Introduction
Chemotherapy is a widely used tumor treatment;
however, it can cause various degrees of ovarian dysfunction, which
can be irreversible. It is therefore important to protect ovaries
during chemotherapy to avoid compromising pregnancy rate,
considering the gradual improvement of women's survival following
chemotherapy.
In 1985, Ataya et al (1) demonstrated that long-acting
gonadotropin releasing hormone (GnRH) agonist (GnRHa) significantly
protects rat ovaries against cyclophosphamide-induced toxicity.
Blumenfeld and Eckman (2) performed
a prospective clinical study on fertile women undergoing
chemotherapy, and reported similar results. However, subsequent
clinical studies have revealed different results and opposite
conclusions (3,4). At present, reports on the protective
effects of GnRHa on ovarian function and whether it alters
chemotherapeutic efficiency are lacking.
In the present meta-analysis, clinical randomized
controlled trials of premenopausal women using GnRHa to protect
ovarian function during chemotherapy were systematically retrieved
and collected. Menstrual function recovery rate, premature ovarian
failure rate and pregnancy rate were analyzed. In addition, the
influence of GnRHa on long-term survival rate was evaluated. This
work may provide an effective strategy to protect ovarian function
in premenopausal women undergoing chemotherapy.
Materials and methods
Strategy for retrieving
literature
By the end of December 2017, the following key words
were used in PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), Embase database
(https://www.embase.com/) and Cochrane library
(https://www.cochranelibrary.com/advanced-search/search-manager)
to retrieve and collect clinical randomized controlled trials in
which GnRHa was administered to premenopausal women undergoing
chemotherapy: ‘GnRH agonist’, ‘GnRH analog’, ‘chemotherapy’,
‘ovarian damage’, ‘ovarian suppression’, ‘ovarian protection’,
‘ovarian function’, ‘ovarian dysfunction’, ‘fertility’ and
‘fertility preservation’.
Literature search
The following inclusion criteria ware applied in the
meta-analysis: i) Premenopausal women with malignant tumors,
systemic lupus erythematous or other diseases requiring
chemotherapy (according to characteristics including age, menstrual
function history, ultrasound and hormone levels); ii) a control
group with the same disease who did not receive GnRHa treatment;
and iii) no limits to ethnicity or language. The following
exclusion criteria were applied in the meta-analysis: i) The test
design in the original reference was not rigorous and the results
were not reliable; ii) the indispensable analytical data were not
provided; iii) case reports; iv) patients with metastatic advanced
malignant disease or malignant tumors; and v) patients who were
under hormone therapy or replacement therapy 3 months prior to
treatment with chemotherapy. The studies consisted of randomized
controlled trials of premenopausal women undergoing treatment with
GnRHa to protect ovarian function during chemotherapy.
Quality assessment and statistical
analysis
Two evaluators independently conducted literature
screening, risk assessment and data extraction. The risk assessment
was conducted according to the clinical randomized controlled trial
evaluation recommended by the Cochrane system evaluator manual 5.1
(5). RevMan 5.3 (http://ims.cochrane.org/revman/download)
provided by Cochrane collaboration, was used to analyze data. The
statistical heterogeneity of each result was analyzed using
χ2 test, and the significance level was set at P=0.1.
P<0.1 was considered to indicate a statistically significant
heterogeneity. I2 was used to quantitatively evaluate
the heterogeneity of the results. I2<25% indicated
that heterogeneity may not be important, I2>50%
indicated heterogeneity and I2>75% indicated high
heterogeneity. When heterogeneity was small, the fixed effect model
was adopted. When heterogeneity was high among the literature, the
random effect model was adopted and subgroup analysis was carried
out. All participants were included in the analysis, and divided
into various treatment groups, including the GnRHa group
(chemotherapy combined with GnRHa) and control group (chemotherapy
not combined with GnRHa) according to the type of intervention.
Relative risk (RR) and 95% confidence interval (CI) were calculated
for the following variables: Menstrual function recovery rate,
pregnancy rate, premature ovarian failure (POF) incidence,
tumor-free survival rate, total survival rate.
Results
Features of the included study
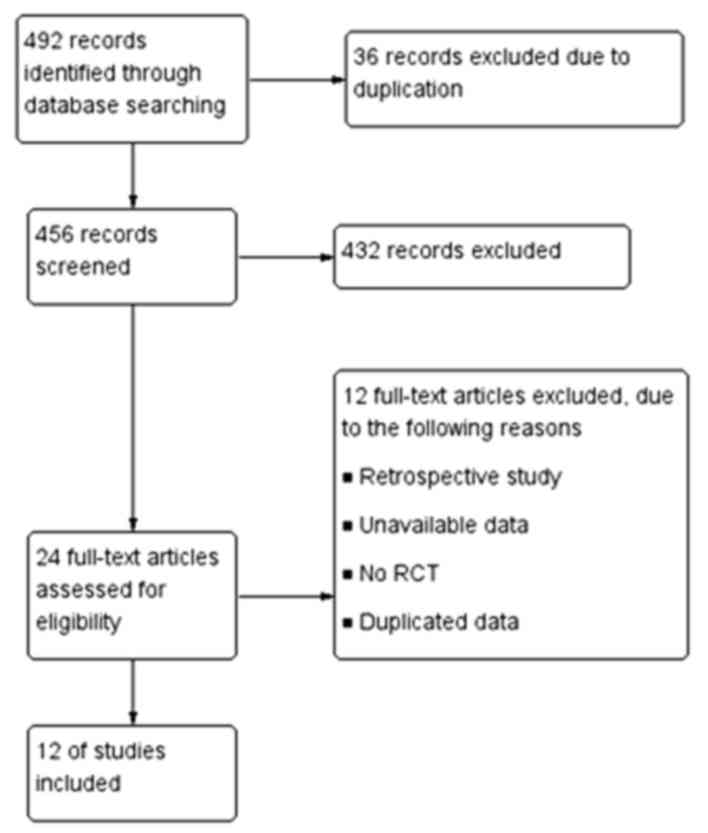
A total of 492 references were retrieved and 12
references were included in the present meta-analysis following
accurate selection (3,4,6–15) (Fig.
