Introduction
Chronic myeloid leukemia (CML) is a
myeloproliferative disorder characterized by the proliferation and
accumulation of mature myeloid cells and their progenitors
(1). The hallmark of the disease is
the presence of the reciprocal translocation (9;22)(q34;q11.2),
resulting in a BCR/ABL1 gene fusion on the derivative
chromosome 22, the so-called Philadelphia (Ph) chromosome (1). Variant translocations are identified in
5–10% of patients with newly diagnosed CML (1). The translocation can be observed either
in a simple form (involving 22q11.2 and one additional breakpoint)
or in a complex form, involving 9q34, 22q11.2, and at least one
additional breakpoint (2,3). Although all chromosomes have been
reported to participate in variants, the distribution of
breakpoints clearly exhibits a non-random pattern, with a marked
clustering to specific chromosome bands (2,3).
Fluorescence in situ hybridization (FISH) has
been commonly used to detect the presence of the BCR/ABL1
fusion gene at disease diagnosis and also to monitor its evolution
during therapy. Different FISH probes can be combined to accurately
determine the complex variant translocations involving more than
two chromosomes when observed by cytogenetic analysis (3).
We report herein the characterization, by
conventional and molecular cytogenetics, of 32 cases with complex
variant Ph translocations, diagnosed in 32 patients with CML.
Materials and methods
Patients' initial features
From 1990 to 2015, 693 patients with CML were
diagnosed in three different centers: Hospital Clínic de Barcelona,
Hospital del Mar de Barcelona, and Hospital Trias i Pujol de
Badalona. Among these, 32 (5%) CML patients exhibited complex
variant Ph translocations. The primary clinical and hematological
parameters of the patients are outlined in Table I. The patients were comprised of 15
females and 17 males, ranging in age from 23 to 81 years. The
ethical approval for the present study, including the written
informed consent of the patients, was granted following the
guidelines of the Ethics Committee of the Hospital Clínic de
Barcelona, Hospital del Mar de Barcelona, and Hospital Trias i
Pujol de Badalona.
 | Table I.Main clinical and hematological data
of 32 CML patients at the time of diagnosis. |
Table I.
Main clinical and hematological data
of 32 CML patients at the time of diagnosis.
| No. | Age | Palpable spleen | WBC (×109/l) | Hb (g/l) | Platelets
(×109/l) | Therapy | Survival
(months) |
|---|
| 1 | 27 | NO | 67 | 97 | 745 | HU, IFN,ALLO-SCT | 9 |
| 2 | 81 | NO | 18.5 | 112 | 884 | IMATINIB | 60 |
| 3 | NA | NA | NA | NA | NA | NA | NA |
| 4 | 38 | YES | 57.9 | 150 | 237 | HU, IFN,
ALLO-SCT | 22 |
| 5 | 51 | YES | 254 | 90 | 498 | HU, IFN,
ALLO-SCT | 23 |
| 6 | NA | NA | NA | NA | NA | NA | NA |
| 7 | 67 | YES | 15 | 111 | 1,075 | IMATINIB,
DASATINIB | NA |
| 8 | 39 | NO | 41 | 145 | 126 | IMATINIB,
DASATINIB | 55 |
| 9 | 23 | NA | 49 | 95 | 349 | NA | NA |
| 10 | NA | NA | NA | NA | NA | NA | NA |
| 11 | NA | NA | NA | NA | NA | NA | NA |
| 12 | 67 | NO | 42.6 | 146 | 326 | IMATINIB | 108 |
| 13 | 72 | YES | 7.9 | 124 | 249 | IMATINIB, DASATINIB,
BOSUTINIB | 108 |
