Introduction
Esophageal cancer (EC), a common malignancy of the
digestive tract, is one of the leading causes for cancer-associated
mortality worldwide (1). Surgery is
not recommended for EC as the majority of patients (60%) present
with advanced EC upon diagnosis (2).
Therefore, radiotherapy-based combined modality is considered a
major treatment option for EC. Unfortunately, a large percentage of
patients with EC (60–70%) exhibit a poor response following
non-surgical treatment, including local recurrence and/or distal
metastasis, with a 5-year survival rate of only 20–40% (3,4).
Thalidomide, initially used as a sedative, has been
reported to be effective for the management of vomiting and nausea
in pregnancy; however, its application is hindered as it can induce
congenital disabilities in neonates (5). In 2006, thalidomide was approved by
Food and Drug Administration for treating newly diagnosed multiple
myeloma as it demonstrated antitumor activity in refractory
multiple myeloma possibly by inhibiting angiogenesis (6). In addition, thalidomide has been
considered as a treatment option for solid tumors due to its
anti-angiogenesis effects (7). It
has been demonstrated that EC cells exhibit increased sensitivity
to a combination of radiotherapy and thalidomide (8–10). Our
previous study revealed that a variation of serum vascular
endothelial growth factor (VEGF) could predict the prognosis of
patients with EC during chemo-radiotherapy (CRT). Additionally, the
overall survival (OS) and progression-free survival (PFS) time in
patients with elevated serum VEGF levels were significantly lower
compared with patients with a decreased serum VEGF level (11). In a retrospective analysis, Yu et
al (12) reported radiotherapy
combined with thalidomide triggered a down-regulation of VEGF
expression, but it caused no OS extension. The present study
evaluated patients with EC with a poor prognosis, who exhibited
elevated serum VEGF level during radiotherapy, with the aim of
comparing the treatment efficiency, prognosis and side-effects
between CRT and a combination of CRT and thalidomide.
Materials and methods
Clinical data
A total of 215 patients (male: 152; female: 63, age:
40–87 years, median age: 66.3±9.9 years) diagnosed with esophageal
squamous carcinoma, who were admitted to the Department of
Radiotherapy, the Affiliated Changzhou No. 2 People's Hospital,
Nanjing Medical University (Changzhou, China) between February 2011
and December 2015, were enrolled in the present study. The
inclusion criteria were as follows: i) An Eastern Cooperative
Oncology Group performance status score of 0 or 1; ii) received no
treatment previously; iii) age ≤75 years; iv) denied surgical
treatment or with contraindications of surgery; v) lesion size ≤10
cm; vi) absence of esophageal perforation; vii) no severe hepatic,
renal and cardiopulmonary disease; viii) no severe cachexia; ix)
Karnofsky performance score (KPS) (13) ≥80; and iix) a survival time of >3
months from the time of diagnosis. Pregnant patients, patients who
were breast-feeding and patients with any other malignancy were
excluded from the study. VEGF elevation was defined as an elevation
of serum VEGF of ≥16.2 ng/l during radiation compared with that
before radiation. The value of 16.2 ng/l was defined according to
the two-fold of the standard (8.1 ng/l). The number of patients
with increased, stable or decreased VEGF during radiotherapy was
61, 88 and 66, respectively. Patients with elevated VEGF during
radiotherapy (n=61) were randomly divided into: i) a test group
(n=31), who received a combination of CRT and thalidomide; and ii)
a control group (n=30), who received CRT only. The criteria for the
EC staging were based on the guidelines proposed by the 7th edition
of the American Joint Committee on Cancer Tumor-Node-Metastasis
(TNM) staging system (14). Written
informed consent was obtained from each patient. The study
protocols were approved by the Ethics Committee of the Second
People's Hospital of Changzhou Affiliated to Nanjing Medical
University (Nanjing, China). This clinical trial was registered in
the United States Trial Registry (ID: NCT01551641).
Treatment regimen
CT simulation was performed in all patients in the
supine position. CT images were obtained at a 5-mm thickness
throughout the entire neck and thorax. Treatment plans were
generated with a three-dimensional planning system (ADAC-Pinnacle
3; Philips Medical Systems, Inc.; version 9.3). Irradiation was
delivered with 6-MV photon energy through three-dimensional
conformal radiotherapy or intensity modulated irradiation therapy.
Gross tumor volume (GTV) was defined as any visible primary tumor
on the CT or esophageal barium study, as well as metastatic lymph
nodes. Metastatic nodes were identified based on the following
radiographic criteria: Nodes ≥1 cm in the shortest axis in the
intra-abdominal and/or intrathoracic regions and nodes beside the
recurrent nerve with the shortest axis of ≥0.5 cm. Clinical target
volume (CTV) was defined as the GTV plus 3 cm of proximal and
distal normal esophagus without lateral margins. Planning target
volume was termed by adding a 1-cm margin around the CTV. For
radiotherapy, patients were treated 5 days per week (1.8–2.0 Gy)
with a total dose of 50–70 Gy. The maximal dose for the spinal cord
was <45 Gy and the mean dose for the lung was ≤13 Gy. The volume
of the whole lung receiving ≥20 Gy and volume of the whole heart
receiving ≥50 Gy was <28 and 45%, respectively. The regimen of
chemotherapy consisted of paclitaxel (Lvye Pharma, www.luyesike.com, Nanjing, China; d1, 135
mg/m2) and cisplatin (Jiangsu Hanson Pharmaceutical Co.,
Ltd., Lianyungang, China; d2-5, 20 mg/m2). Two cycles of
chemotherapy were performed concurrently with the radiotherapy.
Following radiotherapy, two cycles (21–28 days for each cycle) of
maintenance chemotherapy were performed. In the test group, the
patients received thalidomide with an initial dose of 200 mg/day
and then the dose was increased to 300 mg/day in the absence of
moderate or severe side-effects within 1 week.
