Introduction
Breast cancer is the leading type of cancer
affecting females worldwide and accounts for 25% of cancer
incidence among women (1,2). Epidemiological analyses from the
Surveillance, Epidemiology, and End Results Program (SEER) database
in the USA have indicated that the majority (almost 60%) of breast
cancer cases are diagnosed at 65 years or older and >30% of the
cases are diagnosed over the age of 70, thus rendering breast
cancer the leading cause of cancer-related mortality among women
older than 65 years of age (3).
Surgery, radiotherapy and systemic therapy,
including chemotherapy and endocrine therapy, are the most common
treatment methods for patients with breast cancer. However, the
administration of systemic therapy has been shown to be associated
with cognitive dysfunction described as ‘chemobrain’ or
chemotherapy-related cognitive dysfunction (4,5).
‘Chemobrain’ refers to limitations in memory, concentration,
learning ability, processing speed, language and executive function
(4,6). Although the majority of studies
describe cognitive dysfunction related to chemotherapy as a
reversible side-effect that usually occurs within 18 to 36 months
following diagnosis, a large retrospective analysis from the SEER
database including 18,360 patients indicated a long-term increased
risk of dementia (5,7).
Stress, as a consequence of significant
environmental factors, has also been shown to be associated with
poorer cognitive function and has been shown to exert a damaging
effect on brain regions involved in cognitive performance, such as
the prefrontal cortex and the hippocampus (8). Prolonged exposure to stress can
accelerate the aging process and increase the risk of developing
dementia and other cognitive deficits (9,10).
Patients with breast cancer have to cope with several types of
stress during their everyday life as a result of the disease itself
and the side-effects of the applied treatments (e.g., neuropathy,
bone pain, hair loss, etc.) (11,12). As
indicated by case-control studies, patients with breast cancer
undergoing active treatment have elevated stress levels compared to
healthy individuals (13,14). Of interest, recent evidence indicates
that younger patients experience higher stress levels as compared
to older ones (15).
Moreover, it is well known that normal aging affects
a wide range of cognitive domains, such as memory, processing
speed, executive function, shift of attention, visuospatial and
constructional skills (16).
Structural and functional changes mediate this decline, which has
been highlighted by neuropsychological and neuroimaging studies
(16–20). Nevertheless, the prevalence of
cognitive disorders, such as dementia, is higher among the elderly
(16,21). Recent advances in medicine and
technology, have led to major declines in mortality, not only of
younger-age patients, but also of older-age ones, resulting in a
marked increase in the elderly population worldwide. Thus, it has
become a great challenge to safeguard cognitive functions and to
preserve independence and functionality in the aging population.
Geriatric patients with breast cancer have increased supportive
care needs due to age-related functionality decline, more
comorbidities and a lower tolerance to treatment regimens, i.e.,
chemotherapy and radiotherapy (22).
However, more elderly patients are expected to be treated in the
future as, a recent analysis from the SEER database including
160,676 women aged 65 years and older (21,743 aged older than 80
years) indicated an overall survival benefit from chemotherapy,
particularly in those assessed as ‘good risk’ by geriatric
assessment tools (23).
The aim of this review was to shed light on the role
of systemic therapies, stress, and age in the cognitive function of
patients with early-stage breast cancer. In addition, this review
aimed to discuss the differential effects of chemotherapy and
stress on the cognitive function of elderly and younger patients
with breast cancer.
Data collection methods
Study design
For the purpose of this study, we conducted a
systematic review of the literature. A.P. and T.S. participated in
the literature search, study selection process and extraction of
the studies. Disagreements between the authors were resolved
through discussion or with the assistance of A.K. All authors
participated in the appraisal of the extracted studies.
Literature search
A search for articles in the English language