| 14 | 27 | NO | 24 | 133 | 378 | DASATINIB, PONATINIB,
ALLO-SCT | 36 |
| 15 | 36 | YES | 537 | 93 | 503 | HU, IFN ALLO-SCT | 24 |
| 16 | NA | NO | NA | NA | NA | NA | NA |
| 17 | 48 | YES | 234 | 68 | 69 | HU, DASATINIB,
BOSUTINIB | 24 |
| 18 | 61 | YES | 73 | 93 | 30 | IMATINIB, DASATINIB,
ALLO-SCT | 12 |
| 19 | 50 | NO | 149 | 95 | 466 | IMATINIB | 120 |
| 20 | 45 | YES | 93 | 111 | 250 | IMATINIB, DASATINIB
ALLO-SCT, PONATINIB | 24 |
| 21 | 53 | NO | 18 | 108 | 208 | IMATINIB,
NILOTINIB | 96 |
| 22 | 43 | NA | 12 | 116 | 1294 | HU, IFN | 121 |
| 23 | 34 | YES | 228 | 90 | 297 | HU, IFN,
ALLO-SCT | 27 |
| 24 | 39 | NA | 12.9 | 81 | 16 | IMATINIB, ALLO-SCT
DASATINIB | 60 |
| 25 | 74 | NO | 27 | 142 | 347 | IMATINIB,
DASATINIB | 59 |
| 26 | 41 | NO | 178 | 108 | 282 | IMATINIB | 96 |
| 27 | 50 | NO | 88 | 144 | 336 | IMATINIB | 132 |
| 28 | 44 | YES | 130 | 110 | 394 | NILOTINIB | 32 |
| 29 | 42 | NA | 126 | 116 | 260 | IMATINIB | 108 |
| 30 | 50 | NA | 21.6 | 110 | 72 | NA | NA |
| 31 | 38 | YES | 212 | 92 | 122 | HU, IMATINIB | 10 |
| 32 | 64 | NA | 6.7 | 171 | 206 | IMATINIB,
NILOTINIB | 144 |
Conventional cytogenetics
Bone marrow samples were processed for cytogenetic
and FISH analysis. Cytogenetic studies were carried out on G-banded
chromosomes obtained from 24 h un-stimulated bone marrow cultures.
Karyotypes were described according to An International System for
Human Cytogenomic Nomenclature (4).
A300-band ideogram was considered as the standard level of
resolution for the purpose of the present study. Given that three
laboratories were involved in the present study, with a different
chromosome quality, it was agreed that translocations with
breakpoints differing in one band would be considered as the same
translocation.
Molecular cytogenetics (FISH)
FISH probes were used to establish whether the
BCR/ABL1 rearrangement was present, as well as its location,
and to characterize the complex variant translocations. Two
different FISH probes were used in order to detect the
BCR/ABL1 rearrangements: LSI BCR/ABL1.ES and LSI BCR/ABL1
DCDF, as described previously (5).
For the characterization of complex variant translocations, whole
chromosome paint (WCP) probes for chromosomes 1, 5, 11, 12, 20 and
22; Centromeric (CEP) probes for chromosome 9.
All probes were provided by VYSIS (Abbott Products
Operations AG, Allschwil, Switzerland) and the hybridization and
detection were performed according to the manufacturer's protocols.
Images were captured and processed with a Cytovision Ultra System
(v.5.1.22; Leica Biosystems, Wetzlar, Germany).
BCR-ABL determination by the reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
White blood cells were isolated from peripheral
blood or bone marrow samples with a lysis buffer containing 0.144 M
NH4Cl and 0.01 M NH4HCO3. Total
RNA was extracted using TRIzol reagent (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol.
Reverse transcription was performed on 1 µg of RNA with the Moloney
murine leukemia virus (M-MLV) reverse transcriptase (Thermo Fisher
Scientific, Inc.) and random hexamer primers. Briefly, 1 µg of RNA
in 19 µl of RNAse-free water was incubated at 65°C for 5 min.
