Introduction
Chronic lymphocytic leukemia (CLL) is an incurable
disease with extremely variable progression (1,2). CLL is
characterized by a disruption of the homeostasis between the
proliferation and apoptosis of leukemia cells and the accumulation
of neoplastic B lymphocytes that co-express cluster of
differentiation (CD)5 and CD19 antigens (3–6).
However, the mechanism underlying the development of CLL remains
unclear. The role of microRNA-155 (miR-155) in tumor immunity is
receiving increasing attention (7).
Numerous T helper (Th)1 and Th2 microRNA (miRNA)
molecules associated with inflammation, including miRNA-146,
miR-155 and miRNA-324-5p, have been revealed to be conserved in
mouse and human macrophages and are induced by lipopolysaccharide
(LPS) and interleukin (IL)-4 (8).
The signaling pathways associated with genome-wide miRNA expression
levels and cellular functions regulated by miRNAs during
alternative macrophage activation remain largely undetermined. The
expression levels of miRNAs are useful for the prediction of the
clinical manifestation of CLL (9).
Structurally, miRNA are short RNA molecules measuring 19–25
nucleotides in length, processed from hairpin loop structures
(pre-miRNA, 60–110 nucleotides in length), which regulate the
expression of protein-coding genes via complementary binding with
targeted messenger RNA (10).
Previously, several chromosomal abnormalities, including 11q-,
13q-, 17p- and trisomy 12, and other molecular aberrations,
including the degradation or downregulation of miRNA-15a and
miRNA-16-1, and the overexpression of anti-apoptotic genes, have
been identified in patients with CLL (11–14).
Furthermore, a unique miRNA signature was revealed to be
differentially expressed in patients with various immunoglobulin
heavy variable 4-38-2-like (IgVH) and ζ chain of T cell receptor
associated protein kinase 70 (ZAP-70) kinase statuses, and are
composed of the most frequently deregulated miRNA molecules in
different hematological malignancies, including miRNA-15/16, the
miRNA-29 family and miR-155 (15,16).
As signal transducer and activator of transcription
6 (STAT6) and miRNA are associated with tumor growth (17), the present study aimed to determine
the underlying pathways associated with these factors, and to
explore the association between STAT6 and miR-155 in CLL.
Materials and methods
Cell culture
The human CLL MEC-1 cell line was obtained from the
American Tissue Culture Collection (Manassas, VA, USA) and
maintained at 37°C in 5% CO2. Cells were subsequently
cultured in Iscove's modified Dulbecco's medium (HyClone: GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (HyClone; GE Healthcare Life Sciences).
Transfection of cells
MEC-1 cells (2×105 cells/well) were
seeded in 24-well plates and incubated overnight. Subsequently,
cells were transfected with the human miR-155 and negative control
(NC) miRNA (both from Shanghai GenePharma Co., Ltd., Shanghai,
China) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) for 48 h, according to
the manufacturer's protocol. The concentration of all miRNA
molecules transfected was 100 nM. The sequences of the miRNAs used
for transfection were as follows: miR-155 mimic sense,
UUAAUGCUAAUCGUGAUAGGGGU; and antisense, CCCUAUCACGAUUAGCAUUAAUU; NC
mimic sense, 5′-UUCUCCGAACGUGUCACGUTT-3′; and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. The sequences of the miR-155
inhibitors used for transfection were as follows: anti-miR-155;
5′-ACCCCUAUCACGAUUAGCAUUAA-3′; and a anti-miR-155 NC
5′-CAGUACUUUUGUGUAGUACAA-3′. The miR-1555i and respective NC were
purchased from Shanghai GenePharma Co., Ltd.
Knockdown of human STAT6 via RNA
interference
STAT6 expression was knocked down via transfection
with a small interfering (si)RNA complementary to STAT6 mRNA
(5′-AAGCAGGAAGAACUCAAGUUUTT-3′ and 5′-AAACUUGAGUUCUUCCUGCUUTT-3′),
as described previously (18).
pGC-siSTAT6 (0.8 µg) was transfected into MEC-1 cells in 24-well
plates (2×105 cells per well) using
Lipofectamine® 2000 (2 µl; Invitrogen; Thermo Fisher
Scientific, Inc.) for 4 h according to the manufacturer's protocol,
and the medium was subsequently replaced with regular growth
medium. Cells were used in subsequent experiments at 24 h following
transfection.
