Introduction
Pancreatic ductal adenocarcinoma is a one of the
most aggressive types of cancer in the digestive system and is
associated with a poor prognosis, with a 5-year survival rate of
<6% (1,2). Radical resection is currently the best
treatment option for curative outcome and long-term survival rates,
however, only 15–20% of patients are suitable candidates for
curative resection as first-line treatment at initial diagnosis
(3). Some distant micrometastases
are undetectable at first diagnosis, despite thorough preoperative
imaging tests, and may only be confirmed by examination during
planned curative resection or by rapid relapse shortly following
surgery (1,3). For this reason, patients undergoing
curative pancreaticoduodenectomy have a 25–50% risk of recurring
local or distant metastases, and those with metastatic disease have
a median life expectancy of ~3–6 months and a 5-year relative
survival rate of only 1% (1,3,4).
Therefore, efforts to identify factors that predict early
recurrence in patients with pancreatic ductal adenocarcinoma
undergoing surgical resection are warranted.
The pancreatic head is the most common location for
the development of pancreatic ductal adenocarcinoma, and resectable
pancreatic head adenocarcinoma does not involve the main
peripancreatic arteries (5).
Previous studies have described tumor resectability in pancreatic
ductal adenocarcinoma, however, the majority of definitions of
resectable pancreatic head adenocarcinoma are based on the
technical or anatomical resectability of the tumor during
preoperative abdominal contrast-enhanced computed tomography
(CE-CT), with little focus on the potential biological behavior of
the tumor (6). Consequently,
patients with radiologically diagnosed resectable pancreatic cancer
frequently have a poor prognosis, even following radical surgery
(5,6).
The superior mesenteric artery (SMA) is an important
anatomic structure for predicting prognosis in patients with
pancreatic head adenocarcinoma (7).
Tumor involvement of the SMA is defined as one of the criteria for
diagnosing borderline resectable pancreatic cancer in various
guidelines (6,8–10). The
SMA margin is one of the incised margins in pancreatic head
adenocarcinoma, and is described as the soft tissues directly
adjacent to the proximal 3–4 cm of the SMA, mainly containing the
pancreatic head plexus II (PLX–II) and other tissues including
fibrous and adipose tissues, lymphatic vessels and capillaries, all
of which communicate with the uncinate process (7,11,12). The
SMA margin is the surgical margin with the highest frequency of
residual tumor, which leads to early recurrence following tumor
resection (11,13). On preoperative CE-CT, the SMA margin
or the PLX–II is approximately equal to the right side of the SMA
boundary, although the patterns of the SMA boundary on preoperative
CE-CT may vary among patients (14,15). In
clinical experience, patients with obscure SMA boundaries often
present with early recurrence following radical resection.
Therefore, the aim of the present retrospective study was to
evaluate the biological and prognostic implications of the SMA
boundary on preoperative CE-CT, and investigate the characteristics
of extrapancreatic neuropathy.
Patients and methods
Patient recruitment
The present study was approved by the Institutional
Review Board of the Peking University Third Hospital (Beijing,
China). Patients with pancreatic head cancer who underwent radical
surgery at the Department of General Surgery of the Peking
University Third Hospital between January 2010 and December 2015
were included. All patients were radiologically diagnosed with
resectable pancreatic head cancer, without invasion of the main
peripancreatic arteries, and were all treated with surgery.
Postoperative pathological analysis of the tumor confirmed the
diagnosis of pancreatic ductal adenocarcinoma. No distant
metastases were identified prior to or during surgery, and all
fatalities were due to tumor recurrence. The clinicopathological
characteristics and postoperative outcomes of these patients were
recorded. The observation period was between January 2010 and
December 2017.
Patterns of the SMA boundary
Abdominal CE-CT was preoperatively performed with a
64-detector row scanner (Siemens AG, Munich, Germany; Definition
2008 G H-SP) in all patients. The technical parameters were
standardized as follows: 120 kV, 36 mA and 3-mm-thick contiguous
sections. The CE-CT scans were retrospectively analyzed by three
experienced gastroenterologists (DX, CY and YP), who all had at
least 10 years of clinical experience with abdominal CE-CT as part
of their daily clinical and research practice. Results identified
by more than one gastroenterologist were considered the final
results for each patient. All images were reviewed on a Picture
Archiving and Communications System workstation monitor (General
Electric, Co., Ltd.). The reviewers were aware that the patients
had undergone radical surgery for pancreatic cancer, although they
were blinded to all surgical and pathological findings. All phases
of the CE-CT scans were evaluated as a whole.
‘Tumor invasion’ of the SMA referred to
arterial-tumor direct contact, which was confirmed in the arterial
phase on preoperative CE-CT; patients with true SMA invasion were
excluded from the present study. The pathway of the SMA in the
arterial phase of preoperative CE-CT was examined, mainly focusing
on the region located between the SMA and the uncinate process. The
presence of soft tissues in this region was defined as ‘all or
none’, and it was suggested that the normal soft tissue around the
SMA may be distinguished from the tumor invasion in this manner.
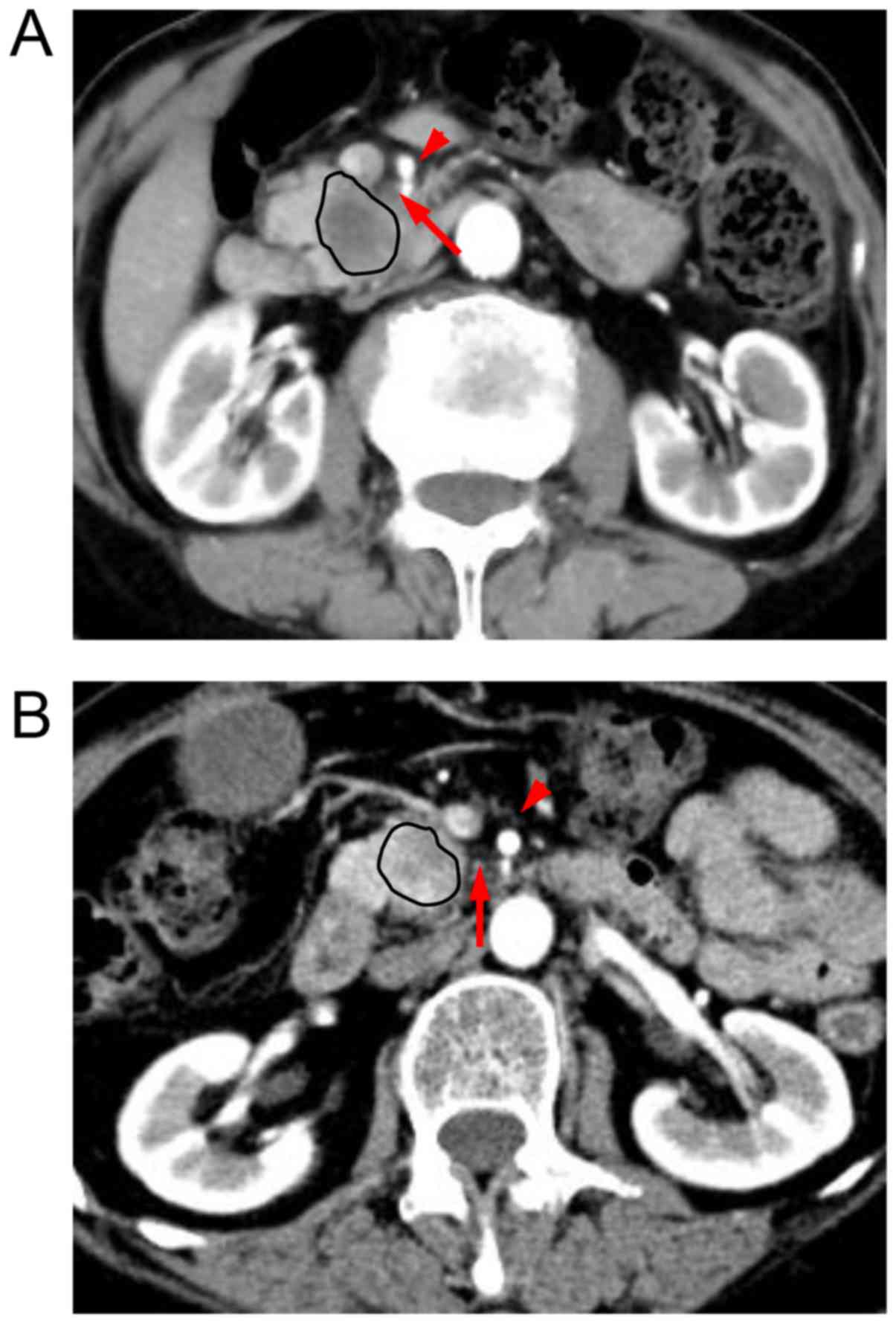
The characteristics of the tissues in this region differed among
patients. If the tissues in this region exhibited fat density on
imaging, then the SMA boundary was defined as clear. If confluent
soft tissue replaced the adipose tissue in this region, the SMA
boundary was defined as obscure (Fig.
