Introduction
Prostate cancer, one of the leading causes of death
in the male population in Europe and the United States, ranks first
among male urogenital diseases (1).
The incidence of prostate cancer is increasing with the continuous
improvement of people's material living standards (2,3). The
5-year survival rate of patients with metastatic prostate cancer is
36% to 54% (4). This may be due to
the difficulty in the treatment of advanced prostate cancer.
Radical resection for treating patients with early prostate cancer
greatly improves their quality of life and survival quality
(5). At present, the cause of
prostate cancer remains unclear. Its treatment is still in the
experimental stage, and the conditions for clinical large-scale
application are not yet available. Therefore, it is especially
important to select the best clinically diagnostic method for the
early diagnosis of prostate cancer (6).
Various advanced science and technology have been
widely used in the diagnosis of clinical diseases due to their
continuous advancement and development. Radiological examinations
such as magnetic resonance imaging (MRI) and computed tomography
(CT) have good diagnostic value for the diagnosis and stage of
prostate cancer. This is particularly true for the multi-parameter
MRI with MRI combined with spectrum analysis and dynamic
diffusion-weighted imaging (DWI), which is increasingly recognized
in clinical practice and has a great value in the early diagnosis
and clinical stage of prostate cancer (7). There is literature showing that the
diagnostic sensitivity of CT is lower than that of MRI in early
prostate cancer (8). Multi-slice
spiral CT (MSCT) scanning is considered to be more sensitive and
specific than traditional CT scanning in detecting lymph node
metastasis of carcinoma of esophagus (9). However, there are relatively few
comparative studies on the use of MSCT and MRI in the diagnosis of
patients with different pathological stages of prostate cancer.
In this investigation, the accuracy of MSCT and MRI
was compared in the detection of stages A/B and C/D of prostate
cancer, and their sensitivity and specificity were also compared,
in order to explore the diagnostic value for the different
pathological stages of prostate cancer.
Patients and methods
General information
A total of 112 patients with prostate cancer who
underwent surgical pathology in The Affiliated Yantai Yuhuangding
Hospital of Qingdao University (Yantai, China) from February 2014
to January 2016 were enrolled as the prostate cancer group. They
were aged 62.34±9.65 years, with a course of disease of 6 months to
4 years and an average course of disease of 2.0±1.2 years. The
serum prostate-specific antigen detection value (PSA) of the
patients was 25.23±9.9 ng/ml, and there were 89 patients with an
increase in single serum PSA (Table
I). Another 100 patients who received physical health
examinations in The Affiliated Yantai Yuhuangding Hospital of
Qingdao University during the same period were collected as the
normal group, aged 62.65±9.57 years.
 | Table I.Patient general information
[n(%)]/(mean ± SD). |
Table I.
Patient general information
[n(%)]/(mean ± SD).
| Variables | Patients with
prostate cancer (n=112) |
|---|
| Age (years) | 62.34±9.65 |
| Ethnicity |
|
| Han | 69 (61.6) |
| Ethnic
minority | 43 (38.4) |
| Course of
disease | 6 months-4 years |
| Average course of
disease (years) | 2.0±1.2 |
| Prostate volume
(ml) | 43.13±19.89 |
| Serum PSA value
(ng/ml) | 25.23±9.9 |
| Increased single
serum PSA (patients) | 89 (79.5) |
| Positive rectal touch
(patients) | 19 (17.0) |
| Infringement of
seminal vesicle (patients) | 15 (13.4) |
| Infringement of the
bladder (patients) | 11 (9.8) |
| Iliac bone metastasis
(patients) | 4 (3.6) |
| Pulmonary metastasis
(patients) | 8 (7.1) |
| Pelvic lymph node
metastasis (patients) | 11 (9.8) |
Inclusion criteria: Patients who did not receive
anti-tumor treatments before the examination; patients with
clinical symptoms that were mainly frequent micturition, urgency of
urination, dysuria and frequent nocturia, and some patients with
hematuria; males aged over 50 years.
Exclusion criteria: Patients with other severe tumor
diseases; patients who did not actively cooperate; patients who did
not undergo routine examination before operation; those previously
suffering from mental disorder and with a family history of mental
illness; those with incomplete clinical data.
The study was approved by the Ethics Committee of
The Affiliated Yantai Yuhuangding Hospital of Qingdao University
and the experimental content of subjects was described in detail.
Subjects agreed to and signed a completed informed consent
form.