Co-treatment and co-culture
experiments
Recombinant human IL-4 (rIL-4) was added to the
MEC-1 cell medium to investigate the effects of extracellular IL-4
on phosphorylated (p)-STAT6 and miR-155 levels in MEC-1 cells. The
optimum concentration of rIL-4 used was 10 ng/ml. Following
co-culture of the cells with rIL-4 for 0, 2, 5, 10, 15 and 20 h,
levels of p-STAT6 and miR-155 were determined via western blot
analysis and reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) assays, respectively.
Western blot analysis
MEC-1 cells were treated with
radioimmunoprecipitation assay lysis buffer containing 1%
phenylmethylsulfonyl fluoride (Shanghai Shenergy Biocolor
BioScience Technology Company, Shanghai, China) to extract total
protein. For cytoplasmic and nuclear extracts, cells were washed
with PBS and were lysed in NE-PER extraction reagent (Pierce;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The protein concentrations of the samples were determined
using a bicinchoninic acid assay (Shanghai Shenergy Biocolor
BioScience & Technology Company). A total of 30 µg protein/lane
was separated by 10% SDS-PAGE and transferred onto a polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Following
blocking with 5% skim milk in TBS with 0.1% Tween-20 (TBST) for at
least 1 h at room temperature, the membranes were subsequently
probed with primary antibodies at 4°C overnight. Following washing
with TBST, a goat anti-rabbit IgG secondary antibody conjugated to
horseradish peroxidase (1:3,000; cat no. TA140003; OriGene
Technologies, Beijing, China) was added to the membranes for at
least 1 h at room temperature. The bands were visualized by using
an enhanced chemiluminescent western blot kit (EMD Millipore). The
GAPDH antibody (1:1,000; cat no. sc-47724) was obtained from Santa
Cruz Biotechnology, Inc., (Dallas, TX, USA), and anti-caspase-3
(1:1,000; cat no. ab32351) and p-STAT6 antibodies (1:1,000; cat no.
ab28829) were obtained from Abcam (Cambridge, MA, USA). The
anti-caspase-3 antibody bound to pro-caspase and cleaved forms of
the protein. Western blot analysis results were analyzed using the
Image J software 1.48 (National Institutes of Health, Bethesda, MD,
USA) and SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
RT-qPCR
TRIzol® reagent (Thermo Fisher
Scientific, Inc.) was used to extract total RNA from the MEC-1
cells. The procedure of RT and qPCR analyses was performed as
described previously (19). Primers
were purchased from BioSune Biotechnology Co., Ltd., (Shanghai,
China), and the sequences are listed in Table I.
 | Table I.Sequences of primers used for the
reverse transcription quantitative polymerase chain reaction. |
Table I.
Sequences of primers used for the
reverse transcription quantitative polymerase chain reaction.
| Gene name | Sequence |
|---|
| miR-155 | Forward
5′-TTAATGCTAATCGTGA-3′ |
|
| Reverse
5′-TTTGGCACTAGCACATT-3′ |
| U6 | Forward
5′-CTCGCTTCGGCAGCACA-3′ |
|
| Reverse
5′-AACGCTTCACGAATTTGCGT-3′ |
Assessment of cell apoptosis
MEC-1 cells (5×103 cells/well) were
seeded into 96-well plates and then transfected with siSTAT6,
miR-155 and anti-miR-155 (16).
anti-miR-155 cells were subsequently incubated for 20 h in the
presence and absence of rIL-4 (10 ng/ml). Staining with an Annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide (PI)
apoptosis detection kit (cat. no. FAK011.100; Neobioscience,
Shenzhen, China) was performed to investigate the effect of rIL-4
on the levels of apoptotic and necrotic MEC-1 cells. MEC-1 cells
(1×106) were incubated with Annexin V-FITC and PI (10
µl) for 10 min in the dark at room temperature. Cells were
immediately analyzed using a flow cytometer (CytExpert version 1.1,
Beckman Coulter, Inc., Brea, CA, USA). Necrotic cells were
identified via positive staining of Annexin V-FITC and PI, whereas
apoptotic cells were identified via positive staining of Annexin
V-FITC and negative staining of PI.
Statistical analysis
All statistical analyses were performed using the
SPSS version 20.0 (IBM Corp.) for Windows. Results are presented as
mean ± standard deviation of at least 3 independent experiments.
