinoma (PC) is one of the most
frequently diagnosed malignancies worldwide and represents the
second most common cause of cancer-associated mortality (1). Treatment outcomes of early stage PC are
usually satisfactory (2); however,
owing to the lack of symptoms of PC, many patients are diagnosed
with existing tumor metastasis, which is a major cause of treatment
failure and poor postsurgical survival (3). Currently, the incidence rate of PC is
increasing, particularly in developing countries, including China,
where PC is becoming a major public health problem (4). The pathogenesis of PC has not yet been
entirely elucidated, and further investigation of the underlying
molecular mechanism of PC development remains crucial to improving
PC treatment.
B cell lymphoma 2 (Bcl-2) and glucose transporter 1
(GLUT-1) are involved in the pathogenesis of various types of
malignancy, including PC (5,6). Long non-coding (lnc) RNAs are a group
of non-coding RNAs that possess crucial functions in normal and
pathological physiological processes involved in numerous human
diseases and malignancies, including different types of cancer
(7). lncRNAs serve their roles by
regulating the expression of downstream target genes that may be
critical factors in human cancer (7). Therefore, the regulation of certain key
lncRNAs may benefit the treatment of disease. Previous studies have
reported that Bcl-2 and GLUT-1 interact with numerous lncRNAs
involved in various biological processes (8,9).
Growth-arrest-associated lncRNA 1 (GASL1) is as newly identified
lncRNA that acts as a tumor suppressor gene in liver cancer
(10). In addition, GASL1 deletion
enhances liver tumor growth, and low GASL1 expression levels are
associated with poor survival of patients with liver cancer
(11). The present study
demonstrated that GASL1 inhibited cancer cell proliferation,
upregulated BCL-2 and downregulated GLUT-1 expression levels in
PC.
Materials and methods
Patients and specimens
The present study examined clinical data from 66
patients diagnosed with PC and treated at Tongji Hospital (Wuhan,
China) between January 2011 and January 2013. Prostate biopsy
tissues and serum samples were obtained from all patients. The
inclusion criteria were as follows: i) Patients were diagnosed with
PC by pathological examinations; ii) patients completed the whole
treatment procedure at Tongji Hospital; iii) patients completed the
follow-up and had complete clinical data record; and iv) patients
received no treatment before admission. The exclusion criteria were
as follows: i) At the time of the study, patients suffered or had
suffered from other prostate diseases; ii) at the time of the
study, patients suffered or had suffered from another cancer; iii)
patients who were treated before admission; iv) patients who failed
to complete the whole treatment procedure; and v) patients who died
of other causes during the follow up. Prostate biopsy tissues and
serum samples were also collected from 56 healthy subjects who
received physiological examinations at Tongji Hospital between
January 2011 and January 2013. These subjects underwent prostate
biopsy to detect potential prostate lesions; prostate diseases were
eventually excluded. The age of patients in the PC group ranged
between 47 and 86 years (age, 68.9±5.1 years), and those in the
healthy Control group ranged between 46 and 85 years (age, 69.1±5.0
years). No significant differences in age, smoking and drinking
habits, and other basic clinical data, including body mass index
and height, were identified between these two groups. The Ethics
Committee of Tongji Medical College, Huazhong University of Science
and Technology approved the present study. Written informed
consents were obtained from all participants and/or their
families.
Cell culture and transfection
The HPrEC human normal prostate epithelial cell line
[cat. no. PCS-440-010™; American Type Culture Collection (ATCC),
Manassas, VA, USA] and the two human PC cell lines 22Rv1 (cat. no.
CRL-2505™; ATCC) and DU145 (cat. no. HTB-81™; ATCC) were used in
the present study. All cells were cultured with RPMI-1640 medium
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal
bovine serum (Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2.
An EcoRI-EcoRI fragment containing
full length GASL1 cDNA was amplified by polymerase chain reaction
(PCR), and a GASL1 expression vector was constructed by inserting
this fragment into the pIRSE2-EGFP vector (Clontech Laboratories,
Inc., Mountainview, CA, USA). GASL1 expression vector or empty
pIRSE2-EGFP vector (10 nM) was transfected into 6×105
cells using Lipofectamine® 2000 reagent (cat. no.
11668-019; Invitrogen, Thermo Fisher Scientific, Inc.) following
the manufacturer's protocol. Incubation with vectors was performed
at 37°C for 6 h. Subsequent experiments were performed 12 h after
transfections. Expression of GASL1 was detected prior to and
following subsequent experiments, and an overexpression rate
>150% (150–280%) was reached every time.
Cell counting Kit-8 (CCK-8) assay
Following transfection, HPrEC, 22Rv1 and DU145 cells
were collected, washed with PBS and resuspended in RPMI-1640 medium
(catalog no. 30-2001; ATCC) to the density of 3×104/ml.
Cell proliferation was measured by CCK-8 assay. The highly
water-soluble tetrazolium salt WST-8 is reduced by cellular
dehydrogenases, which becomes orange in color (formazan); the
amount of formazan is proportional to the number of living cells.
Cells were cultured in a 96 well plate at a density of
3×103 cells/100 µl/well and placed in the incubator.