Detection methods
MSCT examination
In this study, the Toshiba Activion l6-slice spiral
CT instrument was used for MSCT examination. Each patient was
placed in the supine position and scanned, and the scan range
included the prostate, the seminal vesicle and the bladder.
Perfusion scanning was performed centering on the largest central
level of the lesion or the center level of the prostate. Scanning
parameters were as follows: 320 mA in current flow, 120 kV in
voltage, 0.9376 in pitch, with l6×0.5 mm thin layer detector. The
enhanced scanning was performed with a non-ionic contrast media.
The dosage of the contrast agent (Bayer Schering Pharmaceutical
Co., Ltd.; guoyaozhunzi: J20050047) was adjusted to 1.5–2.0 ml/kg
based on the actual situation of the patients, and was injected
from the median vein of the elbow at a flow rate of 3.5–4 m/sec.
Then, dynamic scanning was performed 5 sec later, with a scanning
time of 0.5 sec/16 layers. The data was reconstructed, with 0.8 mm
in reconstruction interval and 1 mm in layer thickness, and then
transmitted to the processing server. The imaging was reconstructed
with multi-planar reconstruction (MPR), maximum density projection
(MIP) and volume rendering technology (VR). The attending
radiologists (at least two) analyzed the data together and made a
conclusion.
MRI examination
The HDXT 1.5T superconducting MRI imager from GE was
used as an MRI diagnostic instrument. Before the scanning, the
patients were told to drink water in order to maintain the filling
of the bladder. During the operation, patients were placed in the
supine position and maintained uniform and gentle breathing. Array
coils were performed through the body phase control of standard 6
units. The scanning sequence was conventional coronal, sagittal,
cross-sectional T1WI, T2WI and DWI images. Specific parameters were
as follows: T1WI was (TR/TE = 250/4.92 msec, matrix 320*256); T2WI
was (TR/TE = 6,000/100 msec, matrix 320*320); the number of
excitations was 2 to 4 times, FOV was 240*240 mm, the layer
thickness was 5 mm, and the interlayer spacing was 5 mm. Chemical
displacement Selection Saturation method was used for lipid
inhibition. DWI was (TR/TE = 8,200/100 msec, matrix 128*128, the
layer thickness was 5 mm; the interlayer spacing was 5 mm, and the
b-value was 1,000 sec/mm2).
Criteria for clinical stage
The Whitmore-Jewett method (10) was used for staging prostate cancer
that was divided into stage A, B, C and D. In stage A, the tumor
was concealed in the prostate and difficult to be detected through
the rectal mouth. In stage B, the tumor could be detected in the
rectum, and there was a tumor in the capsule of the prostate. In
stage C, the tumor had exceeded the capsule of the prostate, but
there was no metastasis. In stage D, the tumor had a distant
metastasis.
Outcome measures
The apparent diffusion coefficient (ADC) plot was
automatically generated after the DWI scanning. The ADC value of
the lesion was measured by manually placing the region of interest
(ROI) on the ADC plot. Calcifications, blood vessels or bleeding
were avoided, and the corresponding ADC values were recorded. The
measurement was repeated twice to calculate the average value.
Clinical outcome measures: The sensitivity and
specificity of MSCT and MRI for the diagnosis of prostate cancer
were calculated based on the results of postoperative puncture
pathological biopsy. The formula was as follows: the sensitivity =
[true positive/(true positive + false negative) × 100%], and the
specificity = [true negative/(true negative + false positive)
×100%]. The diagnostic coincidence rates, misdiagnosis rates and
missed diagnosis rates of MSCT and MRI for prostate cancer staging
were compared.
Statistical analysis
SPSS 17.0 statistical software (SPSS Inc., Chicago,
IL, USA) was used for the statistical analysis of the experimental
data. Enumeration data were expressed as n (%), and Chi-square test
was used for comparison between the two groups. Measurement data
were expressed as (mean ± SD), one-way analysis of variance and LSD
post hoc test were used for the comparison of mean between multiple
groups, and paired t-test was used between the two groups. Spearman
correlation coefficient was used for analyzing the pathological
stage and the ADC value. P<0.05, indicates the difference is
statistically significant.
Results
Comparison of clinical baseline data
between the two groups
The clinical baseline data of age, height, body mass
index, smoking and drinking, presence or absence of diabetes, white
blood cells (WBC), hemoglobin (HB), red blood cell (RBC) count and
platelet (PLT) count were collected from patients in the prostate
cancer group and the normal group. There was no statistically
significant difference in the clinical baseline data of patients
between the two groups (P>0.05), which are comparable (Table II).
 | Table II.Comparison of clinical baseline data
between the two groups. |
Table II.