published from database inception until September 23, 2018 was
carried out on the PubMed, Scopus and Web of Science databases. The
combinations of keywords used were as follows: (‘breast cancer’ OR
‘breast carcin*’ OR ‘breast tumor*’) AND (cognit* OR ‘executive
function’ OR attention OR memory OR orientation OR perception OR
language OR verbal OR visual OR vigilance OR visuospatial OR
‘problem solving’ OR ‘processing speed’) AND chemotherapy AND
(stress OR stressor OR cortisol OR ‘perceived stress’). A second
search was also carried out using the terms (‘breast cancer’ OR
‘breast carcin*’ OR ‘breast tumor*’) AND (cognit* OR ‘executive
function’ OR attention OR memory OR orientation OR perception OR
language OR verbal OR visual OR vigilance OR visuospatial OR
‘problem solving’ OR ‘processing speed’) AND chemotherapy AND
(toxicity OR side-effect* OR ‘adverse event*’ OR chemobrain OR
chemofog OR ‘adverse react*’ OR ‘central nervous system’). In
addition, a snowball technique was utilized in order to include any
potential studies not revealed through this process. Issues of
related journals, reference lists of included studies, and other
relevant articles in the field were rummaged in an attempt to
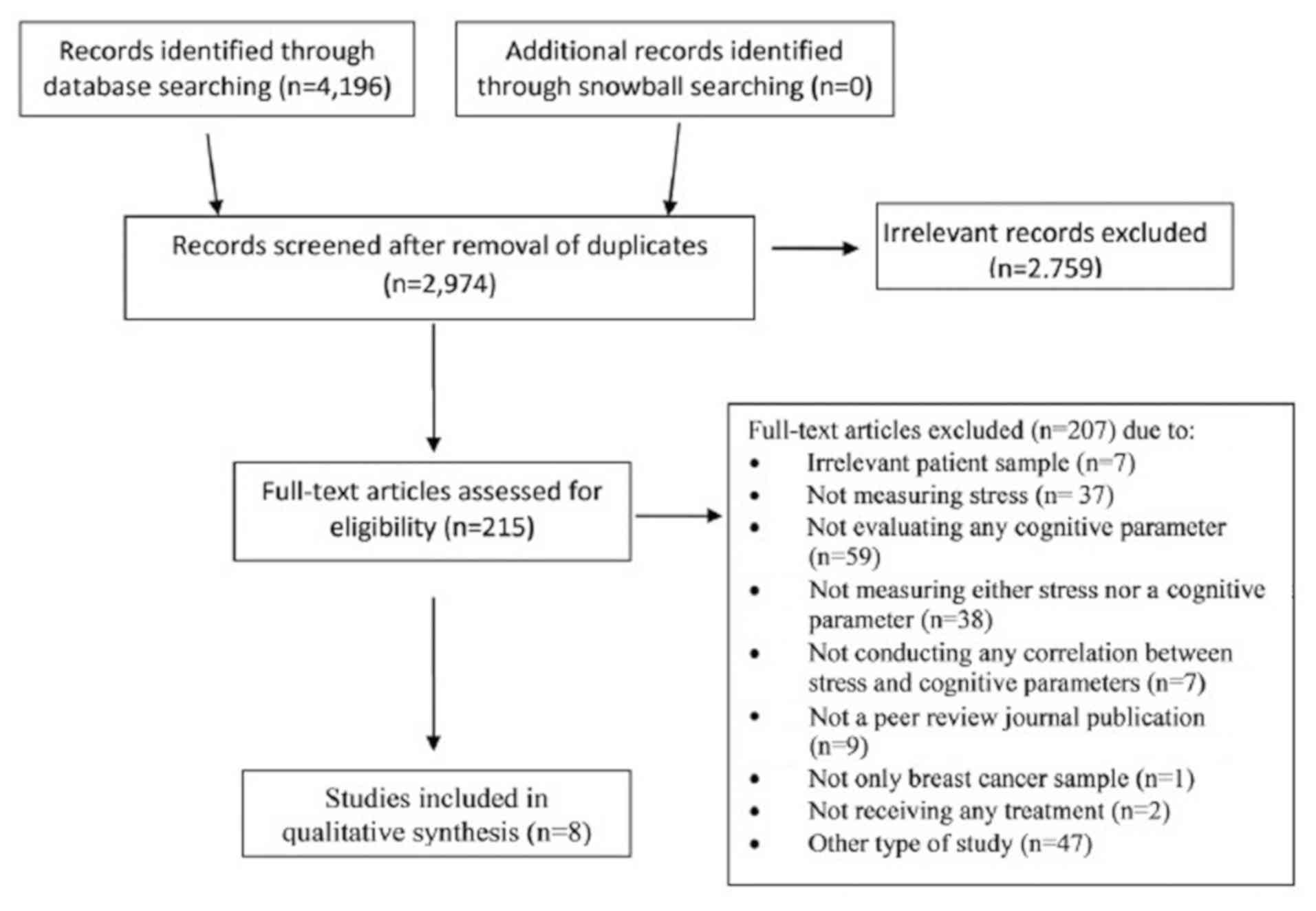
locate possible records. The flow of information from record
identification to inclusion followed the principles of the
Preferred Reporting Items for Systematic review and Meta-Analysis
Protocols statement (PRISMA) (24)
and is presented in Fig. 1.
Study selection
The inclusion criteria were as follows: i) Original
articles published in peer-reviewed journals; ii) studies including
elderly and/or non-elderly patients with breast cancer; and iii)
studies assessing stress and cognitive function of such patients
pre- and/or post-treatment. The exclusion criteria were the
following: i) Preclinical and interventional type of studies; and
ii) diagnosis of brain metastasis.
Extracted data
The extracted data of these articles included the
following: Name of study, country, total number of patients, total
number of participants, sample characteristics, measures of stress,
measures of cognitive function, timing of assessments, and
association of stress with cognitive parameters (Table I).
 | Table I.Extracted data from 8 studies
including a total number of 1,253 participants, of whom 800 were
patients with breast cancer. |
Table I.
Extracted data from 8 studies
including a total number of 1,253 participants, of whom 800 were
patients with breast cancer.
| Study [Author, year
(Ref.)] | Country | Study design | No. of
patients | Sample
characteristics | Measures of
stress | Measures of
cognitive parameters | Time of
measurements | Main findings |
|---|
| Cognitive
impairment in a subset of patients with breast cancer following
systemic therapy - results from a longitudinal study, Menning et
al, 2016 (26) | The
Netherlands | Longitudinal
case-control | 88 | Case BC+SYST group:
n=31, ≥70 years old, 39% post-menopausal. BC group: n=24, 54%
post-menopausal Control NC group: nn=33, ≥70 years old, 55%
post-menopausal | PSS IES (at
T2) | COWA test, BADS-Zoo
Map test, TMT parts A&B, EFT, VRT test, DS of the WAIS-III, VRT
of the WMS-R, HVL-R test, DSC test of the WAIS-III, FT | T1: Before the
initiation of the treatment T2: 6 months after the completion of
the treatment | At secondary
analysis, cognitively impaired patients exhibited statistically
significant higher levels of distress (P=0.019) in comparison with
the non-impaired patients |
| Effects of breast
cancer treatment on the hormonal and cognitive consequences of
acute stress, Andreano et al, 2012 (27) | USA
(California) | Case-control and
field experiment study | 40 | Case n=20, 27–49
years old, female breast cancer survivors treated with Lupron (6
additionally treated with aromatase inhibitors, 4 with tamoxifen
and 1 with chemotherapy). At the initiation total sample
premenopausal. Control n=20, 26–48 years old, healthy and naturally
cycled women | CPT Salivary
cortisol | Subtests from the
WMS-III (working memory, verbal paired associate memory and
narrative recall) | Salivary cortisol:
At baseline, 10, 20 and 30 min and 1 week after CPS test. WMS-III:
At baseline, after 1 week | Logical recall:
Significant decrease in total recall in week 2 but independently of
stress condition [F(1,36)=4.465, P<0.05, η2=0.019]. Significant
interaction between the drug and stress condition when testing
stories separately [F(3,36)=2.792, P<0.05, η2=0.018).