Determination of VEGF
Peripheral blood (2 ml) was collected before, during
weeks 2–4 of radiotherapy and 1 week after radiotherapy. Blood
samples were centrifuged at 999 × g at 4°C for 10 min and the serum
was stored at −70°C for further analyses. The level of VEGF was
determined using a commercial ELISA kit for VEGF (cat. no.,
69-50044; http://www.mskbio.com/search.aspx?word=VEGF;
International Capital Corporation Limited, Beijing, China),
according to the manufacturer's protocol.
Evaluation of treatment efficiency and
side-effects
The efficiency was evaluated by a barium
esophagogram and thoracic computed tomography (CT) scan 1 month
after radiotherapy. The primary lesions in the esophagus were
evaluated by barium esophagogram. Treatment efficiency of the lymph
node metastasizing lesions was evaluated based on the response
evaluation criteria in solid tumors (2.1) guidelines (15). The side-effects of radiotherapy were
evaluated using the guidelines proposed by the Radiation Therapy
Oncology Group (16). The
side-effects of chemotherapy and thalidomide were evaluated using
the toxicity criteria by the World Health Organization (12). Hemoglobin: Grade 0 ≥11.0 g/l; grade
1, 9.5–11.0 g/l; grade 2, 7.5–9.5 g/l; grade 3, 5.0–7.5 g/l; grade
3, <5.0 g/l. White blood cell: Grade 0 ≥4.0×109/l;
grade 1, 3.0–4.0×109/l; grade 2,
2.0–3.0×109/l; grade 3, 1.0–2.0×109/l; grade
4, <1.0×109/l. Platelet: Grade 0
≥100.0×109/l; grade 1, 75.0–100.0×109/l;
grade 2, 50.0–75.0×109/l; grade 3,
25.0–75.0×109/l; grade 4, <25.0×109/l.
Follow-up
Follow-up was performed every 3 months among those
with a survival of <2 years and every 6 months for those with a
survival >2 years, according to the National Comprehensive
Cancer Network guidelines (17).
Data collection in the follow-up included case history, physical
examinations, laboratory tests, electrocardiogram, abdominal
ultrasound examination, and a barium esophagogram and thoracic CT
scan. The OS time, PFS time and local control (LC; no recurrence in
the primary lesion and local lymph node metastasis after
radiotherapy) were used to evaluate the prognosis.
Statistical analysis
The primary endpoint was PFS and the secondary
endpoints were the OS and LC. SPSS 19.0 software (IBM Corp.,
Armonk, NY, USA) was used for the data analysis. Measurement data
are presented as the mean ± standard deviation. χ2 test
or Fisher's exact test were used for the comparison of grouped
data. Mann-Whitney rank sum test was performed for the comparison
of ranked data in two groups. Univariate analysis was performed
using the Kaplan-Meier method and a log rank test. Multivariate
analysis was performed using the Cox proportional hazards model.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
A total of 3 patients in the present study were
recommended to receive other treatment options due to the presence
of distal metastasis during radiotherapy. One case terminated the
radiotherapy due to radiation pneumonitis, one case was transferred
to another department due to presence of gallstones and one case
denied subsequent therapy due to a high fever. In total, 2 patients
denied the four-cycle chemotherapy. In addition, 2 patients
completed the study but were lost during follow-up. Therefore, a
total of 51 cases were included in the analysis (test group, 26;
control group, 25). The characteristics or these patients are
presented in Table I. No statistical
differences were identified in the characteristics at the baseline
level between the test group and control group (P>0.05).
 | Table I.Comparison of the characteristics of
patients with esophageal cancer in the control and test groups. |
Table I.
Comparison of the characteristics of
patients with esophageal cancer in the control and test groups.
| Characteristic | n | Control group
(n=25) | Test group
(n=26) | P-value |
|---|
| Sex |
|
|
| 0.499a |
| Male | 40 | 21 | 19 |
|
|
Female | 11 | 4 | 7 |
|
| Age, years |
|
|
| 0.843b |
|
41–59 | 17 | 8 | 9 |
|
|
60–75 | 34 | 17 | 17 |
|
| Site of tumor |
|
|
| 0.318a |
| Chest,
upper | 10 | 7 | 3 |
|
| Chest,
middle segment | 21 | 10 | 11 |
|
| Chest,
lower | 20 | 8 | 12 |
|
| Type of cancer |
|
|
| 0.977a |
|
Medullary type | 49 | 24 | 25 |
|
| Ulcer
type | 2 | 1 | 1 |
|
| T stage |
|
|
| 0.090a |
| T1 | 2 | 2 | 0 |
|
| T2 | 7 | 2 | 5 |
|
| T3 | 36 | 20 | 16 |
|
| T4 | 6 | 1 | 5 |
|
| N stage |
|
|
| 0.169a |
| N0 | 11 | 6 | 5 |
|
| N1 | 36 | 19 | 17 |
|
| N2 | 4 | 0 | 4 |
|
| TNM stage |
|
|
| 0.343a |
| I | 6 | 3 | 3 |
|
| II | 39 | 21 | 18 |
|
|
III | 6 | 1 | 5 |
|
Side-effects evaluation
The patients demonstrated no significant cardiac
side-effects following radiotherapy. No statistical differences
were revealed in the radiotherapy-associated esophageal injury and
lung injury between the two groups (P>0.05; Table II). The present study also evaluated
chemotherapy-associated adverse events, including blood count,
vomiting, liver function, peripheral nerve injury, constipation and
muscular pain. The data indicated no statistical differences
between the two groups for these factors (P>0.05; Table III). The major side-effects for
thalidomide included hypersomnia (n=3, 5 and 3 for mild, moderate
and severe, respectively). No cardiovascular system issues,
including deep vein thrombosis, hypotension and bradycardia, were
recorded.
 | Table II.Toxicity of radiotherapy in the lung
and esophagus. |
Table II.
Toxicity of radiotherapy in the lung
and esophagus.
|
| Esophageal injury,
% | Lung injury, % |
|---|
|
|
|
|
|---|
| Group | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 |
|---|
| Control (n=25) | 32.0 | 60.0 |
8.0 | 44.0 | 48.0 |
8.0 |
| Test (n=26) | 42.3 | 46.2 | 11.5 | 53.8 | 34.6 | 11.5 |
| Z-value |
| −0.485 |
|
| −0.481 |
|
| P-value |
| 0.628 |
|
|
0.631 |
|
 | Table III.Comparison of chemotherapy-associated
toxicities. |
Table III.
