Introduction
Triptolide (TPL) is an active extract obtained from
the Chinese herb Tripterygium wilfordii that has been used
for centuries to treat autoimmune diseases in China (1). Numerous studies have reported that TPL
has antitumor, anti-inflammatory and immunosuppressive activities.
The antitumor activity of TPL has been investigated in various
types of cancer cells in vitro and in vivo (2). Furthermore, recent studies have
revealed that TPL enhances the sensitivity of cancer cells to
chemotherapy by inducing apoptosis (3,4). To the
best of our knowledge, among all the cancer types that have been
investigated, breast cancer exhibits the highest sensitivity to TPL
(5). Breast cancer is a common type
of cancer in females. A number of distinct molecular subtypes of
breast cancer exist, including luminal A, luminal B, human
epidermal growth factor receptor 2 (HER2)-positive, basal-like and
normal-like (6,7). In total, ~70% of cases of
triple-negative breast cancer exhibit a basal-like subtype
(8). The treatment of breast cancer,
particularly triple-negative breast cancer, is a challenge due to
the lack of targets, including for hormone receptors and amplified
HER2 expression levels (9).
The associated molecular mechanisms by which TPL
inhibits breast cancer cells have been investigated. n In breast
cancer, TPL induces p53-dependent and lysosomal-mediated apoptosis,
and inhibits numerous oncogenic signaling pathways, including the
Wnt/β-catenin, the protein kinase B (Akt) and the focal adhesion
kinase-signaling pathway (3,10,11).
Notably, TPL inhibits key transcriptional factors, including MYC
and estrogen receptor (ER), which suppresses associated target
networks (12,13).
Although TPL exhibits a number of bioactivities and
pharmacological effects, the clinical application of TPL has been
restricted due to a number studies reporting multi-target toxicity
of the extract, including reproductive toxicity, hepatotoxicity and
renal cytotoxicity (14,15). Numerous studies have attempted to
identify a combination of TPL with other regents, which support the
function of TPL at a low dose and reduce the toxicity (16–18). It
has been reported that inhibition of ER-negative breast cancer cell
growth requires a high dose of TPL, which may induce toxicity
(19). The present study
investigated a new strategy to enhance TPL sensitivity in
triple-negative breast cancer cells. Increased expression and
activation of insulin-like growth factor-1 receptor (IGF-1R) and
its associated downstream signaling components has been reported in
clinical breast cancer samples, and has been associated with
disease progression and recurrence (20). Furthermore, overexpression of IGF1R
has been demonstrated to be associated with radio- or
tamoxifen-resistant breast cancer cells. The IGF1R inhibitor AG1024
reduces this resistance by inhibition of IGF1R signaling (21). The present study reported that AG1024
synergistically enhanced apoptosis induced by low doses of TPL and
cisplatin in the triple-negative breast cancer cells MDA-MB-231. In
addition, high amplification of IGF1R in triple-negative tumors may
serve as a potential target of a combination of AG1024 and DNA
damage reagents.
Materials and methods
Chemicals, antibodies and
plasmids
Triptolide (TPL), AG1024 and cisplatin were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Cell
Counting Kit-8 (CCK-8) was obtained from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan). Antibodies against B-cell
lymphoma (Bcl-)2 (sc-7382; 1:1,000) and β-actin (sc-8432, 1:2,500)
were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). The anti-phospho-H2A.X antibody (07–164, 1:1,000) was
purchased from EMD Millipore (Billerica, MA, USA). Cleaved
caspase-3, caspase-3 and IGF1R antibodies were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). The expression
plasmid 3336 pcDNA3 Bcl-2 and the empty vector 336 pcDNA3 were
kindly provided by Dr. Stanley Korsmeyer of the Dana-Farber Cancer
Institute, Inc (cat. no. 8768; Addgene, Boston, MA, USA) (22).
Cell culture
Human breast carcinoma cell lines MDAMB231 and
MDA-MB-436 were obtained from American Type Cell Culture Collection
(Manassas, VA, USA). Cells were grown in Dulbecco's modified
Eagle's medium (Invitrogen; Thermo Fisher Scientific, Waltham, MA,
USA) supplemented with 10% fetal bovine serum (HyClone; GE
Healthcare, Logan, UT, USA), 100 U/ml penicillin and 100 U/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Waltham, MA,
USA) at 37°C in a humidified 5% CO2 atmosphere. Cells
from <20 passages were used for experiments.
Cell viability assay
MDAMB231 and MDA-MB-436 cells were seeded in 96-well
plates at a density of 4×103 cells/well, followed by
treatment with DMSO or increasing doses of TPL (5 to 80 nM) and
AG1024 (1 to 40 µM) for 3 days at 37°C. Cells were then incubated
with 10 µl CCK-8 reagent for 1 h and the optical density value was
measured at 450 nm, according to the manufacturer's protocol.
Cell cycle analysis
Cell cycle analysis was performed to evaluate the
number of cells in the sub-G1 phase. MDA-MB-231 cells were seeded
at 60% confluence and allowed to attach overnight. Cells were then
treated with various concentrations of drugs (10 nM TPL, 10 µM
AG1024, or 1 µM cisplatin) for 96 h at 37°C, trypsinized, washed
with PBS and fixed in 70% ethanol at −20°C overnight. Prior to
analysis, cells were washed with PBS, suspended in cold propidium
iodide (PI; Sigma-Aldrich; Merck KGaA) solution (50 µl/ml in PBS)
and incubated at room temperature in the dark for 30 min. Flow
cytometry was then performed using a flow cytometer (BD FACSAria
system) and analyzed using BD FACSDiva software version 5.0 (BD
Biosciences, USA).
Transient transfection assay
A reverse transfection protocol was used to
knockdown IGF1R by small interfering RNA (siRNA). 25 nM SMARTpool
siRNAs (siIGF1R, L-003012-00-0005; and non-targeting
siRNA;,D-001810-0X; GE Healthcare Dharmacon, Inc., Lafayette, CO.
USA) was transfected into MDA-MB-231 cells using RNAiMAX
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Transfected cells were plated in 96-well
plates at a density of 2×103 cells/well for
proliferation assays at various time points (day 0–5) or plated for
protein collection 72 h after transfection.