CCK-8 solution (10 µl; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was added at 24, 48, 72 and 96 h. Cells were then cultured
for additional 4 h and 10 µl DMSO was added. OD values were
measured at 450 nm using Fisherbrand™ accuSkan™ GO UV/Vis
microplate spectrophotometer (Thermo Fisher Scientific, Inc.).
Experiments were performed in triplicate. At 24, 48, 72 and 96 h,
cells were subjected to RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) to
confirm the overexpression of GASL1.
RT-qPCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific Inc.) was used to extract total RNA from HPrEC,
22Rv1 and DU145 cells (3×104 cells/ml). To achieve
complete cell lysis, tissues (0.05 g) were ground in liquid
nitrogen prior to addition of TRIzol. RT was performed to
synthesize cDNA using SuperScript IV Reverse Transcriptase kit
(Thermo Fisher Scientific, Inc.) with the following conditions:
25°C for 6 min, 55°C for 20 min and 80°C for 10 min. qPCR reaction
systems were prepared using SYBR® Green Real-Time PCR
Master Mixes (Thermo Fisher Scientific, Inc.). All primers were
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The PCR
primers sequences were as follows: GASL1, forward
5′-CTGAGGCCAAAGTTTCCAAC-3′, reverse 5′-CAGCCTGACTTTCCCTCTTCT-3′;
and β-actin, forward 5′-GACCTCTATGCCAACACAGT-3′, reverse
5′-AGTACTTGCGCTCAGGAGGA-3′. Thermocycling conditions were as
follows: 95°C for 50 sec, followed by 40 cycles of 95°C for 15 sec
and 60°C for 35 sec. Expression of GASL1 was normalized to β-actin
endogenous control using the 2−ΔΔCq method (12). This experiment was performed in
triplicate.
Western blotting
Total protein was extracted from HPrEC, 22Rv1 and
DU145 cell lines 12 h after transfection using RIPA Lysis and
Extraction Buffer (Thermo Fisher Scientific Inc.) with a cell
density of 3×104 cells/ml. The bicinchoninic acid assay
was used to measure protein concentration. Proteins (30 µg per
lane) were separated by 10% SDS-PAGE electrophoresis and
transferred to a polyvinylidene fluoride membrane. Membranes were
blocked with 5% skimmed milk at room temperature for 1 h. Membranes
were incubated with primary antibodies, including rabbit anti-Bcl-2
(cat. no. ab59348; 1:1,200; Abcam, Cambridge, UK), rabbit anti-
Bcl-associated X (Bax; cat. no. ab32503; 1:1,200; Abcam, Cambridge,
UK), rabbit anti-GLUT-1 (cat. no. ab15309; 1:1,200; Abcam) and
rabbit anti-GAPDH antibody (cat. no. ab9485; 1:1,400; Abcam) at 4°C
overnight. They were then incubated with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary
antibody (cat. no. MBS435036; 1:1,000; MyBioSource, San Diego, CA,
USA) for 2.5 h at room temperature. Enhanced Chemiluminescence