Comparison of chemotherapy-associated
toxicities.
|
|
| Toxicities, % |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | Group | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Z-value | P-value |
|---|
| Hemoglobin | Control (n=25) | 76.0 | 24.0 | 0.0 | 0.0 | 0.0 | −0.237 | 0.813 |
|
| Test (n=26) | 73.1 | 26.9 | 0.0 | 0.0 | 0.0 |
|
|
| White blood
cell | Control (n=25) | 0.0 | 56.0 | 36.0 | 4.0 | 4.0 | −0.368 | 0.713 |
|
| Test (n=26) | 0.0 | 61.5 | 30.8 | 3.8 | 3.8 |
|
|
| Platelet | Control (n=25) | 64.0 | 28.0 | 8.0 | 0.0 | 0.0 | −0.261 | 0.794 |
|
| Test (n=26) | 69.2 | 19.2 | 11.5 | 0.0 | 0.0 |
|
|
| Nausea and
vomiting | Control (n=25) | 48.0 | 40.0 | 12.0 | 0.0 | 0.0 | −0.738 | 0.461 |
|
| Test (n=26) | 57.7 | 34.6 | 7.7 | 0.0 | 0.0 |
|
|
| Liver function | Control (n=25) | 96.0 | 4.0 | 0.0 | 0.0 | 0.0 | −0.555 | 0.579 |
|
| Test (n=26) | 92.3 | 7.7 | 0.0 | 0.0 | 0.0 |
|
|
| Peripheral nerve
disease | Control (n=25) | 56.0 | 44.0 | 0.0 | 0.0 | 0.0 | −0.153 | 0.878 |
|
| Test (n=26) | 53.8 | 46.2 | 0.0 | 0.0 | 0.0 |
|
|
| Constipation | Control (n=25) | 44.0 | 40.0 | 12.0 | 4.0 | 0.0 | −0.041 | 0.968 |
|
| Test (n=26) | 50.0 | 23.1 | 23.1 | 3.8 | 0.0 |
|
|
| Muscular pain | Control (n=25) | 72.0 | 28.0 | 0.0 | 0.0 | 0.0 | −1.466 | 0.143 |
|
| Test (n=26) | 88.5 | 11.5 | 0.0 | 0.0 | 0.0 |
|
|
Treatment response
In the test group, 16 patients demonstrated a
complete response (CR) and 8 exhibited a partial response (PR). In
the control group, 15 demonstrated a CR and 8 exhibited a PR. The
total treatment effective rate demonstrated no statistical
differences between the two groups (92.3 vs. 92.0%; P>0.05;
Table IV).
 | Table IV.Comparison of the total effectiveness
rates. |
Table IV.
Comparison of the total effectiveness
rates.
| Group | CR, % | PR, % | SD, % |
|---|
| Test (n=26) | 61.5 | 30.8 | 7.7 |
| Control (n=25) | 60.0 | 32.0 | 8.0 |
| Z-value |
| −0.109 |
|
| P-value |
| 0.913 |
|
Prognosis analysis
In all cases, the 1-year and 3-year OS rates were
70.6 and 22.5%, respectively. The 1-year and 3-year PFS rates were
56.9 and 22.0%, respectively. In addition, the 1-year and 3-year LC
rates were 81.0 and 52.6%, respectively. The comparison of these
data between the test and control group was shown in Table V. For the 9 patients with cancer
recurrence, the median time to recurrence was 7.5 months [95%
confidence interval (CI), 1.3–13.7 months], and the median OS and
PFS times were 20.6 months (95% CI, 15.1–26.2 months) and 17.0
months (95% CI, 8.8–25.2 months), respectively. The median OS time
in the test group was 36.8 months (95% CI, 14.0–59.5 months), while
that for the control group was 17.0 months (95% CI, 8.8–25.8
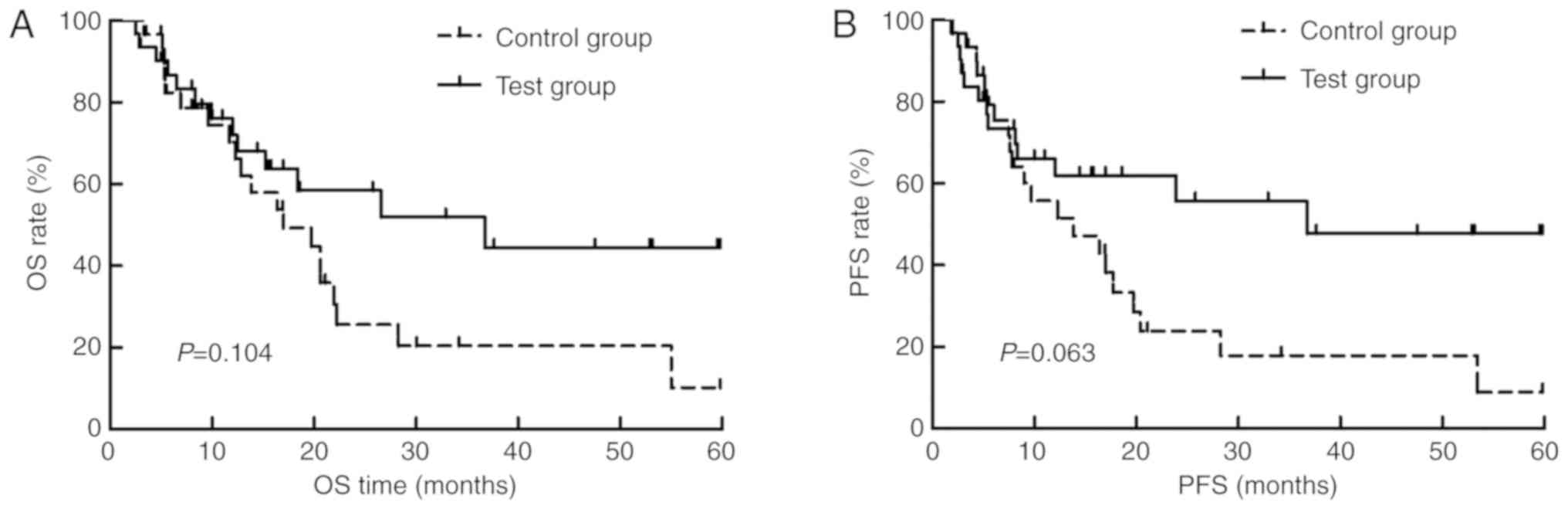
months), respectively (P=0.104; Fig.