Samples were cooled on ice and the following reagents were added to
a final volume of 40 µl: 8 µl 5× RT buffer (250 mM Tris-HCl pH 8.3,
375 mM KCl, 15 mM KCl, 15 nM MgCl2; Thermo Fisher
Scientific, Inc.), 0.4 µl DTT (0.1 M; Thermo Fisher Scientific,
Inc.); 1.6 µl dNTPs (25 mM; GE Healthcare) 1.2 µl pdN6
hexanucleotides (10X; Roche Diagnostics, Basel, Switzerland), 1.5
µl RT enzyme M-MLV (200 U/µl; Thermo Fisher Scientific, Inc.), 0.75
µl RNAsin (40 U/µl; Thermo Fisher Scientific, Inc.) and 7.55 µl
RNAse-free water. Samples were then incubated at 37°C for 80 min,
at 65°C for 10 min and 4°C at the end of RT step. Subsequently,
qPCR was run from 2 µl cDNA as described by Van Dongen et al
(6).
Results
All variant chromosome Ph translocations were
complex and involved 3 chromosomes, except in case 31 where the
translocation included 4 chromosomes. The karyotypes are described
in Table II. The karyotypes of
patients 1, 4, 5, 15, 22, 23 and 31 have already reported in a
previous study (5).
 | Table II.Karyotype, chromosomal region of the
additional chromosome/s involved in the complex variant Ph
translocation and its location in a G-light band for the 32
patients with CML. |
Table II.
Karyotype, chromosomal region of the
additional chromosome/s involved in the complex variant Ph
translocation and its location in a G-light band for the 32
patients with CML.
| Case | Karyotype at
diagnosis | BP | G-light BP |
|---|
| 1 |
46,XX,del(22)(q11.2)[19]/46,XX[1]//a46,XX,t(1;9;22)(p36.1;q34;q11.2)
cryptic | 1p36 | Yes |
| 2 |
46,XX,t(1;9;22)(p36.1;q34;q11.2)[20] | 1p36 | Yes |
| 3 |
46,XX,t(1;9;22)(p11;q34;q11.2)[19]/46,XX[1] | 1p11 | Cen |
| 4 |
46,XY,t(1;9;22)(q21;q34;q11.2)[9]/46,XY[1] | 1q21 | Yes |
| 5 |
46,XX,t(1;9;22)(q21;q34;q11.2)[13]//a46,XX,
der(1)ins(9;1)(q34;q23q44), der(9)t(9;22)(q34;q11.2)ins(9;1) | 1q21 | Yes |
| 6 |
46,XX,t(1;9;22)(q32;q34;q11.2)[16] | 1q32 | Yes |
| 7 |
46,XX,t(9;22)(q34;q11.2)[5]/46,XX,t(1;9;22)(q42;q34;q11.2)[2]/47,
idem, +der(22)t(1;9;22)(q42.1;q34;q11.2)[16]/46,XX[4] | 1q42 | Yes |
| 8 |
46,XY,t(2;9;22)(p13;q34;q11.2)[19]/46,XY[1] | 2p13 | No |
| 9 |
46,XY,t(2;9;22)(p13;q34;q11.2)[20] | 2p13 | Yes |
| 10 |
46,XX,t(3;9;22)(p21;q34;q11.2)[20] | 3p21 | Yes |
| 11 |
46,XY,t(3;9;22)(p13;q34;q11.2)[20] | 3p13 | Yes |
| 12 |
46,XY,der(3)del(3)(p25)t(3;9;22)(q27;q34;q11.2)[20] | 3q27 | Yes |
| 13 |
46,XX,t(5;9,22)(q12;q34;q11.2)[19]/47,XX,t(5;9;22)(q12;q34;q11.2),
+der(22)t(5;9;22)(q12;q34;q11.2)[1] | 5q12 | No |
| 14 |
46,XY,t(5;9,22)(q31;q34;q11.2)[14]/46,XY[6] | 5q31 | Yes |
| 15 |