Following treatment of the MDA-MB-231 cells with
drugs (10 nM TPL and 10 µM AG1024) for 2 days at 37°C, cells were
transfected with the expression plasmid 3336 pcDNA3 Bcl-2 (2 µg) or
empty vector (2 µg) using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) Cells were harvested
48 h after transfection for western blot analysis and cell cycle
analysis.
Western blotting
Cells were harvested and lysed in
radioimmunoprecipitation assay buffer (Invitrogen; Thermo Fisher
Scientific, Inc.). Lysates were centrifuged at 10,000 × g for 15
min at 4°C and supernatants were collected as whole cell extracts.
The protein concentration was determined using a Pierce™ Coomassie
Plus (Bradford) assay kit (Thermo Fisher Scientific, Inc.).
Equivalent amounts (20 µg) of protein were separated by SDS-PAGE
(4–12%) and transferred to a PVDF membrane. Following blocking with
5% milk at room temperature for 1 h, the membranes were incubated
with the corresponding primary antibodies for 2 h and horseradish
peroxidase-conjugated secondary antibodies at room temperature
(Santa Cruz Biotechnology, Inc.; cat. no. sc-2005 and sc-2004,
1:8,000) for 1 h. Protein bands were detected using an ECL reagent
(Thermo Fisher Scientific, Inc.). ImageJ software (version 1.41;
National Institutes of Health, Bethesda, MD, USA) was used to
quantify the blots.
Bioinformatics analysis
The amplification of IGF1R in different subtypes of
breast tumor was analyzed using data regarding breast invasive
carcinoma from The Cancer Genome Atlas (TCGA) (23,24) with
the online cBioPortal for Cancer Genomics platform (http://www.cbioportal.org) (25). The ER status determined by
immunohistochemistry was selected for the analysis of the
association between IGF1R expression and ER status. A list of the
overexpressed proteins associated with genomic alterations of IGF1R
in TCGA cohort was downloaded using cBioPortal. Gene ontology and
pathway analysis of the overexpressed proteins were performed using
the ToppGene online tool (26).
Statistical analysis
Experiments were replicated a minimum of three
times. Data are presented as the mean ± standard error of the mean.
GraphPad Prism software, version 7 (GraphPad Software, Inc., La
Jolla, CA, USA) was used to conduct statistical analyses; unpaired
t-test and one-way ANOVA (followed by Dunnett's test) were used to
compare the experimental groups with the control group. P<0.05
was considered to indicate a statistically significant
difference.
Results
TPL and AG1024 synergistically inhibit
the proliferation of triple-negative breast cancer cells
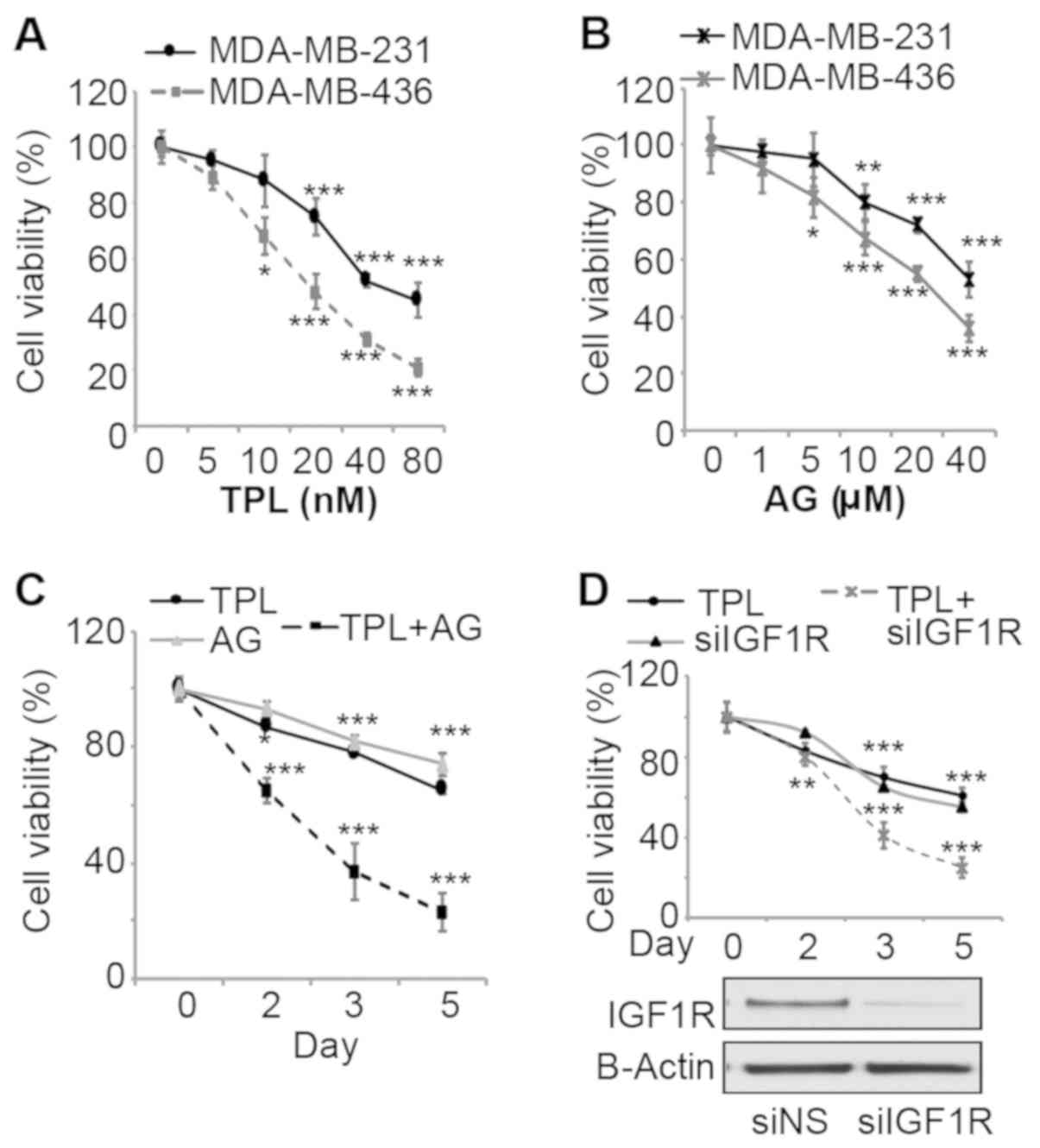
To evaluate effects of TPL and AG1024 on
triple-negative breast cancer cell growth, MDA-MB-231 and
MDA-MB-436 cells were treated with various doses of the two drugs
and the viability was measured. After 3 days of treatment, TPL and
AG1024 inhibited cell viability in a dose dependent-manner for the
two cell lines (Fig. 1A and B). The
sensitivity of MDA-MB-436 cells to TPL and AG1024 was higher
compared with MDA-MB-231 cells, potentially due to the presence of
a mutant BRCA1 (27). To evaluate
synergistic effects, a combination of low doses (10 nM TPL and 10
mM AG1024) at which TPL or AG1024 alone cannot markedly inhibit
cell growth was selected for cell proliferation assays with
MDA-MB-231 cells. Notably, this combination of TPL and AG1024
inhibited cell viability by ~60% after 3 days of treatment
(Fig. 1C). TPL and AG1024 at a low
dose (10 nM TPL and 10 mM AG1024) alone only inhibited cell growth
by ~20% (Fig. 1A and B). After 5
days, a combined treatment inhibited cell growth by ~80%. To
specifically inhibit IGF1R, cells were transfected with siIGF1R and
the transfection efficiency was confirmed by western blot analysis
(Fig. 1D). siIGF1R alone inhibited
cell viability by ~30% by day 3, while a combination of TPL and
siIGF1R inhibited cell viability by ~60% (Fig. 1D). These results are consistent with
the results of TPL and AG1024-treatment, which suggested that the
synergistic effect was specific to the inhibition of IGF1R rather
than other targets of AG1024.