Significant positive correlation between recall of story A and 20
min′ salivary cortisol post-stressor (r=0.544, P<0.05, df=9).
The cortisol/memory association for story A differed significantly
between control and breast cancer groups by Fisher's z test
(z=2.18, P<0.05) |
| Measures of
cognitive function and work in occupationally active breast cancer
survivors, Calvio et al, 2010 (28) | USA (Washington
DC) | Case-control | 235 | Case n=122, 18–65
years old, female breast cancer survivors (non metastatic), working
≥1 year prior to the study, between 1 and 10 years of breast cancer
treatment completion (surgery, chemotherapy, HT, BT). Control
n=113, 18–65 years old, female non-cancer participants, without a
prior diagnosis of any type of cancer | Behavioral risk
factor surveillance survey (1 item used to assess job stress in
this study) | Perceived cognitive
function: CSC-modified performance-based cognitive function:
CNSVS | A single
measurement following a screening to determine the eligibility of
the participants | Case: Job stress
had a significant impact on the cognitive limitations at work
either according to performance (β=0.29, P<0.01), or according
to the patients' reports (β=0.29, P<0.01). Control: β indicator
did not show a statistically significant correlation (β=−0.06 and
β=0,06, respectively) |
| Cognitive function
and quality of life after surgery for early breast cancer in North
Jutland, Denmark, Debess et al, 2009 (29) | Denmark (North
Jutland) | Case-control | 348 | Case n=124, <60
years old female, right after primary breast cancer surgery, no
metastasis. Control n=224, female, age matched, not prior history
of any type of cancer | POMS | Four questions
about memory, concentration/attention, mental fatigue and vitality
from the ISPOCD 2 and 4 tests: VL test, CS test, SCWI test, LDC
test. DART | All tests were
administered in a mean 34.5 days after surgery (19–75 days) for the
cases and at the date of inclusion for the controls | Case: Psychological
stress was significantly correlated with subjective cognitive
function (Spearman's rho: −0.3 to −0.4, P<0.05). Control: Fewer
correlations between POMs' subscales and subjective cognitive
function were found (Spearman's rho: −0.2 to −0.3, P<0.05) |
| Symptom cluster of
emotional distress, fatigue and cognitive difficulties among young
and older breast cancer survivors: The mediating role of subjective
stress, Levkovich et al, 2018, (30) | Israel |
Cross-sectional | 170 | Case N=120 BCS aged
20–59 and N=50 BCS aged 60–82, stages I–III, 1–12 months
post-chemotherapy, patients receiving HT and BT included, with no
current evidence of disease | Subjective stress
scale BSI 18 | Cognitive
difficulties scale (four-item subscale taken from the EORTC
QLQ) | All tests were
administered within 1–12 months post-chemotherapy | The older-aged
survivors reported lower levels of subjective stress (M=4.59,
SD=2.38) and cognitive difficulties (M=1.17, SD=1.07), compared to
the younger-aged survivors (M=5.38, SD=2.84 and M=1.66, SD=1.23,
P<0.01–0.05). The association between age and cognitive
difficulties was linear and negative, and statistically
significant, meaning that the higher the age, the lower the
symptoms. Subjective stress mediated the effect of age on cognitive
difficulties |
| Modifiable
correlates of perceived cognitive function in breast cancer
survivors up to 10 years after chemotherapy completion, Henneghan
et al, 2018, (31) | USA (Texas) |
Cross-sectional | 90 | Case Ν=90 BCS 6
months to 10 years post-chemotherapy (average 3 years), patients
receiving HT included. Age 20–65 years, stages I–III. | PSS | FACT-Cog | All tests were
administered within 6 months to 10 years post-chemotherapy | Mediation analyses
revealed that stress both directly and indirectly impacts perceived
cognitive function (P<0.02) according to the SEM model
(P<0.01–0.05) |
| Effects of
childhood trauma exposure and cortisol levels on cognitive
functioning among breast cancer survivors, Kamena et al,
2017 (32) | USA |
Cross-sectional | 56 | Case n=56 BCS, aged
≥21 years, mean age, 53.6 years, SD=9.8 ≥2 weeks post treatment
(except HT), stages I–IV | TES Salivary
cortisol levels | FACT-Cog | T1: Upon waking T2:
30 min after waking; T3: at 9:00 p.m. for 2 days | Childhood trauma
exposure is associated with self-reported cognitive functioning
among breast cancer survivors and is mediated by cortisol
dysregulation, as shown by steeper cortisol slope in the exposed
patients (P=0.02) |
| Chemotherapy and
post-traumatic stress in the causation of cognitive dysfunction in
breast cancer patients, Hermelink et al, 2017 (33) | Germany | Longitudinal,
case-control | 226 | Case n=166 BC
patients, aged 18 and 65 years, female sex, diagnosed with yet
untreated stage 0 to IIIc BC, scheduled to receive appropriate
treatment Control n=60, female, age matched, negative BC imaging or
not prior history of any type of cancer | Structured clinical
interview according to DSM-IV criteria for PTSD | Alternate forms of
the VLMT cognitive difficulties scale (four-item subscale taken
from the EORTC QLQ) FEDA | T1: Prior to
primary surgery or neoadjuvant chemotherapy for breast cancer
patients and a minimum of one week after negative breast imaging
for control subjects; T2: ≥1 week after completion of chemotherapy
or at matched intervals after T1 T3: 1 year after T1. | Case: Patients
demonstrated overall cognitive decline (group*time effect on
composite z-score: −0.13, P ¼ .04) and scored consistently worse on
Go/Nogo errors. The latter effect was mediated by PTSD symptoms
(mediation effect: B ¼ 0.15, 95% confidence interval ¼ 0.02 to
0.38). Only patients receiving chemotherapy exhibited a declined
reaction time on a computerized alertness test at 1 year. Overall,
cognitive performance correlated with self-reported cognitive
problems at one year (T3) (T ¼ −0.11, P ¼ .02). Control: Controls
performed consistently better than patients on a computerized
measure of behavioral control and Go/No go errors |
Appraisal of the quality of the
studies
The quality of the included studies was evaluated
using the Newcastle-Ottawa Scale proposed by the Cochrane
Non-Randomized Studies Methods Working Group (25). This instrument was selected as it can
be applied to evaluate studies involving different methodological
study designs in a single assessment process. In particular, as
regards the assessment of case-control studies, the
Newcastle-Ottawa Scale includes 8 items which control for the
selection of the cases and controls (adequate definition and
representativeness of the cases, selection and definition of
controls), the comparability between cases and controls with
respect to certain factors (i.e., age, sex, etc.) and the degree of
exposure (method of ascertainment of exposure, similarity of method
for cases and controls, non-response rates for groups). A maximum
of 4 stars can be given for the selection subscale, 2 for the
comparability subscale and 3 for the exposure subscale, summing up
to a maximum of 9 stars (Table
II).
