Introduction
Tongue cancer is a common cancer in the mouth,
accounting for 30–50% of all oral cancers. The most common type of
tongue cancer is squamous cell carcinoma. Statistics indicated that
the incidence is significantly higher for men compared to women
(1,2). As the population in China is aging, the
incidence of tongue cancer among elderly people is increasing year
by year. Because blood vessels and lymphatic vessels are rich in
the tongue and the tongue muscle is frequently squeezed due to
structural reasons, tongue squamous cell carcinoma is prone to
metastasize even in its early stage and therefore has a poor
prognosis (3,4). Early diagnosis and early treatment of
tongue squamous cell carcinoma is the key to patient recovery. At
present, commonly used clinical treatment options for tongue
squamous cell carcinoma are surgery, chemotherapy, radiotherapy and
comprehensive treatment. However, therapeutic outcomes of patients
with early tongue squamous cell carcinoma using various surgical
protocols are still open to debate, and few related studies can be
found in literature (5,6). In this study, retrospective analysis
was performed on clinical records of 128 patients with early tongue
squamous cell carcinoma who were treated in Henan Province Hospital
of TCM (Zhengzhou, China) from June, 2010 to June, 2013.
Therapeutic outcomes of these patients with early tongue squamous
cell carcinoma were compared between three treatment options, i.e.
surgical therapy alone, preoperative radiotherapy and postoperative
radiotherapy. This study aimed to provide a more precise treatment
plan for patients with tongue cancer in different stages and to
improve treatment.
Materials and methods
Subjects
Clinical records of 128 patients with early tongue
squamous cell carcinoma who were treated in Henan Province Hospital
of TCM from June, 2010 to June, 2013 were retrospectively analyzed.
According to adopted treatment plan, the patients were divided into
3 groups: 92 patients in surgical therapy alone group, 13 patients
in preoperative radiotherapy group, and 23 patients in
postoperative radiotherapy group.
The study was approved by the Εthics Committee of
Henan Province Hospital of TCM. Patients who participated in this
research had complete clinical data. The signed informed consents
were obtained from the patients or the guardians.
Inclusion criteria
Patients who met the following criteria were
eligible for the study: i) Patients who were diagnosed to have
primary tongue squamous cell carcinoma in the early stages
(cT1-2N0); ii) patients who received treatment for the first time;
iii) patients whose lesion was on the forward portion of the
tongue; and iv) patients who had complete clinical records.
Exclusion criteria
Patients who met the following criteria were
excluded from this study: i) patients who also had other kinds of
primary cancers; ii) patients who also had lymph node metastasis;
iii) patients who underwent cervical lymph node dissection; iv)
patients with pathologically positive surgical margins; and v)
patients who had a survival of less than two months.
Treatment procedures
Patients in the surgical therapy alone group
underwent primary lesion resection, which was performed by
physicians who had been in clinical practice for more than 5 years.
The diseased tissue was removed along a cut edge 1–2 cm away from
the lesion, resulting in cancer-free resection margins. Patients in
the preoperative radiotherapy group received preoperative
radiotherapy in addition to the above-mentioned primary lesion
resection. First, the lesion location was determined by imaging
techniques and the results of clinical diagnosis. Then,
radiotherapy was given by external beam radiation alone or
combination of external beam radiation and internal radiation by
inserting a radiation source inside the tissue (combined
radiation). The total dose of external beam radiation therapy was
50–72 Gy (64.5±4.7 Gy), and the energy range of external radiation
source was 5–6 MV. The total dose of combined radiation therapy was
<60 Gy. Radiotherapy was completed within 15–70 days before
surgery. The radiotherapy protocol in the postoperative
radiotherapy group was similar to that in the preoperative
radiotherapy group mentioned above. The total dose of external beam
radiation therapy was 50–72 Gy (66.1±5.3 Gy), and the total dose of
combined radiation therapy was <70 Gy. Radiotherapy was
completed within 15–75 days after surgery.