TPL and AG1024 synergistically induce
apoptosis of triple-negative breast cancer cells
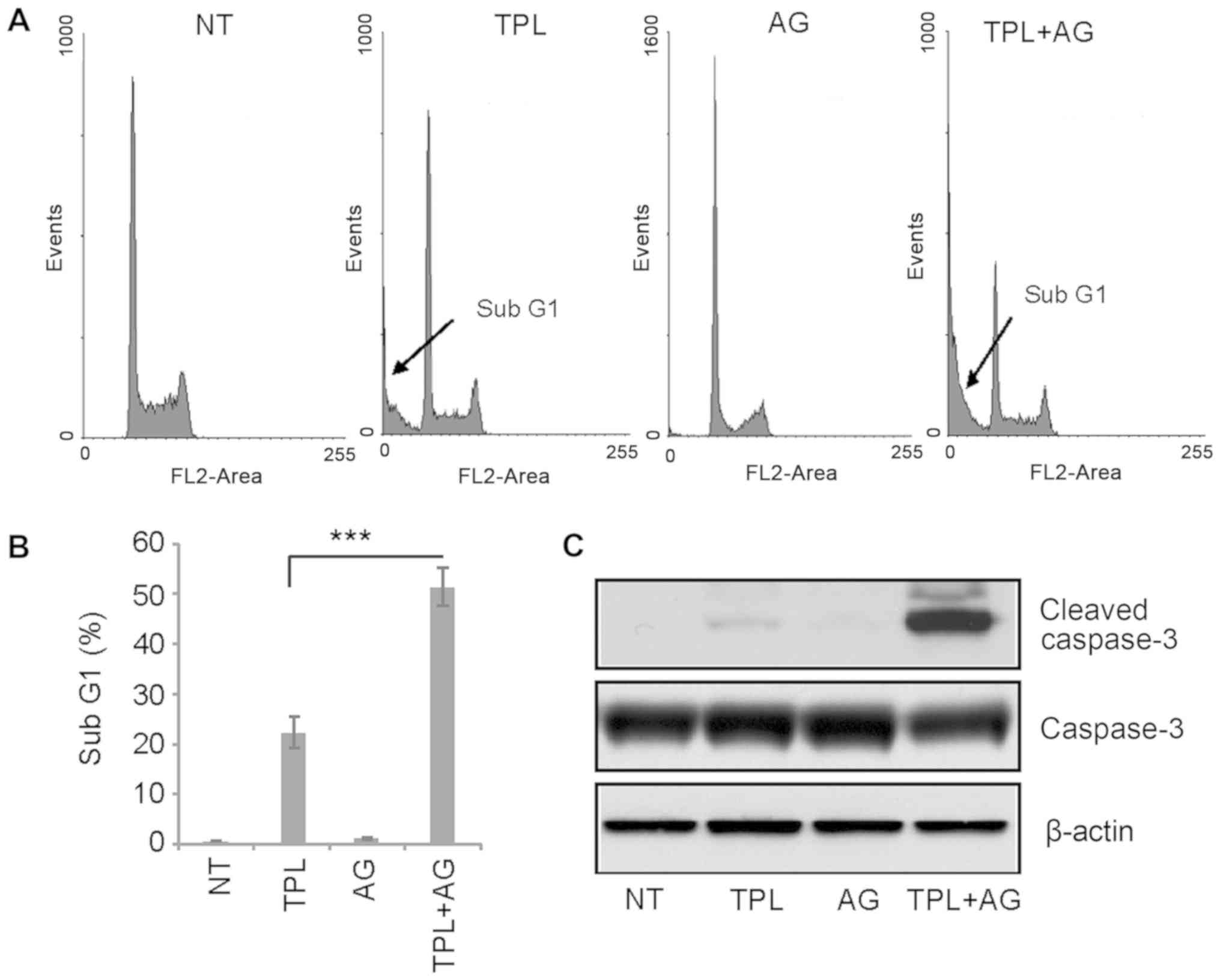
TPL has been reported to induce apoptosis of
MDA-MB-231 cells (2). Therefore,
flow cytometry was used in the present study to evaluate the
effects of a combination of drugs on apoptosis. The sub-G1 cell
population is understood to represent apoptotic cells. Following 4
days of treatment, 10 nM TPL increased the percentage of cells in
the sub-G1 population to ~20%, while 10 µM AG1024 did not induce
apoptosis (Fig. 2A and B). However,
after 4 days of treatment with TPL and AG1024, the percentage of
cells in the sub-G1 cell population was ~50% (Fig. 2A and B). These results were confirmed
by western blotting. Notably, a high expression of cleaved
caspase-3 was detected in TPL and AG1024-treated cells, while only
a small amount of cleaved caspase-3 was detected in TPL-treated
cells (Fig. 2C). In summary, these
results suggested a synergistic effect of TPL and AG1024 in
MDA-MB-231 cells.