 | Table II.Newcastle-Ottawa scale (NOS) for the
assessment of the quality of case control studies (adjusted to the
criteria of the present systematic review). |
Table II.
Newcastle-Ottawa scale (NOS) for the
assessment of the quality of case control studies (adjusted to the
criteria of the present systematic review).
| NOS scale | Studies presented
according to fulfilled criterion (★); Author, year (Ref.) |
|---|
|
|---|
| A, Selection
(maximum 4) | Control group
characteristics | Menning et
al, 2016 (26) | Andreano et
al, 2011 (27) | Calvio et
al, 2010 (28) | Debess et
al, 2009 (29) | Levkovich et
al, 2018 (30) | Henneghan et
al, 2018 (31) | Kamena et
al, 2017 (32) | Hermenlik et
al, 2017 (33) |
|---|
| 1. Case definition
adequate | With independent
validation | ★ | ★ |
| ★ |
| ★ | ★ | ★ |
| 2.
Representativeness of the cases | Consecutive or
obviously representative series of cases | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
| 3. Selection of
controls | Community
controls |
|
| ★ | ★ |
|
|
| ★ |
| 4. Definition of
control | No history of
breast cancer | ★ |
| ★ | ★ |
|
|
| ★ |
|
| B, Comparability
(maximum 2) | Control group
characteristics | Menning et
al, 2016 (26) | Andreano et
al, 2011 (27) | Calvio et
al, 2010 (28) | Debess et
al, 2009 (29) | Levkovich et
al, 2018 (30) | Henneghan et
al, 2018 (31) | Kamena et
al, 2017 (32) | Hermenlik et
al, 2017 (33) |
|
| 1. Comparability of
cases and controls on the basis of the design or analysis | Study controls
matched for age and sex | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
| 2. Study controls
for any additional factor | Study controls
matched for age of menarche, menopausal status, age at any
childbirth, lifetime estrogen exposure, working status, educational
status, marital status, premorbid IQ, income, current occupation,
primary occupation, medication for other physical or psychiatric
diseases and/or contraceptives use etc. | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
|
| C. Exposure
(maximum 3) | Control group
characteristics | Menning et
al, 2016 (26) | Andreano et
al, 2011 (27) | Calvio et
al, 2010 (28) | Debess et
al, 2009 (29) | Levkovich et
al, 2018 (30) | Henneghan et
al, 2018 (31) | Kamena et
al, 2017 (32) | Hermenlik et
al, 2017 (33) |
|
| 1. Ascertainment of
exposure | Secure record or
structured interview where blind to case/control status |
|
|
| ★ |
|
| ★ |
|
| 2. Same method
cases and controls | Yes |
|
| ★ |
| ★ |
|
| ★ |
| 3. Non-response
rate | Same rate for both
groups | ★ |
|
| ★ |
|
|
| – |
| Total quality score
(maximum 9) |
| 6 | 4 | 6 | 8 | 5 | 4 | 5 | 7 |
Interpretation of the collected data
Of the 4,196 study records retrieved through
database searching, 8 met the pre-set eligibility criteria and were
included in this review (Fig. 1)
(26–33). Of these, 6 were case-control studies
(26–30,33) and
2 that had no control group used literature data for comparative
analysis (31,32). Only 2 of the studies were
longitudinal and assessed the participants both before and at
standard times following the completion of chemotherapy (26,33).
From the point of view of age, 2 studies included patients under
and over 65 years of age (26,30), but
only one analyzed them as a separate group (30). With respect to systemic treatment, 1
study examined patients just prior to the initiation of adjuvant
treatment (29), 2 studies included
patients on active chemotherapy (26,33) and
5 studies described patients who had completed chemotherapy from 2
weeks up to 10 years earlier (27,28,30–32). Two
studies included groups of patients who had only received surgery
at the time of the assessment, either prior to the initiation of
chemotherapy or not requiring further treatment (26,33). In
all the studies, the study participants could have received
additional types of treatment (i.e., endocrine treatment and
radiotherapy), apart from surgery and chemotherapy
In total, 1,253 women were included in the analysis.