Comparison of clinical baseline data
between the two groups.
|
| Prostate cancer group
(n=112) | Normal group
(n=100) | χ2/t | P-value |
|---|
| Age (years) | 62.34±9.65 | 62.65±9.57 | 0.234 | 0.815 |
| Height (cm) | 167.23±7.87 | 167.36±6.98 | 0.127 | 0.899 |
| Body mass index
(kg/m2) | 25.37±2.87 | 25.76±2.65 | 1.024 | 0.307 |
| Smoking |
|
| 0.017 | 0.897 |
| Yes | 82 (73.2) | 74 (74.0) |
|
|
| No | 30 (26.8) | 26 (26.0) |
|
|
| Drinking |
|
| 1.087 | 0.297 |
| Yes | 76 (67.9) | 61 (61.0) |
|
|
| No | 36 (32.1) | 39 (39.0) |
|
|
| History of diabetes
mellitus |
|
| 0.325 | 0.569 |
| Yes | 48 (42.9) | 39 (39.0) |
|
|
| No | 64 (57.1) | 61 (61.0) |
|
|
| WBC
(×109/l) | 6.19±3.24 | 6.23±3.35 | 0.088 | 0.930 |
| HB (gm/dl) | 12.24±2.09 | 12.47±2.11 | 0.796 | 0.427 |
| PLT
(×109/l) | 161.17±20.98 | 159.98±21.09 | 0.411 | 0.681 |
| RBC
(1012/l) | 4.54±0.59 | 4.57±0.61 | 0.363 | 0.716 |
Comparison of ADC values between
different stages of prostate cancer
Based on the ADC values generated by DWI imaging in
MRI (Table III), the ADC value of
stage A was significantly higher than that of stage B, C and D
(P<0.05), that of stage B was significantly higher than that of
stage C and D (P<0.05), and that of stage C was significantly
higher than of stage D (P<0.05). The pathological stage was
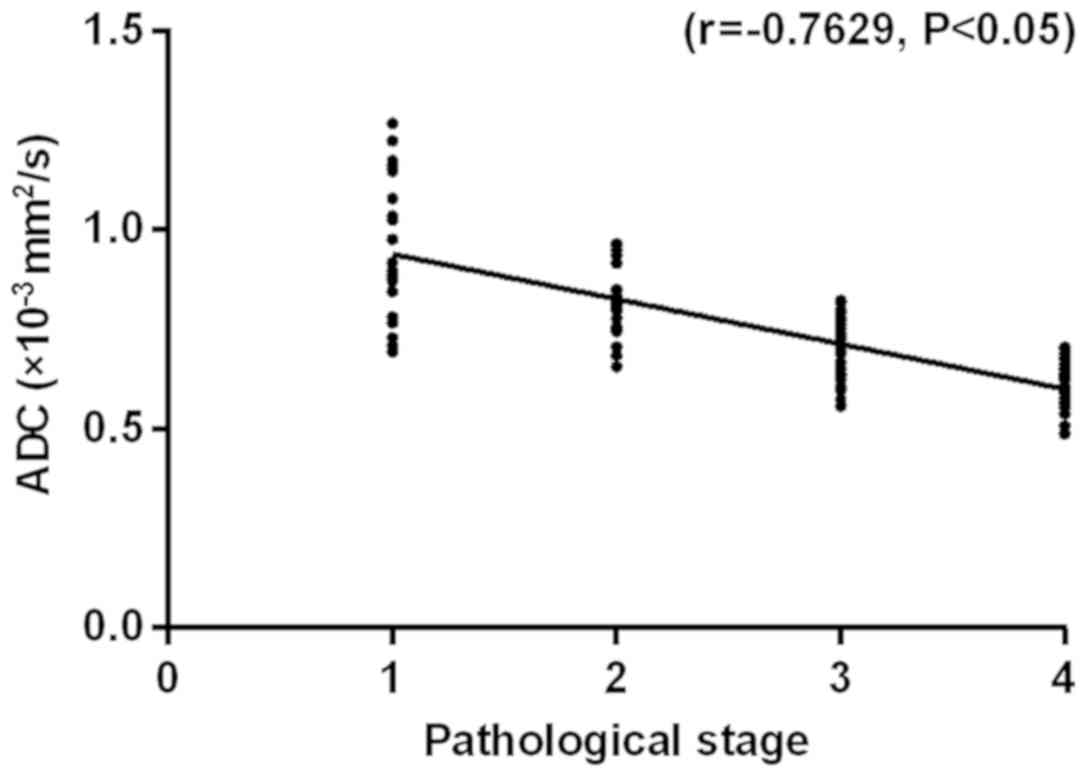
negatively correlated with the ADC value (r=−0.7629, P<0.05),
indicating that the ADC value correlates well with the tumor stage,
and the higher the stage is, the lower the ADC value is (Fig. 1).