46,XY,t(5,9;22)(q31;q34;q11.2),-21,+mar[20]/46,XY[1] | 5q31 | Yes |
| 16 |
47,XX,add(1)(q42),t(5;9;22)(q35;q34;q11,2),+8[20] | 5q35 | Yes |
| 17 |
46,XY,t(5;9;22)(q35;q34;q11.2)[20] | 5q35 | Yes |
| 18 |
45,X,-Y[13]/46,XY,t(6;9;22)(p23;q34;q11.2)[7] | 6p23 | Yes |
| 19 | 46,XY,
t(6;9;22)(p21;q34;q11.2)[20] | 6p21 | Yes |
| 20 |
46,XX,t(7;9;22)(q36;q34;q11.2)[20] | 7q36 | Yes |
| 21 |
46,XX,t(9;22;11)(q34;q11.2;q11)[20] | 11q11 | Cen |
| 22 |
46,XX,t(9;22;11)(q34;q11.2;q13)[20] | 11q13 | Yes |
| 23 |
46,XX,t(9;22;11)(q34;q11.2;q13)[15] | 11q13 | Yes |
| 24 |
44,XY,dic(7;9)(q11;q11),t(9;22;12)(q34;q11.2;p13),-18,
add[20](q13)(18)/46,XY[2] | 12p13 | Yes |
| 25 |
46,XY,t(9;22;12)(q34;q11.2;p13)[3] | 12p13 | Yes |
| 26 |
46,XY,t(9;22;13)(q34;q11.2;q14)[20] | 13q14 | Yes |
| 27 |
46,XY,t(9;22;15)(q34;q11.2;q22)[20] | 15q22 | Yes |
| 28 |
46,XY,t(9;22;15)(q34;q11.2;q22)[6] | 15q22 | Yes |
| 29 |
46,XX,t(9;22;17)(q34;q11.2;q12)[20] | 17q12 | Yes |
| 30 |
47,XX,+8,t(9;22;19)(q34;q11.2;p13)[15] | 19p13 | Yes |
| 31 |
46,XY,t(9;22;12)(q34;q11.2;p13)[20]//a46,XY,t(9;22;20;12)(q34;q11.2;q12;p13) | 20q12/12p13 | Yes/yes |
| 32 |
46,XY,t(9;22;21)(q34;q11.2;q21)[25] | 21q21 | No |
The most frequent variants were t(1;9;22)
(p36;q34;q11.2), t(1;9;22)(q21;q34;q11.2),
t(2;9;22)(p13;q34;q11.2), t(5;9,22)(q31; q34;q11.2),
t(5;9;22)(q35;q34;q11,2), t(9;22;11)(q34;q11.2;q13),
t(9;22;12)(q34;q11.2;p13) and t(9;22;15)(q34;q11.2;q22), as they
were identified twice.
The chromosomes included in the translocations were:
1 (n=7), 5 (n=5), 3, 11 and 12 (n=3), 2, 6 and 15 (n=2), and 7, 13,
17, 19, 20 and 21 (one each). Chromosomes 4, 8, 9, 10, 14, 16, 18,
22, X and Y were not included in any translocations. A total of 33
breakpoints were described in 32 translocations, and 17 of those
were recurrent, being 12p13 (n=3), and 1p36, 1q21, 2p13, 5q31,
5q35, 11q13 and 15q22 (n=2). The q chromosome arm was more
frequently involved in the translocations (n=20; 60%) than the p
arm. The breakpoints were located in the G-light bands in the
majority of cases (n=28; 85%), while the remaining breakpoints
werein the dark bands (5q12, 17q12 and 21q21) and in the
centromeric areas (1p11 and 11q11) (Table II).
Additional chromosomal abnormalities were observed
in 6 out of 32 (19%) patients, including: der(22)/ der(22)/
−21,+mar/add(1)(q42), +8/dic(7;9)(q11;q11),-18 and add(20)(q13)/
+8. Clinical information on possible progression was available in 3
out of the 6 cases with additional chromosomal abnormalities,
(cases 7, 15 and 30), and none of these patients were in the blast
crisis phase (Table II).