1). A total of 1,413 premenopausal patients with breast cancer
or lymphoma undergoing chemotherapy were included in the selected
studies. All studies selected included a comparison between the
GnRHa group (705 patients) and control group (708 patients). No
significant difference in baseline data between the GnRHa and
control groups was observed in these 12 studies. The GnRHa drugs
used were triptorelin, goserelin or leuprolide. GnRHa was used
either at the beginning of chemotherapy or prior to it. Basic
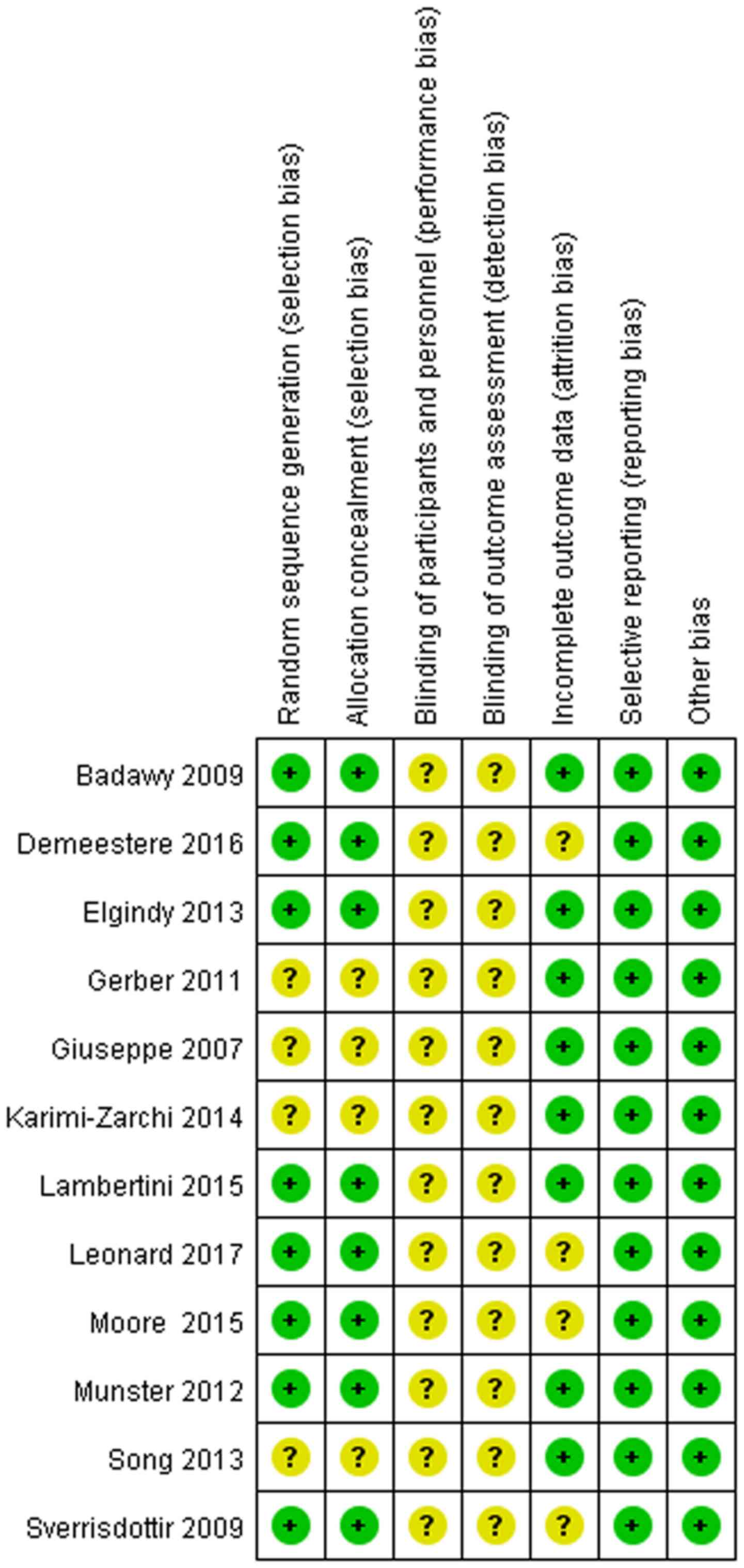
features of the included literature are presented in Table I. Quality assessment is shown in
Fig. 2.
 | Table I.Basic features of the literature
included in the present meta-analysis. |
Table I.
Basic features of the literature
included in the present meta-analysis.
| First author,
year | Disease | Results | Drugs | Dose and
interval | Start time | Follow-up time | (Refs.) |
|---|
| Badawy, 2009 | Breast cancer | Menstrual recovery,
POF | Goserelin | 3.6 mg/28 days | 2 weeks before
chemotherapy | 8 months | (3) |
| Demeestere, 2016 | Lymphoma | POF, pregnancy,
tumor-free survival, overall survival | Triptorelin | 3.75 mg/28 days | 2±0.51 days before
chemotherapy | GnRHa group: 5.33
years Control group: 5.58 years | (4) |
| Elgindy, 2013 | Breast cancer | Menstrual recovery,
pregnancy | Triptorelin | 3.75 mg/28 days | 10 days before
chemotherapy | 1 year | (6) |
| Gerber, 2011 | Breast cancer | Menstrual recovery,
pregnancy | Goserelin | 3.6 mg/28±3 days | At least 2 weeks
before chemotherapy | 2 years | (7) |
| Giuseppe, 2007 | Lymphoma | Menstrual recovery,
POF, pregnancy | Triptorelin | Triptorelin 3.25
mg/month Or Triptorelin 11.25 mg/3 months | Immediately after
diagnosis | GnRHa group: 2.42±1.7
years Control group: 5.93±4.47 years | (8) |
| Karimi-Zarchi,
2014 | Breast cancer | Menstrual recovery,
POF | Triptorelin | 3.75 mg/28
days | Same time as
chemotherapy | 6 months | (9) |
| Lambertini,