 | Table III.Comparison of ADC values between
different stages of prostate cancer. |
Table III.
Comparison of ADC values between
different stages of prostate cancer.
| Stage | Number of
cases | ADC value
(×10−3 mm2/sec) |
|---|
| A | 22 | 0.92±0.18 |
| B | 23 |
0.83±0.11a |
| C | 38 |
0.71±0.08a,b |
| D | 29 |
0.61±0.05a–c |
| F-value |
| 40.56 |
| P-value |
| <0.0001 |
Diagnostic results of prostate cancer
by MSCT, MR and pathology
Altogether 112 patients with prostate cancer were
detected by puncture pathological biopsy. In total 113 positives
were detected by MRI, of which 103 positives were true positives
and 10 positives were false positives. Altogether 119 positives
were detected by MSCT, of which 89 positives were true positives
and 30 positives were false positives (Table IV). As can be seen from Table V, the diagnostic sensitivity of MRI
was significantly higher than that of MSCT (92.0 vs. 79.5%,
P<0.05), and the diagnostic specificity of MRI was significantly
higher than that of MSCT (90.0 vs. 70.0%, P<0.05), with
statistically significant differences.
 | Table IV.Diagnostic results of prostate cancer
by MSCT, MR and pathology (patients). |
Table IV.
Diagnostic results of prostate cancer
by MSCT, MR and pathology (patients).
| MSCT/MRI
results | Pathologic findings
(+) | Pathologic findings
(−) | Total |
|---|
|
MRI+ | 103 | 10 | 113 |
|
MRI− | 9 | 90 | 99 |
| Total | 112 | 100 | 212 |
|
MSCT+ | 89 | 30 | 119 |
|
MSCT− | 23 | 70 | 93 |
| Total | 112 | 100 | 212 |
 | Table V.Comparison of sensitivity and
specificity between the two groups/%. |
Table V.
Comparison of sensitivity and
specificity between the two groups/%.
|
| Sensitivity | Specificity |
|---|
| MRI | 92.0 | 90.0 |
| MSCT | 79.5 | 70.0 |
| χ2 | 6.66 | 12.50 |
| P-value | 0.01 | <0.001 |
Comparison of the diagnostic accuracy
of stage A and B of prostate cancer in patients
The operation was performed on 112 patients with
prostate cancer. A total of 22 patients with stage A of prostate
cancer and 23 patients with stage B of prostate cancer were
diagnosed by pathological biopsy. A total of 26 patients with
stages A and B of prostate cancer were diagnosed by MSCT, with a
misdiagnosis rate of 17.8% and a missed diagnosis rate of 24.4%. A
total of 39 patients with stages A and B of prostate cancer were
diagnosed by MRI, with a misdiagnosis rate of 4.4% and a missed
diagnosis rate of 8.9%. There was a significant difference in the
diagnostic coincidence rate between MSCT and MRI (P<0.05). The
misdiagnosis rate and missed diagnosis rate of MRI were
significantly lower than those of MSCT (P<0.05) (Table VI).
 | Table VI.Comparison of the diagnostic accuracy
of stage A and B of prostate cancer in patients [n(%)]. |
Table VI.