Research method
Statistical analysis was performed on patient
general data and clinical records, as well as the patient's 5-year
survival rate and recurrence rate. The general data and clinical
records included sex ratio, age distribution, disease course
(referring to the time from onset to operation), pathological type,
and clinical stage. The statistics of patients' clinical data were
obtained by follow-up. The starting time of follow-up is the
discharge time of patients, and the end time was 30 June 2018 or
the death of patients. The forms of follow-up mainly include
questionnaire, telephone, and outpatient follow-up.
Statistical analysis
Data were processed using SPSS 19.0 (IBM Corp.,
Armonk, NY, USA) statistics software. The χ2 test was
used for comparison of quantitative data which were expressed as
(mean ± SD). The t-test was used for comparison of measurement
data. Differences in survival rate between groups were obtained by
using the Kaplan-Meier test and log rant test. Results of
multivariate analysis were obtained by using the COX test. A
difference was considered statistically significant at
P<0.05.
Results
Comparison of patient general
data
As shown in Table I,
there were no statistically significant differences in sex ratio,
age distribution, disease course, pathological type, clinical
stage, and tumor location among the three groups.
 | Table I.Comparison of patient general
data. |
Table I.
Comparison of patient general
data.
| Variant | Surgical therapy
alone group (n=92) | Preoperative