Reagent (Sigma-Aldrich, Merck KGaA) was used to develop signal,
which was detected using MYECL™ Imager (Thermo Fisher Scientific,
Inc.). Expression levels of Bcl-2 and GLUT-1 were normalized to
GAPDH endogenous control using ImageJ v1.6 software (National
Institutes of Health, Bethesda, MD, USA). This experiment was
performed in triplicate.
Statistical analysis
SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was
used to perform all statistical analysis. Expression levels of
proteins, mRNA and cell proliferation data were expressed as the
mean ± standard deviation, and compared using unpaired Student's
t-test (between 2 groups) or one-way analysis of variance followed
by least significant difference test (among multiple groups).
χ2 test was used to analyze the associations between
GASL1 (in prostate tissues and serum) and patients'
clinicopathological data. Receiver operating characteristic (ROC)
curve was performed using patients with PC as true positive
subjects and healthy controls as true negative subjects, and all
parameters were default [95% confidence interval (CI)]. Patients
were divided into high and low expression group according to the
median serum levels of GASL1 (1.64). Kaplan-Meier (KM) analysis was
performed to plot survival curves, which were compared by log-rank
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of expression levels of
lncRNA GASL1 in prostate tissues and sera between patients with PC
and healthy controls
mRNA expression levels of lncRNA GASL1 in prostate
tissues and sera of patients with PC and healthy controls were
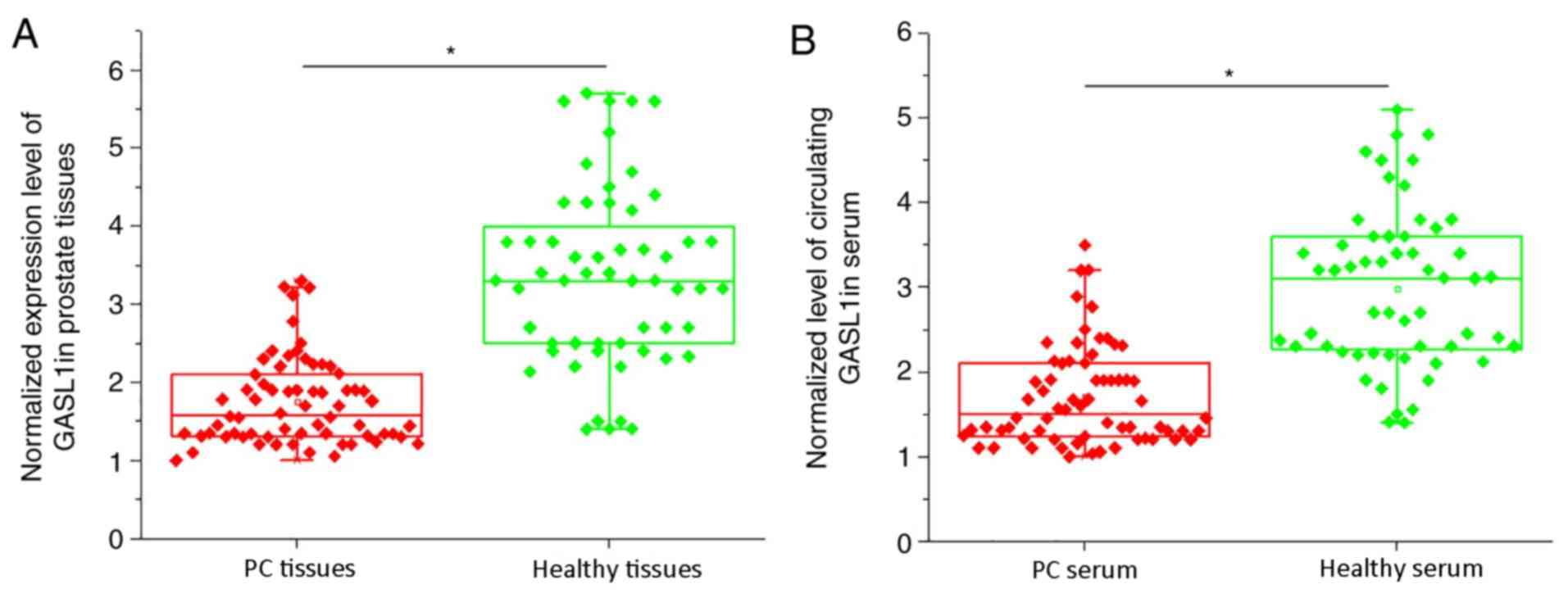
detected by RT-qPCR. As shown in Fig.
1A, the expression levels of lncRNA GASL1 in PC tissues were
significantly lower compared with healthy control tissues
(P<0.05). In addition, the expression levels of circulating
lncRNA GASL1 in serum were also significantly lower in PC tissues
compared with healthy controls (P<0.05; Fig. 1B). These data suggested that
downregulation of lncRNA GASL1 may be involved in pathogenesis of
PC.
Diagnostic values of lncRNA GASL1 for
PC
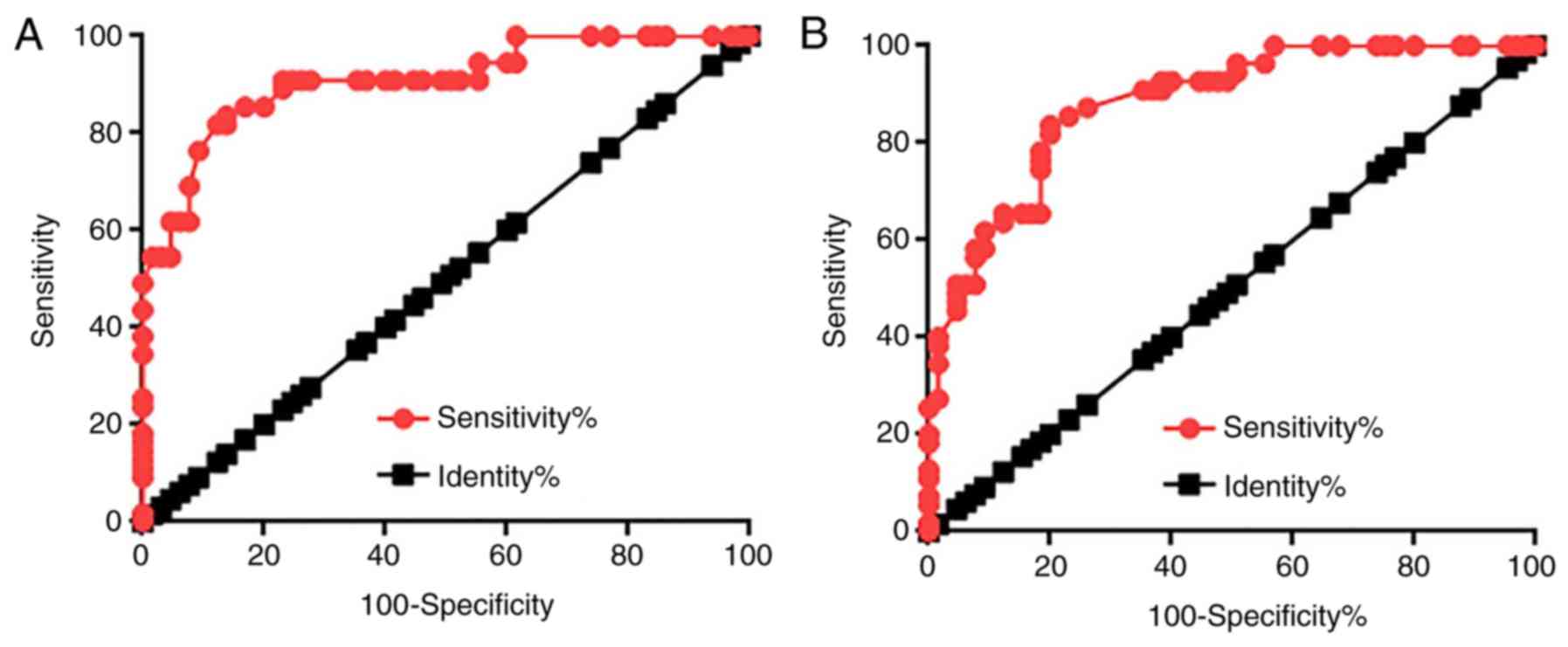
Diagnostic values of lncRNA GASL1 expression in
prostate tissues and sera for patients with PC were evaluated using
ROC curve analysis. As demonstrated in Fig. 2A, the area under the curve (AUC) for
GASL1 expression in prostate tissues used for PC diagnosis was
0.9076 with a standard error of 0.02718 and 95% CI of
0.8543–0.9608. In addition, the AUC of GASL1 expression in serum
used for PC diagnosis was 0.8811 with a SE of 0.02976 and 95% CI of
0.8228–0.9359 (Fig. 2B). These
results suggested that lncRNA GASL1 may serve as a potential
biomarker for PC.