2015 | Breast cancer | Menstrual recovery,
pregnancy, tumor-free survival | Triptorelin | 3.75 mg/28
days | At least 1 week
before chemotherapy | 7.3 years | (10) |
| Leonard, 2017 | Breast cancer | Menstrual recovery,
POF, pregnancy outcomes, overall survival | Goserelin | 3.6 mg/28 days | At least 1–2 weeks
before chemotherapy | 2 years | (11) |
| Moore, 2015 | Breast cancer | Menstrual recovery,
POF, pregnancy, tumor-free, survival overall survival | Goserelin | 3.6 mg/28 days | 1 week before
chemotherapy | 5–7 years | (12) |
| Munster, 2012 | Breast cancer | Menstrual recovery,
POF, pregnancy | Triptorelin | 3.75 mg/28–30
days | At least 7 days
before chemotherapy | GnRHa group: 4.96
years Control group: 5.82 years | (13) |
| Song, 2013 | Breast cancer | Menstrual recovery,
POF | Leuprolide | 3.75 mg/28
days | Before chemotherapy
(if ovarian suppression was confirmed, patients started to receive
chemotherapy) | 1 year | (14) |
| Sverrisdotti,
2009 | Breast cancer | POF | Goserelin | 3.6 mg/28 days | Same time as
chemotherapy | 3 years | (15) |
Meta-analysis results
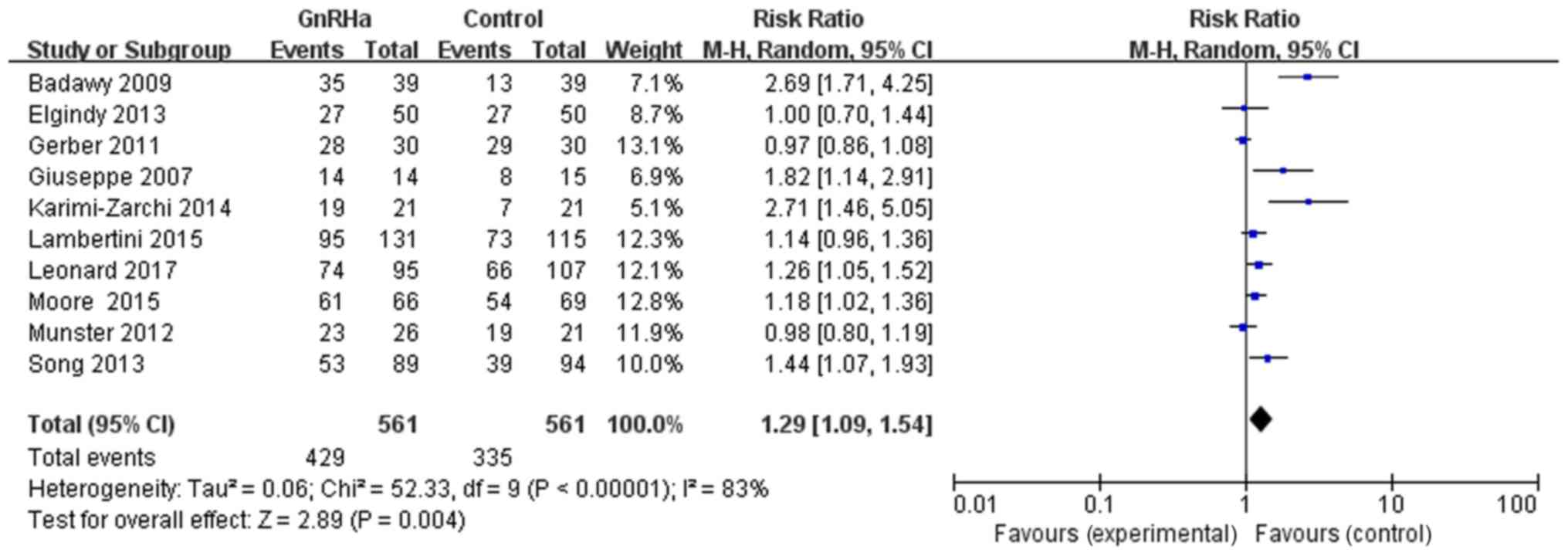
With regards to the effect of GnRHa on the menstrual
function recovery rate, 10 references provided evidence of
menstrual function recovery in both the GnRHa and control groups.
In the GnRHa group (561 patients), 429 presented menstrual function
recovery. In the control group (561 patients), 335 had menstrual
function recovery. The results exhibited the following RR and CI:
RR=1.29, 95% CI=1.09–1.54, P=0.004, with a statistically
significant difference, suggesting that chemotherapy combined with
GnRHa may significantly improve menstrual function recovery rate
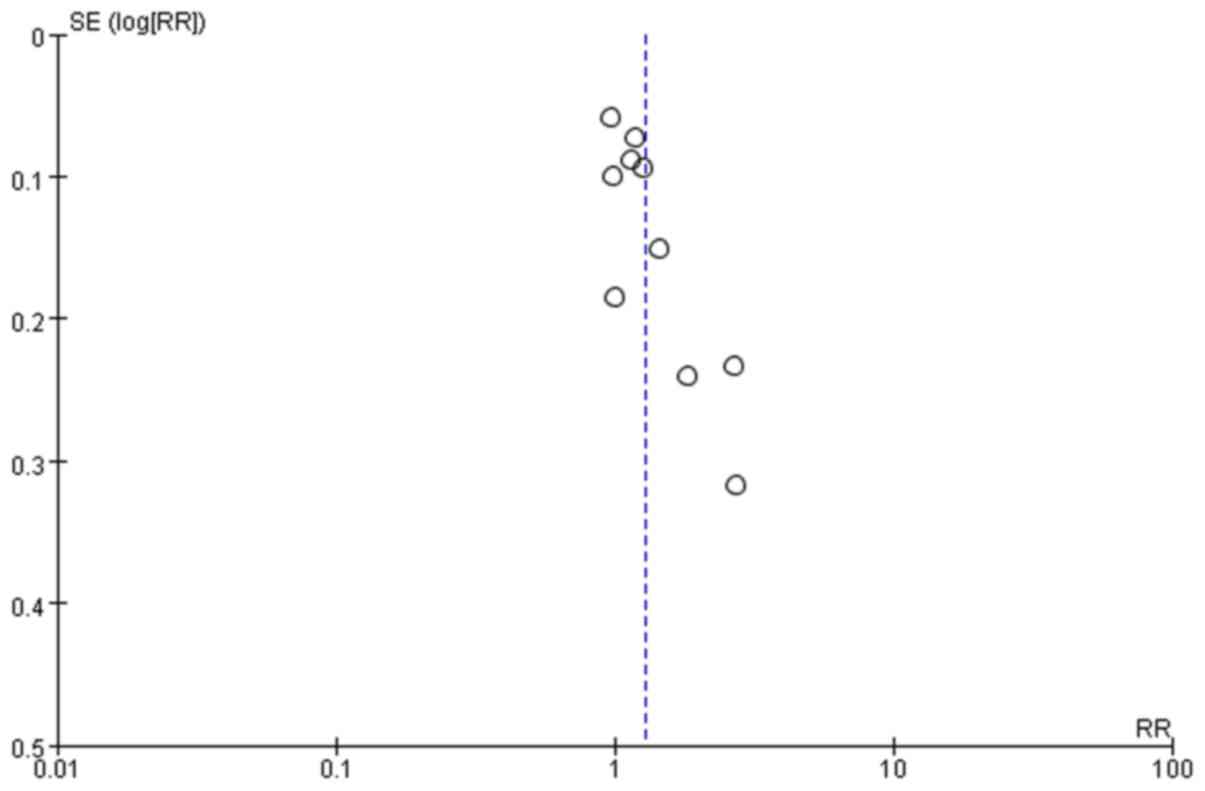
(Fig. 3). The scattered points of
the inverted funnel plot were less symmetrical and the aggregation
was more concentrated; therefore, some publication bias may be
present as certain negative results may not be published, as
presented in Fig. 4.