1). All patients were classified into these two groups
according to the patterns of the SMA boundary.
Tissue processing
All patients underwent standard
pancreaticoduodenectomy according to tumor location and lymph node
metastasis. The Kocher maneuver (16) was performed to expose the junction of
the left renal vein and the inferior vena cava, as it helped to
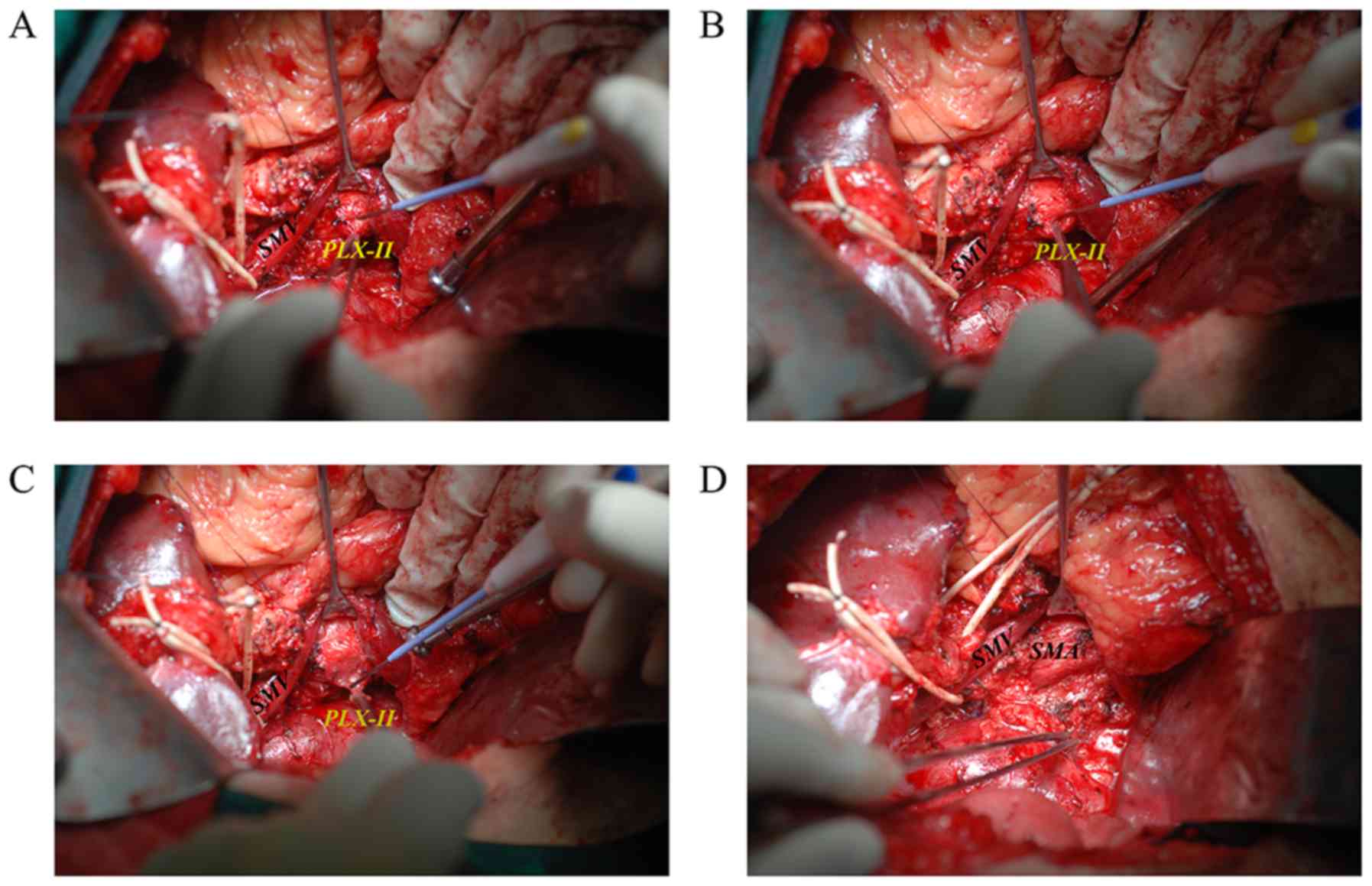
confirm the position of the SMA. The pancreatic neck was dissected
in front of the superior mesenteric vein (SMV). Following gentle
pushing of the SMV to the inner side using a vascular retractor,
the tissues in the SMA margin were exposed, followed by right-sided
semi-circumferential dissection of the PLX–II, which is the plexus
surrounding the SMA (Fig. 2). The
SMV or portal vein (PV) was partially resected and reconstructed,
either by end-to-end anastomosis or by insertion of a venous graft,
if tumor infiltration was identified.
Immunohistochemical staining
The PLX–II tissues in the SMA margin were
pathologically examined individually. Five paraffin-embedded 3-µm
sections of PLX–II tissues were obtained from each patient, and
immunohistochemical staining with a rabbit polyclonal antibody
against the vesicular acetylcholine transporter (VAChT; 1:100; cat.
no. ab-68984; Abcam, Cambridge, MA, USA) was performed in each
section to observe the parasympathetic nerves in the SMA margin.
Briefly, all sections were deparaffinized with xylene and
rehydrated, and antigen retrieval was achieved via heat treatment
in an autoclave in 10 mmol/l sodium citrate buffer (pH 6.0) for 30
min. All sections were further treated with methanol and 3%
hydrogen peroxide prior to washing with phosphate-buffered saline
(PBS). Bovine serum albumin (5%; cat. no. A8850-5; Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) was used for 1
h at room temperature to block background staining, following which
the membrane was incubated overnight with the primary antibody at
4°C. The detection of the antigen-antibody complex was performed
using the Super Sensitive™ Polymer-HRP IHC Detection system
(BioGenex Laboratories, Fremont, CA, USA), according to the
manufacturer's protocol. PBS was used instead of a primary antibody
for the negative control (17). One
pathologist, who was blinded to the clinical outcome, independently
scored the results of the staining.