radiotherapy group (n=13) | Postoperative
radiotherapy group (n=23) | χ2 | P-value |
|---|
| Sex |
| Male | 56 | 8 | 15 | 0.146 | 0.929 |
|
Female | 36 | 5 | 8 |
|
|
| Age (years) | 53.1±6.9 | 55.4±7.1 | 54.9±7.3 | 0.663 | 0.794 |
| Smoking |
|
|
| 0.024 | 0.988 |
| Yes | 51 | 7 | 13 |
|
|
| No | 41 | 6 | 10 |
|
|
| Disease course |
| >6
months | 52 | 6 | 11 | 0.903 | 0.637 |
| ≤6
months | 40 | 7 | 12 |
|
|
| Pathological
type |
| Well
differentiated | 70 | 9 | 16 | 0.470 | 0.791 |
|
Moderately differentiated | 14 | 3 | 5 |
|
|
| Poorly
differentiated | 8 | 1 | 2 |
|
|
| Clinical stage |
| Stage
I | 53 | 8 | 9 | 2.787 | 0.248 |
| Stage
II | 39 | 5 | 14 |
|
|
| Tumor location |
| Lateral
tongue | 65 | 9 | 17 | 0.076 | 0.963 |
| Ventral
tongue | 21 | 3 | 4 |
|
|
| Dorsal
tongue | 6 | 1 | 2 |
|
|
Comparison of survival rate
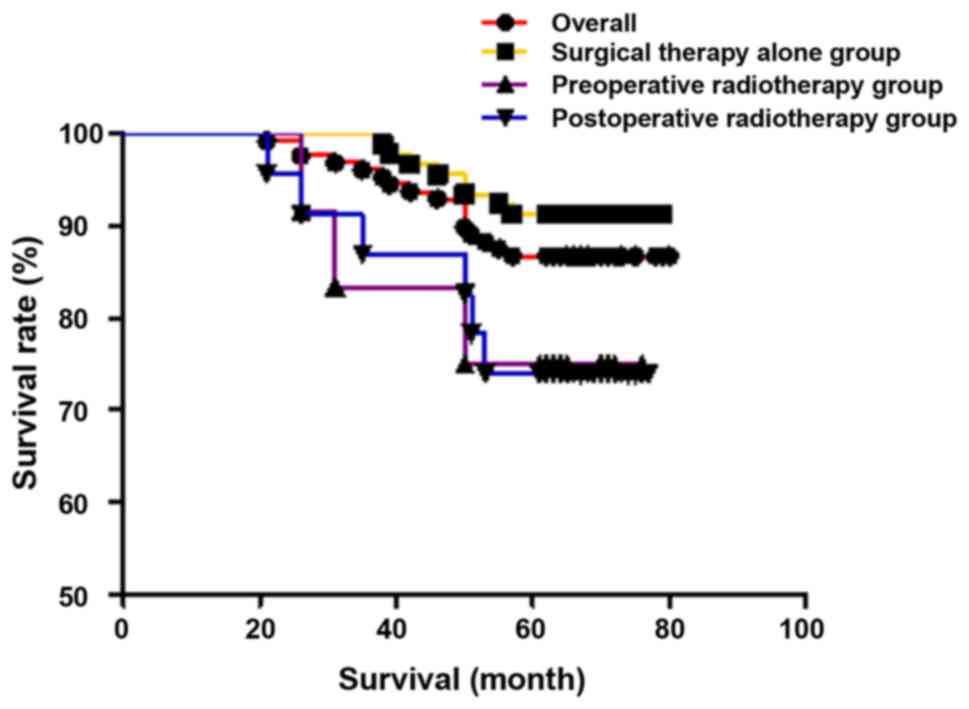
The overall 5-year survival rate for all patients
was 86.7% (111/128), that of stage I was 88.5% (62/70), and that of
stage II was 84.5% (49/58). The 5-year disease-specific survival
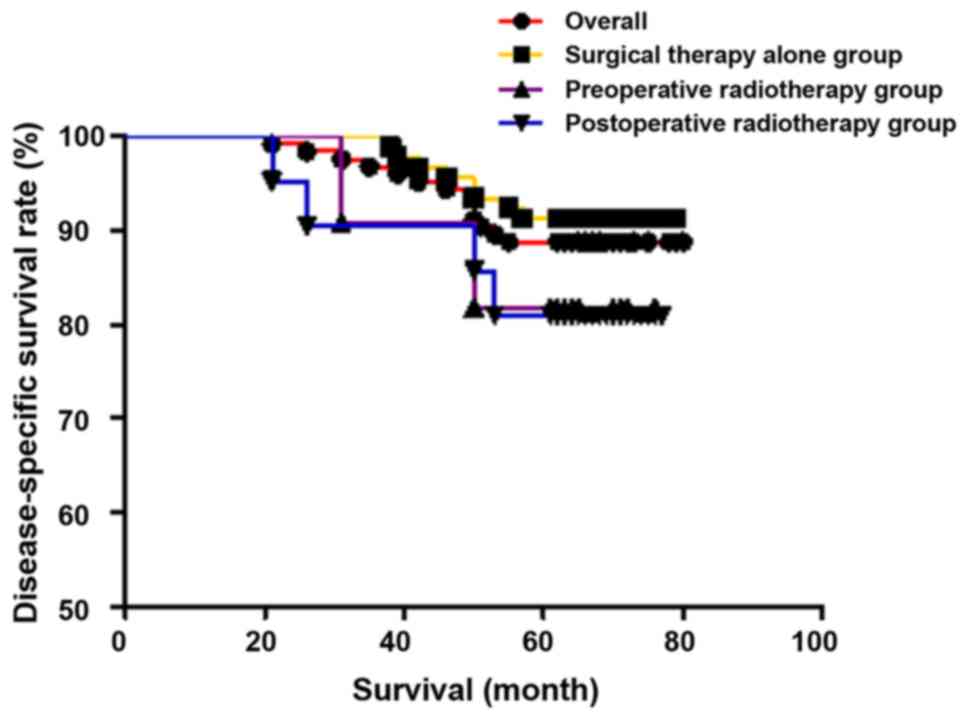
rate was 88.8% (111/125), which was 91.2% (62/68) in stage I, and
85.9% (49/57) in stage II. In individual groups, the 5-year
survival rate and the 5-year disease-specific survival rate were
91.3% (84/92) and 91.3% (84/92), respectively, in the surgical
therapy alone group 76.9% (10/13) and 83.3% (10/12), respectively,
in the preoperative radiotherapy group, and 73.9% (17/23) and 81.0%
(17/21), respectively, in the postoperative radiotherapy group.