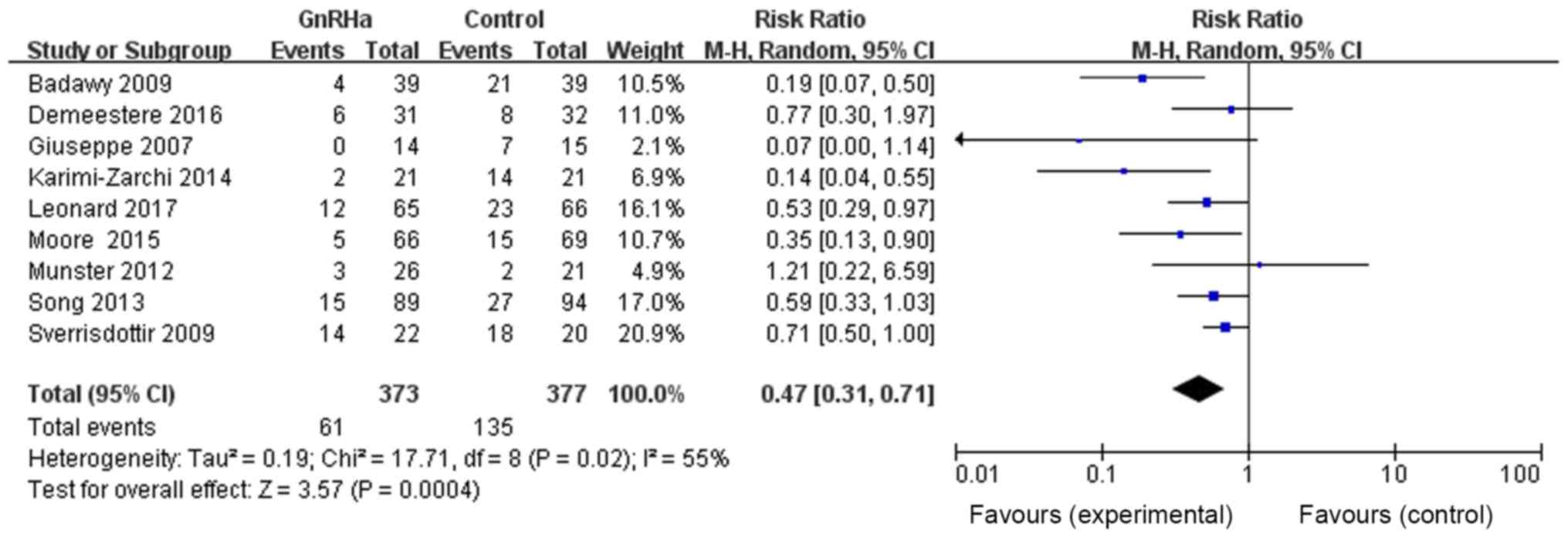
The effects of GnRHa on POF incidence were then
analyzed. Among the 12 references, nine provided evidence of POF
incidence in both GnRHa and control groups. The total number of
patients in the GnRHa group was 373, with 61 patients with POF,
whereas the total number of patients in the control group was 377,
with 135 patients with POF. The results were as follows: RR=0.47,
95% CI=0.31–0.71, P=0.0004, with statistically significant
differences, suggesting that chemotherapy combined with GnRHa may
significantly reduce POF incidence (Fig.
5).
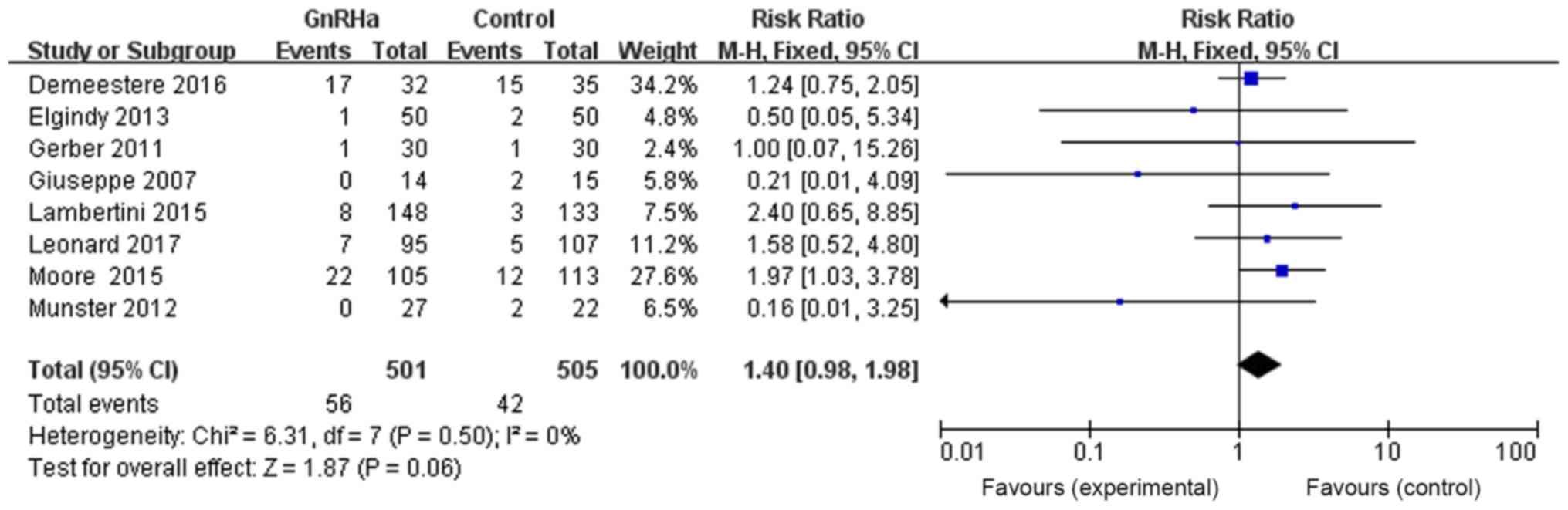
The effects of GnRHa on pregnancy rate were also
assessed. Eight references provided evidence of pregnancy in both
the GnRHa and control groups. The total number of patients in the
GnRHa group was 501, with 56 pregnant patients, whereas the total
number of patients in the control group was 505, with 42 pregnant
patients. The results were as follows: RR=1.40, 95% CI=0.98–1.98,
P=0.06, without any statistically significant difference,
suggesting that chemotherapy combined with GnRHa may have no effect
on pregnancy rate (Fig. 6).