Pathological analysis
The surgical margins were considered to be
microscopically positive (R1) if carcinoma was present at the
transection lines or in the dissected peripancreatic soft tissue
margins within 1 mm. R0 resection was defined as no microscopic
evidence of cancer cells along all margins (18). The final stage of pancreatic head
adenocarcinoma was determined pathologically according to the
tumor-node-metastasis classification system of malignant tumors
published by the Union for International Cancer Control (UICC), 8th
edition (19).
Parasympathetic neurogenesis is a morphological
phenomenon in the tumor microenvironment, involving the
distribution of abnormal parasympathetic nerve fibers in the tumor
stroma. Nerve fibers of various shapes and other constituents,
including adipose and fibrous tissue, were evaluated.
Parasympathetic neurogenesis was assessed by immunohistochemical
staining of tissue sections. The number of parasympathetic nerve
fibers was calculated in each section, and the mean number of five
sections from each patient was compared among the different
groups.
Follow-up observations
All patients were followed up by physical
examination and abdominal CE-CT every 3–6 months. The follow-up
time was defined as the time from the date of the first surgery to
the date of the final follow-up, and was recorded in months.
Follow-up information was obtained from office charts, hospital
records and telephone interviews. Overall survival (OS) was defined
as the time from the date of the first surgery to the date of
patient mortality due to recurrence. Disease-free survival (DFS)
was defined as the time from the date of the first surgery to the
date of the first evidence of recurrence [loco-regional recurrence
(LR) or distant metastasis]. Early recurrence was defined as
relapse within 12 months. The patterns of recurrence sites were
subdivided into LR and liver metastasis (LM); the former indicated
a recurrence presumably due to occult residual tumor cells left
behind at the time of resection, whereas the latter is the most
common cause of mortality among patients with pancreatic ductal
adenocarcinoma who undergo radical surgery.
Statistical analysis
All statistical analyses were conducted using SPSS
software (version 23.0 for Windows; IBM Corp., Armonk, NY, USA).
Correlation analyses among various groups and different biological
factors were performed using the χ2 test and independent
sample t-tests. Survival analysis was performed by constructing
Kaplan-Meier curves for OS, DFS, LR and LM. The log-rank test was
used to compare survival differences among the groups. Multivariate
Cox regression models (backward stepwise likelihood ratio) were
used to analyze factors affecting survival rate, and reported with
a hazard ratio of 1.0 as the baseline and 95% confidence intervals.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinicopathological
characteristics
According to the inclusion and exclusion criteria,
121 patients who underwent radical tumor resection were included in
the present study. A total of 67 men and 54 women were included,
ranging in age between 23 and 84 years, with a mean age of 62.0
years. All diagnoses were ultimately confirmed clinically and
pathologically. Of the 121 patients, 68 (56.2%) had clear SMA
boundaries on preoperative CE-CT, whereas 53 (43.8%) had obscure
boundaries. Increased cancer antigen 19-9 (CA19-9)
levels were observed in 83.5% (101/121) of the patients, and
increased total bilirubin (TBIL) levels were found in 72.7%
(88/121) of the patients. In 46 (38.0%) patients, venous (SMV and
PV) invasion was identified during surgery, and 20 (16.5%) patients
received venous resection and reconstruction. Lymph node invasion
was observed in 65 patients (53.7%), among whom 51 (42.1%) were
classified as N1 stage and 14 (11.6%) were classified as N2 stage
patients. Based on the most recent UICC classification system, 48
(39.7%) cases were defined as stage III carcinoma. R0 resections
were achieved in 58 (47.9%) patients, and a positive SMA margin
(R1) was observed in 36 (29.8%) patients. Cancer emboli in the
vessels were observed in 35 (28.9%) patients, and intrapancreatic
neural invasion (IPNI) was identified in 90 (74.4%) patients. In
addition, the carcinoma was poorly differentiated in 54.5% (66/121)
of the cases. Within 12 months, 63.6% (77/121) of the patients had
developed recurrence and 47.1% (57/121) succumbed to the disease.
Of the patients with early recurrence, 57 (47.1%) had LM and 32
(26.4%) had LR. All other characteristics are summarized in
Table I.
 | Table I.Clinical and pathological
characteristics of 121 patients. |
Table I.
Clinical and pathological
characteristics of 121 patients.