FISH studies using the LSI BCR/ABL1 were performed
in 23 out of 32 cases, allowing for the detection of the
BCR/ABL1 fusion gene in the Ph chromosome in all cases. In
the 7 cases (cases 1, 5, 14, 15, 16, 31 and 32) where WCP and CEP
FISH probes were used, the complex variant translocations were
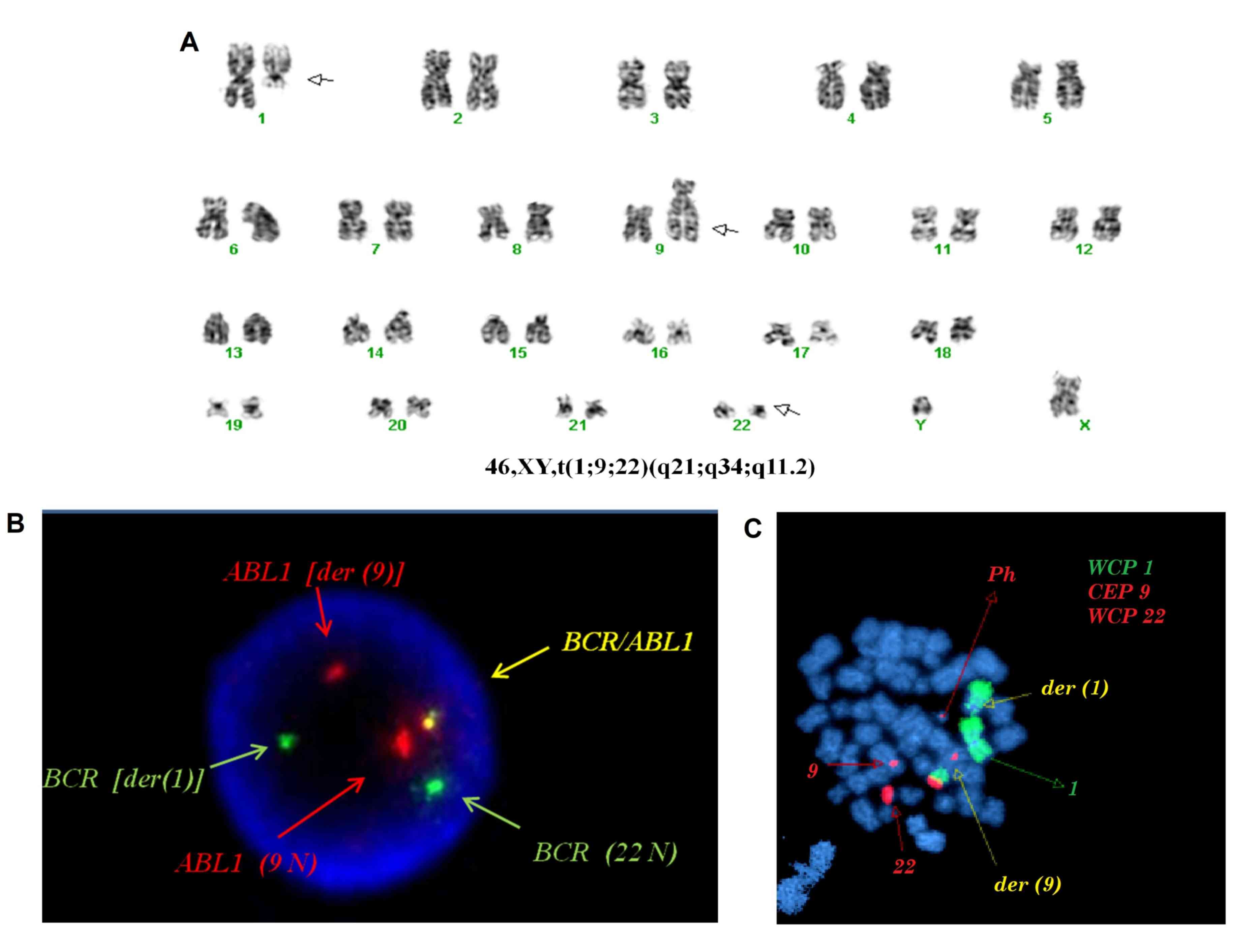
confirmed. Characterization of the t(1;9;22)(q21;q34;q11.2), using
G-banded karyotype and LSI and WCP FISH probes for chromosomes 1, 9
and 22 in interphase nuclei and metaphases, are shown in Fig. 1.