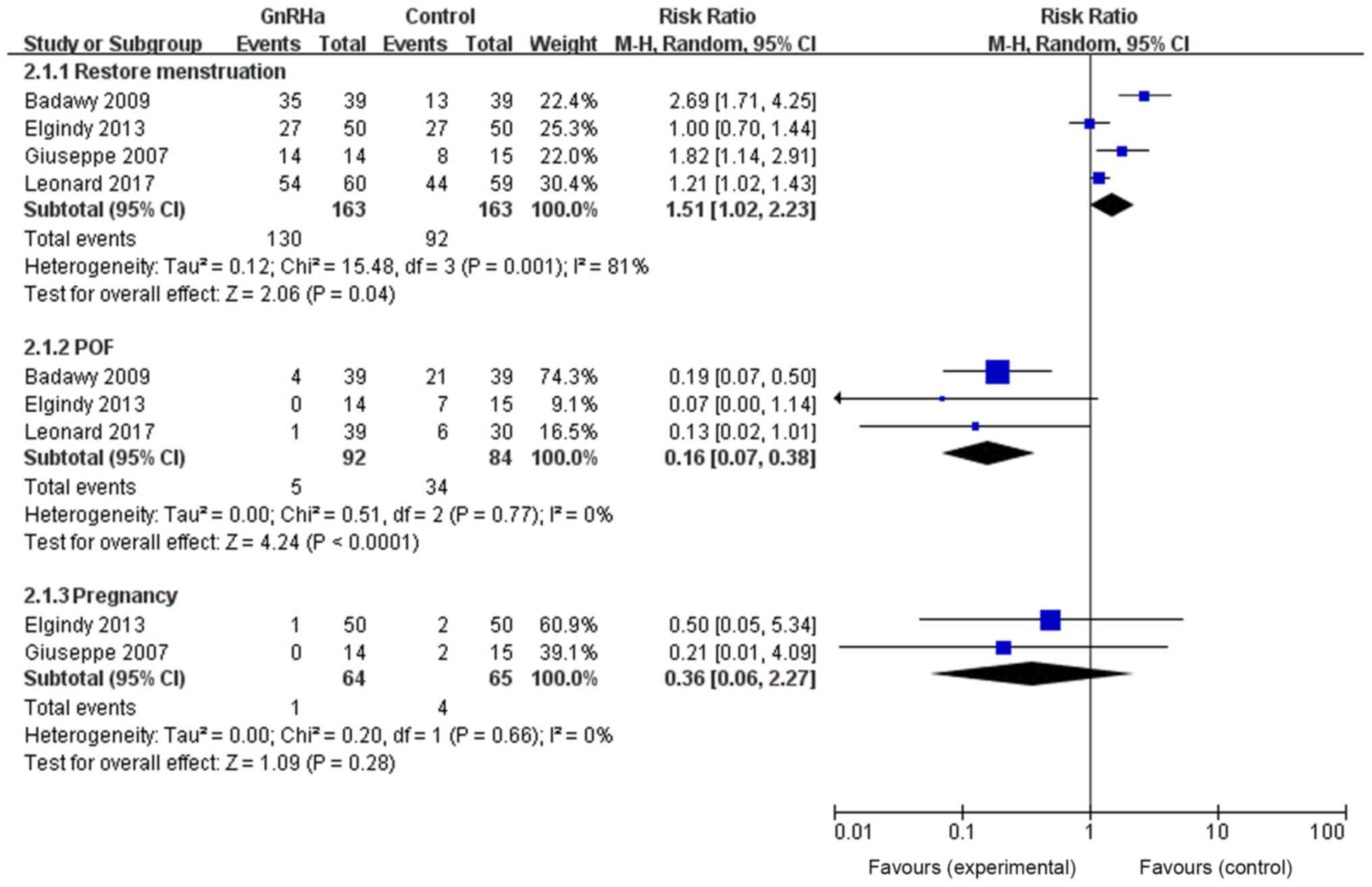
The effects of GnRHa on the menstrual function
recovery rate, POF incidence and pregnancy rate were also
determined on patients <40 years old. This subgroup analysis was
conducted because the literature was heterogenous. Results revealed
that GnRHa improved the menstrual function recovery rate of
patients undergoing chemotherapy (RR=0.16, 95% CI=0.07–0.38,
P<0.0001) and reduced POF incidence (RR=1.51, 95% CI=1.02–2.23,
P=0.04), with no effect on pregnancy rate (RR=0.36, 95%
CI=0.06–2.27, P=0.28; Fig. 7).