| Characteristic | Variable | N (%) | Clear SMA boundary
(n=68) | Obscure SMA
boundary (n=53) | P-value |
|---|
| Clinical
characteristic |
| Age (years) |
|
|
|
| 0.934a |
|
| ≤65 | 69 (57.0) | 39 (57.4) | 30 (56.6) |
|
|
| >65 | 52 (43.0) | 29 (42.6) | 23 (43.4) |
|
| Gender |
|
|
|
| 0.898a |
|
| Male | 67 (55.4) | 38 (55.9) | 29 (54.7) |
|
|
| Female | 54 (44.6) | 30 (44.1) | 24 (45.3) |
|
| CA19-9
(U/ml) |
|
|
|
| 0.173a |
|
| ≤39 | 20 (16.5) | 14 (20.6) | 6 (11.3) |
|
|
| >39 | 101 (83.5) | 54 (79.4) | 47 (88.7) |
|
| TBIL (µmol/L) |
|
|
|
| 0.067a |
|
| ≤17.1 | 33 (27.3) | 23 (33.8) | 10 (18.9) |
|
|
| >17.1 | 88 (72.7) | 45 (66.2) | 43 (81.1) |
|
| Venous
invasion |
| 46 (38.0) | 19 (27.9) | 27 (50.9) | 0.010a |
| Venous
resection |
| 20 (16.5) | 10 (14.7) | 10 (18.9) | 0.541a |
| Adjuvant
therapy |
| 56 (46.3) | 32 (47.1) | 24 (45.3) | 0.846a |
| Early
mortality |
| 57 (47.1) | 24 (35.3) | 33 (62.3) | 0.003a |
| Early
recurrence |
| 77 (63.6) | 34 (50.0) | 43 (81.1) |
<0.001a |
| Early LM |
| 57 (47.1) | 23 (33.8) | 34 (64.2) | 0.001a |
| Early LR |
| 32 (26.4) | 14 (20.6) | 18 (34.0) | 0.098a |
| Median OS time
(months) |
|
| 24.9±3.6 | 13.9±1.7 | 0.005b |
| Median DFS time
(months) |
|
| 22.1±3.6 | 9.0±1.1 | 0.003b |
| Median time without
LR (months) |
|
| 40.1±5.6 | 23.6±3.6 | 0.182b |
| Median time without
LM (months) |
|
| 36.5±4.6 | 11.2±1.5 | 0.001b |
| Pathological
characteristics |
| Surgical
margin |
|
|
|
| 0.212a |
|
| R0 | 58 (47.9) | 36 (52.9) | 22 (41.5) |
|
|
| R1 | 63 (52.1) | 32 (47.1) | 31 (58.5) |
|
| SMA margin |
|
|
|
|
<0.001a |
|
| R0 | 85 (70.2) | 57 (83.8) | 28 (52.8) |
|
|
| R1 | 36 (29.8) | 11 (16.2) | 25 (47.2) |
|
| Intrapancreatic
neural invasion |
| 90 (74.4) | 48 (70.6) | 42 (79.2) | 0.279a |
| Cancer embolus in
vessel |
| 35 (28.9) | 25 (36.8) | 10 (18.9) | 0.031a |
| Poor
differentiation |
| 66 (54.5) | 37 (54.4) | 29 (54.7) | 0.973a |
| T stage |
|
|
|
|
<0.001a |
|
| T1 | 4 (3.3) | 4 (5.9) | 0 (0) |
|
|
| T2 | 65 (53.7) | 48 (70.6) | 17 (32.1) |
|
|
| T3 | 12 (9.9) | 8 (11.8) | 4 (7.5) |
|
|
| T4 | 40 (33.1) | 8 (11.8) | 32 (60.4) |
|
| N stage |
|
|
|
| 0.556a |
|
| N0 | 56 (46.3) | 32 (47.1) | 24 (45.3) |
|
|
| N1 | 51 (42.1) | 30 (44.1) | 21 (39.6) |
|
|
| N2 | 14 (11.6) | 6 (8.8) | 8 (15.1) |
|
| UICC stage |
|
|
|
|
<0.001a |
|
| I | 30 (24.8) | 24 (35.3) | 6 (11.3) |
|
|
| II | 43 (35.5) | 31 (45.6) | 12 (22.6) |
|
|
| III | 48 (39.7) | 13 (19.1) | 35(66.0) |
|
| Size of largest
tumor (cm) |
| 3.1±1.9 | 3.4±2.5 | 3.3±1.4 | 0.831c |
The clinicopathological parameters were examined in
the two groups (Table I). There was
no correlation between the pattern of the SMA boundary and age
(P=0.934), gender (P=0.898), preoperative levels of
CA19-9 (P=0.173) or TBIL (P=0.067), venous resection
(P=0.541) or adjuvant therapy (P=0.846). In addition, there was no
association between the pathological characteristics, including
IPNI (P=0.279), surgical margin (P=0.212), tumor differentiation
(P=0.973) and N stage (P=0.556), and the SMA boundary. Venous
invasion occurred predominantly in patients with obscure SMA
boundaries (P=0.010), who were also more likely to have positive
SMA margins (P<0.001) and poorer pathological UICC stages
(P<0.001).
Survival analysis
The median follow-up time was 13 months; 121 cases
of tumor recurrence were observed in 98 (81.0%) patients at the
final follow-up. The details of recurrence sites are summarized in
Table II. For all patients, the
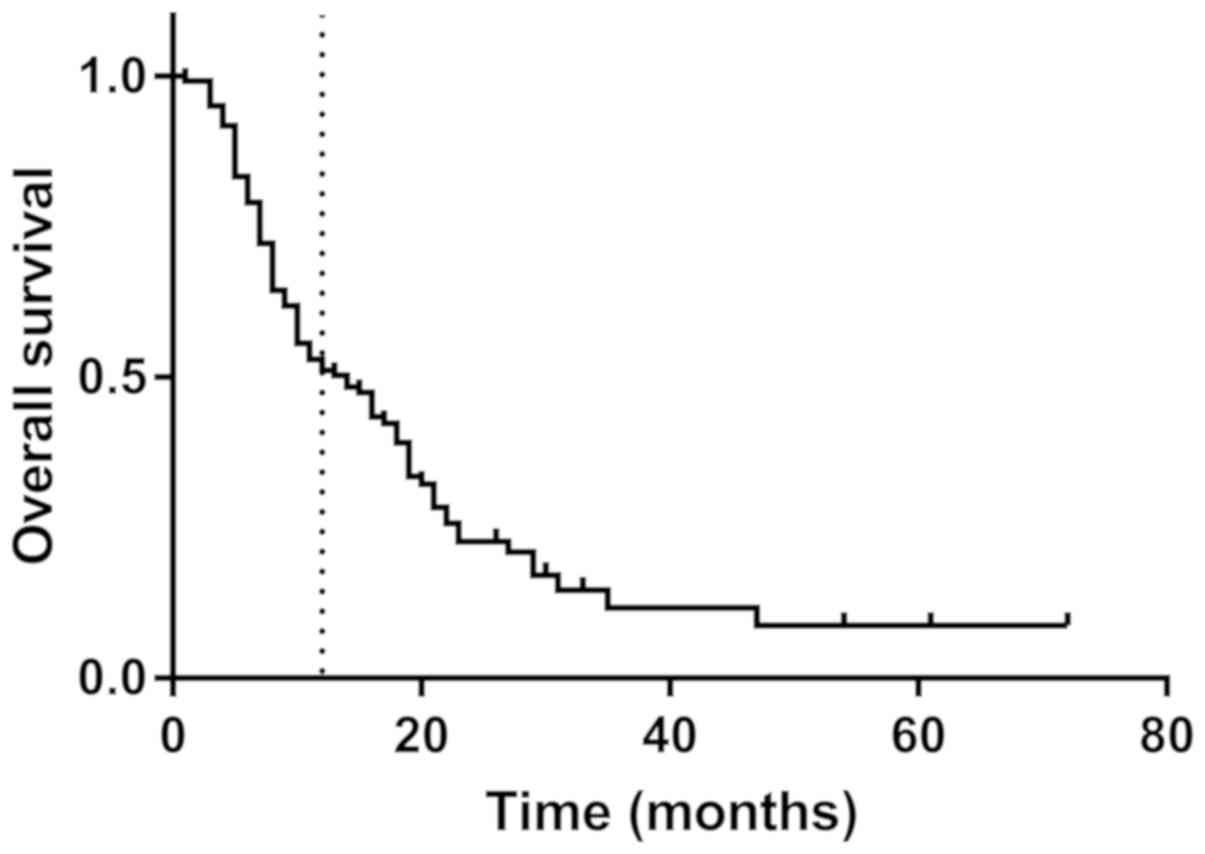
mean OS was 14.4 months, the mean DFS was 11.1 months and the
1-year survival rate was 52.9% (Fig.
3). The univariate survival analysis of prognostic factors is
summarized in Table III. The
pattern of the SMA boundary on preoperative CE-CT (P=0.005), venous
invasion (P<0.001) or resection (P=0.011), surgical margins
(P=0.005), pathological N stage (P=0.015) and UICC stage (P=0.004)
were all found to be significantly associated with OS. The
prognostic factors for DFS included the SMA boundary (P=0.003),
venous invasion or resection (P=0.001 and P=0.027 respectively),
surgical margins (P=0.014) and UICC stage (P=0.019). Pathological T
stage (P=0.021) and UICC stage (P=0.046) were associated with LR.
The SMA boundary (P=0.001), venous invasion (P=0.004) and surgical
margins (P=0.001) were risk factors for LM.
 | Table II.Sites of recurrence following radical
surgery. |
Table II.
Sites of recurrence following radical
surgery.
| Sites of
recurrence | No. of
patients |
|---|
| Loco-regional
recurrence | 43 |
| Liver
metastasis | 68 |
| Others |
|
|
Lung | 2 |
|
Bone | 1 |
|
Spleen | 1 |
| Peritoneum | 6 |
| Total | 21 |
 | Table III.Univariate survival analysis of
prognostic factors. |
Table III.