Molecular studies (RT-PCR) revealed e14a2 chimeric
BCR/ABL mRNA in 15 cases and e13a2 chimeric BCR/ABL mRNA in 12
cases. In the remaining 5 cases the molecular studies were not
performed.
Discussion
The karyotype and the combination of different FISH
probes are essential to characterize complex variant Ph
translocations (3,5). The use of the FISH probe for detecting
the BCR/ABL1 rearrangement in interphase nuclei and
metaphases is crucial to determine not only the presence of the
rearrangement and its localization, but also whether further events
have occurred, such as the presence of a double fusion gene, which
may be relevant to interpret the clinical course and the prognosis
of the disease. In our series, conventional and molecular
cytogenetic studies have allowed the characterization of the 32
complex variant Ph translocations.
At present, in spite of its high genetic complexity,
it is widely accepted that the clinical, prognostic and
hematological features of patients with CML with complex variant
translocations are not different from those with the classical
t(9;22) translocation because it is accepted that the key
pathological event is the formation of the BCR/ABL1 fusion
gene (2).
Although all chromosomes have been described in the
complex variants, some regions are more frequently involved
(3). In our series of experiments,
the chromosomes most frequently involved were chromosomes 1 and 5,
while the more frequent breakpoint was 12p13. The q chromosome arm
participated more frequently (60%) than the p-arm. It may be
hypothesized that the longer the arm, the higher the probability of
recombination. Chromosomes 4, 8, 9, 10, 14, 16, 18, 22, X and Y
were not identified in our translocations. All 32 variant
translocations identified in our experiments have been previously
described in complex variant Ph translocations (3,7).
The present study observed that the breakpoints of
the variant 9,22 translocations locate preferentially, with 85% of
them in the G-light bands (CG-richest areas). Fisher et al
(8), reported this association in
relation with that the CG richness areas reflect increases in the
density of the CpG islands, genes, repetitive elements, and
recombination.
Variant translocations may be caused by different
mechanisms. Some variants are originated by multiple simultaneous
breaks (one-step) and some arise as a result of two, or even more,
genetic events in close succession (two-step or multiple-step)
(9–11). In our series, the complex variant
t(9;22;V) was identified in 30 out of 32 cases at the time of
diagnosis suggesting that the t(9;22;V) originated in a stem cell,
probably as the result of a one-step translocation. In two cases
(cases 5 and 7), a two-step translocation could explain the complex
variant translocations. In case 5, the insertion of material from
chromosome 1 into the der (9)
involved a second breakpoint in 9q34. In case 7, the identification
of the two cell lines, t(9;22) and t(1;9;22), at the time of
diagnosis suggests a two-step translocation.
Clonal evolution typically coincides with or
precedes the accelerate phase or blast crisis of CML (9,10).
Therefore, an inherent implication of the two-step mechanism is
that variant translocations might be associated with a poorer
prognosis (9,10).
Secondary abnormalities in the chronic phase of CML
have been reported between 10–20% of cases, with the frequency
being similar in t(9;22) or its variants. In our series, 19% of the
cases have a secondary abnormality, which is concordant with the
reported rates (1).