Prognostic values of serum level of
lncRNA GASL1 for PC
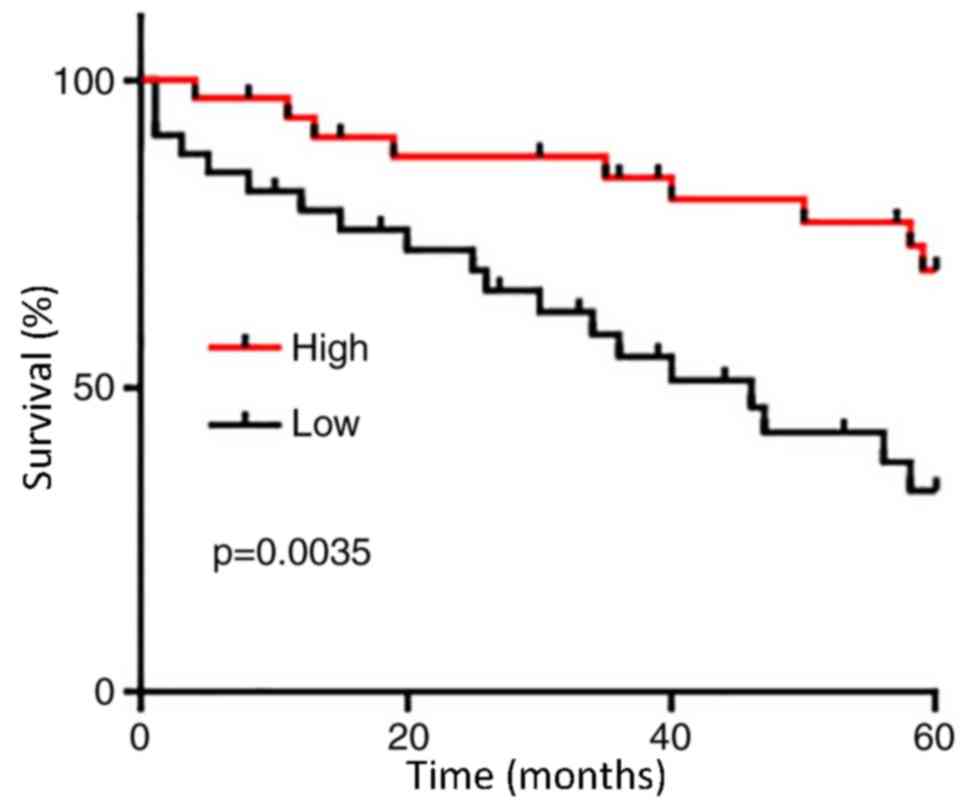
The 66 patients with PC were divided into high
(n=33) and low (n=33) expression groups according to the median
serum level of GASL1 (1.64). KM was used to plot survival curves,
which were compared using log rank test. As presented in Fig. 3, the overall survival of patients
with low serum level of GASL1 was significantly poorer compared
with patients with high serum level of GASL1 (P=0.0035). These data
suggested that serum levels of GASL1 may serve as a potential
prognostic biomarker for PC.
Association of lncRNA GASL1 expression
levels with clinicopathological data of patients with PC
Associations between expression levels of lncRNA
GASL1 in prostate tissues and sera from patients with PC
clinicopathological data were analyzed by χ2 test. As
presented in Tables I and II, expression levels of lncRNA GASL1 in
prostate tissues and sera were not associated with patients age,
lifestyle habits (including drinking and smoking) and existing of
tumor distant metastasis. By contrast, expression levels of lncRNA
GASL1 in prostate tissues and sera were associated with tumor
size.
 | Table I.Expression levels of
growth-arrest-associated lncRNA 1 in prostate tissues were not
associated with patients age, lifestyle habits including drinking
and smoking and tumor distant metastasis. |
Table I.