Different aspects of stress were investigated in different studies,
namely generalized stress (26,29–31),
job-related stress (28),
trauma-related stress (32,33) and physiological response to stress
(27). Accordingly, stress
assessment methods varied among the studies. Five studies used
validated questionnaires (26,29–32). One
study used a single item of a battery to assess the responders'
job-related stress (28). One study
used a structured clinical interview for post-traumatic stress
disorder symptoms (33) and 2
studies measured cortisol levels during the day and under a
laboratory controlled task respectively (27,32). The
fields of cognitive function examined in the 8 studies were
attention (26,28,29,33)
cognitive flexibility (28,29) executive function (26,28,29,33)
concentration (29,30,33)
verbal and visual memory (26,28,29,33)
episodic memory (29), working
memory (27,28), verbal paired associated memory
(27) processing speed (26,29,33)
functional and verbal tasks (26,33)
stress-enhanced recall (27),
general intelligence (29),
perceived cognitive impairments, comments from others, perceived
cognitive abilities, impact on quality of life (31,32) and
work output (28).
All the studies demonstrated a statistically
significant association of stress with at least one cognitive
parameter in patients with breast cancer. One study reported a
significant association of perceived cognitive deficits with
psychological stress in chemotherapy-treated patients, although a
control group was not included (31). However, similar results were reported
from a study comparing non-chemotherapy-treated patients with a
healthy control group (29).
Specifically, a worse perceived cognitive function associated with
psychological distress in patients was also highlighted in a study
comparing non-chemotherapy-treated patients with a healthy control
group (29) despite the absence of
differences between patients and controls in the neuropsychological
testing. Moreover, Menning et al demonstrated that patients
with cognitive dysfunction experienced higher levels of anxiety
compared to the non-impaired, irrespectively of whether they were
receiving or adjuvant therapy not (26). Specifically, the authors found that
impaired patients exhibited a worse physical (P=0.002) and social
functioning (P=0.004), and more symptoms of anxiety and depression
(P=0.008) at 6 months post-chemotherapy, compared to the
non-impaired patients. With respect to job-related stress, this was
found to be significantly associated with both objective and
subjective cognitive limitations at work, compared to the controls
(28). In a study with an
experimental stressor task, the patients with breast cancer
exhibited neither cortisol release nor memory enhancement in
response to the stressor, unlike the healthy controls (27). Two studies looking into the
trauma-related stress found a stress-mediated association between
cognitive dysfunction in chemotherapy-treated breast cancer
patients, as reported in both subjective (32,33) and
objective cognitive assessments (33). As regards older-aged patients treated
with chemotherapy, it was demonstrated that lower levels of
subjective stress mediated the inverse association of age with
self-rated cognitive deficits, as opposed to younger-aged patients
(30).
As for the quality appraisal of the included
studies, it was found to be modest as for the selection subscale,
perfect as for the comparability scale, and insufficient as for the
exposure subscale. The total scoring of the Newcastle-Ottawa Scale
for the eight studies ranged from 4 to 8 stars. A detailed display
of the qualitative analysis of the eligible studies of this
systematic review according to the criteria of the Newcastle-Ottawa
Scale is presented in Table II.
Involvement of stress, age and adjuvant
therapy on cognitive function of patients with breast cancer
This review aimed to search for the association
between stress and cognitive function in systematically treated
patients with breast cancer, bearing in mind the role of age in
this association. The results obtained from our search indicated
that research to date has mainly focused on non-elderly patients
with the exception of one study (30). The scarcity of such results
downgrades the comparability of the findings, but does not debar a
critical appraisal of the results.
In total, all of the included studies revealed an
association of some aspect of stress with some cognitive
parameters. Both generalized stress, job stress and post-traumatic
stress disorder symptoms do seem to mediate cognitive deficits in
systemically treated patients, although such difficulties were
reported, yet to a lesser degree, to breast cancer patients not
receiving systematic treatment (26,29,33). Age
was found to be ass with lower stress levels in patients with
breast cancer >65 years of age (30), which is in line with previous studies
(14,34–36) and
the lower stress levels were in turn associated with better
cognitive function, as shown in the studies included in this
review.
Depression is an important confounding factor that
should be considered, keeping in mind the well-known burdening
effect it has on cognitive function and the increased prevalence of
affective disorders in oncologic patients (37,38).
Only one study did not conduct an assessment of depression
(27). Five studies assessed and
included depression in a regression analysis/linear mixed effects
model, with no significant changes of the main findings (28,30,31–33). In
2 studies (26,29), depression as a covariate was not
associated with objective tests of cognitive function. Thus, we
have grounds to believe that our results were not affected by the
presence of a mood disorder.
Moreover, a factor increasing the internal validity
of our findings is the use of neuropsychological assessments to
evaluate cognitive function, which are considered highly reliable
(39).
Future research should focus on the investigation of
the stress-mediated cognitive dysfunction in elderly patients with
breast cancer, since as indicated by the results of this systematic
review, only studies for the non-elderly population were located.
Particular emphasis should be placed on the cognitive domains that
tend to decline with age, i.e., memory, processing speed, etc., as
opposed to those which remain relatively intact, such as language
skills. Studies have shown that cognitive function is impaired for
at least 2 years following diagnosis and treatment in elderly
patients, whereas follow-up measurements in studies with younger
patients have not shown such a maintained effect. A large
longitudinal Chinese study of 1,300 chemotherapy-treated patients
with breast cancer between the ages of 20 and 75 demonstrated
significant cognitive improvement at the 18 and 36 months of
follow-up; however, this improvement was significantly lower for
older patients (4). Likewise, a
Canadian study of 100 patients with breast cancer with a median age
of 48 years revealed a significant reduction in adjuvant
therapy-related cognitive deficits at 1 and 2 years post-treatment
(40). By contrast, cognitive
dysfunction seemed to persist for at least 2 years among a
tamoxifen-treated population of 179 Dutch post-menopausal patients
with breast cancer with a mean age of 68 years (41). Still, a long-term dysfunction in
brain areas related to cognitive function has been shown in
Positron Emission Tomography studies of younger patients; however,
since there are no neuroimaging studies investigating long-term
cognitive function in older patients, a comparison between the two
age groups is of greater difficulty (42,43).