Univariate survival analysis of
prognostic factors.
|
| P-value |
|---|
|
|
|
|---|
| Characteristic | OS | DFS | LR | LM |
|---|
| Gender | 0.389 | 0.746 | 0.450 | 0.940 |
| Age | 0.600 | 0.496 | 0.828 | 0.568 |
| Venous
invasion | 0.000 | 0.001 | 0.135 | 0.004 |
| Venous
resection | 0.011 | 0.027 | 0.301 | 0.309 |
| SMA boundary | 0.005 | 0.003 | 0.182 | 0.001 |
|
CA19-9 | 0.615 | 0.528 | 0.570 | 0.739 |
| TBIL | 0.501 | 0.619 | 0.308 | 0.937 |
| Adjuvant
therapy | 0.843 | 0.437 | 0.807 | 0.257 |
| Surgical
margin | 0.005 | 0.014 | 0.529 | 0.001 |
| SMA margin | 0.184 | 0.211 | 0.611 | 0.067 |
| Poor
differentiation | 0.290 | 0.565 | 0.526 | 0.274 |
| Cancer embolus in
vessel | 0.577 | 0.342 | 0.627 | 0.977 |
| Intrapancreatic
neural invasion | 0.325 | 0.642 | 0.757 | 0.863 |
| T stage | 0.139 | 0.158 | 0.021 | 0.520 |
| N stage | 0.015 | 0.262 | 0.936 | 0.068 |
| UICC stage | 0.004 | 0.019 | 0.046 | 0.146 |
The results of the multivariate survival analysis
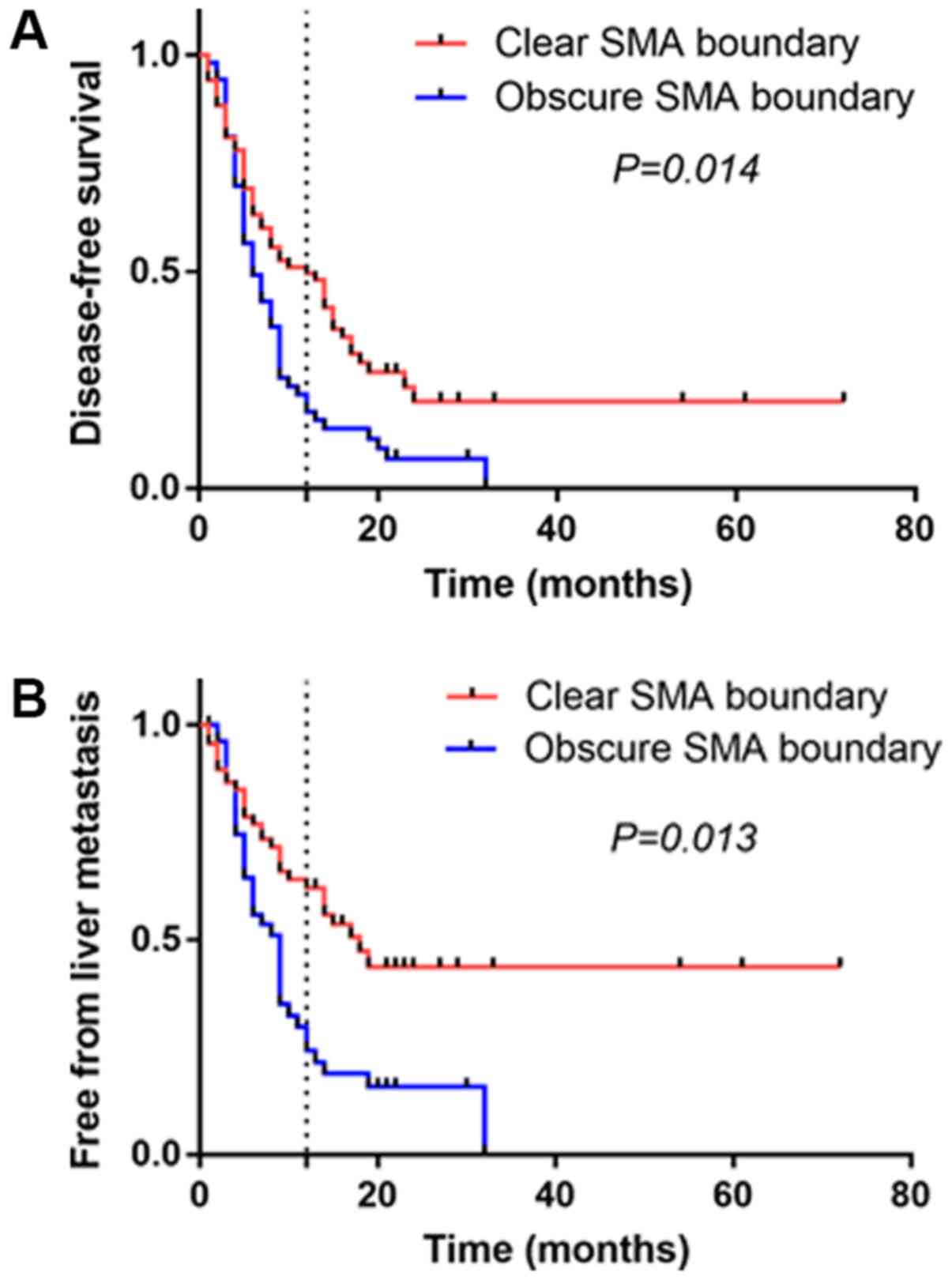
are presented in Table IV. The SMA
boundary was independently associated with DFS (P=0.014) and LM
(P=0.013). Patients with an obscure SMA boundary had a higher rate
of recurrence and LM following radical resection, compared with
those with a clear SMA boundary (Fig. 4A
and B). Early recurrence occurred in 50% (34/68) of patients
with clear SMA boundaries and in 81.1% (43/53) of patients with
obscure SMA boundaries (P<0.001). LM occurred within 12 months
in 33.8% (23/68) of patients with clear SMA boundaries, whereas the
rate of LM was 64.2% (34/53) in patients with obscure SMA
boundaries (P=0.001).
 | Table IV.Multivariate survival analysis of
prognostic factors. |
Table IV.
Multivariate survival analysis of
prognostic factors.
| Characteristic | Variable | n | HR | 95% CI | P-value |
|---|
| Overall
survival |
| N stage | N0 | 56 | 1 |
|
|
|
| N1 | 51 | 1.283 | 0.805–2.046 | 0.295 |
|
| N2 | 14 | 2.507 | 1.280–4.910 | 0.007 |
| Venous
invasion | No | 75 | 1 |
|
|
|
| Yes | 46 | 2.106 | 1.331–3.333 | 0.001 |
| Disease-free
survival |
| SMA boundary | Clear | 68 | 1 |
|
|
|
| Obscure | 53 | 1.664 | 1.109–2.496 | 0.014 |
| Surgical
margin | R0 | 58 | 1 |
|
|
|
| R1 | 63 | 1.539 | 1.024–2.313 | 0.038 |
| Venous
resection | No | 101 | 1 |
|
|
|
| Yes | 20 | 1.828 | 1.067–3.131 | 0.028 |
| Liver
metastasis |
| SMA boundary | Clear | 68 | 1 |
|
|
|
| Obscure | 53 | 1.878 | 1.142–3.087 | 0.013 |
| Surgical
margin | No | 58 | 1 |
|
|
|
| Yes | 63 | 1.847 | 1.108–3.078 | 0.019 |
Pathological analysis of the SMA
boundary
The pathological analysis of PLX–II revealed that
extrapancreatic neural invasion (EPNI) occurred in 16.2% (11/68) of
patients with clear SMA boundaries and in 13.2% (7/53) of patients
with obscure SMA boundaries (Table
V). Therefore, EPNI was not significantly associated with the
pattern of the SMA boundary (P=0.649). VAChT-positive
parasympathetic nerve fibers were observed in the SMA margins of
all patients with pancreatic ductal adenocarcinoma, and the median
number of parasympathetic nerve fibers was 24 (range, 7–55) per
section. The number of parasympathetic nerve fibers in the SMA
margin differed significantly between the two groups (P=0.014).