There were no statistically significant differences in 5-year
survival rate (χ2=5.990, P=0.051) and 5-year
disease-specific survival rate (χ2=2.223, P=0.329) among
the three groups. The results are shown in Figs. 1 and 2.
Comparison of recurrence rate and
metastatic rate in the three groups
As shown in Table
II, there were 25 cases of recurrence in total during
follow-up. The recurrence rate was 19.5%; the local recurrence rate
was 11.7% (15/128); and the regional recurrence rate was 7.8%
(10/128). There were 5 cases of metastasis, and the metastatic rate
was 4.7%. There were no statistically significant differences in
recurrence rate and metastatic rate among the three groups.
 | Table II.Comparison of recurrence rate and
metastatic rate in the three groups. |
Table II.
Comparison of recurrence rate and
metastatic rate in the three groups.
| Variant | Surgical therapy
alone group | Preoperative
radiotherapy group | Postoperative
radiotherapy group | χ2 | P-value |
|---|
| Patient number | 92 | 13 | 23 |
|
|
| Recurrence | 18 | 2 | 5 | 0.282 | 0.868 |
| Local recurrence | 11 | 1 | 3 | 0.225 | 0.880 |
| Regional
recurrence | 7 | 1 | 2 | 0.087 | 0.958 |
| Metastasis | 3 | 1 | 1 | 0.743 | 0.690 |
Discussion
Surgical therapy is the primary treatment option for
tongue cancer, while radiotherapy and chemotherapy are adjuvant
therapy. Patients rarely receive radiotherapy or chemotherapy alone
for tongue cancer. Radiotherapy and chemotherapy have proven
systemic toxic side effects, and physicians are unable to reach a
consensus on whether they have an impact on patient survival
(7,8). All the subjects in this study underwent
surgical treatment and included the majority of patients diagnosed
with tongue squamous cell carcinoma. This study aimed to provide a
scientific reference for the treatment of tongue cancer.
Patients with tongue cancer have a poor prognosis.
The 5-year survival rate is about 27% for mid-stage/late stage
patients. The subjects included in this study were all patients
with early tongue squamous cell carcinoma, for whom few research
reports appear in literature. In this study, the overall 5-year
survival rate and the 5-year disease-specific survival rate were
86.7 and 88.8%, respectively, which were all higher compared with
similar studies in literature (9,10). The
causes of this exceptional result could be that i) the patients
selected in this study had milder symptoms or ii) continuous
advancement of medical technology led to overall improvement of
treatment outcomes such as overall survival rate of patients with
early tongue squamous cell carcinoma (11,12).
There were no statistically significant differences in 5-year
survival rate among patients in the three groups. To get more
accurate results, a larger sample size may be needed because the
number of subjects in this study reached the threshold. However,
some studies have pointed out that (13) functional and aesthetic deficiencies
caused by radical surgery can reduce the quality of life of
patients, so conservative treatment is better than radical
treatment for elderly patients aged over 60 years. Therefore, the
average age of patients selected into this study is lower than 60
years.
The main purpose of preoperative radiotherapy was to
attenuate tumor cell activity and reduce the risk of tumor cell
implantation into benign lesions, thereby lowering cancer
recurrence rate and metastatic rate. In addition, preoperative
radiotherapy can significantly decrease tumor cell volume, making
the surgical procedures a little easier to perform. The
postoperative radiotherapy was to give patients more precise
radiotherapy in terms of location and dose because related
information can be more accurately obtained after surgery and
verified using imaging techniques. Postoperative radiotherapy was
expected to improve the treatment (13,14). In
this study, it was found that adjuvant radiotherapy did not improve
the 5-year survival rate of patients with early tongue cancer. Our
finding was opposite to reports in literature (15,16), and
the possible reason could be that the recruited patients with early
tongue cancer had relatively milder symptoms. Surgery caused great
tissue injury to the patient's body. In addition, systemic side
effects of radiotherapy can seriously affect the immune system.