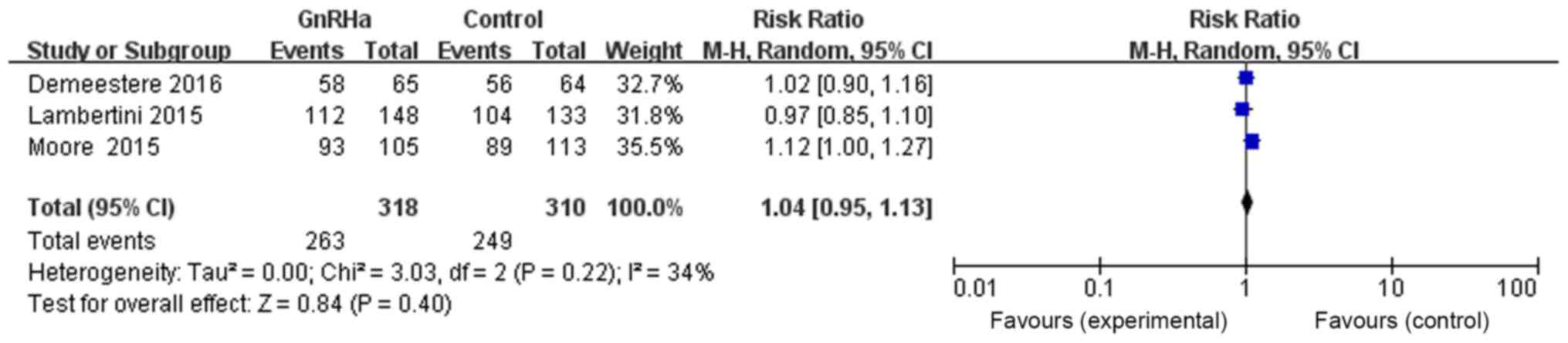
With regards to the effects of GnRHa on the
long-term tumor-free survival rate, three references provided
evidence of long-term tumor-free survival in both the GnRHa and
control groups. The total number of patients in the GnRHa group was
318, with 263 cases of survival without tumor, and 310 in the
control group, with 249 cases of survival without tumor. The
results were as follows: RR=1.04, 95% CI=0.95–1.13, P=0.40, with no
statistically significant difference, suggesting that the
combination of chemotherapy and GnRHa may have no effect on
long-term tumor-free survival rate (Fig.
8).
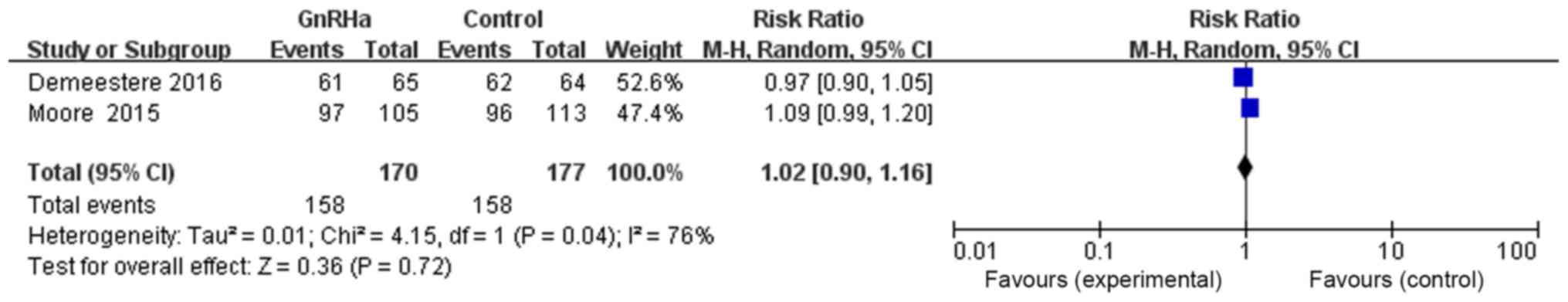
With regards to the effect of GnRHa on long-term
overall survival rate, two references provided evidence of
long-term survival in both the GnRHa and control groups. The total
number of patients in the GnRHa group was 170, of which 158
survived, whereas the number of patients in the control group was
177, of which 158 survived. The results of the meta-analysis were
as follows: RR=1.02, 95% CI=0.90–1.16, P=0.72, without any
statistically significant difference, suggesting that chemotherapy
combined with GnRHa may have no effect on long-term overall
survival rate (Fig. 9).
Discussion
The results of the present meta-analysis revealed
that GnRHa may reduce ovarian function damage caused by
chemotherapy-induced toxicity, and may significantly improve the
menstrual function recovery rate and reduce POF incidence in
patients undergoing chemotherapy. A previous study (16) has also analyzed the influence of
GnRHa on the therapeutic effects of chemotherapeutic drugs. Cuzick
et al (16) performed a
meta-analysis on 16 randomized controlled trials, and evaluated a
total of 11,906 premenopausal women who required chemotherapy for
early breast cancer. The study revealed that GnRHa as an adjuvant
chemotherapy for cancer patients does not affect chemotherapy. The
present meta-analysis analyzed long-term tumor-free survival rate
and overall survival rate of patients, and demonstrated that GnRHa
had no effect on long-term chemotherapy.
Chemotherapy can cause several collateral effects to
the ovaries, resulting in damage, including irreversible ovarian
dysfunction, amenorrhea and infertility, thus compromising the
health and quality of life of patients. There are three main types
of chemical drugs that can cause damage to ovarian function
(3). The first group comprises
nitrogen mustard, cyclophosphamide and other alkylating agents,
which have effects on cells in any cell cycle phase; these are the
most harmful drugs. The second group of chemotherapeutic drugs
includes cisplatin and adriamycin, which mainly affect
proliferative cells. These drugs have minor effects on the
primordial follicle, do not induce ovarian damage and only result
in short-term amenorrhea. The third chemotherapeutic drugs group,
including the methotrexate-treated group, exerts only minor or no
damage to the ovaries. Overall, the effects of chemotherapy on
ovarian function are influenced by numerous factors: i) The
concentration of chemotherapeutic drugs; ii) the duration of
chemotherapy; iii) drug superposition; and iv) age of the patient
at the beginning of chemotherapy and the type of disease.
The protective effects of GnRHa on ovarian function
have been extensively studied. GnRHa can be combined with the GnRH
receptor, which inhibits the secretion of lutein hormone and
follicle-stimulating hormone (FSH), thus inhibiting gonadotropin.