Overexpression of Bcl-2 partially
inhibits TPL and AG1024-induced apoptosis
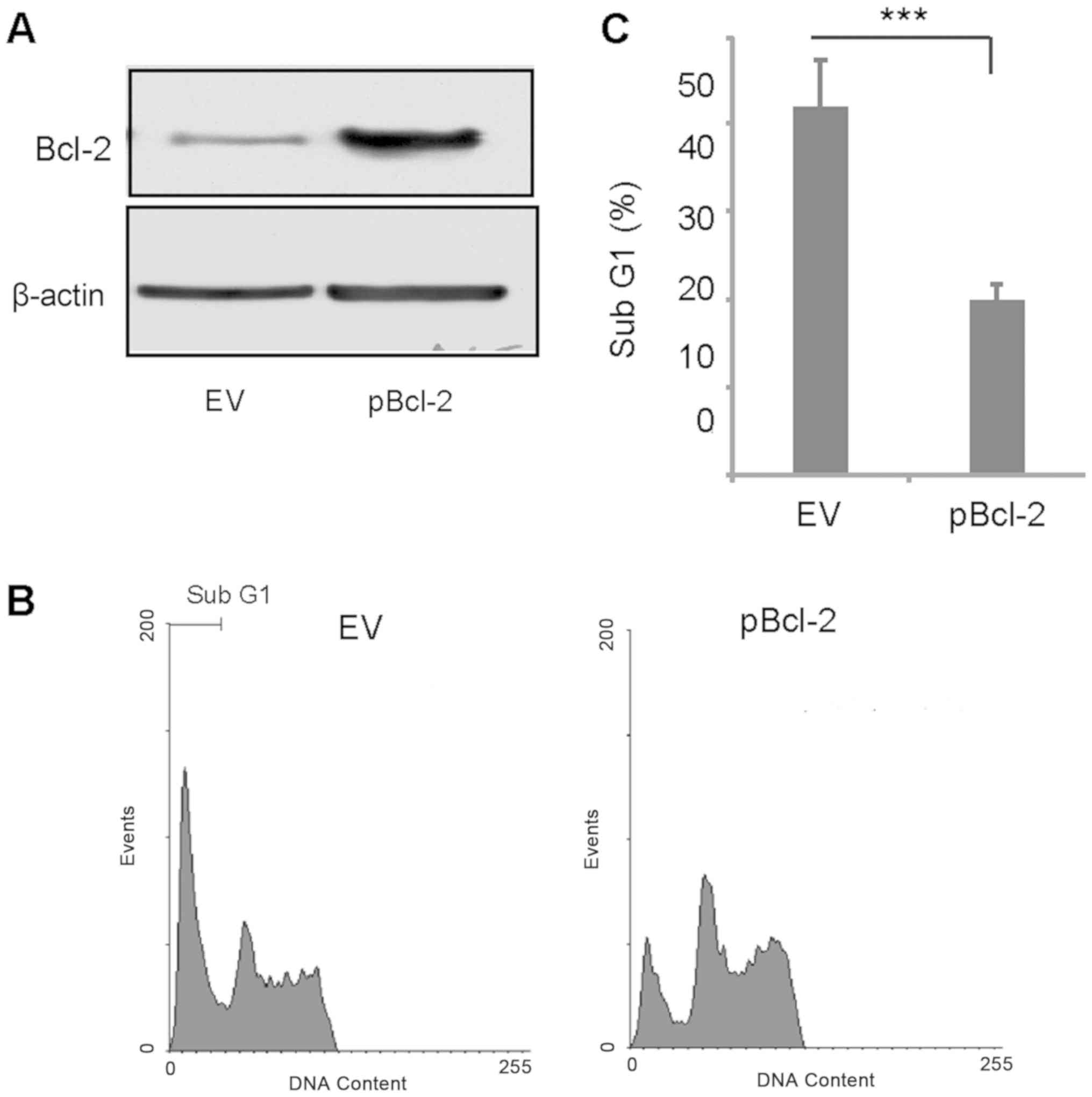
It has been reported that TPL induces
caspase-mediated Bcl-2 cleavage, which contributes to TPL-induced
apoptosis in MDA-MB-231 cells (28).
Therefore, the present study transiently overexpressed Bcl-2 and
treated the transfected cells with a combination of TPL and AG1024.
Bcl-2 was successfully overexpressed in MDA-MB-231 cells (Fig. 3A). After 3 days of treatment with TPL
and AG1024, the percentage of apoptotic cells in the Bcl-2
overexpressing group was significantly lower compared with the
control group (Fig. 3B and C). This
suggested cleavage of Bcl-2 may serve an important role in TPL and
AG1024-induced apoptosis.