Surgical therapy alone would be enough to achieve the treatment
goal. In order to avoid overtreatment and reduce complexity of
treatment procedures, the authors suggest that surgical therapy
alone be adopted to treat patients with early tongue squamous cell
carcinoma.
There were 25 cases of recurrence in total during
follow-up. The recurrence rate was 19.5%; the local recurrence rate
was 11.7% (15/128); and the regional recurrence rate was 7.8%
(10/128). There were 6 cases of metastasis, and the metastatic rate
was 4.7%. It was found that neither preoperative radiotherapy nor
postoperative radiotherapy can reduce the 5-year recurrence rate.
The possible reason could be that both preoperative radiotherapy
and postoperative radiotherapy had a balance between effectiveness
in killing cancer cells and aggressiveness in causing damage to the
body (17–19). Radiotherapy caused great damage to
the patient's body, and was probably too aggressive for treating
early tongue squamous cell carcinoma. Preoperative and
postoperative radiotherapy should be avoided as much as
possible.
In conclusion, compared with surgical therapy alone,
radiotherapy combined with surgical therapy neither improved 5-year
survival rate nor reduced recurrence rate. Therefore, surgical
therapy alone is suggested to be the preferred option for treating
patients with early tongue squamous cell carcinoma.
Acknowledgements
Not applicable.
Funding
This study was supported by 2016 Henan Science
andTechnology Research Program Fund Project (162102310183).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and YW worked on treatment procedures. AL and RL
collected and analyzed general data and clinical records. XZ and CZ
were responsible for patient's 5-year survival rate and recurrence
rate analysis. LZ and XX contributed to statistical analysis. LZ
wrote the manuscript. All the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Εthics Committee of
Henan Province Hospital of TCM (Zhengzhou, China). Patients who
participated in this research had complete clinical data. The
signed informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kelner N, Rodrigues PC, Bufalino A,
Fonseca FP, Santos-Silva AR, Miguel MC, Pinto CA, Leme AF, Graner
E, Salo T, et al: Activin A immunoexpression as predictor of occult
lymph node metastasis and overall survival in oral tongue squamous
cell carcinoma. Head Neck. 37:479–486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aparna M, Rao L, Kunhikatta V and
Radhakrishnan R: The role of MMP-2 and MMP-9 as prognostic markers
in the early stages of tongue squamous cell carcinoma. J Oral
Pathol Med. 44:345–352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maxwell JH, Thompson LD, Brandwein-Gensler
MS, Weiss BG, Canis M, Purgina B, Prabhu AV, Lai C, Shuai Y,
Carroll WR, et al: Early oral tongue squamous cell carcinoma:
Sampling of margins from tumor bed and worse local control. JAMA
Otolaryngol Head Neck Surg. 141:1104–1110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thiagarajan S, Nair S, Nair D, Chaturvedi
P, Kane SV, Agarwal JP and D'Cruz AK: Predictors of prognosis for
squamous cell carcinoma of oral tongue. J Surg Oncol. 109:639–644.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ren ZH, Wu HJ, Zhang S, Wang K, Gong ZJ,
He ZJ and Peng J: A new surgical strategy for treatment of tongue
squamous cell carcinoma based on anatomic study with preliminary
clinical evaluation. J Craniomaxillofac Surg. 43:1577–1582. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Janakiraman H, House RP, Talwar S,
Courtney SM, Hazard ES, Hardiman G, Mehrotra S, Howe PH, Gangaraju
V and Palanisamy V: Repression of caspase-3 and RNA-binding protein
HuR cleavage by cyclooxygenase-2 promotes drug resistance in oral
squamous cell carcinoma. Oncogene. 36:3137–3148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abu-Ghanem S, Yehuda M, Carmel NN, Leshno
M, Abergel A, Gutfeld O and Fliss DM: Elective neck dissection vs
observation in early-stage squamous cell carcinoma of the oral
tongue with no clinically apparent lymph node metastasis in the
neck: A systematic review and meta-analysis. JAMA Otolaryngol Head
Neck Surg. 142:857–865. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsushima N, Sakashita T, Homma A,
Hatakeyama H, Kano S, Mizumachi T, Kakizaki T, Suzuki T and Fukuda
S: The role of prophylactic neck dissection and tumor thickness
evaluation for patients with cN0 tongue squamous cell carcinoma.