1A). The median PFS time in the test group was 36.2 months (95%
CI, 13.2–58.8 months), while that in the control group was 13.9
months (95% CI, 3.7–24.0 months), respectively (P=0.063; Fig. 1B).
 | Table V.Comparison of the OS, PFS and LC
rates for patients with esophageal cancer. |
Table V.
Comparison of the OS, PFS and LC
rates for patients with esophageal cancer.
|
| 1-yr OS | 3-yr OS | 1-yr PFS | 3-yr PFS | 1-yr LC | 3-yr LC |
|---|
|
|
|
|
|
|
|
|
|---|
| VEGF type | Survival | Dead | Survival | Dead | Progression
free | Progression | Progression
free | Progression | No local
recurrence | Local
recurrence | No local
recurrence | Local
recurrence |
|---|
| Test group | 19 | 7 | 7 | 12 | 16 | 10 | 7 | 12 | 19 | 2 | 4 | 7 |
| Control group | 17 | 8 | 2 | 19 | 13 | 12 | 2 | 20 | 15 | 6 | 9 | 2 |
| χ2
value | 0.158 | – | 0.473 | – | – | – |
| P-value | 0.764a | 0.060b | 0.577a | 0.057b | 0.238b | 0.070b |
Prognosis analysis in locally advanced
patients
Among the 61 cases, 55 were confirmed with locally
advanced EC (test group, n=23; control group, n=22). Locally
advanced EC referred to primary lesions of ≥T3 or positive for
lymph nodes. Table VI summarizes
the OS, PFS and LC rates in the test and control groups of patients
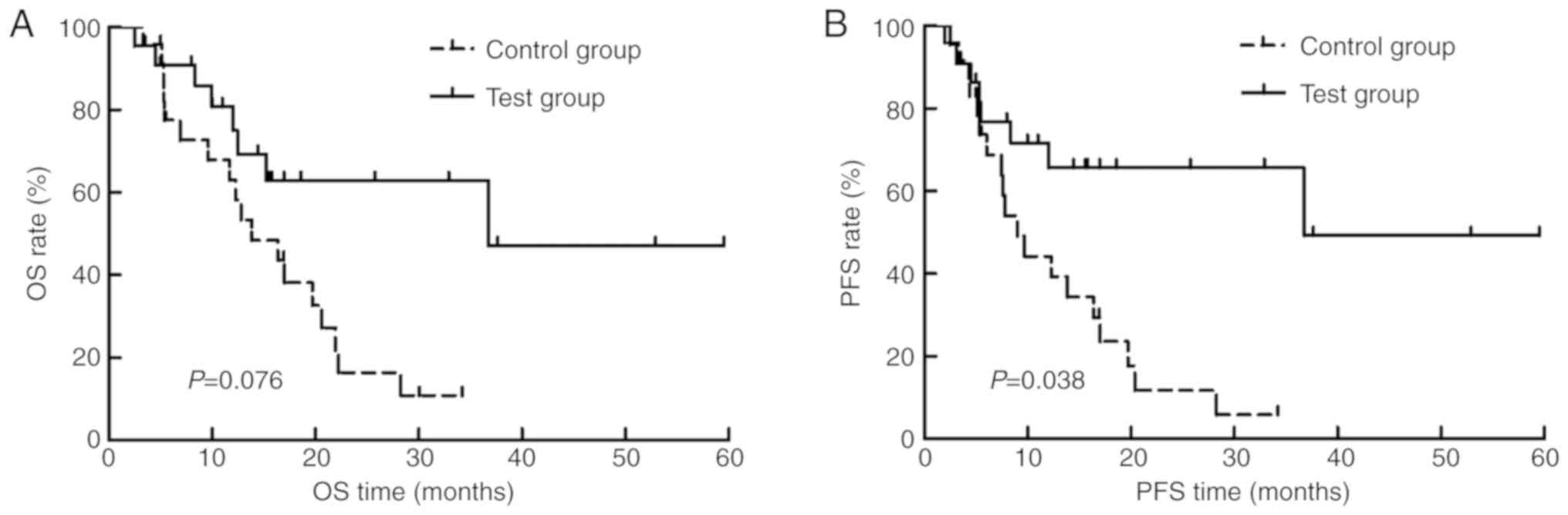
with locally advanced EC. The 3-year OS, PFS and LC rates in the
test group were higher than that of the control. The median OS
times in the test and control groups were 36.8 months (95% CI,
21.2–46.3 months) and 16.4 months (95% CI, 10.5–22.3 months),
respectively (P=0.076; Fig. 2A). The
median PFS time was 36.8 months (95% CI, 20.2–47.3 months) in the
test group, while that for the control group was 9.7 months (95%
CI, 3.6–15.9 months), respectively (P=0.038; Fig. 2B).
 | Table VI.Comparison of the OS, PFS and LC
rates for patients with locally advanced esophageal cancer. |
Table VI.