Differences between two groups were compared using Student's
t-test. Multiple group comparisons were performed using one-way
analysis of variance with a Student-Newman-Keuls post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
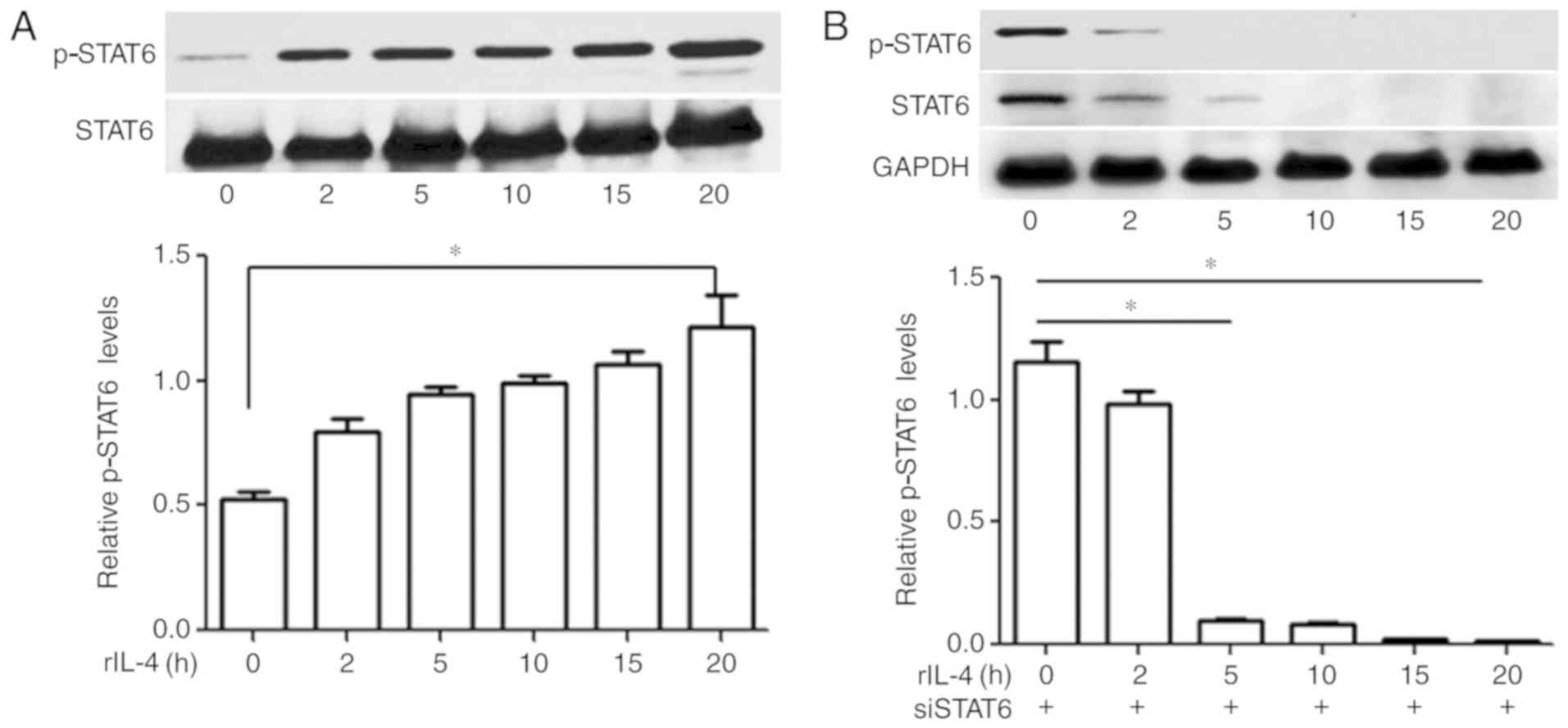
IL-4 induces activation of STAT6 at
various time intervals
Expression levels of p-STAT6 in MEC-1 cells were
investigated via western blot analysis, and the presence of p-STAT6
was determined by the visualization of a single band at ~100 kDa
position. The results revealed that MEC-1 cells that were not
treated with IL-4 did not exhibit marked levels of p-STAT6; whereas
increased levels of p-STAT6 were demonstrated in MEC-1 cells
treated with 10 ng/ml IL-4 in a time-dependent manner (Fig. 1A).