Comparison of the diagnostic accuracy
of stage A and B of prostate cancer in patients [n(%)].
| Diagnostic
methods | Number of
pathological findings | Diagnostic
coincidence rate | Missed diagnosis
rate | Misdiagnosis
rate |
|---|
| MSCT | 45 | 26 (57.8) | 11 (24.4) | 8 (17.8) |
| MRI | 45 | 39 (86.7) | 4 (8.9) | 2 (4.4) |
| χ2 |
| 9.36 | 3.92 | 4.05 |
| P-value |
| 0.002 | 0.048 | 0.044 |
Comparison of the diagnostic accuracy
of stage C and D of prostate cancer in patients
The operation was performed on 112 patients with
prostate cancer. A total of 38 patients stage C of prostate cancer
and 29 patients with stage D of prostate cancer were diagnosed by
pathological biopsy. A total of 63 patients with stage C and D of
prostate cancer were diagnosed by MSCT, with a misdiagnosis rate of
6.0%. There was no missed diagnosis in MSCT and MRI. A total of 64
patients with stage C and D of prostate cancer were diagnosed by
MRI, with a misdiagnosis rate of 4.5%. There was no statistically
significant difference in the diagnostic coincidence rate and
misdiagnosis rate between the two groups (P>0.05) (Table VII).
 | Table VII.Comparison of the diagnostic accuracy
of stage C and D of prostate cancer in patients [n(%)]. |
Table VII.
Comparison of the diagnostic accuracy
of stage C and D of prostate cancer in patients [n(%)].
| Diagnostic
methods | Number of
pathological findings | Diagnostic
coincidence rate | Misdiagnosis
rate |
|---|
| MSCT | 67 | 63 (94.0) | 4 (6.0) |
| MRI | 67 | 64 (95.5) | 3 (4.5) |
| χ2 |
| 0.15 | 0.15 |
| P-value |
| 0.698 | 0.698 |
Discussion
Prostate cancer is a male malignant tumor and its
incidence is the highest among all male malignant tumors (11). It has been reported that its
incidence in male tumors is 9.7% (12,13). The
cause of prostate cancer currently remains unclear (14). Although China has a low incidence of
prostate cancer, the incidence has shown a certain upward trend in
recent years (3). Early detection,
diagnosis and treatment can significantly improve the prognosis of
prostate cancer (15).
At present, PSA is a commonly used method for
detecting prostate cancer, but some studies have found that it can
only be used as a preliminary screening method, and the detection
results often cause unnecessary iatrogenic injuries (16). The key to the treatment and prognosis
of prostate cancer is early diagnosis and stage (17). Imaging plays an important role. The
spiral CT has improved the sharpness of the image, but there are
sometimes artifacts during the CT examination, which interferes
with the diagnosis (18). As a
commonly used imaging method, MRI images in multiple directions. It
has a high diagnostic value for finding the primary lesion of
prostate cancer and determining the lesion size, local involvement
(whether the tumor had broken through the capsule and whether it
involved the seminal vesicle) range, and pelvic lymph node
metastasis. It also shows lesions of bone metastasis (19). Being a new MRI functional imaging
technology, DWI was developed in the early and middle 1990s. It is
the only non-invasive method that reflects the phenomenon of the
diffusion of the living tissue, and characterized by its
sensitivity to molecular motion (20).
There is usually no obvious symptom in the early
stage of prostate cancer. Once found, prostate cancer is in the
advanced stage, so its clinical treatment effect is unsatisfactory
(6). Prostate cancer is specifically
manifested on MRI, CT and other examinations (21). Therefore, the comparison of the
differences between MSCT and MRI in the diagnosis of different
stages of prostate cancer provides a certain reference value for
clinical research. In this investigation, the basic information of
patients with prostate cancer was compared with that of healthy
controls in the normal control group, with no significant
difference. The ADC value reflects the degree of diffusion of water
molecules in the tissue, and the ADC value increases as the tissue
signal with fast diffusion decays (22). This study found a negative
correlation between the pathological stage and the ADC value. The
higher the stage was, the lower the ADC value was. In the study by
Anwar et al (23), the ADC
value of prostate cancer was 0.57±0.08–0.94±0.25×103
mm2/sec, poorly differentiated prostate cancer had a low
ADC value, and well differentiated prostate cancer had a high ADC
value. These findings indicate that the more severe the tumor is,
the lower the ADC value is. The data of our study are similar to
the results of that study and furthermore found that the number of
true positives detected by MRI was significantly higher than that
detected by MSCT, and the sensitivity and specificity were
significantly higher in the MRI group than those in the MSCT group,
with statistically significant differences (P<0.05). There is
literature showing that the resolution of dynamic enhanced MRI in
scanning soft tissue is higher than that of CT (8). In the diagnosis of stage A and B
prostate cancer, the diagnostic coincidence rate was 86.7% in the
MRI group, and 57.8% in the MSCT group, with a significant
difference between the two groups (P<0.05). The misdiagnosis
rate and missed diagnosis rate was significantly lower in the MRI
group than those in the MSCT group (P<0.05). This may be because
there is an increase in prostate volume in stage A and B of
prostate cancer, but no significant change in density. Besides, the
blurring of the edge affects CT diagnosis (23). As a result, the accuracy is low.