Numerous mechanisms explain ovarian protection. Primordial follicle
maturation and growth depends on FSH, and it has been demonstrated
that these follicles contain mRNA able to express FSH and lutein
hormone receptors; this expression is dependent on gonadotropins
(17). Furthermore, the chemical
structure of GnRHa is similar to GnRH; however it has a stronger
affinity to the receptors. When GnRHa is combined with the
pituitary gland receptors, it can induce an increase in
gonadotropin release, known as the flare-up effect. The number of
GnRH receptors then decreases, blocking the
hypothalamus-pituitary-ovarian axis and subsequently decreasing the
amount of FSH released, reducing the maturity and growth of
original follicles, and reducing the sensitivity of the ovaries to
chemotherapy (18). Badaru et
al (19) demonstrated that this
inhibition is positively correlated with the dosage of GnRHa, and
that the inhibitory effect of GnRHa on the
hypothalamus-pituitary-ovarian axis is increased when administered
at 7.5 mg/month compared with at 3.75 mg/month. Previous studies
have suggested that GnRHa reduces the amount of blood flowing
through the ovaries, leading to a reduced concentration of topical
drugs. However, few studies are available on this subject, and the
results are contradictory. Kitajima et al (20) suggested that high levels of estrogen
can significantly increase ovarian hyperstimulation and ovarian
blood flow in a mouse model, whereas these effects are inhibited by
GnRHa, and the degree of inhibition is positively associated with
GnRHa dosage. A prospective study completed by Reinsch et al
(21) revealed that after 3 months
of continuous use of leuprorelin acetate, the blood flow to the
uterus decreases by 21% and the signal of blood flow to the ovaries
disappears. Conversely, Ng et al (22) and Jarvela et al (23) discovered that there is no alteration
in ovarian blood flow before or after GnRHa treatment. The effects
of GnRHa on ovarian blood flow remain unclear and require further
investigation.
There are two main types of GnRH receptors: GnRH
receptor-I and GnRH receptor-II. Choi et al (24) discovered that GnRH receptors are
present in ovarian cancer cell lines, ovarian surface epithelium,
preovulatory follicles and corpus luteal cells, but are not
detectable in the original follicles and early sinus follicles.
Imai et al (25) reported
that GnRHa acts directly on the granulosa cells, thus reducing the
toxic effect of chemotherapy drugs.
Recent studies (26,27) have
suggested that the damage induced by chemotherapy to female
reproduction and endocrine function is due to cell apoptosis. GnRHa
can be used to increase secretion of the gonadal protective
molecule sphingosine-1-phosphate (S-1-P), which can prevent
follicle injury or reproductive cell apoptosis. It has been
demonstrated that S-1-P application to patients undergoing
radiotherapy reduces ovarian damage (28).
The number follicles contained in the ovaries can
reach 7,000,000 in a 28-week-old fetus, after which the follicles
gradually die. No new cells are produced in the ovaries, and all
cells stored will eventually disappear, resulting in perimenopausal
symptoms. However, a recent study (29) has presented opposite results,
indicating that the ovaries contain ovarian stem cells. Johnson
et al (29) reported that rat
ovaries have active germ line stem cells, which can continuously
replace the immature ovarian follicles, allowing primordial
follicular pool regeneration. Therefore, some researchers have
hypothesized that GnRHa may preserve ovarian function by protecting
the undifferentiated germ line stem cells. This hypothesis requires
further investigation.
The present study had some limitations. Firstly, the
12 references included in this study had different definitions of
POF and the follow-up time was markedly different, which
potentially affects the results of this work. Secondly, eight
references provided evidence of pregnancy in the GnRHa and control
groups; however, no information was given on the use of
contraception following cessation of chemotherapy and during
follow-up. It was therefore difficult to evaluate the effects of
chemotherapy combined with GnRHa on fertility. Thirdly, only three
of the 12 references provided long-term tumor-free survival rate,
which represented a small sample size that could potentially have
led to a wrong conclusion. In addition, the 12 clinical randomized
controlled trials included had an overall heterogeneity, and their
differences in disease, chemotherapy, follow-up time and POF
definition may have affected the results. A larger sample size,
longer follow-up period and well-designed clinical randomized
controlled trials are therefore required to further study and/or
confirm the protective effects of GnRHa on ovarian damage induced
by chemotherapy.
In conclusion, the present meta-analysis
demonstrated that GnRHa may reduce ovarian function damage caused
by chemotherapy. GnRHa significantly increased the rate of
menstrual function recovery and reduced POF incidence; however, it
had no effect on pregnancy rate, tumor-free survival rate and
overall survival rate.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
FZ and BZ designed the study and performed the
literature review. YL and YW interpreted the data and wrote the
manuscript. QF and LW performed the literature review, data
collection and analysis, and wrote the manuscript. YC designed the
the study, performed data analysis and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ataya KM, McKanna JA, Weintraub AM, Clark
MR and LeMaire WJ: A luteinizing hormone-relasing hormone agnist
for the prevention of chemotherapy-induced ovarian fllicular loss
in rats. Cancer Res. 45:3651–3656. 1985.PubMed/NCBI
|
|
2
|
Blumenfeld Z and Eckman A: Preservation of
fertility and ovarian function and minimization of
chemotherapy-induced gonadotoxicity in young women by GnRH-a. J
Nail Cancer Inst Monogr. 34:40–43. 2005. View Article : Google Scholar
|
|
3
|
Badawy A, Elnashar A, El-Ashry M and
Shahat M: Gonadotropin-releasing hormone agonists for prevention of
chemotherapy-induced ovarian damage: Prospective randomized study.
Fertil Steril. 91:694–697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Demeestere I, Brice P, Peccatori FA,
Kentos A, Dupuis J, Zachee P, Casasnovas O, Van Den Neste E,
Dechene J, De Maertelaer V, et al: No evidence for the benefit of
gonadotropin-releasing hormone agonist in preserving ovarian
function and fertility in lymphoma survivors treated with
chemotherapy: Final long-term report of a prospective randomized
trial. J Clin Oncol. 34:2568–2574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions. John Wiley & Sons.
(Ltd., Chichester). 2008. View Article : Google Scholar
|
|
6
|
Elgindy EA, El-Haieg DO, Khorshid OM,
Ismail EI, Abdelgawad M, Sallam HN and Abou-Setta AM: Gonadatrophin
suppression to prevent chemotherapy-induced ovarian damage: A
randomized controlled trial. Obstet Gynecol. 121:78–86. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gerber B, von Minckwitz G, Stehle H,
Reimer T, Felberbaum R, Maass N, Fischer D, Sommer HL, Conrad B,
Ortmann O, et al: Effect of luteinizing hormone-releasing hormone
agonist on ovarian function after modern adjuvant breast cancer
chemotherapy: the GBG 37 ZORO study. J Clin Oncol. 29:2334–2341.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giuseppe L, Attilio G, Edoardo DN,
Loredana G, Cristina L and Vincenzo L: Ovarian function after
cancer treatment in young women affected by Hodgkin disease (HD).