STAT6 knockdown in MEC-1 cells
suppresses IL-4-induced phosphorylation of STAT6
Levels of p-STAT6 in the MEC-1 cells transfected
with siSTAT6 were determined via western blot analysis at various
time intervals. Following incubation with rIL-4 for 20 h, levels of
p-STAT6 in the siSTAT6-transfected groups were almost undetectable.
The results demonstrated that IL-4-induced phosphorylation of STAT6
was significantly attenuated following transfection with siSTAT6
(P>0.05; Fig. 1B).
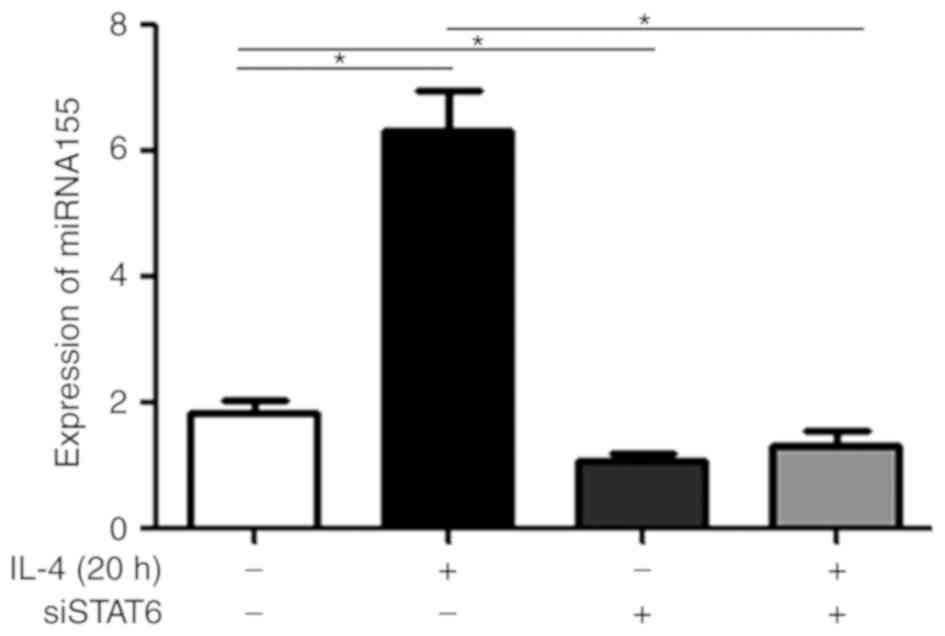
Treatment with IL-4 upregulates
miR-155 levels in MEC-1 cells, and transfection with siSTAT6
attenuates this effect
To additionally investigate the molecular mechanisms
underlying the STAT6 signaling pathway, MEC-1 cells were treated
with rIL-4 for 20 h. As presented in Fig. 2, treatment with rIL-4 increased
miR-155 expression in the MEC-1 cells (P<0.05). Furthermore,
this effect was revealed to be significantly attenuated in cells
treated with rIL-4 and also transfected with siSTAT6 (P<0.05;
Fig. 2). MEC-1 cells transfected
with siSTAT6 alone, exhibited significantly decreased expression
levels of miR-155 compared with MEC-1 cells not treated with
anything (P<0.05; Fig. 2). These
results suggested that rIL-4 promoted the expression of miR-155 via
STAT6 phosphorylation.
miR-155 inhibits MEC-1 cell
apoptosis
As STAT6 was revealed to markedly upregulate miR-155
expression in MEC-1 cells, the roles of STAT6 and miR-155 in the
apoptosis of MEC-1 cells were investigated. As demonstrated in
Fig. 3A and B, inhibition of STAT6
in MEC-1 cells promoted cell apoptosis, and this was partly
attenuated by treatment with rIL-4. Overexpression of miR-155 was
revealed to significantly decrease MEC-1 cell apoptosis
(P<0.001, Fig. 3B); whereas
overexpression of anti-miR-155 enhanced cells apoptosis despite the
presence of rIL-4 (P<0.001, Fig.
3B). It is therefore reasonable to suggest that treatment with
rIL-4 may inhibit apoptosis in CLL cells. Transfection with siSTAT6
partly attenuated the anti-apoptosis effects of rIL-4 on MEC-1
cells (P<0.05; Fig. 3B).
miR-155-treatment provided modest protection against apoptosis in
CLL cells despite the presence of rIL-4 (P<0.001, Fig. 3B). In addition, knockdown of STAT6
increased caspase3 activation, which was attenuated by rIL-4
treatment (Fig. 3C).