Furthermore, since cognitive dysfunction is a
partially stress-mediated treatment side-effect, future research
should look into a potentially beneficial effect of stress
management techniques on the cognitive function of patients with
breast cancer. These types of methods, i.e., progressive muscle
relaxation, either on their own or as a part of combinational
programs including different techniques, have been shown to
decrease the psychological burden and stress levels in this patient
group (44,45). Therefore, clinicians should keep a
low threshold for offering psychotherapy and medication treatment
for stress in these patients. An integrative model of medical care,
in which the various patients' needs (e.g., medical, nursing,
psychosocial) are compiled, and has been shown to improve their
quality of life and, possibly, clinical outcomes (46).
Limitations to the data interpretation
Despite the clinical importance of stress management
in patients with breast cancer, there are several limitations to
take into account as regards the interpretation of our results,
particularly given the heterogeneity of the included studies.
Significant differences were noted regarding treatments used or
combined (chemotherapy, endocrine treatment and radiotherapy, with
or without surgery), patient traits (as per age and various
demographic data, i.e., level of education) and methodologies
utilized.
First, in all groups treated with chemotherapy,
patients receiving other types of systematic treatment (i.e.,
endocrine therapy ± radiotherapy) were not excluded; therefore, any
outcome on cognition cannot be solely interpreted as a direct
effect of chemotherapy. It is well known that beyond chemotherapy,
endocrine therapy with or without radiotherapy have also been
linked to the impairment of the cognitive function of patients with
breast cancer (47–49). Still, the majority of patients, in
the publications included in this review, were either under
chemotherapy at the time of the study or had received chemotherapy
from 2 weeks up to 10 years earlier had long-lasting cognitive
impairment (50–52).
In addition, there may be a possible bias in the
assessment of cognitive function, given that 3 of the studies
(30–32) used self-rated questionnaires, whereas
4 of them used both objective and subjective methods (26,28,29,33).
Therefore, recall bias and an ‘over-alertness’ of patients
regarding cognitive symptoms, in addition to psychological reasons,
may have affected the cognitive function assessment. However,
interestingly enough, 2 two of the studies that used both methods,
subjective evaluation correlated with objective performance
(28,33).
Moreover, all studies including neuropsychological
assessments are vulnerable to external validity limitations. These
batteries may be both valid and reliable, but they do not reflect
the responder's performance in the ‘real world’ and thus the use of
such measurements decreases the generalizability of the findings.
In other words, they burden a small effect size (53). In this review, this limitation was
obvious in the study by Calvio et al (28), where the objective cognitive
assessment failed to predict a decline in work output of patients,
in contrast to the self-evaluated cognitive function
instrument.
As far as the internal validity is concerned,
according to the Newcastle-Ottawa Scale scoring, the ascertainment
of exposure in patients and healthy individuals was not adequate in
6 out of 8 studies (26–28,30,31,33).
Furthermore, only 3 have reported same method of exposure
ascertainment in patients and a healthy population (28,30,33).
These findings indicate that there is a high risk of bias for the
results of the majority of studies (26,27,29–32).
Finally, there is also a concern regarding the use
of cortisol levels to assess stress in 2 of the studies (27,32).
Previous data have yielded inconsistent results of a correlation
between self-reported measures and stress biomarkers, including
cortisol (54). Furthermore, a
relevant study on patients with breast cancer assessing stress
through the perceived stress scale and cortisol levels found no
association between these measurements (55). Taken together, all the above are
questioning the reliability of cortisol as a stress-related
indicator in this patient group.
Hence, our findings are better interpreted as a
general tendency to a correlation between stress and cognitive
function in breast cancer patients, rather than a firm
deduction.
In conclusion, this review supports that the
cognitive dysfunction of patients with breast cancer may be
partially stress-related, at least among the non-elderly
population. Future research should investigate a potential effect
of stress on the cognitive function of elderly patients,
considering their increased risk for age-related cognitive
disorders. Moreover, oncologists should be aware that even though
chemotherapy has a considerable impact on the cognitive function of
patients with breast cancer, this phenomenon may be partially
stress-related, and therefore it should be addressed promptly.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
All authors (AP, TS, ER, KK, AN, NA, CT, DAS and AK)
contributed to the data analysis and the writing of the manuscript.
AK was also involved in the conception of the study. AP, TS and AK
were also involved in the literature search for this systematic
review. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Ward E and Thun MJ: Recent trends
in breast cancer incidence rates by age and tumor characteristics
among U.S. women. Breast Cancer Res. 9:R282007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moore HC: An overview of
chemotherapy-related cognitive dysfunction, or ‘chemobrain’.
Oncology (Williston Park). 28:797–804. 2014.PubMed/NCBI
|
|
5
|
Zheng Y, Luo J, Bao P, Cai H, Hong Z, Ding
D, Jackson JC, Shu XO and Dai Q: Long-term cognitive function
change among breast cancer survivors. Breast Cancer Res Treat.