Therefore, MRI has an advantage in the diagnosis of early prostate
cancer, with a low error rate. In the diagnosis of stage C and D of
prostate cancer, there were no statistically significant
differences in the diagnostic coincidence rate and misdiagnosis
rate between the two groups (P>0.05). Both MRI and MSCT can
accurately detect stage C and D of prostate cancer, considering
that it is clearly related to the fact that the cancer tissue has
penetrated the capsule, and the morphological changes of the
prostate. MRI judges whether the capsule is attacked by cancer
cells (24). Therefore, MRI is
important for the diagnosis of different clinical stages, and shows
clearly bone metastasis and the lesion invasion of pelvic lymph
nodes (25), which has a great
application value.
In the study, the sensitivity, specificity and
accuracy of MSCT and MRI were compared. However, there is no
unified diagnosis of patients with prostate cancer in clinical
practice. Therefore, the research in this direction should be
increased in the future, and the prognosis of patients should be
discussed in depth, to improve the diagnosis rate and reduce the
deterioration of the disease.
The accuracy of MRI is higher than that of MSCT in
the diagnosis of patients with stage A and B of prostate cancer,
but that of MSCT and MRI is similar in the diagnosis of patients
with stage C and D of prostate cancer. The sensitivity and
specificity of MRI diagnosis are higher than those of MSCT, and the
ADC value in MRI has great clinical significance for judging the
risk of a tumor. Therefore, MRI is more valuable than MSCT in the
diagnosis of patients with different pathological stages of
prostate cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS and JL conceived and designed the study. CH
collected the patients' data. ZZ and YS analyzed and interpreted
the patient data regarding the different pathological stages of
prostate cancer. YS, JL, ZZ and YS were the major contributors in
writing the manuscript. CH reviewed the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Affiliated Yantai Yuhuangding Hospital of Qingdao University
(Yantai, China). Patients who participated in this research had
complete clinical data. Signed informed consents were obtained from
the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Osimani M, Bellini D, Di Cristofano C,
Palleschi G, Petrozza V, Carbone A and Laghi A: Perfusion MDCT of
prostate cancer: Correlation of perfusion CT parameters and
immunohistochemical markers of angiogenesis. AJR Am J Roentgenol.
199:1042–1048. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Resnick MJ, Koyama T, Fan KH, Albertsen
PC, Goodman M, Hamilton AS, Hoffman RM, Potosky AL, Stanford JL,
Stroup AM, et al: Long-term functional outcomes after treatment for
localized prostate cancer. N Engl J Med. 368:436–445. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maresca KP, Hillier SM, Femia FJ, Keith D,
Barone C, Joyal JL, Zimmerman CN, Kozikowski AP, Barrett JA,
Eckelman WC, et al: A series of halogenated heterodimeric
inhibitors of prostate specific membrane antigen (PSMA) as
radiolabeled probes for targeting prostate cancer. J Med Chem.
52:347–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mottet N, Bellmunt J, Bolla M, Briers E,
Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau
S, et al: EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1:
Screening, Diagnosis, and Local Treatment with Curative Intent. Eur
Urol. 71:618–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen G, Deng H, Hu S and Jia Z: Comparison
of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the
diagnosis of bone metastases in patients with prostate cancer: A
meta-analysis. Skeletal Radiol. 43:1503–1513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stephenson SK, Chang EK and Marks LS:
Screening and detection advances in magnetic resonance image-guided
prostate biopsy. Urol Clin North Am. 41:315–326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Afshar-Oromieh A, Haberkorn U, Schlemmer
HP, Fenchel M, Eder M, Eisenhut M, Hadaschik BA, Kopp-Schneider A
and Röthke M: Comparison of PET/CT and PET/MRI hybrid systems using
a 68Ga-labelled PSMA ligand for the diagnosis of recurrent prostate
cancer: Initial experience. Eur J Nucl Med Mol Imaging. 41:887–897.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan R, Yao SZ, Huang ZQ, Li J, Li X, Tan
HH and Liu QW: Combination of FDG PET/CT and contrast-enhanced MSCT
in detecting lymph node metastasis of esophageal cancer. Asian Pac
J Cancer Prev. 15:7719–7724. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tobisu K: Clinical and pathological
staging of prostate cancer. Nihon Rinsho. 63:225–230. 2005.(In
Japanese). PubMed/NCBI
|
|
11
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Gao X, Deeb D, Zhang Y, Shaw J,
Valeriote FA and Gautam SC: Mycotoxin verrucarin A inhibits
proliferation and induces apoptosis in prostate cancer cells by
inhibiting prosurvival Akt/NF-κB/mTOR signaling. J Exp Ther Oncol.