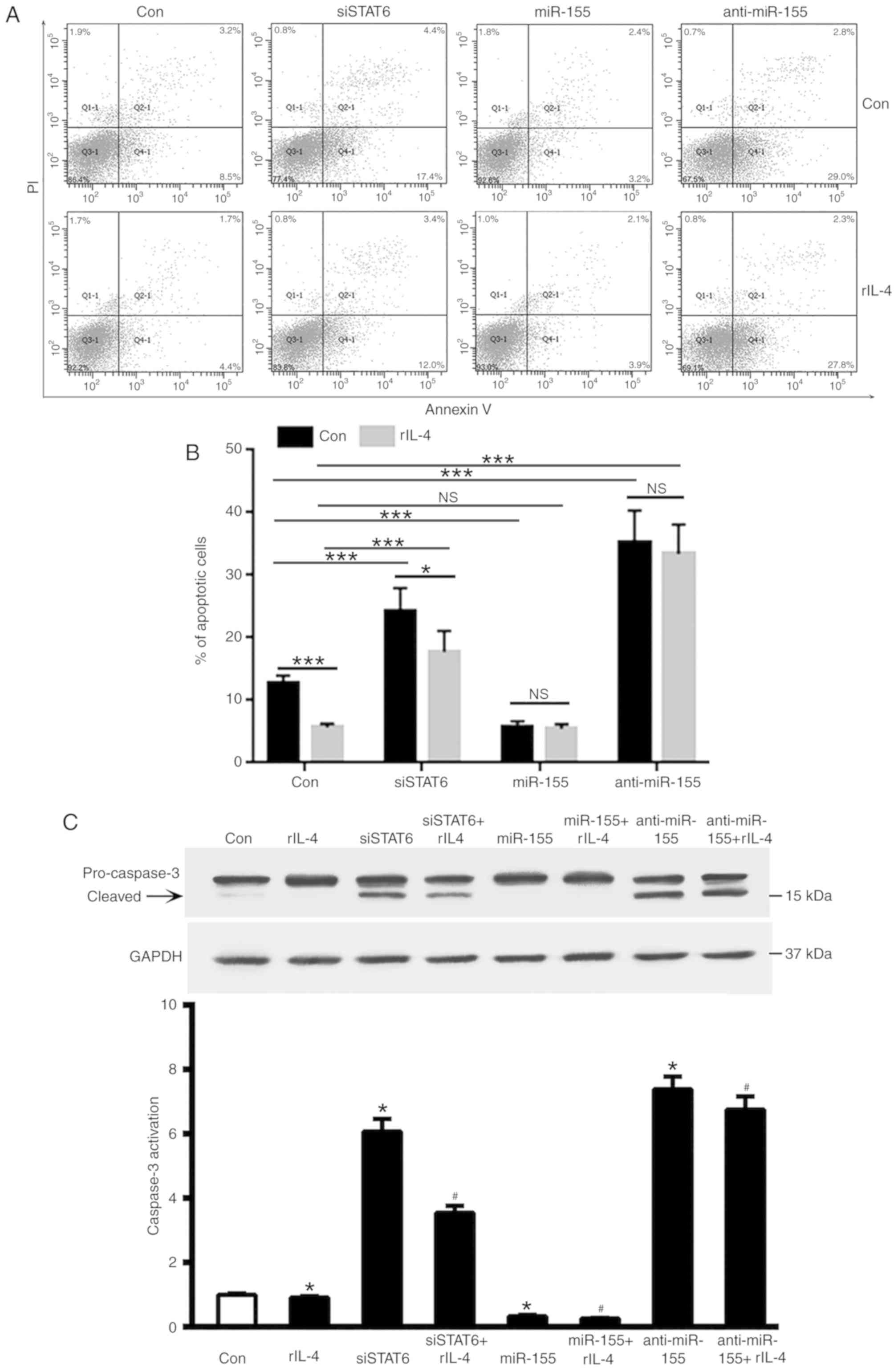 | Figure 3.Effects of miR-155 on the apoptosis of
MEC-1 cells. (A) MEC-1 cells were transfected with siSTAT6, miR-155
and anti-miR-155, and subsequently treated with rIL-4 for 20 h.
Apoptosis and necrosis levels of MEC-1 cells were determined using
flow cytometry. (B) Quantification of the flow cytometry apoptosis
data. Columns indicate the mean apoptosis rate of three repeats.