Expression levels of
growth-arrest-associated lncRNA 1 in prostate tissues were not
associated with patients age, lifestyle habits including drinking
and smoking and tumor distant metastasis.
| Characteristic | Cases | High-expression | Low-expression | χ2 | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
>70 | 31 | 14 | 17 | 0.55 | 0.46 |
| ≤70 | 35 | 19 | 16 |
|
|
| Drinking |
|
|
|
|
|
| Yes | 50 | 23 | 27 | 1.32 | 0.25 |
| No | 16 | 10 | 6 |
|
|
| Smoking |
|
|
|
|
|
| Yes | 44 | 20 | 24 | 2.58 | 0.11 |
| No | 22 | 13 | 9 |
|
|
| Primary tumor
diameter (cm) |
|
|
|
|
|
|
>5 | 30 | 10 | 20 | 6.11 | 0.01 |
| ≤5 | 36 | 23 | 13 |
|
|
| Tumor distant
metastasis |
|
|
|
|
|
| Yes | 23 | 13 | 10 | 0.6 | 0.44 |
| No | 43 | 20 | 23 |
|
|
 | Table II.Expression levels of
growth-arrest-associated lncRNA 1 in serum were significantly
associated with tumor size. |
Table II.
Expression levels of
growth-arrest-associated lncRNA 1 in serum were significantly
associated with tumor size.
| Characteristic | Cases | High-expression | Low-expression | χ2 | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
>70 | 31 | 15 | 16 | 0.1 | 0.81 |
| ≤70 | 35 | 18 | 17 |
|
|
| Drinking |
|
|
|
|
|
| Yes | 50 | 22 | 28 | 3 | 0.08 |
| No | 16 | 11 | 5 |
|
|
| Smoking |
|
|
|
|
|
|
Yes | 44 | 19 | 25 | 2.5 | 0.12 |
| No | 22 | 14 | 8 |
|
|
| Primary tumor
diameter (cm) |
|
>5 | 30 | 11 | 19 | 3.9 | 0.047 |
| ≤5 | 36 | 22 | 14 |
|
|
| Tumor distant
metastasis |
|
|
|
|
|
|
Yes | 23 | 13 | 10 | 0.6 | 0.44 |
| No | 43 | 20 | 23 |
|
|
Effects of GASL1 overexpression on
cell proliferation
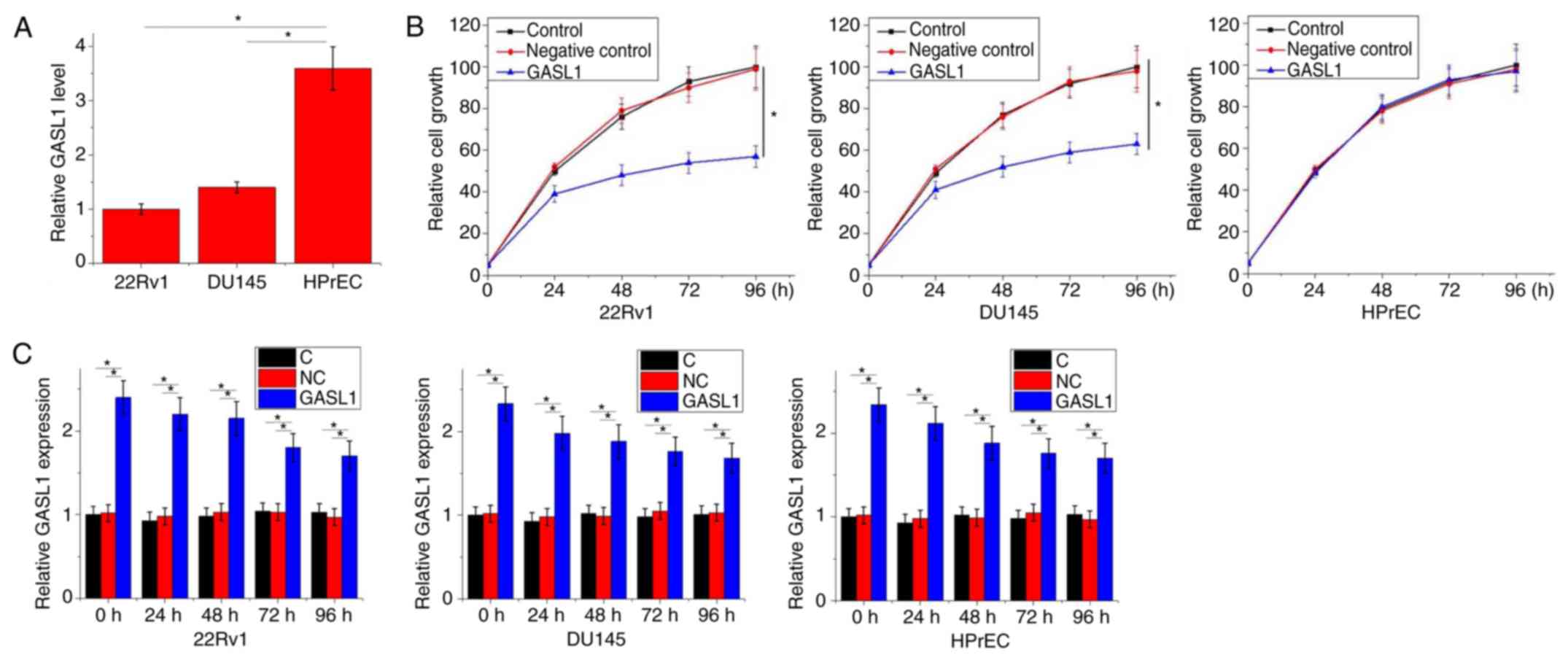
Significantly lower expression levels of GASL1 were
observed in 22Rv1 and DU145 cell lines compared with the HPrEC cell
line (P<0.05; Fig. 4A). According
to the data in Tables I and II, GASL1 could hypothetically be involved
in PC tumor growth. The effects of GASL1 overexpression on cell
proliferation were assessed by CCK-8 assay. As demonstrated in
Fig. 4B, GASL1 overexpression
significantly inhibited 22Rv1 and DU145 cell proliferation compared
with the Control groups at 96 h; however, GASL1 overexpression
exhibited no observable effect on HPrEC cell proliferation. In
addition, compared with the control and negative control groups,
significant GASL1 overexpression was observed at different time
points, which supports the effects of GASL1 overexpression on
cancer cell proliferation (P<0.05; Fig. 4C).