Comparison of the OS, PFS and LC
rates for patients with locally advanced esophageal cancer.
|
| 1-year OS | 3-year OS | 1-year PFS | 3-year PFS | 1-year LC | 3-year LC |
|---|
|
|
|
|
|
|
|
|
|---|
| Group | Survival | Died | Survival | Died | PFS | Progression | PFS | Progression | No local
recurrence | Local
recurrence | No local
recurrence | Local
recurrence |
|---|
| Test | 16 | 7 | 5 | 11 | 13 | 10 | 5 | 11 | 16 | 2 | 5 | 2 |
| Control | 14 | 8 | 0 | 19 | 10 | 12 | 0 | 22 | 12 | 6 | 1 | 8 |
|
χ2-value | 0.178 | – | – | – | – | – |
| P-value | 0.758 | 0.013a | 0.556 | 0.012a | 0.228 | 0.041a |
Effects of thalidomide on the change
of serum VEGF
The changes of VEGF level were classified as
decrease, stable or increase compared with the level during
radiotherapy. Compared with the control group, statistical
differences were identified in the changes of VEGF between the test
group and control group (P<0.05; Table VII).
 | Table VII.Changes of VEGF in control and test
groups post-radiotherapy compared with during radiotherapy. |
Table VII.
Changes of VEGF in control and test
groups post-radiotherapy compared with during radiotherapy.
| Group | Decreased VEGF,
% | Stable VEGF, % | Elevated VEGF,
% |
|---|
| Test (n=26) | 53.8 | 38.5 |
7.7 |
| Control (n=25) | 16.0 | 64.0 | 20.0 |
| Z-value |
| −2.745 |
|
| P-value |
| 0.006a |
|
Associations between VEGF changes and
prognosis
Subsequently, the associations between VEGF changes
and the OS, PFS and LC rates for patients with EC were evaluated.
Patients with increased VEGF following radiotherapy all died within
1 year. A significant increase was identified in the 1-year OS,
1-year PFS, 1-year LC and 3-year LC rates for patients with stable
or decreased VEGF compared with those with an increase of VEGF
(P<0.05; Table VIII).
 | Table VIII.Comparison of the OS, PFS, and LC for
patients of the test group with various changes of VEGF. |
Table VIII.
Comparison of the OS, PFS, and LC for
patients of the test group with various changes of VEGF.
|
| 1-year OS | 3-year OS | 1-year PFS | 3-year PFS | 1-year LC | 3-year LC |
|---|
|
|
|
|
|
|
|
|
|---|
| VEGF change | Survival | Died | Survival | Died | PFS | Progression | PFS | Progression | No local
recurrence | Local
recurrence | No local
recurrence | Local
recurrence |
|---|
| Decrease | 13 | 1 | 6 | 4 | 12 | 2 | 6 | 4 | 6 | 0 | 6 | 6 |
| Stable | 6 | 4 | 1 | 6 | 10 | 4 | 1 | 6 | 3 | 0 | 1 | 0 |
| Elevated | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 |
| P-value | 0.013a | 0. 110 | 0.006a | 0.110 | 0.005a | 0.028a |
Analysis of prognostic factors
Univariate analysis revealed that the OS time was
not associated with sex, age, site and type of tumors, KPS,
pre-treatment cancer length, tumor diameter, N stage, radiotherapy
dose, administration of thalidomide or the presence of residual
cancer after radiotherapy (Table
IX). However, T stage (P=0.028) and TNM stage (P=0.024) were
identified to be significantly associated with OS time (Table IX). Cox multivariate analysis
demonstrated that compared with stage I patients, the risk of
mortality increased in stage II [relative risk (RR), 5.613; 95% CI,
1.161–27.127; P<0.05] and III patients (RR, 8.097; 95% CI,
1.312–49.972; P<0.05) after adjusting for age, sex and
administration of thalidomide (Table
X). For the PFS time, univariate analysis revealed age, sex,
site and type of tumor, KPS, pre-treatment tumor length, tumor
diameter, N stage, dose of radiotherapy and administration of
thalidomide were not significant associated. By contrast, the
presence of residual cancer cell after radiotherapy (P=0.006), T
stage (P=0.025) and TNM stage (P=0.019) were identified to be
associated with PFS time (Table
XI). Cox multivariate analysis demonstrated that compared with
stage I patients, the risk of disease progression increased in
stage II (RR, 4.190; 95% CI, 0.630–27.853; P<0.05) and III
patients (RR, 5.693; 95% CI, 1.117–29.025; P<0.05) after
adjusting for age, sex and administration of thalidomide (Table XII). Compared with those with CR
following radiotherapy, patients with the presence of residue EC
cells demonstrated a significant increase in disease progression
after radiotherapy (RR, 1.910; 95% CI, 1.246–2.930; P<0.05;
Table XII).
 | Table IX.Univariate analysis of the overall
survival rate of patients with esophageal cancer. |
Table IX.
Univariate analysis of the overall
survival rate of patients with esophageal cancer.
| Variable | β-value | Standard error | Wald-value | RR | 95% CI | P-value |
|---|
| Sex | 0.261 | 0.429 | 0.371 | 1.298 | 0.560–3.009 | 0.543 |
| Age | −0.025 | 0.027 | 0.897 | 0.975 | 0.926–1.027 | 0.343 |
| KPS | 0.038 | 0.038 | 0.991 | 1.039 | 0.964–1.121 | 0.320 |
| Lesion
position | −0.132 | 0.248 | 0.286 | 0.876 | 0.539–1.423 | 0.593 |
| Lesion type | 0.421 | 0.749 | 0.315 | 1.523 | 0.351–6.606 | 0.574 |
| T stage | 0.710 | 0.324 | 4.808 | 2.033 | 1.078–3.834 | 0.028a |
| N stage | 0.670 | 0.345 | 3.779 | 1.955 | 0.994–3.844 | 0.052 |
| TNM stage | 0.634 | 0.282 | 5.066 | 1.885 | 1.085–3.275 | 0.024a |
| Cancer length
before treatment | 0.134 | 0.088 | 2.358 | 1.144 | 0.964–1.358 | 0.125 |
| Lesion
diameter | 0.033 | 0.197 | 0.027 | 1.033 | 0.702–1.520 | 0.868 |
| Residual cancer
cells after radiotherapy | 0.365 | 0.194 | 3.541 | 1.441 | 0.985–2.109 | 0.060 |
| Radiotherapy
dose | −0.058 | 0.035 | 2.774 | 0.944 | 0.882–1.010 | 0.096 |
| Thalidomide | −0.575 | 0.359 | 2.568 | 0.562 | 0.278–1.137 | 0.109 |
 | Table X.Cox multivariate analysis of the
overall survival rate of patients with esophageal cancer. |
Table X.