Microscopic evaluation revealed that the parasympathetic nerve
fibers were densely distributed in patients with obscure SMA
boundaries, with a mean number of fibers was 30.2±11.5 per section.
By contrast, the mean number of such fibers in patients with clear
SMA boundaries was 16.1±12.8 per section, and these parasympathetic
nerve fibers were sparsely distributed (Fig. 5A and B).
 | Table V.Pathological characteristics of
extrapancreatic plexus among patients with different SMA
boundaries. |
Table V.
Pathological characteristics of
extrapancreatic plexus among patients with different SMA
boundaries.
| Characteristic | Mean ± SD/N
(%) | Clear SMA boundary
(n=68) | Obscure SMA
boundary (n=53) | P-value |
|---|
| Parasympathetic
nerves (number of fibers/section) | 23.2±14.1 | 16.1±12.8 | 30.2±11.5 | 0.014a |
| Extrapancreatic
neural invasion | 18 (14.9) | 11 (16.2) | 7 (13.2) | 0.649b |
Discussion
Pancreatic head adenocarcinoma is characterized by
early recurrence following radical resection and the guidelines
that define borderline resectable pancreatic cancer are used to
identify those patients who would not benefit from first-line
surgery (5). However, even patients
with resectable pancreatic head adenocarcinoma may succumb to early
local recurrence or LM following first-line surgery (4). Previous research has revealed that,
among patients with early (within 6 months) recurrence following
surgery, the median survival time is poor (8–9 months) and the
5-year survival rate is 0% (20);
however, the mechanism underlying early recurrence and metastasis
remains to be fully elucidated.
All patients included in the present study had
resectable tumors that did not appear to invade the main arteries
on preoperative abdominal CE-CT and, thus, received standard
pancreaticoduodenectomy. The pattern of the SMA boundary on CE-CT
revealed the potential in indicating the prognosis of patients with
resectable pancreatic head adenocarcinoma; patients with obscure
SMA boundaries presented with poorer prognosis than patients with
clear SMA boundaries. The SMA boundary was an independent
prognostic factor of DFS (P=0.014) and LM (P=0.013) following
radical surgery. Early recurrence within 12 months (all recurrence
sites) occurred in 50.0% of patients with clear SMA boundaries,
with a median DFS of 22.1±3.6 months; by contrast, early recurrence
occurred in 81.1% of patients with obscure SMA boundaries and the
median DFS was only 9.0±1.1 months. Early LM occurred in 33.8% of
patients with clear SMA boundaries and 64.2% of patients with
obscure SMA boundaries, with a median LM-free duration of 36.5±4.6
and 11.2±1.5 months, respectively. This suggests that the pattern
of the SMA boundary reflected the biological behavior of the
pancreatic head adenocarcinoma, with an obscure SMA boundary on
CE-CT appearing to be associated with a higher degree of
malignancy.
The obscure SMA boundary in CE-CT may be a
radiological manifestation of EPNI (13–15).
However, the results of the present study revealed that the obscure
SMA boundary had no statistically significant association with
neural invasion of the PLX–II located at the right-hand side of the
SMA (P=0.649). Notably, previous studies have revealed tumor-nerve
interactions in pancreatic cancer (21,22). The
influence of tumor cells on nerves may lead to neuropathies,
including sympathetic or parasympathetic neurogenesis (23,24),
neuritis (25), nerve hypertrophy
(26) or neural invasion (27). Similarly, neuropathy may also serve a
key role in pancreatic neoplastic transformation, including tumor
budding, which may occur prior to tumor invasion (28–30).
Therefore, parasympathetic neurogenesis is a neuropathy that occurs
in the tumor microenvironment (23).
Immunohistological staining demonstrated that the pattern of the
SMA boundary was associated with the grade of parasympathetic
neurogenesis in the SMA margin (P=0.014); patients with obscure SMA
boundaries exhibited more parasympathetic nerve fibers (30.2±11.5
fibers/per section) in the SMA margin compared with those with
clear SMA boundaries (16.1±12.8 fibers/per section).
However, how the autonomic nervous system, including
parasympathetic nerves, affects the prognosis of pancreatic head
adenocarcinoma remains to be elucidated. Several studies have
demonstrated that the peripheral nervous system may be considered
as a neuronal circuit, which connects all organs to the central
nervous system, and the internal organs may interact with the tumor
microenvironment via the autonomic nerves (22). For example, sympathetic and
parasympathetic nerves are reportedly necessary throughout all
phases of prostate cancer progression in mice (31). The autonomic nerves are also
essential components of the tumor microenvironment; they can
regulate tissue homeostasis and promote cancer growth or metastasis
by interacting with the majority of internal organs (32). Therefore, the sympathetic nervous
system serves an important role in pancreatic cancer, and
intratumoral parasympathetic neurogenesis may also be associated
with tumor budding (23,33).
Sympathetic and parasympathetic nerves may regulate
tumor growth via direct innervation and the release of
neurotransmitters (33). Nerves
infiltrate the tumor microenvironment and release neurotransmitters
directly into the cancer cell environment to stimulate their
survival, proliferation and ability to spread; in turn, tumor cells
stimulate nerve outgrowth (21,22,34).
This may explain why obscure SMA boundaries were associated with a
positive SMA margin (P<0.001); it was suggested that
parasympathetic neurogenesis may allow tumor cells to infiltrate
connective tissue, thereby resulting in a positive SMA margin.
Although the actions of the sympathetic and parasympathetic nervous
systems are classically in opposition, they are in fact
complementary in cancer, where sympathetic nerves stimulate early
tumorigenesis and parasympathetic nerves activate the late
metastatic process (22,35). Prostate tumors with parasympathetic
cholinergic fiber infiltration have a tendency for dissemination,
and the density of nerves has been found to be directly associated
with tumor aggressiveness (31). An
increasing body of preclinical evidence has demonstrated that the
seeding of pancreatic cancer cells in distant organs often occurs
even prior to tumor formation at the primary site (36). In the present study, the density of
parasympathetic nerves was associated with the SMA boundary;
patients with a high number of parasympathetic nerves were more
likely to have an obscure SMA boundary on preoperative CE-CT, which
is predictive of early LM.
Early metastasis is responsible for early mortality,
thereby providing a rationale for the administration of systemic
therapy to patients with resectable pancreatic head adenocarcinoma
or early-stage disease (37). The
theory of nerve-cancer interactions has led to the development of
innovative anticancer therapies; denervation treatment, for
example, has been found to slow down cancer progression (38). Therefore, there is a rationale for
neoadjuvant therapy in this setting, including that for the early
treatment of potential micrometastatic disease that is responsible
for postoperative recurrence, although this requires further
validation in well-designed randomized trials (39). Patients with obscure SMA boundaries
may develop early LM following radical surgery and may benefit from
adjuvant hepatic arterial infusion chemotherapy following
resection, which may effectively and safely prevent LM and improve
prognosis (40); however, further
investigation is required to confirm this hypothesis.