146:599–609. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bompaire F, Durand T, Léger-Hardy I,
Psimaras D and Ricard D: Chemotherapy-related cognitive impairment
or ‘chemobrain’: concept and state of art. Geriatr Psychol
Neuropsychiatr Vieil. 15:89–98. 2017.PubMed/NCBI
|
|
7
|
Heck JE, Albert SM, Franco R and Gorin SS:
Patterns of dementia diagnosis in surveillance, epidemiology, and
end results breast cancer survivors who use chemotherapy. J Am
Geriatr Soc Sep. 56:1687–1692. 2008. View Article : Google Scholar
|
|
8
|
Jay TM, Rocher C, Hotte M, Naudon L,
Gurden H and Spedding M: Plasticity at hippocampal to prefrontal
cortex synapses is impaired by loss of dopamine and stress:
Importance for psychiatric diseases. Neurotox Res. 6:233–244. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pecori Giraldi F, Moro M and Cavagnini F;
Study Group on the Hypothalamo-Pituitary-Adrenal Axis of the
Italian Society of Endocrinology, : Gender-related differences in
the presentation and course of Cushing's disease. J Clin Endocrinol
Metab. 88:1554–1558. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsolaki M: Post traumatic stress dementia:
A new target for cholinesterase inhibitors. Neurobiol Aging.
25:26–27. 2004.
|
|
11
|
Boehmke MM and Dickerson SS: Symptom,
symptom experiences, and symptom distress encountered by women with
breast cancer undergoing current treatment modalities. Cancer Nurs.
28:382–389. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Günüşen NP, İnan FŞ and Üstün B:
Experiences of Turkish women with breast cancer during the
treatment process and facilitating coping factors. Asian Pac J
Cancer Prev. 14:3143–3149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hinnen C, Ranchor AV, Sanderman R,
Snijders TA, Hagedoorn M and Coyne JC: Course of distress in breast
cancer patients, their partners, and matched control couples. Ann
Behav Med. 36:141–148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hoskins CN: Breast cancer
treatment-related patterns in side effects, psychological distress,
and perceived health status. Oncol Nurs Forum. 24:1575–1583.
1997.PubMed/NCBI
|
|
15
|
Berhili S, Kadiri S, Bouziane A, Aissa A,
Marnouche E, Ogandaga E, Echchikhi Y, Touil A, Loughlimi H, Lahdiri
I, et al: Associated factors with psychological distress in
Moroccan breast cancer patients: A cross-sectional study. Breast.
31:26–33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murman DL: The impact of age on cognition.
Semin Hear. 36:111–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oh H, Mormino EC, Madison C, Hayenga A,
Smiljic A and Jagust WJ: β-amyloid affects frontal and posterior
brain networks in normal aging. Neuroimage. 54:1887–1895. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dennis EL and Thompson PM: Functional
brain connectivity using fMRI in aging and Alzheimer's disease.
Neuropsychol Rev. 24:49–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wisdom NM, Mignogna J and Collins RL:
Variability in Wechsler Adult Intelligence Scale-IV subtest
performance across age. Arch Clin Neuropsychol. 27:389–397. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oosterman JM, Vogels RL, van Harten B,
Gouw AA, Poggesi A, Scheltens P, Kessels RP and Scherder EJ:
Assessing mental flexibility: Neuroanatomical and
neuropsychological correlates of the Trail Making Test in elderly
people. Clin Neuropsychol. 24:203–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park DC and Festini SB: Theories of memory
and aging: A look at the past and a glimpse of the future. J
Gerontol B Psychol Sci Soc Sci. 72:82–90. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gosain R, Pollock Y and Jain D:
Age-related disparity: Breast cancer in the elderly. Curr Oncol
Rep. 18:692016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sinha S, Panebianco L, Wu X, Wang D, Huang
D and Sivapiragasam A: Abstract GS2-02: Efficacy and utilization
trends of adjuvant chemotherapy for stage I, II, and III breast
cancer in the elderly population: A National Cancer Database (NCDB)
analysis. San Antonio Breast Cancer Symposium (San Antonio).
2018.
|
|
24
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; the PRISMA Group, : Preferred reporting items for systematic
reviews and meta-analyses: The PRISMA statement. PLoS Medicine.
6:e10000972009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in metaanalyses.
Ottawa Health Research Institute. (Ottawa). 1999.
|
|
26
|
Menning S, de Ruiter MB, Kieffer JM,
Agelink van Rentergem J, Veltman DJ, Fruijtier A, Oldenburg HS,
Boven E, van der Meij S, Lustig V, et al: Cognitive impairment in a
subset of breast cancer patients after systemic therapy-results
from a longitudinal study. J Pain Symptom Manage. 52:560–569.e1.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Andreano JM, Waisman J, Donley L and
Cahill L: Effects of breast cancer treatment on the hormonal and
cognitive consequences of acute stress. Psychooncology.
21:1091–1098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Calvio L, Peugeot M, Bruns GL, Todd BL and
Feuerstein M: Measures of cognitive function and work in
occupationally active breast cancer survivors. J Occup Environ Med.