In conclusion, the combination of conventional and
molecular cytogenetics studies has allowed us: i) To detect and
quantify the BCR/ABL1 fusion gene; ii) to characterize the complex
variant translocations and detect cryptic translocations; iii) to
confirm that the breakpoints are commonly localized in the
CG-richest regions of the genome; (iv) to confirm that the genesis
of variant translocations could be via either the one-step or
two-step mechanisms; and v) to report new cases of complex variant
translocations, which can involve new breakpoints that can
eventually be recurrent and important for the understanding of this
leukemia.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DC conceived and designed the study. JG, BE and DC
were responsible for the data acquisition, selection and analysis.
AA, CG and MLG were responsible for the analysis and interpretation
of the conventional (karyotype) and molecular (FISH and RT-qPCR)
studies. MN and FC were responsible for the analysis and
interpretation of data, and critically revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of the Hospital Clínic de Barcelona, Hospital del Mar de
Barcelona and Hospital Trias i Pujol de Badalona. Written informed
consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Heim S and Mitelman F: Cancer
Cytogenetics: Chromosomal and Molecular Genetic Aberrations of
Tumor Cells. (4th). Wiley-Blackwell. (New Jersey). 2015. View Article : Google Scholar
|
|
2
|
Johansson B, Fioretos T and Mitelman F:
Cytogenetic and molecular genetic evolution of
Philadelphia-chromosome-positive chronic myeloid leukaemia. Chronic
Myeloproliferative Disorders. Cytogenetic and molecular genetic
abnormalities. Bain BJ: Karger. (Basel). 44–61. 2003.
|
|
3
|
Mitelman F, Johansson B and Mertens F:
Mitelman Database of Chromosome Aberrations and Gene Fusions in
Cancer. http://cgap.nci.nih.gov/Chromosomes/Mitelman
|
|
4
|
McGowan-Jordan J, Simons A and Schmid M:
ISCN 2016: An International System for Human Cytogenomic
Nomenclature. Karger. (Basel). 2016.
|
|
5
|
Costa D, Carrió A, Madrigal I, Arias A,
Valera A, Colomer D, Aguilar JL, Teixido M, Camós M, Cervantes F,
et al: Studies of complex Ph translocations in cases with chronic
myelogenous leukemia and one with acute lymphoblastic leukemia.
Cancer Genet Cytogenet. 166:89–93. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Dongen JJM, Macintyre EA, Gabert JA,
Delabesse E, Rossi V, Saglio G, Gottardi E, Rambaldi A, Dotti G,
Griesinger F, et al: Standardized RT-PCR analysis of fusion gene
transcripts from chromosome aberrations in acute leukemia for
detection of minimal residual disease. Report of the BIOMED-1
Concerted Action: Investigation of minimal residual disease in
acute leukemia. Leukemia. 13:1901–1928. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Atlas of Genetics and Cytogenetics in
Oncology and Haematology. http://atlasgeneticsoncology.orgMarch
26–2018
|
|
8
|
Fisher AM, Strike P, Scott C and Moorman
AV: Breakpoints of variant 9;22 translocations in chronic myeloid
leukemia locate preferentially in the CG-richest regions of the
genome. Genes Chromosomes Cancer. 43:383–389. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gorusu M, Benn P, Li Z and Fang M: On the
genesis and prognosis of variant translocations in chronic myeloid
leukemia. Cancer Genet Cytogenet. 173:97–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bennour A, Sennana H, Laatiri MA, Elloumi
M, Khelif A and Saad A: Molecular cytogenetic characterization of
variant Philadelphia translocations in chronic myeloid leukemia:
Genesis and deletion of derivative chromosome 9. Cancer Genet
Cytogenet. 194:30–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bennour A, Saad A and Sennana H: Chronic
myeloid leukemia: Relevance of cytogenetic and molecular assays.
Crit Rev Oncol Hematol. 97:263–274. 2016. View Article : Google Scholar : PubMed/NCBI
|