Error bars represent the standard deviation. *P<0.05 and
***P<0.001. (C) Caspase-3 activation, measured by relative
levels of cleaved protein, was determined by western blot analysis.
*P<0.05 vs. control group; #P<0.05 vs. rIL-4
treated group. Data are presented as the mean ± standard deviation
of at least 3 independent experiments, and the exact values were as
follows: Con Group=1±0.0153 (n=5); IL-4 group=0.9146±0.01809 (n=5);
siSTAT6 group=6.069±0.1752 (n=5); siSTAT6+IL-4 group=3.543±0.09789
(n=5); miR-155 group=0.3274±0.01877 (n=5); miR-155+IL-4
group=0.2609±0.008641 (n=5); anti-miR-155 group=7.39±0.1753 (n=5);
and anti-miR-155 + IL-4 group=6.753±0.1842 (n=5). miR, microRNA;
si, small interfering; STAT6, signal transducer and activator of
transcription 6; rIL-4, recombinant interleukin 4; PI, propidium
iodide; Con, control; NS, not significant. |
Discussion
Unique miRNA signatures are differentially expressed
in patients with various IgVH and ZAP-70 kinase statuses and are
composed of the most frequently deregulated miRNAs in different
hematological malignancies, including miR-15/16, the miR-29 family
and miR-155 (13,14). Increased expression levels of miR-155
in leukemia cells are associated with a more aggressive disease
phenotype in patients with CLL (20). miR-155 is an important regulator of
post-transcriptional gene expression in B cells (21). miR-155 has been previously
demonstrated to be conserved in both mouse and human macrophages
and is induced by LPS and IL-4 (22). A previous study revealed that the
phosphorylation and activation of STAT6 in CLL cells may be rapidly
induced by IL-4 in vitro (23). In the present study, the results
demonstrated that a novel signaling pathway, the p-STAT6-mediated
extracellular IL-4 upregulation of miR-155, is involved in CLL
pathology.
IL-4-driven alternative macrophage activation and
proliferation are characteristic features of anti-helminthic immune
responses, which primarily occur during inflammatory responses
(23,24). The signaling pathways associated with
genome-wide miRNA expression and cellular functions regulated by
miRNAs during alternative macrophage activation remain largely
unknown. Numerous studies have investigated the roles of
IL-4-regulated miRNAs in human and mouse alternative macrophage
activation (25–27). Furthermore, ~1,000 miRNAs are present
in the human genome; however, little is known about the
transcriptional regulation of miRNAs (28,29).
Considering that miRNA expression levels are deregulated in CLL,
the present study aimed to determine whether STAT6 affected the
transcription levels of miRNAs in CLL cells (30,31). Our
previous study indicated that following treatment with rIL-4,
levels of p-STAT6 in MEC-1 cells increased in a time-dependent
manner (17). Based on these data,
the present study aimed to determine whether miR-155 was involved
in the development of CLL. The results demonstrated that
pretreatment with rIL-4 promoted the expression of miR-155;
however, this effect was attenuated following transfection with
siSTAT6. These results suggested that rIL-4 induced the expression
of miR-155 via STAT6 phosphorylation.
The effects of miR-155 were determined to
additionally investigate the function of miR-155 in CLL
pathogenesis. The results revealed that miR-155 decreased apoptosis
levels in MEC-1 cells, which suggested that the upregulation of
miR-155 may be associated with the pathogenesis of CLL.
In conclusion, the results of the present study
demonstrated that IL-4 induced miR-155 expression and STAT6
phosphorylation. STAT6 knockdown was revealed to suppress
IL-4-induced miR-155 expression. Overexpression of miR-155 was
demonstrated to decrease the apoptotic rate of MEC-1 cells; whereas
overexpression of anti-miR-155 was demonstrated to enhance MEC-1
cell apoptosis. The results of the present study suggested that
miR-155 expression was induced by IL-4, which subsequently enhanced
p-STAT6 levels to regulate CLL cell survival. The results of the
present study have provided an improved understanding regarding the
molecular mechanism of CLL pathogenesis and identified a novel
therapeutic target for the treatment of patients with CLL.
Acknowledgements
Not applicable.