The present study had certain limitations. First, a
number of patients were lost to follow-up, resulting in a median
follow-up time of only 13 months. However, as the focus was on
early recurrence (within 12 months), the results are considered
accurate. Second, the present study only observed the association
between parasympathetic neurogenesis in the SMA margin and the
pattern of the SMA boundary, whereas other types of extrapancreatic
neuropathy were not investigated. Finally, the present study was
conducted in a single institution, and it was not
population-based.
In conclusion, resectable pancreatic head
adenocarcinoma is associated with a high risk for early relapse
following surgery due to the intrinsic biological characteristics
of the tumor. This suggests that first-line surgery is the optimal
approach for only a minority of the patients. The SMA boundary on
preoperative CE-CT may be associated with extrapancreatic
neuropathies, including parasympathetic neurogenesis; an obscure
SMA boundary may be a consequence of high-grade parasympathetic
neurogenesis, and was identified as a predictive factor of a higher
risk of recurrence and LM. Therefore, high-grade parasympathetic
neurogenesis in the extrapancreatic plexus may be associated with a
more aggressive phenotype of pancreatic head adenocarcinoma, and
may assist in stratifying patients into prognostic subgroups to
support surgeons in determining the optimal therapeutic strategies
for individual patients.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from National
Natural Science Foundation of China (grant no. 81672862) and the
Capital Characteristic Clinical Application Research and
Achievement Promotion Project (grant no. Z171100001017121).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DX, CY and ML conceived and designed the present
study. LZ and LG performed the HE staining and
immunohistochemistry. LG analyzed the results of the
immunohistochemical experiment. ML, CY and LT performed statistical
analysis and data interpretation; YP, ML and GL performed clinical
data collection and samples collection; ML, CY and DX wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was exempted of informed consent.
This study was approved by the Institutional Review Board of Peking
University Third Hospital.
Patient consent for publication
Informed consent was waived in the present study, as
all identifying information was removed, public interest
considerations outweigh the potential harm, it was impossible to
obtain permission and a reasonable individual would be unlikely to
object to publication.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CE-CT
|
contrast-enhanced computed
tomography
|
|
SMA
|
superior mesenteric artery
|
|
PLX–II
|
pancreatic head plexus II
|
|
SMV
|
superior mesenteric vein
|
|
PV
|
portal vein
|
|
VAChT
|
vesicular acetylcholine
transporter
|
|
PBS
|
phosphate-buffered saline
|
|
UICC
|
Union for International Cancer
Control
|
|
OS
|
overall survival
|
|
DFS
|
disease-free survival
|
|
LR
|
locoregional recurrence
|
|
LM
|
liver metastasis
|
|
CA19-9
|
cancer antigen 19-9
|
|
TBIL
|
total bilirubin
|
|
IPNI
|
intrapancreatic neural invasion
|
|
EPNI
|
extrapancreatic neural invasion
|
|
ASCO
|
American Society of Clinical
Oncology
|
References
|
1
|
Nipp RD, Zanconato A, Zheng H, Ferrone CR,
Lillemoe KD, Wo JY, Hong TS, Clark JW, Ryan DP and Fernández-del
Castillo C: Predictors of early mortality after surgical resection
of pancreatic adenocarcinoma in the era of neoadjuvant treatment.
Pancreas. 46:183–189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu X, Xiu LJ, Jiao JP, Zhao J, Zhao Y, Lu
Y, Shi J, Li YJ, Ye M, Gu YF, et al: Traditional Chinese medicine
integrated with chemotherapy for stage IV non-surgical gastric
cancer: A retrospective clinical analysis. J Integr Med.
15:469–475. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi H, Jin C and Fu D: Preoperative
evaluation of pancreatic ductal adenocarcinoma with synchronous
liver metastasis: Diagnosis and assessment of unresectability.
World J Gastroentero. 22:10024–10037. 2016. View Article : Google Scholar
|
|
4
|
Stark AP, Sacks GD, Rochefort MM, Donahue
TR, Reber HA, Tomlinson JS, Dawson DW, Eibl G and Hines OJ:
Long-term survival in patients with pancreatic ductal
adenocarcinoma. Surgery. 159:1520–1527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Isaji S, Mizuno S, Windsor JA, Bassi C,
Fernández-Del Castillo C, Hackert T, Hayasaki A, Katz MHG, Kim SW,
Kishiwada M, et al: International consensus on definition and
criteria of borderline resectable pancreatic ductal adenocarcinoma
2017. Pancreatology. 18:2–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zaky AM, Wolfgang CL, Weiss MJ, Javed AA,
Fishman EK and Zaheer A: Tumor-Vessel relationships in pancreatic
ductal adenocarcinoma at multidetector CT: Different classification
systems and their influence on treatment planning. Radiographics.
37:93–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu L, Katz MH, Lee SM, Fischer LK,
Prakash L, Parker N, Wang H, Varadhachary GR, Wolff RA, Lee JE, et
al: superior mesenteric artery margin of posttherapy
pancreaticoduodenectomy and prognosis in patients with pancreatic
ductal adenocarcinoma. Am J Surg Pathol. 39:1395–1403. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Denbo JW and Fleming JB: Definition and
management of borderline resectable pancreatic cancer. Surg Clin
North Am. 96:1337–1350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lopez NE, Prendergast C and Lowy AM:
Borderline resectable pancreatic cancer: Definitions and
management. World J Gastroentero. 20:10740–10751. 2014. View Article : Google Scholar
|
|
10
|
Bockhorn M, Uzunoglu FG, Adham M, Imrie C,
Milicevic M, Sandberg AA, Asbun HJ, Bassi C, Büchler M, Charnley
RM, et al: Borderline resectable pancreatic cancer: A consensus
statement by the International Study Group of Pancreatic Surgery
(ISGPS). Surgery. 155:977–988. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng Y, Xiu D, Jiang B, Ma Z, Yuan C, Su
J, Shi X, Li L and Tao M: A clinical study about applying different
R1 criteria to evaluate pancreatic head ductal adenocarcinoma
specimens. Zhonghua Wai Ke Za Zhi. 52:834–838. 2014.(In Chinese).