Cox multivariate analysis of the
overall survival rate of patients with esophageal cancer.
| Variable | RR | 95% CI | P-value |
|---|
| Sex |
|
Male | 1.000 | – |
|
|
Female | 0.745 | 0.311–1.785 | 0.509 |
| Age | 1.006 | 0.952–1.063 | 0.838 |
| TNM stage |
| Stage
I | 1.000 | – |
|
| Stage
II | 5.613 | 1.161–27.127 | 0.032a |
| Stage
III | 8.097 | 1.312–49.972 | 0.024a |
| Thalidomide |
| No | 1.000 | – |
|
|
Yes | 0.523 | 0.248–1.101 | 0.088 |
 | Table XI.Univariate analysis of the
progression-free survival rate of patients with esophageal
cancer. |
Table XI.
Univariate analysis of the
progression-free survival rate of patients with esophageal
cancer.
| Variable | β-value | Standard error | Wald-value | RR | 95% CI | P-value |
|---|
| Sex | −0.312 | 0.427 | 0.534 | 0.732 | 0.317–1.691 | 0.465 |
| Age | −0.042 | 0.026 | 2.580 | 0.959 | 0.911–1.009 | 0.108 |
| KPS | 0.048 | 0.038 | 1.572 | 1.049 | 0.973–1.131 | 0.210 |
| Lesion
position | −0.069 | 0.242 | 0.082 | 0.933 | 0.581–1.499 | 0.774 |
| Lesion type | 0.184 | 0.742 | 0.061 | 1.202 | 0.281–5.146 | 0.804 |
| T stage | 0.727 | 0.325 | 5.022 | 2.070 | 1.096–3.910 | 0.025a |
| N stage | 0.706 | 0.360 | 3.849 | 2.027 | 1.001–4.105 | 0.050 |
| TNM stage | 0.658 | 0.282 | 5.468 | 1.932 | 1.112–3.354 | 0.019a |
| Cancer length
before treatment | 0.155 | 0.090 | 2.997 | 1.168 | 0.980–1.393 | 0.083 |
| Lesion
diameter | 0.198 | 0.195 | 1.028 | 1.219 | 0.831–1.787 | 0.311 |
| Residual cancer
cells after radiotherapy | 0.535 | 0.194 | 7.627 | 1.707 | 1.168–2.495 | 0.006a |
| Radiotherapy
dose | -.045 | 0.036 | 1.608 | 0.956 | 0.891–1.025 | 0.205 |
| Thalidomide | -.652 | 0.357 | 3.340 | 0.521 | 0.259–1.048 | 0.068 |
 | Table XII.Cox multivariate analysis of the
progression-free survival rate of patients with esophageal
cancer. |
Table XII.
Cox multivariate analysis of the
progression-free survival rate of patients with esophageal
cancer.
| Variable | RR | 95% CI | P-value |
|---|
| Sex |
|
Male | 1.000 | – |
|
|
Female | 0.853 | 0.346–2.108 | 0.731 |
| Age | 0.962 | 0.909–1.018 | 0.178 |
| TNM stage |
| Stage
I | 1.000 | – |
|
| Stage
II | 4.190 | 0.630–27.853 | 0.138 |
| Stage
III | 5.693 | 1.117–29.025 | 0.036a |
| Thalidomide |
| No | 1.000 | – |
|
Yes | 0.473 | 0.221–1.014 | 0.054 |
| Residual cancer
cells after radiotherapy | 1.910 | 1.246–2.930 | 0.003a |
Discussion
Surgery is not recommended for the majority of
patients who present with advanced EC upon diagnosis. In a previous
study, CRT demonstrated a comparable efficiency to surgery for
patients with EC (18). Therefore,
concurrent CRT is recommended by the National Cancer Institute
Network as a standard treatment option for EC (18). The present study compared the
treatment efficiency, prognosis and side-effects between CRT and a
combination of CRT and thalidomide.
VEGF, a mitogen-activated factor secreted by
vascular endothelial cells, serves important roles in several
biological processes, including differentiation of endothelial
cells, elevation of capillary permeability, endothelial cell
migration and angiogenesis (19).
VEGF has been identified to be expressed in vascular endothelial
cells, esophageal endothelial cells and mononuclear macrophages. In
addition, it is expressed in several types of malignant cell
including cancer cells and tumor vascular endothelial cell.
Furthermore, it has been detected in serum and exudate (20). Generally, the expression of VEGF in
the normal tissues was relatively low, as it could only maintain
vascular density and basic osmosis, which contributed to the
nutrition delivery (21). The
expression of VEGF in cancer tissues is markedly higher compared
with normal tissues as the growth of cancer depends on the
transport of nutrients mediated by blood vessels (11). VEGF has been reported to serve a
pivotal role in vascularization (11). In addition, VEGF is considered as an
independent prognosis factor and is closely associated with the
recurrence and metastasis of cancer (22). Serum VEGF has been reported to be
closely associated with tumor load, infiltration and lymph node
metastasis. The expression of VEGF was identified to be
significantly higher in patients with a large tumor volume and
lymph node metastasis compared with those with a small tumor volume
and the absence of lymph node metastasis (22). Our previous study (19) demonstrated that VEGF level is
associated with the sensitivity to radiotherapy and prognosis of
chemo-radiotherapy, and the treatment efficiency in patients with
downregulated VEGF was higher compared with those with an increased
level of VEGF. In patients with EC with a satisfactory prognosis,
the VEGF level during radiotherapy was demonstrated to be decreased
compared with the baseline level. The elevation of VEGF induced by
radiotherapy may be associated with the self-protection of cancer
cells to encounter the toxicity of radiotherapy.