Synergy between AG1024 and DNA
damage-inducing reagents enhances apoptosis in MDA-MB-231
cells
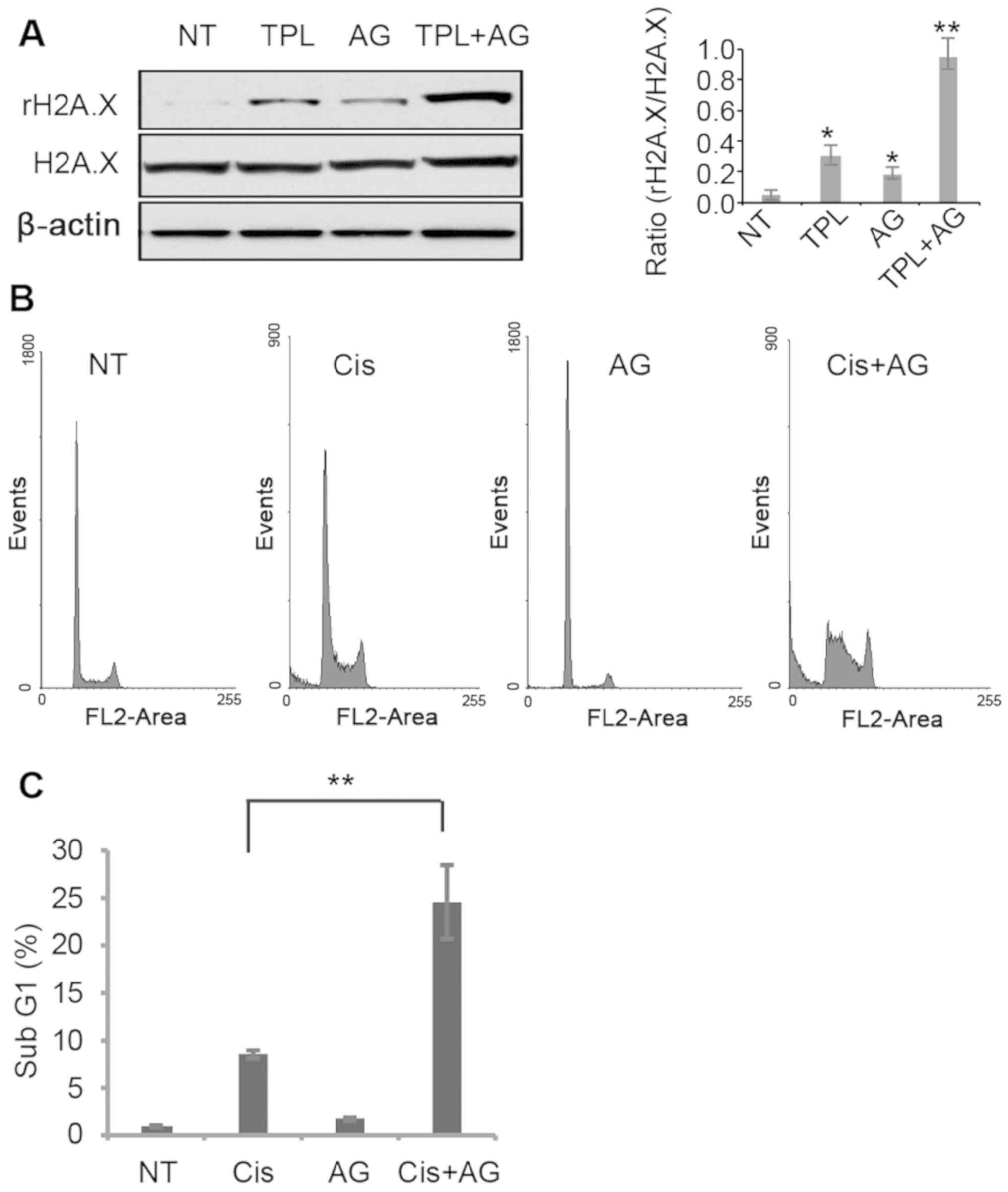
TPL has been reported to induce DNA damage in cancer
cells (29). In MDA-MB-231 cells,
TPL and AG1024 induced DNA damage, evidenced by a higher
phosphorylation level of the DNA damage marker H2A.X (Fig. 4A). Notably, treatment with TPL and
AG1024 increased H2A.X phosphorylation to a greater extent compared
with treatment with TPL or AG1024 alone (Fig. 4A). It is hypothesized that AG1024
served a synergistic role with other DNA damage reagents. The DNA
damage inducer cisplatin was selected to test this hypothesis. A
low dose of cisplatin (1 µM) resulted in 8.3% of apoptotic cells,
while a combined treatment of cisplatin (1 µM) and AG1024 (10 µM)
significantly increased the number of apoptotic cells compared with
cisplatin alone (Fig. 4B and C).
These results suggest that a combined use of AG1024 and DNA damage
reagents may provide a new approach for breast cancer
treatment.
High level of IGF1R in ER-negative
breast cancer
A high expression level of IGF1R has been reported
in triple-negative MDA-MB-231 cells (30). Therefore, it was hypothesized that
inhibition of IGF1R may increase the sensitivity of MDA-MB-231
cells to TPL and cisplatin, due to a higher activity of IGF1R
signaling. The present study evaluated whether increased IGF1R
expression was observed in patients with ER-negative breast cancer
compared with patients with ER-positive breast cancer in TCGA
clinical cohort (Fig. 5). Notably,
genomic alteration of the IGF1R gene was observed in 18% of cases
in the breast invasive TCGA cohort (Fig.
5A). In addition, a higher frequency of IGF1R amplification
(Amp) and copy number gain (Gain) was observed in patients with
ER-negative breast cancer compared with ER-positive breast
cancer.
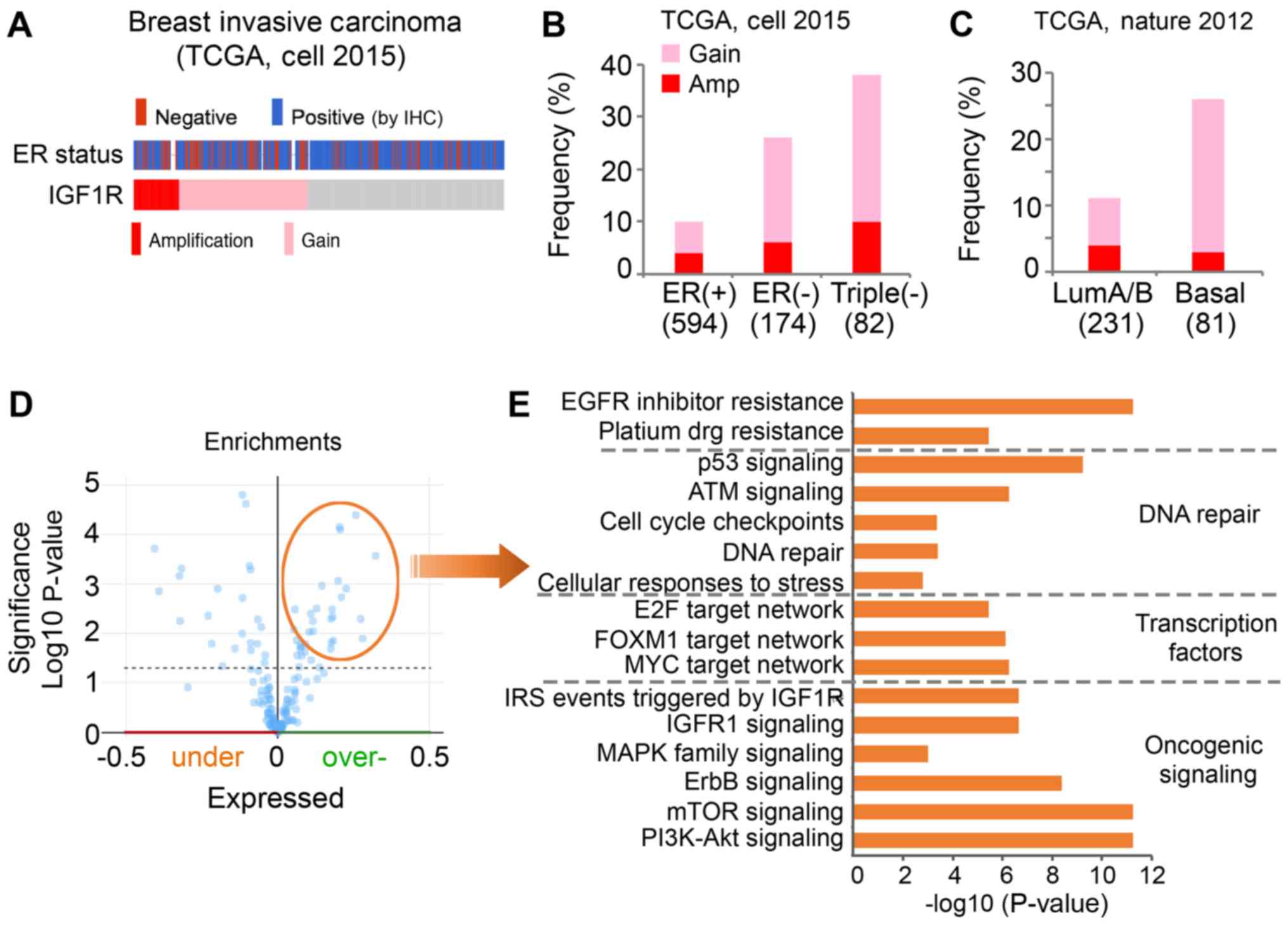 | Figure 5.A higher frequency of Amp and Gain of
IGF1R is present in ER-negative breast tumors compared with
ER-positive breast tumors. (A) Genomic alteration of IGF1R and ER
status of breast invasive carcinoma cases of TCGA cohort. Gray
represents individual tumors without an IGF1R alteration. (B) The
frequency of IGF1R alteration in differential subtypes of tumors
was analyzed in TCGA (cell, 2015) cohort. (C) The frequency of
IGF1R alteration in differential subtypes of tumors was analyzed in
TCGA (Nature, 2012) cohort. (D) Overexpressed proteins associated
with genomic alterations of IGF1R in the Cell 2015 TCGA cohort were
identified. (E) Pathway analysis was performed with the
significantly overexpressed genes using the ToppGene online tool.
The x-axis indicates the significance of the enrichment of the
pathway. The y-axis presents the pathway term. TCGA, The Cancer
Genome Atlas; ER, estrogen receptor; IGF1R, insulin-like growth
factor 1 receptor; IHC, immunohistochemistry; Gain, copy number
gain; Amp, amplification; LumA/B, Luminal A/B-like A/B; EGFR,