52:219–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Debess J, Riis JØ, Pedersen L and Ewertz
M: Cognitive function and quality of life after surgery for early
breast cancer in North Jutland, Denmark. Acta Oncol. 48:532–540.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Levkovich I, Cohen M, Alon S, Kuchuk I,
Nissenbaum B, Evron E, Pollack S and Fried G: Symptom cluster of
emotional distress, fatigue and cognitive difficulties among young
and older breast cancer survivors: The mediating role of subjective
stress. J Geriatr Oncol. 9:469–475. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Henneghan A, Stuifbergen A, Becker H,
Kesler S and King E: Modifiable correlates of perceived cognitive
function in breast cancer survivors up to 10 years after
chemotherapy completion. J Cancer Surviv. 12:224–233. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kamen C, Scheiber C, Janelsins M, Jo B,
Shen H and Palesh O: Effects of childhood trauma exposure and
cortisol levels on cognitive functioning among breast cancer
survivors. Child Abuse Negl. 72:163–171. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hermelink K, Bühner M, Sckopke P, Neufeld
F, Kaste J, Voigt V, Münzel K, Wuerstlein R, Ditsch N, Hellerhoff
K, et al: Chemotherapy and post-traumatic stress in the causation
of cognitive dysfunction in breast cancer patients. J Natl Cancer
Inst. May 3–2017.(Epub ahead of print). doi:
https://doi.org/10.1093/jnci/djx057. View Article : Google Scholar
|
|
34
|
Reyes-Gibby CC, Anderson KO, Morrow PK,
Shete S and Hassan S: Depressive symptoms and health-related
quality of life in breast cancer survivors. J Womens Health
(Larchmt). 21:311–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ehlers DK, Aguiñaga S, Cosman J, Severson
J, Kramer AF and McAuley E: The effects of physical activity and
fatigue on cognitive performance in breast cancer survivors. Breast
Cancer Res Treat. 165:699–707. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang H, Brand JS, Fang F, Chiesa F,
Johansson AL, Hall P and Czene K: Time-dependent risk of
depression, anxiety, and stress-related disorders in patients with
invasive and in situ breast cancer. Int J Cancer. 140:841–852.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McDermott LM and Ebmeier KP: A
meta-analysis of depression severity and cognitive function. J
Affect Disord. 119:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim JI, Song Y, Lee JH, Kim HJ, Hong EJ,
Kim SA, Jun YS and Chang OJ: Depression, stress and self-esteem
according to treatment phase in patients with breast cancer. Indian
J Sci Technol. 8:1–7. 2015. View Article : Google Scholar
|
|
39
|
Franzen MD: Practical and methodological
considerations regarding reliability. In: Reliability and Validity
in Neuropsychological Assessment. Springer Science and Business
Media. (New York, NY). 15–26. 2013.
|
|
40
|
Fan HG, Houédé-Tchen N, Yi QL, Chemerynsky
I, Downie FP, Sabate K and Tannock IF: Fatigue, menopausal
symptoms, and cognitive function in women after adjuvant
chemotherapy for breast cancer: 1- and 2-year follow-up of a
prospective controlled study. J Clin Oncol. 23:8025–8032. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schilder CM, Seynaeve C, Beex LV, Boogerd
W, Linn SC, Gundy CM, Huizenga HM, Nortier JW, van de Velde CJ, van
Dam FS, et al: Effects of tamoxifen and exemestane on cognitive
functioning of postmenopausal patients with breast cancer: Results
from the neuropsychological side study of the tamoxifen and
exemestane adjuvant multinational trial. J Clin Oncol.
28:1294–1300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Silverman DH, Dy CJ, Castellon SA, Lai J,
Pio BS, Abraham L, Waddell K, Petersen L, Phelps ME and Ganz PA:
Altered frontocortical, cerebellar, and basal ganglia activity in
adjuvant-treated breast cancer survivors 5–10 years after
chemotherapy. Breast Cancer Res Treat. 103:303–311. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
de Ruiter MB, Reneman L, Boogerd W,
Veltman DJ, Caan M, Douaud G, Lavini C, Linn SC, Boven E, van Dam
FS, et al: Late effects of high-dose adjuvant chemotherapy on white
and gray matter in breast cancer survivors: Converging results from
multimodal magnetic resonance imaging. Hum Brain Mapp.
33:2971–2983. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pelekasis P, Matsouka I and Koumarianou A:
Progressive muscle relaxation as a supportive intervention for
cancer patients undergoing chemotherapy: A systematic review.
Palliat Support Care. 15:465–473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pelekasis P, Zisi G, Koumarianou A,
Marioli A, Chrousos G, Syrigos K and Darviri C: Forming a stress
management and health promotion program for women undergoing
chemotherapy for breast cancer: A pilot randomized controlled
trial. Integr Cancer Ther. 15:165–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Maizes V, Rakel D and Niemiec C:
Integrative medicine and patient-centered care. Explore (NY).
5:277–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cimprich B, So H, Ronis DL and Trask C:
Pre-treatment factors related to cognitive functioning in women
newly diagnosed with breast cancer. Psychooncology. 14:70–78. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wefel JS, Lenzi R, Theriault R, Buzdar AU,
Cruickshank S and Meyers CA: ‘Chemobrain’ in breast carcinoma?: A
prologue. Cancer. 101:466–475. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hermelink K, Untch M, Lux MP, Kreienberg
R, Beck T, Bauerfeind I and Münzel K: Cognitive function during
neoadjuvant chemotherapy for breast cancer: Results of a
prospective, multicenter, longitudinal study. Cancer.
109:1905–1913. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schagen SB, Muller MJ, Boogerd W,
Mellenbergh GJ and van Dam FS: Change in cognitive function after
chemotherapy: a prospective longitudinal study in breast cancer
patients. J Natl Cancer Inst. 98:1742–5. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Stewart A, Bielajew C, Collins B,
Parkinson M and Tomiak E: A meta-analysis of the neuropsychological
effects of adjuvant chemotherapy treatment in women treated for
breast cancer. Clin Neuropsychol. 20:76–89. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Héry C, Ferlay J, Boniol M and Autier P:
Quantification of changes in breast cancer incidence and mortality
since 1990 in 35 countries with Caucasian-majority populations. Ann
Oncol. 19:1187–1194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Franzen MD: Validity as applied to
neuropsychological assessment. In: Reliability and Validity in
Neuropsychological Assessment. Springer Science and Business Media.
(New York, NY). 33–54. 2013.
|
|
54
|
Hellhammer DH, Wüst S and Kudielka BM:
Salivary cortisol as a biomarker in stress research.
Psychoneuroendocrinology. 34:163–171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wu SM, Yang HC, Thayer JF and Andersen BL:
Association of the physiological stress response with depressive
symptoms in patients with breast cancer. Psychosom Med. 76:252–256.
2014. View Article : Google Scholar : PubMed/NCBI
|