Effects of GASL1 overexpression on
Bcl-2, GLUT-1 and Bax expression
Bcl-2, GLUT-1 and Bax are associated with
development of various types of malignancy (8,9). In the
present study, GASL1 overexpression (12 h following transfection)
significantly increased Bcl-2 expression and significantly
inhibited GLUT-1 expression in 22Rv1 and DU145 cells (Fig. 5A and B, respectively). However, GASL1
overexpression had no effect on Bcl-2 and GLUT-1 expression in the
HPrEC cell line (Fig. 5C). GASL1
overexpression had no effect Bax expression in any of the three
cell lines (Fig. 5A-C).
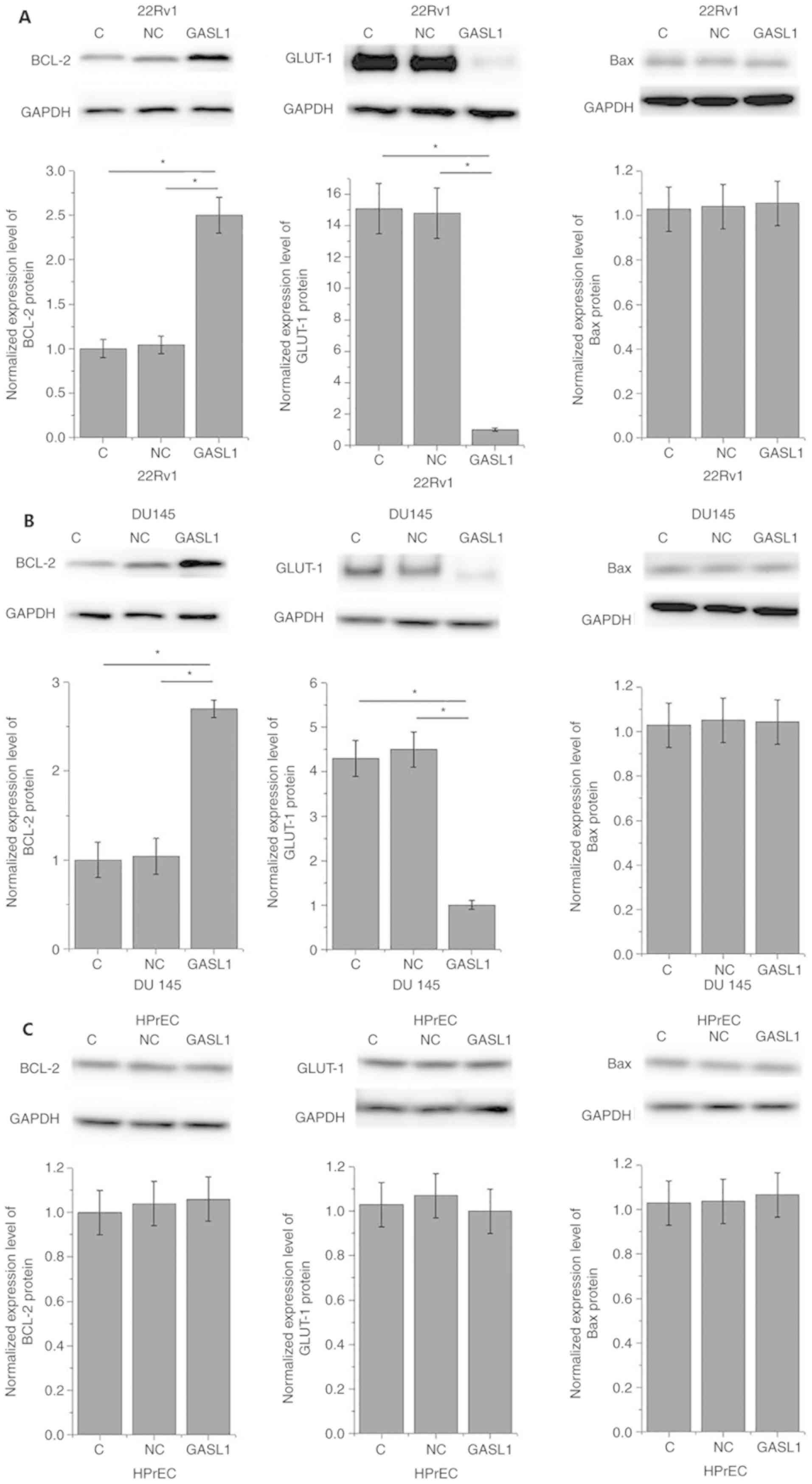 | Figure 5.Effects of lncRNA GASL1 overexpression
on Bcl-2, GLUT-1 and Bax expression. (A-C) GASL1 overexpression (12
h post-transfection) significantly increased Bcl-2 expression, but
inhibited GLUT-1 expression in (A) 22Rv1 and (B) DU145 cells, but
not in the (C) HPrEC cell line. GASL1 overexpression did not
significantly affect Bax expression in the three cell lines. Each
data represent the mean of three biological replicates; *P<0.05.