PubMed/NCBI
|
|
12
|
Patel BN, Giacomini C, Jeffrey RB,
Willmann JK and Olcott E: Three-dimensional volume-rendered
multidetector CT imaging of the posterior inferior
pancreaticoduodenal artery: Its anatomy and role in diagnosing
extrapancreatic perineural invasion. Cancer Imaging. 13:580–590.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Noto M, Miwa K, Kitagawa H, Kayahara M,
Takamura H, Shimizu K and Ohta T: Pancreas head carcinoma:
frequency of invasion to soft tissue adherent to the superior
mesenteric artery. Am J Surg Pathol. 29:1056–1061. 2005.PubMed/NCBI
|
|
14
|
Mochizuki K, Gabata T, Kozaka K, Hattori
Y, Zen Y, Kitagawa H, Kayahara M, Ohta T and Matsui O: MDCT
findings of extrapancreatic nerve plexus invasion by pancreas head
carcinoma: Correlation with en bloc pathological specimens and
diagnostic accuracy. Eur Radiol. 20:1757–1767. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zuo HD, Tang W, Zhang XM, Zhao QH and Xiao
B: CT and MR imaging patterns for pancreatic carcinoma invading the
extrapancreatic neural plexus (Part II): Imaging of pancreatic
carcinoma nerve invasion. World J Radiol. 4:13–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bonnichon P, Rossat-Mignod JC, Corlieu P,
Aaron C, Yandza T and Chapuis Y: Surgical approach to the superior
mesenteric artery by the Kocher Maneuver: Anatomy study and
clinical applications. Ann Vasc Surg. 1:505–508. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen SJ, Zhang YH, Gu XX, Jiang SJ and Xu
LJ: Yangfei Kongliu Formula, a compound Chinese herbal medicine,
combined with cisplatin, inhibits growth of lung cancer cells
through transforming growth factor-β1 signaling pathway. J Integr
Med. 15:242–251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strobel O, Hank T, Hinz U, Bergmann F,
Schneider L, Springfeld C, Jäger D, Schirmacher P, Hackert T and
Büchler MW: Pancreatic cancer surgery: The new R-status counts. Ann
Surg. 265:565–573. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Allen PJ, Kuk D, Castillo CF, Basturk O,
Wolfgang CL, Cameron JL, Lillemoe KD, Ferrone CR, Morales-Oyarvide
V, He J, et al: Multi-institutional validation study of the
American Joint Commission on Cancer (8th edition) Changes for T and
N staging in patients with pancreatic adenocarcinoma. Ann Surg.
265:185–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsumoto I, Murakami Y, Shinzeki M, Asari
S, Goto T, Tani M, Motoi F, Uemura K, Sho M, Satoi S, et al:
Proposed preoperative risk factors for early recurrence in patients
with resectable pancreatic ductal adenocarcinoma after surgical
resection: A multi-center retrospective study. Pancreatology.
15:674–680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Demir IE, Friess H and Ceyhan GO:
Nerve-cancer interactions in the stromal biology of pancreatic
cancer. Front Physiol. 3:972012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jobling P, Pundavela J, Oliveira SM,
Roselli S, Walker MM and Hondermarck H: Nerve-cancer cell
cross-talk: A novel promoter of tumor progression. Cancer Res.
75:1777–1781. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, Guo L, Tao M, Fu W and Xiu D:
Parasympathetic neurogenesis is strongly associated with tumor
budding and correlates with an adverse prognosis in pancreatic
ductal adenocarcinoma. Chin J Cancer Res. 28:180–186. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Demir IE, Friess H and Ceyhan GO: Neural
plasticity in pancreatitis and pancreatic cancer. Nat Rev
Gastroenterol Hepatol. 12:649–659. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ceyhan GO, Bergmann F, Kadihasanoglu M,
Altintas B, Demir IE, Hinz U, Müller MW, Giese T, Büchler MW, Giese
NA and Friess H: Pancreatic neuropathy and neuropathic pain-a
comprehensive pathomorphological study of 546 cases.
Gastroenterology. 136:177–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ceyhan GO, Demir IE, Rauch U, Bergmann F,
Müller MW, Büchler MW, Friess H and Schäfer K: Pancreatic
neuropathy results in ‘neural remodeling’ and altered pancreatic
innervation in chronic pancreatitis and pancreatic cancer. Am J
Gastroenterol. 104:2555–2565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mitsunaga S, Hasebe T, Kinoshita T,
Konishi M, Takahashi S, Gotohda N, Nakagohri T and Ochiai A: Detail
histologic analysis of nerve plexus invasion in invasive ductal
carcinoma of the pancreas and its prognostic impact. Am J Surg
Pathol. 31:1636–1644. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chouat E, Zehani A, Chelly I, Njima M,
Maghrebi H, Bani MA, Njim L, Zakhama A, Haouet S and Kchir N: Tumor
budding is a prognostic factor linked to epithelial mesenchymal
transition in pancreatic ductal adenocarcinoma. Study report and
literature review. Pancreatology. 18:79–84. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ceyhan GO, Demir IE, Maak M and Friess H:
Fate of nerves in chronic pancreatitis: Neural remodeling and
pancreatic neuropathy. Best Pract Res Clin Gastroenterol.
24:311–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
O'Connor K, Li-Chang HH, Kalloger SE,
Peixoto RD, Webber DL, Owen DA, Driman DK, Kirsch R, Serra S,
Scudamore CH, et al: Tumor budding is an independent adverse
prognostic factor in pancreatic ductal adenocarcinoma. Am J Surg
Pathol. 39:472–478. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Magnon C, Hall SJ, Lin J, Xue X, Gerber L,
Freedland SJ and Frenette PS: Autonomic nerve development
contributes to prostate cancer progression. Science.
341:12363612013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Demir IE, Ceyhan GO, Rauch U, Altintas B,
Klotz M, Muller MW, Büchler MW, Friess H and Schäfer KH: The
microenvironment in chronic pancreatitis and pancreatic cancer
induces neuronal plasticity. Neurogastroenterol Motil. 22:480–490,
e112-e113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo K, Ma Q, Li J, Wang Z, Shan T, Li W,
Xu Q and Xie K: Interaction of the sympathetic nerve with
pancreatic cancer cells promotes perineural invasion through the
activation of STAT3 signaling. Mol Cancer Ther. 12:264–273. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kiba T: Relationships between the
autonomic nervous system and the pancreas including regulation of
regeneration and apoptosis: Recent developments. Pancreas.
29:e51–e58. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stopczynski RE, Normolle DP, Hartman DJ,
Ying H, DeBerry JJ, Bielefeldt K, Rhim AD, DePinho RA, Albers KM
and Davis BM: Neuroplastic changes occur early in the development
of pancreatic ductal adenocarcinoma. Cancer Res. 74:1718–1727.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sohal DP, Shrotriya S, Glass KT, Pelley
RJ, McNamara MJ, Estfan B, Shapiro M, Wey J, Chalikonda S,
Morris-Stiff G, et al: Predicting early mortality in resectable
pancreatic adenocarcinoma: A cohort study. Cancer. 121:1779–1784.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sinn M, Bahra M, Denecke T, Travis S,
Pelzer U and Riess H: Perioperative treatment options in resectable
pancreatic cancer-how to improve long-term survival. World J
Gastrointest Oncol. 8:248–257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Saloman JL, Albers KM, Li D, Hartman DJ,
Crawford HC, Muha EA, Rhim AD and Davis BM: Ablation of sensory
neurons in a genetic model of pancreatic ductal adenocarcinoma
slows initiation and progression of cancer. Proc Natl Acad Sci USA.
113:3078–3083. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khorana AA, Mangu PB, Berlin J,
Engebretson A, Hong TS, Maitra A, Mohile SG, Mumber M, Schulick R,
Shapiro M, et al: Potentially curable pancreatic cancer: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 34:2541–2556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kurosaki I, Kawachi Y, Nihei K, Tsuchiya
Y, Aono T, Yokoyama N, Shimizu T and Hatakeyama K: Liver perfusion
chemotherapy with 5-Fluorouracil followed by systemic gemcitabine
administration for resected pancreatic cancer: Preliminary results
of a prospective phase 2 study. Pancreas. 38:161–167. 2009.
View Article : Google Scholar : PubMed/NCBI
|