Funding
The present study was partly supported by the
National Natural Science Foundation (grant nos. 81500124 and
81600121), Natural Science Foundations of Shandong Province (grant
nos. Y2007C053, 2009ZRB14176 and ZR2016HQ46), Technology
Development Projects of Shandong Province (grant nos. 2007GG10 and
2010GSF10250), Major Research Projects of Shandong Province (grant
nos. 2016GSF201029 and 2017GSF218007), Program of Shandong Medical
Leading Talent and Taishan Scholar Foundation of Shandong
Province.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author upon reasonable
request.
Authors' contributions
NC and XW conceived and designed the study. NC, LF,
KL, XL and PL performed the experiments. NC and XL wrote the paper.
NC, PL and XW reviewed and edited the manuscript. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lu K and Wang X: Therapeutic advancement
of chronic lymphocytic leukemia. J Hematol Oncol. 5:552012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bilous N, Abramenko I, Saenko V, Chumak A,
Dyagil I, Martina Z and Kryachok I: Clinical relevance of TP53
polymorphic genetic variations in chronic lymphocytic leukemia.
Leuk Res. 58:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nasr R, Marçais A, Hermine O and
Bazarbachi A: Overview of targeted therapies for adult T-cell
leukemia/lymphoma. Methods Mol Biol. 1582:197–216. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin UH, Cheng Y, Zhou B and Safe S:
Bardoxolone methyl and a related triterpenoid downregulate cMyc
expression in leukemia cells. Mol Pharmacol. 91:438–450. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quintanilla-Martinez L, Preffer F, Rubin
D, Ferry JA and Harris NL: CD20+ T-cell lymphoma: Neoplastic
transformation of a normal T-cell subset. Am J Clin Pathol.
102:483–489. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moens L, Verbinnen B, Covens K, Wuyts G,
Johnson M, Roalfe L, Goldblatt D, Meyts I and Bossuyt X:
Anti-pneumococcal capsular polysaccharide antibody response and CD5
B lymphocyte subsets. Infect Immun. 83:2889–2896. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balatti V, Tomasello L, Rassenti LZ,
Veneziano D, Nigita G, Wang HY, Thorson JA, Kipps TJ, Pekarsky Y
and Croce CM: miR-125a and miR-34a expression predicts Richter
syndrome in chronic lymphocytic leukemia patients. Blood.
132:2179–2182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papageorgiou SG, Kontos CK, Diamantopoulos
MA, Bouchla A, Glezou E, Bazani E, Pappa V and Scorilas A:
MicroRNA-155-5p overexpression in peripheral blood mononuclear
cells of chronic lymphocytic leukemia patients is a novel,
independent molecular biomarker of poor prognosis. Dis Markers.
2017:20465452017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zitzer NC, Snyder K, Meng X, Taylor PA,
Efebera YA, Devine SM, Blazar BR, Garzon R and Ranganathan P:
MicroRNA-155 modulates acute graft-versus-host disease by impacting
T cell expansion, migration, and effector function. J Immunol.
200:4170–4179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou B, Zhu H, Luo H, Gao S, Dai X, Li Y
and Zuo X: MicroRNA-202-3p regulates scleroderma fibrosis by
targeting matrix metalloproteinase 1. Biomed Pharmacother.
87:412–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oscier D, Else M, Matutes E, Morilla R,
Strefford JC and Catovsky D: The morphology of CLL revisited: The
clinical significance of prolymphocytes and correlations with
prognostic/molecular markers in the LRF CLL4 trial. Br J Haematol.
174:767–775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kriston C, Bödör C, Szenthe K, Bánáti F,
Bánkuti B, Csernus B, Reiniger L, Csomor J, Matolcsy A and Barna G:
Low CD23 expression correlates with high CD38 expression and the
presence of trisomy 12 in CLL. Hematol Oncol. 35:58–63. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Underbayev C, Kasar S, Ruezinsky W,
Degheidy H, Schneider JS, Marti G, Bauer SR, Fraidenraich D,
Lightfoote MM, Parashar V, et al: Role of mir-15a/16-1 in early B
cell development in a mouse model of chronic lymphocytic leukemia.
Oncotarget. 7:60986–60999. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong C, Ji M and Ji C: microRNAs and their
potential target genes in leukemia pathogenesis. Cancer Biol Ther.