Eur Arch Otorhinolaryngol. 273:3987–3992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goepfert RP, Kezirian EJ and Wang SJ: Oral
tongue squamous cell carcinoma in young women: A matched comparison
- do outcomes justify treatment intensity? ISRN Otolaryngol.
2014:5293952014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tong XJ, Tang ZG, Shan ZF and Guo XC: The
anterolateral thigh flap for soft tissue reconstruction in patients
with tongue squamous cell carcinoma. World J Surg Oncol.
14:2132016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goto M, Hanai N, Ozawa T, Hirakawa H,
Suzuki H, Hyodo I, Kodaira T, Ogawa T, Fujimoto Y, Terada A, et al:
Prognostic factors and outcomes for salvage surgery in patients
with recurrent squamous cell carcinoma of the tongue. Asia Pac J
Clin Oncol. 12:e141–e148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cohen EE, Karrison TG, Kocherginsky M,
Mueller J, Egan R, Huang CH, Brockstein BE, Agulnik MB, Mittal BB,
Yunus F, et al: Phase III randomized trial of induction
chemotherapy in patients with N2 or N3 locally advanced head and
neck cancer. JClin Oncol. 32:2735–2743. 2014. View Article : Google Scholar
|
|
13
|
Zhang P, Zhang L, Liu H, Zhao L, Li Y,
Shen JX, Liu Q, Liu MZ and Xi M: Clinicopathologic characteristics
and prognosis of tongue squamous cell carcinoma in patients with
and without a history of radiation for nasopharyngeal carcinoma: A
matched Case-Control Study. Cancer Res Treat. 49:695–705. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sung KW, Kim SM, Myoung H, Kim MJ and Lee
JH: The effectiveness of elective neck dissection on early (stage
I, II) squamous cell carcinoma of the oral tongue. J Korean Assoc
Oral Maxillofac Surg. 43:147–151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Toporcov TN, Znaor A, Zhang ZF, Yu GP,
Winn DM, Wei Q, Vilensky M, Vaughan T, Thomson P, Talamini R, et
al: Risk factors for head and neck cancer in young adults: A pooled
analysis in the INHANCE consortium. Int J Epidemiol. 44:169–185.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meng J, Gu QP, Meng QF, Zhang J, Li ZP, Si
YM, Guo W and Zhuang QW: Efficacy of nimotuzumab combined with
docetaxel-cisplatin-fluorouracil regimen in treatment of advanced
oral carcinoma. Cell Biochem Biophys. 68:181–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Katz O, Nachalon Y, Hilly O, Shpitzer T,
Bachar G, Limon D and Popovtzer A: Radiotherapy in early-stage
tongue squamous cell carcinoma with minor adverse features. Head
Neck. 39:147–150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu K, Li S and Zhang C: Lymph node
transfer characteristics and clinical assessment and treatment of
early tongue squamous cell carcinoma. Int J Stomatology. 42:30–47.
2015.
|
|
19
|
Hakeem AH, Pradhan SA, Kannan R and
Tubachi J: Clinical outcome of surgical treatment of T1-2 N0
squamous cell carcinoma of oral tongue with observation for the
neck: Analysis of 176 cases. Ann Maxillofac Surg. 6:235–240. 2016.
View Article : Google Scholar : PubMed/NCBI
|