epidermal growth factor receptor; E2F, E2 factor; FOXM1, forkhead
box M1; IRS, insulin receptor substrate; MAPK, mitogen-activated
protein kinase pathway; mTOR, mammalian target of rapamycin; PI3K,
phosphatidylinositol 3-kinase. |
To further evaluate, the present study investigated
the frequency of IGF1R alterations, including Amp and Gain, in
patients with ER-positive (n=594), ER-negative (n=174) and
triple-negative (n=82) cancer. IGF1R alteration was observed in 10%
of the ER-positive group (4% Amp, 6% Gain), 26% of the ER-negative
group (6% Amp, 20% Gain) and 38% of the triple-negative group (10%
Amp, 28% Gain; Fig. 5B). It has been
reported that basal-like breast cancers are more aggressive
compared with luminal A/B-like tumors, and the majority of
basal-like subtypes are typically negative for ER, PR and HER2
(24). Similarly, the present study
observed genomic alteration of IGF1R in 26% of basal-like tumors
(3% Amp, 23% Gain) and only 11% of luminal A/B-like tumors (4% Amp,
7% Gain; Fig. 5C). In summary, these
data suggested IGF1R was highly amplified and expressed in
ER-negative and basal-like breast cancer.
To understand the pathways associated with genomic
alterations of IGF1R in clinical samples, the present study
downloaded a list of overexpressed proteins that are positively
associated with an alteration of IGF1R (Amp/Gain) using the
cBioPortal (Fig. 5D). Pathway
analysis was performed with these proteins using the ToppGene
online tool. The overexpressed proteins associated with IGF1R
alteration were identified to be significantly enriched in multiple
oncogenic signaling pathways (Fig.
5E). As expected, proteins were revealed to be significantly
enriched in IGF1R signaling. Furthermore, enrichment of
phosphatidylinositol 3-kinase (PI3K)/Akt and mammalian target of
rapamycin signaling activation was observed. Networks of key
transcription factors, including E2 factor, forkhead box M1 and
MYC, were revealed to be activated in the cases with IGF1R
alteration. In addition, the proteins associated with IGF1R were
enriched in DNA repair pathways. Certain proteins were identified
to be enriched in the platinum drug resistance pathway, which may
explain the synergistic effects of cisplatin and AG1024 observed in
the present study.
Discussion
TPL has been reported to exert potent anticancer
effects via multiple molecular targets and signaling pathways.
However, the side effects of TPL limit its clinical application for
the treatment of cancer (2). The
present study demonstrated that the IGF1R inhibitor AG1024
increased the sensitivity of triple-negative breast cancer cells to
TPL. Therefore, it was suggested that inhibition of IGF1R signaling
and induction of DNA damage may have synergistic effects in the
treatment of ER-negative or triple-negative breast cancer. Notably,
the current study identified a higher amplification of IGF1R in
ER-negative and basal-like breast cancer.