11:251–260. 2016.PubMed/NCBI
|
|
13
|
Shen YC, Kang CH and Chiang PH: Efficacy
of switching therapy of luteinizing hormone-releasing hormone
analogue for advanced prostate cancer. Kaohsiung J Med Sci.
32:567–571. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsai YC, Chen WY, Siu MK, Tsai HY, Yin JJ,
Huang J and Liu YN: Epidermal growth factor receptor signaling
promotes metastatic prostate cancer through microRNA-96-mediated
downregulation of the tumor suppressor ETV6. Cancer Lett. 384:1–8.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Turkbey B, Mani H, Shah V, Rastinehad AR,
Bernardo M, Pohida T, Pang Y, Daar D, Benjamin C, McKinney YL, et
al: Multiparametric 3T prostate magnetic resonance imaging to
detect cancer: Histopathological correlation using prostatectomy
specimens processed in customized magnetic resonance imaging based
molds. J Urol. 186:1818–1824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bell N, Connor Gorber S, Shane A, Joffres
M, Singh H, Dickinson J, Shaw E, Dunfield L and Tonelli M; Canadian
Task Force on Preventive Health Care, : Recommendations on
screening for prostate cancer with the prostate-specific antigen
test. CMAJ. 186:1225–1234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Quan X, Lu S, Huang F, Yang J,
Chan Q and Lin T: The clinical value of dynamic contrast-enhanced
magnetic resonance imaging at 3.0T to detect prostate cancer. J Int
Med Res. 42:1077–1084. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Frauscher F, Halpern EJ and Klauser A: Use
of MRI to detect lymph-node metastases in prostate cancer. N Engl J
Med. 349:1185–1186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eiber M, Holzapfel K, Ganter C, Epple K,
Metz S, Geinitz H, Kübler H, Gaa J, Rummeny EJ and Beer AJ:
Whole-body MRI including diffusion-weighted imaging (DWI) for
patients with recurring prostate cancer: Technical feasibility and
assessment of lesion conspicuity in DWI. J Magn Reson Imaging.
33:1160–1170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Volkin D, Turkbey B, Hoang AN,
Rais-Bahrami S, Yerram N, Walton-Diaz A, Nix JW, Wood BJ, Choyke PL
and Pinto PA: Multiparametric magnetic resonance imaging (MRI) and
subsequent MRI/ultrasonography fusion-guided biopsy increase the
detection of anteriorly located prostate cancers. BJU Int.
114:E43–E49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tanimoto A, Nakashima J, Kohno H, Shinmoto
H and Kuribayashi S: Prostate cancer screening: The clinical value
of diffusion-weighted imaging and dynamic MR imaging in combination
with T2-weighted imaging. J Magn Reson Imaging. 25:146–152. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Anwar SS, Anwar Khan Z, Shoaib Hamid R,
Haroon F, Sayani R, Beg M and Khattak YJ: Assessment of apparent
diffusion coefficient values as predictor of aggressiveness in
peripheral zone prostate cancer: Comparison with Gleason score.
ISRN Radiol. 2014:2634172014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eschmann SM, Pfannenberg AC, Rieger A,
Aschoff P, Müller M, Paulsen F, Anastasiadis A, Claussen CD, Bares
R and Schlemmer HP: Comparison of 11C-choline-PET/CT and whole
body-MRI for staging of prostate cancer. Nuklearmedizin.
46:161–168; quiz N47-N48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Panebianco V, Sciarra A, Lisi D, Galati F,
Buonocore V, Catalano C, Gentile V, Laghi A and Passariello R:
Prostate cancer: 1HMRS-DCEMR at 3T versus [(18)F]choline PET/CT in
the detection of local prostate cancer recurrence in men with
biochemical progression after radical retropubic prostatectomy
(RRP). Eur J Radiol. 81:700–708. 2012. View Article : Google Scholar : PubMed/NCBI
|