C, control; Bax, Bcl-2-associated X; Bcl-2, B cell lymphoma 2;
GASL1, growth-arrest-associated lncRNA 1; GLUT-1, glucose
transporter 1; lncRNA, long non-coding RNA; NC, negative
control. |
Discussion
GASL1 is a novel lncRNA characterized only in liver
cancer (10). The present study
demonstrated that GASL1 may serve a role as a tumor suppressor gene
in PC by inhibiting cancer cell proliferation. The underlying
mechanism of GASL1 in PC may be through GLUT-1 downregulation.
Genetic factors serve a central role in the
pathogenesis of PC. Genetic polymorphism of glutathione
S-transferase M1 and glutathione S-transferase θ1 genes is closely
associated with PC risk (11). A
previous study reported that lncRNA polymorphisms may affect the
occurrence of PC (12). In addition,
numerous lncRNAs exhibit different expression patterns during PC
progression and serve various roles as tumor suppressor genes or
oncogenes (13). GASL1 is
downregulated in liver cancer, indicating that it may be a tumor
suppressor gene (10). In the
present study, expression levels of lncRNA GASL1 were significantly
lower in patients with PC compared with healthy controls in
prostate tissues and in sera, which indicated that GASL1 may serve
a role as a tumor suppressor gene in PC.
Blood biomarkers are crucial for the diagnosis of
numerous human diseases (14). In
the present study, GASL1 was differentially expressed in patients
with PC and healthy controls, and ROC curve analysis revealed that
expression levels of lncRNA GASL1 in prostate tissues and sera may
be used to distinguish patients with PC from healthy controls. The
results also revealed that low expression level of GASL1 was
closely associated with poor postoperative survival. Furthermore,
expression levels of lncRNA GASL1 in prostate tissues and sera were
not associated with patient age or lifestyle habits, which are
factors known to affect the expression of certain lncRNAs (15–17).
These results indicated that GASL1 may serve as a potential
reliable and effective diagnostic and prognostic biomarker for PC.
Compared with prostate biopsy, serum collection from patients is a
less invasive procedure that can be performed in some cases when
biopsy is not applicable. Notably, lncRNA GASL1 has not been
characterized in other diseases. Multiple biomarkers may thus be
combined to improve the diagnosis and prognosis.
In the present study, GASL1 expression was
significantly associated with tumor size but not distant tumor
metastasis, which suggested that GASL1 may be associated with tumor
growth but not with metastasis in PC. Furthermore, CCK-8 assays
revealed that GASL1 may be an inhibitor of cancer cell
proliferation in PC. Bcl-2 is a member of the Bcl-2 protein family
that regulates cell proliferation by inhibiting pro-apoptotic
pathways or inducing anti-apoptotic pathways (18). GLUT-1 serves a major role in glucose
metabolism and is usually upregulated in tumor tissues to promote
tumor growth (19). In the present
study, GASL1 overexpression increased Bcl-2 expression but
inhibited GLUT-1 expression in PC cells. In addition, Bax is an
anti-apoptotic protein (18). In the
present study, GASL1 overexpression had no effect on Bax expression
in PC and normal cell lines. GASL1 may therefore interact with
multiple pathways and have various roles; however, the underlying
mechanisms of action of GASL1 remain unclear. GASL1 may inhibit PC
by downregulating GLUT-1. The stimulating role of GASL1 on Bcl-2
may reveal the complexity of the regulatory role of GASL1 on cell
proliferation and apoptosis. In addition, previous studies reported
that GASL1 regulates cell cycle and apoptosis (10). Therefore, the inhibition of cell
proliferation observed after GASL1 expression may be due to
apoptosis stimulation or cell cycle progression inhibition.
GASL1 overexpression had no effect on growth or
Bcl-2 and GLUT-1 protein expressions in the HPrEC normal prostate
epithelial cell line, which suggested that GASL1 may serve as a
potential therapeutic target for treatment of PC. The present study
had some limitations. Cell apoptosis and cell cycle were not
examined, although they may have revealed functions of GASL1 on
other aspects of PC; these analyses will be performed in a future
study. However, results from the present study may provide guidance
for future studies investigating the functionality of GASL1 in PC
and other malignancies.
In conclusion, GASL1 was significantly downregulated
in patients with PC compared with healthy controls, which suggested
that GASL1 may serve as a potential diagnostic and prognostic
biomarker in PC. In addition, expression levels of GASL1 were
significantly associated with tumor size. GASL1 overexpression
inhibited PC cell proliferation, upregulated Bcl-2 expression and
downregulated GLUT-1 expression. Taken together, these data
suggested that lncRNA GASL1 may inhibit PC cell proliferation by
targeting GLUT-1.