A previous study reported that the ER-positive
breast cancer cells (MCF-7) were more sensitive to TPL treatment
due to repression of the ER pathway by TPL, and that TPL-induced
apoptosis of MDA-MB-231 cells was ER-independent (19). In addition, it has been reported that
MDA-MB-231 cells possess a high IGF1R expression (30), which indicates IGFR1 may serve a role
in cell survival. Consistent with previous studies, the present
study observed that inhibition of IGF1R signaling by AG1024
inhibited cell viability. A combined use of TPL and AG1024 at low
doses demonstrated a high potency compared with single drug
application. This may be due to a higher level of IGFR1 signaling
in ER-negative breast cancer cells (30). It has been reported that suppression
of the IGF system with AG1024 augments cisplatin-induced DNA damage
(30). Consistent with these
findings, the present study identified that AG1024 enhanced
cisplatin-induced apoptosis of MDA-MB-231 cells. Similarly, TPL has
been revealed to induce DNA damage in cancer cells (31). These results suggested a possible
synergistic function of IGF1R signaling inhibition and inducement
of DNA damage.
Previously, it has been reported that patients with
IGF1R-postive/ER-negative breast cancer have a worse prognosis
compared with patients with IGF1R-negative/ER-negative breast
cancer (32). However, the
association of IGF1R signaling with ER status and the
aggressiveness of breast cancer remains controversial. A previous
study reported the differing effects of IGF1R expression on the
prognosis of patients with ER-positive vs. triple-negative invasive
ductal breast carcinoma (IDC) (33).
IGF1R expression in ER-positive IDC is strongly associated with a
favorable disease-free survival (DFS) time; however, IGF1R
expression is associated with a shorter DFS for patients with
TN-IDC. The aforementioned studies were all performed based on
immunohistochemical staining of IGF1R to determine its protein
expression. To the best of our knowledge, the present study was the
first to identify a higher Amp and Gain of IGF1R in ER-negative
breast cancer compared with ER-positive cancer. Furthermore, a
difference in the amplification frequency of IGF1R was revealed
between luminal A/B-like and basal-like breast tumors.
IGF1R signaling activates several downstream
signaling pathways, including the PI3K/Akt signaling pathway and
mitogen-activated protein kinase pathway (34,35). In
the enrichment analysis performed in the current study, a number of
oncogenic signaling pathways were identified to be associated with
alterations of IGF1R, which may contribute to the IGF1R-driven
aggressiveness. The EGFR inhibitor resistance pathway was also
associated with the alteration of IGF1R. This observation was
consistent with a previous study in which AG1024 has been
demonstrated to enhance the apoptotic effects of EGFR inhibitor in
human breast cancer cells (36).
Therefore, a high alteration of IGF1R in ER-negative tumors may
indicate a greater extent of activated oncogenic signaling compared
with other tumors. Inhibition of IGF1R signaling indirectly targets
multiple key pathways (37). The
synergistic effect of TPL and AG1024 may be due to shared targets,
including the PI3K/Akt signaling pathway and MYC. DNA repair
pathways were revealed to be associated with the alteration of
IGF1R, which suggests tumors with an IGF1R alteration may
demonstrate a higher resistance to chemo- or radiotherapy.
Therefore, a combination of IGF1R inhibitor and DNA damage
reagents, including TPL and cisplatin, may serve as an effective
strategy for the treatment of ER-negative or basal-like breast
cancer.
In conclusion, the present identified a synergistic
role of TPL and AG1024 in breast cancer cells. These results
suggested that a potential new treatment strategy for ER-negative
breast cancer may involve a combination of TPL and AG1024 at low
doses, which may reduce the toxicity of the two drugs. Furthermore,
a similar synergistic effect was revealed for AG1024 and cisplatin.
Notably, a high amplification of IGF1R was identified in aggressive
subtypes of breast cancer, including ER-negative and basal-like
breast cancer. In summary, the results of the present study
suggested that a combination of DNA damage reagents and inhibitors
of IGF1R signaling may be a promising approach for the treatment of
cancer types with activated IGF1R signaling.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HW designed the study, performed the experiments and
analysis, visualized the data, wrote the manuscript and provides
supervision. TS and RB performed the experiments and data analysis.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Corson TW and Crews CM: Molecular
understanding and modern application of traditional medicines:
Triumphs and trials. Cell. 130:769–774. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meng C, Zhu H, Song H, Wang Z, Huang G, Li
D, Ma Z and Ma J, Qin Q, Sun X and Ma J: Targets and molecular
mechanisms of triptolide in cancer therapy. Chin J Cancer Res.
26:622–626. 2014.PubMed/NCBI
|
|
3
|
Xiong J, Su T, Qu Z, Yang Q, Wang Y, Li J
and Zhou S: Triptolide has anticancer and chemosensitization
effects by down-regulating Akt activation through the MDM2/REST
pathway in human breast cancer. Oncotarget. 7:23933–23946. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng X, Shi W, Zhao C, Zhang D, Liang P,
Wang G and Lu L: Triptolide sensitizes human breast cancer cells to
tumor necrosis factor-α-induced apoptosis by inhibiting activation
of the nuclear factor-κB pathway. Mol Med Rep. 13:3257–3264. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ateba SB, Ngeu ST, Mvondo MA, Tchoumtchoua
J, Awounfack CF, Krenn L and Njamen D: Natural terpenoids against
female breast cancer: A 5-year recent research. Curr Med Chem.
25:3162–3213. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sims AH, Howell A, Howell SJ and Clarke
RB: Origins of breast cancer subtypes and therapeutic implications.
Nat Clin Pract Oncol. 4:516–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peddi PF, Ellis MJ and Ma C: Molecular
basis of triple negative breast cancer and implications for
therapy. Int J Breast Cancer. 2012:2171852012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shao H, Ma J, Guo T and Hu R: Triptolide
induces apoptosis of breast cancer cells via a mechanism associated
with the Wnt/β-catenin signaling pathway. Exp Ther Med. 8:505–508.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan BJ, Tan BH and Chiu GN: Effect of
triptolide on focal adhesion kinase and survival in MCF-7 breast
cancer cells. Oncol Rep. 26:1315–1321. 2011.PubMed/NCBI
|
|
12
|
Yang A, Qin S, Schulte BA, Ethier SP, Tew
KD and Wang GY: MYC inhibition depletes cancer stem-like cells in
triple-negative breast cancer. Cancer Res. 77:6641–6650. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang Y, Wang J, Cheng J and Wang L:
Antiestrogenic Activity of Triptolide in Human Breast Cancer Cells
MDA-MB-231 and Immature Female Mouse. Drug Dev Res. 78:164–169.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xi C, Peng S, Wu Z, Zhou Q and Zhou J:
Toxicity of triptolide and the molecular mechanisms involved.