Thalidomide was initially utilized for the
management of lepra reactions and was then demonstrated to be
effective for attenuating vomiting and nausea in early gestation.
However, the application of thalidomide is limited as it causes
congenital defects in neonates (10,12).
Since then, studies involving thalidomide predominantly focused on
the immune system, anti-inflammation and anti-angiogenesis. In
1999, Singhal et al (23)
reported that thalidomide exhibited antitumor activity in
refractory multiple myeloma with a CR rate of 32%. Subsequently,
thalidomide has been demonstrated to be effective for the treatment
of various types pf hematologic neoplasm (24–26).
Thalidomide has been regarded as a treatment option for solid
tumors (27) and the majority of
studies have been focused on anti-angiogenesis, chemotherapy
sensitivity and attenuation of the chemotherapy-associated
side-effects (28,29). To the best of our knowledge, no
previous studies have investigated the efficiency of thalidomide
for concomitant therapy with CRT. Previously, thalidomide has been
reported to increase the radiotherapy sensitivity of EC cells
(30). Our previous study (22) demonstrated that a combination of
thalidomide and radiotherapy could trigger a downregulation of
VEGF, specifically patients receiving thalidomide demonstrated
satisfactory tolerance to radiotherapy.
In the present study, patients with EC with an
elevated VEGF level were randomly divided into the test group and
control group to investigate the effects of thalidomide on VEGF and
prognosis. The data revealed that the short-term treatment
efficiency was similar between the two groups, and the 1-year and
3-year OS, PFS and LC rates, and median OS and PFS times
demonstrated no statistical differences. The stratified analysis
revealed that the patients with locally advanced EC in the test
group exhibited significantly higher 3-year OS, PFS and LC rates
and median PFS time compared with the control group, which
indicates that a combination of thalidomide and chemo-radiotherapy
contributes to the prognosis of patients with locally advanced EC
with elevated VEGF. Compared with the control group, the
post-treatment VEGF level demonstrated a significant decrease in
the test group. This indicates that thalidomide contributes to the
decrease of serum VEGF level in patients with EC with elevated VEGF
during the concurrent CRT. Furthermore, the OS, PFS and LC rates
for patients with decreased VEGF following administration of
thalidomide were higher compared with those with increased VEGF,
which indicates that the benefits of thalidomide may be associated
with the inhibition of VEGF in cancer tissues. Additionally,
multivariate analysis demonstrated that TNM stage was significantly
associated with OS time, while the presence of residual cancer
cells and TNM stage were significantly associated with the PFS time
following radiotherapy.
There were certain limitations of the present study.
Firstly, the sample size was too small. To improve this, more data
from a larger number of patients should be included in future
studies. In addition, no significant differences were identified in
the 1-year OS and PFS rates for the patients with locally advanced
EC between the two groups. This may be due to the small sample
size. Furthermore, the present study only focused on the
side-effects, treatment efficiency and prognosis of thalidomide
combined with CRT. Therefore, potential mechanisms should be
investigated in future studies.
In conclusion, the current study demonstrated that
thalidomide contributes to an improvement of prognosis for patients
with locally advanced EC with elevated serum VEGF during
radiotherapy. Furthermore, the toxicities observed were tolerable.
It was concluded that the benefits of thalidomide in clinical
practice may be associated with the inhibition of VEGF in cancer
cells.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 11705095), the Key Science
& Technology Program of Changzhou Municipal Commission of
Health and family planning (grant no. ZD201710), the Changzhou
Scientific and Technological Support Social Development Project
(grant no. CE20165024) and the Changzhou Municipal Science and
Technology Bureau Basic Research Project (grant no.
CJ20159050).
Availability of data and materials
The raw data were available upon appropriate
requests.
Authors' contributions
JW was responsible for data collection and analysis,
as well as manuscript writing. JY, JLW, XN, ZS and WS were
responsible for data collection and analysis. SS and YL designed
the study and revised the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from each
patient. The study protocols were approved by the Ethics Committee
of the Second People's Hospital of Changzhou Affiliated to Nanjing
Medical University (Nanjing, China). This clinical trial was
registered in the United States Trial Registry (ID:
NCT01551641).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EC
|
esophageal cancer
|
|
CRT
|
chemo-radiotherapy
|
|
VEGF
|
vascular endothelial growth factor
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
LC
|
local control
|
|
CR
|
complete response
|
|
PR
|
partial response
|
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DaVee T, Ajani JA and Lee JH: Is
endoscopic ultrasound examination necessary in the management of
esophageal cancer? World J Gastroenterol. 23:751–762. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cooper JS, Guo MD, Herskovic A, Macdonald
JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler
JJ, Spencer S, et al: Chemoradiotherapy of locally advanced
esophageal cancer: Long-term follow-up of a prospective randomized
trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA.
281:1623–1627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao KL, Liu G, Jiang GL, Wang Y, Zhong
LJ, Wang Y, Yao WQ, Guo XM, Wu GD, Zhu LX and Shi XH: Association
of haemoglobin level with morbidity and mortality of patients with
locally advanced oesophageal carcinoma undergoing radiotherapy-a
secondary analysis of three consecutive clinical phase III trials.
Clin Oncol (R Coll Radiol). 18:621–627. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maouris PG and Hirsch PJ: Pregnancy in
women with thalidomide-induced disabilities. Case report and a
questionnaire study. Br J Obstet Gynaecol. 95:717–719. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou S, Wang F, Hsieh TC, Wu JM and Wu E:
Thalidomide-a notorious sedative to a wonder anticancer drug. Curr
Med Chem. 20:4102–4108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rades D, Golke H, Schild SE and Kilic E:
Impact of VEGF and VEGF receptor 1 (FLT1) expression on the
prognosis of stage III esophageal cancer patients after
radiochemotherapy. Strahlenther Onkol. 184:416–420. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoon MS, Nam TK, Lee JS, Cho SH, Song JY,
Ahn SJ, Chung IJ, Jeong JU, Chung WK and Nah BS: VEGF as a
predictor for response to definitive chemoradiotherapy and COX-2 as
a prognosticator for survival in esophageal squamous cell
carcinoma. J Korean Med Sci. 26:513–520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu J, Liu F, Sun M, Sun Z and Sun S:
Enhancement of radiosensitivity and the potential mechanism on
human esophageal carcinoma cells by tetrandrine. Cancer Biother
Radiopharm. 26:437–442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu J, Liu F, Sun Z, Sun M and Sun S: The
enhancement of radiosensitivity in human esophageal carcinoma cells
by thalidomide and its potential mechanism. Cancer Biother
Radiopharm. 26:219–227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Yu JP, Wang JL, Ni XC, Sun ZQ, Sun
W, Nie B, Jiang JT, Sun SP and Wu CP: Pathologic response and
changes of serum VEGF during chemoradiotherapy may predict
prognosis in non-surgical patients with esophageal carcinoma.