Acknowledgements
Not applicable.
Funding
The study was supported by The National Natural
Science Foundation (grant no. 30872924).
Availability of data and materials
All the data generated or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
ZL and HL performed experiments. ZL, HL, WJ, YX, XZ
and JY assisted with the experiments and statistical analysis. ZL,
HL and JY wrote the manuscript. JY revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Tongji Hospital, Tongji
Medical College, Huazhong University of Science and Technology
(Wuhan, China) approved the protocol. Written informed consent was
provided by patients and healthy people involved.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lepor H: Surgical treatment of prostate
carcinoma. J Urol 197 (2S). S41–S42. 2017.
|
|
3
|
Gundem G, Van Loo P, Kremeyer B,
Alexandrov LB, Tubio JMC, Papaemmanuil E, Brewer DS, Kallio HML,
Högnäs G, Annala M, et al: The evolutionary history of lethal
metastatic prostate cancer. Nature. 520:353–357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan W, Li H, Zhou Y, Huang Z, Rong S, Xia
M, Ji Z, Chen J and Jiang Y: Prostate carcinoma spatial
distribution patterns in Chinese men investigated with systematic
transperineal ultrasound guided 11-region biopsy. Urol Oncol.
27:520–524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lian J, Wu X, He F, Karnak D, Tang W, Meng
Y, Xiang D, Ji M, Lawrence TS and Xu L: A natural BH3 mimetic
induces autophagy in apoptosis-resistant prostate cancer via
modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum. Cell
Death Differ. 18:60–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Effert P, Beniers AJ, Tamimi Y, Handt S
and Jakse G: Expression of glucose transporter 1 (Glut-1) in cell
lines and clinical specimens from human prostate adenocarcinoma.
Anticancer Res. 24:3057–3063. 2004.PubMed/NCBI
|
|
7
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmidt LH, Görlich D, Spieker T, Rohde C,
Schuler M, Mohr M, Humberg J, Sauer T, Thoenissen NH, Huge A, et
al: Prognostic impact of Bcl-2 depends on tumor histology and
expression of MALAT-1 lncRNA in non-small-cell lung cancer. J
Thorac Oncol. 9:1294–1304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu X and Gan B: lncRNA NBR2 modulates
cancer cell sensitivity to phenformin through GLUT1. Cell Cycle.
15:3471–3481. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gasri-Plotnitsky L, Ovadia A, Shamalov K,
Nizri-Megnaji T, Meir S, Zurer I, Cohen CJ and Ginsberg D: A novel
lncRNA, GASL1, inhibits cell proliferation and restricts E2F1
activity. Oncotarget. 8:23775–23786. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malik SS, Kazmi Z, Fatima I, Shabbir R,
Perveen S and Masood N: Genetic polymorphism of GSTM1 and GSTT1 and
risk of prostatic carcinoma-a meta-analysis of 7,281 prostate
cancer cases and 9,082 healthy controls. Asian Pac J Cancer Prev.
17:2629–2635. 2016.PubMed/NCBI
|
|
12
|
Jin G, Sun J, Isaacs SD, Wiley KE, Kim ST,
Chu LW, Zhang Z, Zhao H, Zheng SL, Isaacs WB and Xu J: Human
polymorphisms at long non-coding RNAs (lncRNAs) and association
with prostate cancer risk. Carcinogenesis. 32:1655–1659. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou W, Wang Z, Tao Z, Ji L, Zhou B, Shen
M and Tu H: Differential expression of long non-coding RNAs in
prostatic carcinoma cell line LNCaP after occurrence of androgen
independent transformation. Int J Clin Exp Pathol. 10:6665–6673.
2017.
|
|
14
|
Hori SS and Gambhir SS: Mathematical model
identifies blood biomarker-based early cancer detection strategies
and limitations. Sci Transl Med. 3:109ra1162011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grammatikakis I, Panda AC, Abdelmohsen K
and Gorospe M: Long noncoding RNAs(lncRNAs) and the molecular
hallmarks of aging. Aging (Albany NY). 6:992–1009. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mayfield RD: Emerging roles for ncRNAs in
alcohol use disorders. Alcohol. 60:31–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Qiu M, Xu Y, Li M, Dong G, Mao Q,
Yin R and Xu L: Long noncoding RNA CCAT2 correlates with smoking in
esophageal squamous cell carcinoma. Tumour Biol. 36:5523–5528.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H, Li X, Bai M, Suo Y, Zhang G and Cao
X: Matrine inhibited proliferation and increased apoptosis in human
breast cancer MCF-7 cells via upregulation of Bax and
downregulation of Bcl-2. Int J Clin Exp Pathol. 8:14793–14799.
2015.PubMed/NCBI
|
|
19
|
Kurahara H, Maemura K, Mataki Y, Sakoda M,
Iino S, Kawasaki Y, Arigami T, Mori S, Kijima Y, Ueno S, et al:
Significance of glucose transporter type 1 (GLUT-1) expression in
the therapeutic strategy for pancreatic ductal adenocarcinoma. Ann
Surg Oncol. 25:1432–1439. 2018. View Article : Google Scholar : PubMed/NCBI
|