Biomed Pharmacother. 90:531–541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li XJ, Jiang ZZ and Zhang LY: Triptolide:
Progress on research in pharmacodynamics and toxicology. J
Ethnopharmacol. 155:67–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao H, Shi P, Deng M, Jiang Z, Li Y,
Kannappan V, Wang W, Li P and Xu B: Low dose triptolide reverses
chemoresistance in adult acute lymphoblastic leukemia cells via
reactive oxygen species generation and DNA damage response
disruption. Oncotarget. 7:85515–85528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SH, Kang JG, Kim CS, Ihm SH, Choi MG,
Yoo HJ and Lee SJ: Synergistic cytotoxicity of BIIB021 with
triptolide through suppression of PI3K/Akt/mTOR and NF-κB signal
pathways in thyroid carcinoma cells. Biomed Pharmacother. 83:22–32.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiao Z, He M, He MU, Li W, Wang X, Wang Y,
Kuai Q, Li C, Ren S and Yu Q: Synergistic antitumor activity of
gemcitabine combined with triptolide in pancreatic cancer cells.
Oncol Lett. 11:3527–3533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Pan GF, Jiang ZZ, Yang J, Sun LX and
Zhang LY: Triptolide inhibits human breast cancer MDA-MB-231 cell
growth via downregulation of the ERα-mediated signaling pathway.
Acta Pharmacol Sin. 36:606–613. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peiró G, Adrover E, Sánchez-Tejada L,
Lerma E, Planelles M, Sánchez-Payá J, Aranda FI, Giner D and
Gutiérrez-Aviñó FJ: Increased insulin-like growth factor-1 receptor
mRNA expression predicts poor survival in immunophenotypes of early
breast carcinoma. Mod Pathol. 24:201–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Knowlden JM, Hutcheson IR, Barrow D, Gee
JM and Nicholson RI: Insulin-like growth factor-I receptor
signaling in tamoxifen-resistant breast cancer: a supporting role
to the epidermal growth factor receptor. Endocrinology.
146:4609–4618. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamamoto K, Ichijo H and Korsmeyer SJ:
BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal
protein kinase pathway normally activated at G(2)/M. Mol Cell Biol.
19:8469–8478. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ciriello G, Gatza ML, Beck AH, Wilkerson
MD, Rhie SK, Pastore A, Zhang H, McLellan M, Yau C, Kandoth C, et
al: TCGA research network, perou cm. comprehensive molecular
portraits of invasive lobular breast cancer. Cell. 163:506–519.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumours. Nature. 90:61–70.
2012. View Article : Google Scholar
|
|
25
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: an open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen J, Bardes EE, Aronow BJ and Jegga AG:
ToppGene Suite for gene list enrichment analysis and candidate gene
prioritization. Nucleic Acids Res 37(Web Server issue). W305–W311.
2009. View Article : Google Scholar
|
|
27
|
Elstrodt F, Hollestelle A, Nagel JH, Gorin
M, Wasielewski M, van den Ouweland A, Merajver SD, Ethier SP and
Schutte M: BRCA1 mutation analysis of 41 human breast cancer cell
lines reveals three new deleterious mutants. Cancer Res 1:.
66:41–45. 2006. View Article : Google Scholar
|
|
28
|
Wan CK, Wang C, Cheung HY, Yang M and Fong
WF: Triptolide induces Bcl-2 cleavage and mitochondria dependent
apoptosis in p53-deficient HL-60 cells. Cancer Lett. 241:31–41.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chueh FS, Chen YL, Hsu SC, Yang JS, Hsueh
SC, Ji BC, Lu HF and Chung JG: Triptolide induced DNA damage in
A375.S2 human malignant melanoma cells is mediated via reduction of
DNA repair genes. Oncol Rep. 29:613–618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Davison Z, de Blacquière GE, Westley BR
and May FEB: Insulin-like Growth Factor-Dependent Proliferation and
Survival of Triple-Negative Breast Cancer Cells: Implications for
Therapy. Neoplasia. 13:504–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeon JH, Kim SK, Kim HJ, Chang J, Ahn CM
and Chang YS: Insulin-like growth factor-1 attenuates
cisplatin-induced gammaH2AX formation and DNA double-strand breaks
repair pathway in non-small cell lung cancer. Cancer Lett.
272:232–241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Railo MJ, von Smitten K and Pekonen F: The
prognostic value of insulin-like growth factor-I in breast cancer
patients. Results of a follow-up study on 126 patients. Eur J
Cancer 30A. 307–311. 1994. View Article : Google Scholar
|
|
33
|
Hartog H, Horlings HM, van der Vegt B,
Kreike B, Ajouaou A, van de Vijver MJ, Marike Boezen H, de Bock GH,
van der Graaf WT and Wesseling J: Divergent effects of insulin-like
growth factor-1 receptor expression on prognosis of estrogen
receptor positive versus triple negative invasive ductal breast
carcinoma. Breast Cancer Res Treat. 129:725–736. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martin JL and Baxter RC: Expression of
insulin-like growth factor binding protein-2 by MCF-7 breast cancer
cells is regulated through the phosphatidylinositol
3-kinase/AKT/mammalian target of rapamycin pathway. Endocrinology.
148:2532–2541. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Y, Moerkens M, Ramaiahgari S, de
Bont H, Price L, Meerman J and van de Water B: Elevated
insulin-like growth factor 1 receptor signaling induces
antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling
routes. Breast Cancer Res. 13:R522011. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jones HE, Goddard L, Gee JM, Hiscox S,
Rubini M, Barrow D, Knowlden JM, Williams S, Wakeling AE and
Nicholson RI: Insulin-like growth factor-I receptor signalling and
acquired resistance to gefitinib (ZD1839; Iressa) in human breast
and prostate cancer cells. Endocr Relat Cancer. 11:793–814. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Riedemann J and Macaulay VM: IGF1R
signalling and its inhibition. Endocr Relat Cancer. 13 (Suppl
1):S33–S43. 2006. View Article : Google Scholar : PubMed/NCBI
|