Zhonghua Zhong Liu Za Zhi. 38:589–595. 2016.(In Chinese).
PubMed/NCBI
|
|
12
|
Yu JP, Sun SP, Sun ZQ, Ni XC, Wang J, Li
Y, Hu LJ and Li DQ: Clinical trial of thalidomide combined with
radiotherapy in patients with esophageal cancer. World J
Gastroenterol. 20:5098–5103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Milstein JM, Cohen ME and Sinks LF: The
influence and reliability of neurologic assessment and Karnofsky
performance score on prognosis. Cancer 56 (7 Suppl). S1834–S1836.
1985. View Article : Google Scholar
|
|
14
|
Strong VE, D'Amico TA, Kleinberg L and
Ajani J: Impact of the 7th Edition AJCC staging classification on
the NCCN clinical practice guidelines in oncology for gastric and
esophageal cancers. J Natl Compr Canc Netw. 11:60–66. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coche E: Recist and beyond. JBR-BTR.
96:167–171. 2013.PubMed/NCBI
|
|
16
|
Zhang TR, Zhao T, Xu X, Gu XW and Pan YK:
Efficacy and side-effects of docetaxel combined with cisplatin on
the treatment of local advanced esophageal cancer with concomitant
radiation therapy. Zhonghua Zhong Liu Za Zhi. 32:791–794. 2010.(In
Chinese). PubMed/NCBI
|
|
17
|
National Comprehensive Cancer Network, .
NCCN. 2016.[EB/OL]. https://www.nccn.org/August 20–2016
|
|
18
|
Crehange G, Maingon P, Peignaux K, N'guyen
TD, Mirabel X, Marchal C, Verrelle P, Roullet B, Bonnetain F and
Bedenne L; Federation Francophone de Cancerologie Digestive 9102, :
Phase III trial of protracted compared with split-course
chemoradiation for esophageal carcinoma: Federation francophone de
cancerologie digestive 9102. J Clin Oncol. 25:4895–4901. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu JP, Lu WB, Wang JL, Ni XC, Wang J, Sun
ZQ and Sun SP: Pathologic response during chemo-radiotherapy and
variation of serum VEGF levels could predict effects of
chemo-radiotherapy in patients with esophageal cancer. Asian Pac J
Cancer Prev. 16:1111–1116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu JJ, Ma J, Miao R, Gu Y and Zhong FT:
Expression of vascular endothelial growth factor D in human
esophageal squamous cell carcinoma tissue and its significance.
Zhonghua Wei Chang Wai Ke Za Zhi. 16:1191–1194. 2013.(In Chinese).
PubMed/NCBI
|
|
21
|
Bedoya F, Meneu JC, Macías MI, Moreno A,
Enríquez-De-Salamanca R, Gonzalez EM and Vegh I: Mutation in CNR1
gene and VEGF expression in esophageal cancer. Tumori. 95:68–75.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Srivastava VK, Gara RK, Rastogi N, Mishra
DP, Ahmed MK, Gupta S, Goel MM and Bhatt ML: Serum vascular
endothelial growth factor-A (VEGF-A) as a biomarker in squamous
cell carcinoma of head and neck patients undergoing
chemoradiotherapy. Asian Pac J Cancer Prev. 15:3261–3265. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singhal S, Mehta J, Desikan R, Ayers D,
Roberson P, Eddlemon P, Munshi N, Anaissie E, Wilson C, Dhodapkar
M, et al: Antitumor activity of thalidomide in refractory multiple
myeloma. N Engl J Med. 341:1565–1571. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Steins MB, Bieker R, Padró T, Kessler T,
Kienast J, Berdel WE and Mesters RM: Thalidomide for the treatment
of acute myeloid leukemia. Leuk Lymphoma. 44:1489–1493. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kay NE, Shanafelt TD, Call TG, Wu W and
Laplant BR: N9986: A phase II trial of thalidomide in patients with
relapsed chronic lymphocytic leukemia. Leuk Lymphoma. 50:588–592.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Townsend W, Johnson RJ, Pottinger BT,
Counsell N, Smith P, Chadwick H, Evans K, Wickham C and Rudin CE;
UK NCRI Lymphoma Clinical Studies Group, : A phase II clinical
trial of fludarabine and cyclophosphamide followed by thalidomide
for angioimmunoblastic T-cell lymphoma. An NCRI clinical trial.
CRUK number C17050/A5320. Leuk Lymphoma. 57:2232–2234. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar S, Witzig TE and Rajkumar SV:
Thalidomid: Current role in the treatment of non-plasma cell
malignancies. J Clin Oncol. 22:2477–2488. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rehman W, Arfons LM and Lazarus HM: The
rise, fall and subsequent triumph of thalidomide: Lessons learned
in drug development. Ther Adv Hematol. 2:291–308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sauer H, Günther J, Hescheler J and
Wartenberg M: Thalidomide inhibits angiogenesis in embryoid bodies
by the generation of hydroxyl radicals. Am J Pathol. 156:151–158.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goel S, Wong AH and Jain RK: Vascular
normalization as a therapeutic strategy for malignant and
nonmalignant disease. Cold Spring Harb Perspect Med. 2:a0064862012.
View Article : Google Scholar : PubMed/NCBI
|