Hematology. 12:141–147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karimi-Zarchi M, Forat-Yazdi M,
Vafaeenasab MR, Nakhaie-Moghadam M, Miratashi-Yazdi A, Teimoori S
and Dehghani-Tafti A: Evaluation of the effect of GnRH agonist on
menstrual reverse in breast cancer cases treated with
cyclophosphamide. Eur J Gynaecol Oncol. 35:59–61. 2014.PubMed/NCBI
|
|
10
|
Lambertini M, Boni L, Michelotti A,
Gamucci T, Scotto T, Gori S, Giordano M, Garrone O, Levaggi A,
Poggio F, et al: Ovarian suppression with triptorelin during
adjuvant breast cancer chemotherapy and long-term ovarian function,
pregnancies, and disease-free survival: A randomized clinical
trial. JAMA. 314:2632–2640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leonard RCF, Adamson DJA, Bertelli G,
Mansi J, Yellowlees A, Dunlop J, Thomas GA and Coleman RE; Anglo
Celtic Collaborative Oncology Group and National Cancer Research
Institute Trialists, : GnRH agonist for protection against ovarian
toxicity during chemotherapy for early breast cancer: The Anglo
Celtic Group OPTION trial. Ann Oncol. 28:1811–1816. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moore HC, Unger JM, Phillips KA, Boyle F,
Hitre E, Porter D, Francis PA, Goldstein LJ, Gomez HL, Vallejos CS,
et al: Goserelin for ovarian protection during breast-cancer
adjuvant chemotherapy. N Engl J Med. 372:923–932. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Munster PN, Moore AP, Ismail-Khan R, Cox
CE, Lacevic M, Gross-King M, Xu P, Carter WB and Minton SE:
Randomized trial using gonadotropin-releasing hormone agonist
triptorelin for the preservation of ovarian function during
(neo)adjuvant chemotherapy for breast cancer. J Clin Oncol.
30:533–538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song G, Gao H and Yuan Z: Effect of
leuprolide acetate on ovarian function after
cyclophosphamide-doxorubicin-based chemotherapy in premenopausal
patients with breast cancer: Results from a phase II randomized
trial. Med Oncol. 30:6672013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sverrisdottir A, Nystedt M, Johansson H
and Fornander T: Adjuvant goserelin and ovarian preservation in
chemotherapy treated patients with early breast cancer: Results
from a randomized trial. Breast Cancer Res Treat. 117:561–567.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
LHRH-agonists in Early Breast Cancer
Overview Group, ; Cuzick J, Ambroisine L, Davidson N, Jakesz R,
Kaufmann M, Regan M and Sainsbury R: Use of
luteinising-hormone-releasing hormone agonists as adjuvant
treatment in premenopausal patients with hormone-receptor-positive
breast cancer: A meta-analysis of individual patient data from
randomised adjuvant trials. Lancet. 369:1711–1723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng W, Magid MS, Kramer EE and Chen YT:
Follicle-stimulating hormone receptor is expressed in human ovarian
surface epithelium and fallopian tube. Am J Pathol. 148:47–53.
1996.PubMed/NCBI
|
|
18
|
Periti P, Mazzei T and Mini E: Clinical
pharmacokinetics of depot leuprorelin. Clin Pharmacokinet.
41:485–504. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Badaru A, Wilson DM, Bachrach LK, Fechner
P, Gandrud LM, Durham E, Wintergerst K, Chi C, Klein KO and Neely
EK: Sequential comparisons of one-month and three-month depot
leuprolide regimens in central precocious puberty. J Clin
Endocrinol Metab. 91:1862–1867. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kitajima Y, Endo T, Nagasawa K, Manase K,
Honnma H, Baba T, Hayashi T, Chiba H, Sawada N and Saito T:
Hyperstimulation and a gonadotropin releasing hormone agonist
modulate ovarian vascular permeability by altering expression of
the tight junction protein claudin-5. Endocrinology. 147:694–699.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reinsch RC, Murphy AA, Morales AJ and Yen
SS: The effects of RU 486 and leuprolide acetate on uterine artery
blood flow in the fibroid uterus: A prospective, randomized study.
Am J Obstet Gynecol. 170:1623–1627. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ng EH, Tang OS, Chan CC and Ho PC: Ovarian
stromal blood flow in the prediction of ovarian response during in
vitro fertilization treatment. Hum Reprod. 20:3147–3151. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jarvela IY, Sladkevicius P, Kelly S, Ojha
K, Campbell S and Nargund G: Effect of pituitary down-regulation on
the ovary before in vitro fertilization as measured using
threedimensional power Doppler ultrasound. Fertil Steril.
79:1129–1135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi JH, Gilks CB, Auersperg N and Leung
PC: Immunolocalization of gonadotropin-releasing hormone(GnRH)-I,
GnRH-II and type I GnRH receptor during follicular development in
the human ovary. J Clin Endocrinol Metab. 91:4562–4570. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Imai A, Sugiyama M, Furui T, Tamaya T and
Ohno T: Direct protection by a gonadotropin-releasing hormone
analog from doxorubicin-induced granulosa cell damage. Gynecol
Obstet Invest. 63:102–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morita Y and Tilly JL: Oocyte apoptosis:
Like sand through an hourglass. Dev Biol. 213:1–17. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reynolds T: Cell death genes may hold
clues to preserving fertility after Chemotherapy. Natl Cancer Inst.
91:664–666. 1999. View Article : Google Scholar
|
|
28
|
Morita Y, Perez GI, Paris F, Miranda SR,
Ehleiter D, Haimovitz-Friedman A, Fuks Z, Xie Z, Reed JC, Schuchman
EH, et al: Oocyte apoptosis is suppressed by disruption of the acid
sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat
Med. 6:1109–1114. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Johnson J, Canning J, Kaneko T, Pru JK and
Tilly JL: Germline stem cells and follicular renewal in the
postnatal mammalian ovary. Nature. 428:145–150. 2004. View Article : Google Scholar : PubMed/NCBI
|