8:200–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smolej L, Vroblova V, Motyckova M,
Jankovicova K, Schmitzova D, Krejsek J and Maly J: Quantification
of ZAP-70 expression in chronic lymphocytic leukemia: T/B-cell
ratio of mean fluorescence intensity provides stronger prognostic
value than percentage of positive cells. Neoplasma. 58:140–145.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferrajoli A, Shanafelt TD, Ivan C, Shimizu
M, Rabe KG, Nouraee N, Ikuo M, Ghosh AK, Lerner S, Rassenti LZ, et
al: Prognostic value of miR-155 in individuals with monoclonal
B-cell lymphocytosis and patients with B chronic lymphocytic
leukemia. Blood. 122:1891–1899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan C, Chen Y, Kong W, Fu L, Liu Y, Yao Q
and Yuan Y: PVT1-derived miR-1207-5p promotes breast cancer cell
growth by targeting STAT6. Cancer Sci. 108:868–876. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen N, Lu K, Li P, Lv X and Wang X:
Overexpression of IL-9 induced by STAT6 activation promotes the
pathogenesis of chronic lymphocytic leukemia. Int J Clin Exp
Pathol. 7:2319–2323. 2014.PubMed/NCBI
|
|
19
|
Chen N, Lv X, Li P, Lu K and Wang X: Role
of high expression of IL-9 in prognosis of CLL. Int J Clin Exp
Pathol. 7:716–721. 2014.PubMed/NCBI
|
|
20
|
Kneitz C, Goller M, Seggewiss R, Yaman A,
Serfling E and Tony HP: STAT6 and the regulation of CD23 expression
in B-chronic lymphocytic leukemia. Leuk Res. 24:331–337. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeon JI, Ko SH, Kim YJ, Choi SM, Kang KK,
Kim H, Yoon HJ and Kim JM: The flavone eupatilin inhibits eotaxin
expression in an NF-κB-dependent and STAT6-independent manner.
Scand J Immunol. 81:166–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eigsti RL, Sudan B, Wilson ME and Graff
JW: Regulation of activation-associated microRNA accumulation rates
during monocyte-to-macrophage differentiation. J Biol Chem.
289:28433–28447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rückerl D, Jenkins SJ, Laqtom NN,
Gallagher IJ, Sutherland TE, Duncan S, Buck AH and Allen JE:
Induction of IL-4Rα-dependent microRNAs identifies PI3K/Akt
signaling as essential for IL-4-driven murine macrophage
proliferation in vivo. Blood. 120:2307–2316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gautherot I, Burdin N, Seguin D, Aujame L
and Sodoyer R: Cloning of interleukin-4 delta2 splice variant
(IL-4delta2) in chimpanzee and cynomolgus macaque: phylogenetic
analysis of delta2 splice variant appearance, and implications for
the study of IL-4-driven immune processes. Immunogenetics.
54:635–644. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei Y and Schober A: MicroRNA regulation
of macrophages in human pathologies. Cell Mol Life Sci.
73:3473–3495. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Czimmerer Z, Varga T, Kiss M, Vázquez CO,
Doan-Xuan QM, Rückerl D, Tattikota SG, Yan X, Nagy ZS, Daniel B, et
al: The IL-4/STAT6 signaling axis establishes a conserved microRNA
signature in human and mouse macrophages regulating cell survival
via miR-342-3p. Genome Med. 8:632016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ying W, Tseng A, Chang RC, Morin A, Brehm
T, Triff K, Nair V, Zhuang G, Song H, Kanameni S, et al:
MicroRNA-223 is a crucial mediator of PPARγ-regulated alternative
macrophage activation. J Clin Invest. 125:4149–4159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Friedländer MR, Lizano E, Houben AJ,
Bezdan D, Báñez-Coronel M, Kudla G, Mateu-Huertas E, Kagerbauer B,
González J, Chen KC, et al: Evidence for the biogenesis of more
than 1,000 novel human microRNAs. Genome Biol. 15:R572014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Srivastava AK, Sablok G, Hackenberg M,
Deshpande U and Suprasanna P: Thiourea priming enhances salt
tolerance through co-ordinated regulation of microRNAs and hormones
in Brassica juncea. Sci Rep. 7:454902017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pallasch CP, Patz M, Park YJ, Hagist S,
Eggle D, Claus R, Debey-Pascher S, Schulz A, Frenzel LP, Claasen J,
et al: miRNA deregulation by epigenetic silencing disrupts
suppression of the oncogene PLAG1 in chronic lymphocytic leukemia.
Blood. 114:3255–3264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abroun S, Saki N, Ahmadvand M, Asghari F,
Salari F and Rahim F: STATs: An old story, yet mesmerizing. Cell J.
17:395–411. 2015.PubMed/NCBI
|