Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed type of cancer and was the second leading cause of
cancer-associated mortalities in men and women in 2018 globally.
Annually, >1.2 million patients are diagnosed with CRC, whereas
>600,000 mortalities occur due to the disease (1). The development of CRC is primarily
associated with the western lifestyle. The prevalence of CRC is
higher in males and in individuals aged >70 years (2,3). Despite
marked hereditary components, the majority of cases of CRC are
sporadic and account for ~70% of all cases. The disease develops
slowly over several years through the adenoma-carcinoma sequence.
Patients with CRC are primarily diagnosed at an advanced stage of
the disease and distant metastases may also be present. Surgically,
the procedure for treatment is challenging and the survival rate
remains low post-surgery (4). Thus,
novel prognostic biomarkers are required to improve the early
diagnosis and prognosis of patients with CRC.
That the characteristics and prognoses are different
between patients with right-side colon cancer and those with
left-side colon cancer remains debatable. Previous studies have
identified contrasts in the epidemiology, peri-operative course,
pathology and prognosis between patients with cancer of the left
side of the colon and those with cancer on the right side (5–7).
Patients with right-side colon cancer were typically older and more
often female, with tumors of more advanced stages, increased size,
poorer differentiation and different molecular patterns, and with a
poorer prognosis compared with patients with left-side colon cancer
(7). Differences in the embryonic
development of the two sides of the colon may partially explain the
differences (8,9). In contrast, several studies could not
identify any association between tumor location within the colon
and the overall survival time (5).
The flotillin (FLOT)/reggie protein family consists
of the major constituent proteins of lipid rafts and contains two
isoforms: FLOT1 and FLOT2 (10). The
isoforms form hetero-oligomeric complexes that participate in
various cellular functions, including cell adhesion, actin
cytoskeleton reorganization, endocytosis, phagocytosis and the
transduction of cellular signals (11). FLOT1 is associated with various types
of cancer, including non-small cell lung, endometrial and breast
cancer, renal cell carcinoma, neuroblastoma, gastric, bladder,
mouth, cervical and prostate cancer, and hepatocellular carcinoma
(12–22). In breast cancer, the FLOT1 expression
level is associated with clinical staging and prognosis, and its
silencing inhibits the proliferation and tumorigenicity of breast
cancer cells in vitro and in vivo (17). In endometrial cancer, the
upregulation of FLOT1 is associated with the tumor grade (12), whereas in bladder cancer it leads to
cancer progression and recurrence post-surgery (14). In prostate cancer, increased
expression of FLOT1 influences the proliferation of cancer cells
(21). These results suggest that
FLOT1 may serve an important function in the progression and
development of malignant carcinomas. To date, the effect of FLOT1
dysregulation on the pathogenesis of CRC is not
well-documented.
Therefore, the aim of the present study was to
investigate the expression of FLOT1 in left- and right-side CRC
tissue samples, and evaluate its prognostic significance by
analyzing the association between the level of FLOT1 expression and
clinicopathological features and survival time outcomes from CRC
biopsies.
Materials and methods
Patients and tissue samples
The present study included patients with CRC who
were admitted to Qilu Hospital of Shandong University between
November 2009 and May 2010. In total, 81 tumor biopsies and
adjacent tissues samples were obtained from the patients. The study
population consisted of 45 male and 36 female patients with a mean
age of 66.7±14.0 years (range, 22–93 years). The biopsies were
pathologically classified, and the tumor stage was defined
according to the World Health Organization (2000) and the Union of
International Cancer Control/American Joint Committee on Cancer
Tumor-Node-Metastasis (6th edition) classification (23). Following surgical removal, the tumor
biopsy samples were formalin-fixed (10%) for 24 h at 4°C,
paraffin-embedded and stored at 4°C for further histological
analysis and immunohistological studies. The post-surgery clinical
follow-up of the patients took place in the surgical outpatient
clinic biannually for ≤68 months. The age, sex, tumor location,
tumor size, histological type, tumor-node-metastasis stage, and
presence of lymph node and distant metastases were obtained from
the medical records of the patients. The Ethics Committee of
Shandong University, China approved the study. Written informed
consent was obtained from each patient.
Immunohistochemistry
The paraffin-embedded tissue sections were cut into
4–5-µm sections, dewaxed and hydrated. The antigen retrieval was
performed by heating the tissue sections at 95°C in 0.01 M citrate
buffer solution, pH 6.0, for 2–3 min in a stainless steel pressure
cooker. The endogenous peroxidase was blocked for 10 min using 3%
hydrogen peroxide solution at room temperature. The sections were
incubated with goat serum (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) for 15 min at 37°C for
blocking and then immunostained with rabbit antibody against FLOT1
(cat. no. ab41927; dilution 1:100; Abcam) at 4°C overnight.
Incubation with horseradish peroxidase-conjugated anti-rabbit
immunoglobulin G (cat. no. A0208; dilution 1:50; Beyotime Institute
of Biotechnology, Haimen, China) for 1 h at 37°C, followed by
incubation with 3,3′-diaminobenzidine (DAB-0031; Fuzhou Maixin
Biotech Co., Ltd., Fuzhou, China) for 1 min at room temperature for
secondary staining, according to the manufacturer's protocols.
Hematoxylin was used for counterstaining for 1 min at room
temperature.
Evaluation of immunohistochemical
staining
Immunostaining was assessed independently by two
experienced pathologists in a blinded manner. The concurrence ratio
was >95% and when discrepancies appeared, a third pathologist
analyzed the results. Overall, ~500 cells from three randomly
chosen fields under the light microscope (magnification, ×200) were
counted for each sample. The scoring for immunostaining was
performed semi-quantitatively: A combined score of the staining
intensity (0, negative; 1, weak; 2, moderate; and 3, strong) and
the percentage of positively stained cells (0, 0; 1, 1–25; 2,
26–50; 3, 51–75; and 4, 76–100%) was calculated, giving the final
score of FLOT1 protein expression. The cut-off point for the
definition of the expression level was as follows: 0–5, low
expression; and 6–8, high expression.
Statistical analysis
The associations between FLOT-1 expression and
clinicopathological parameters were analyzed using χ2
and Fisher's exact tests. Quantitative data are presented as the
mean ± standard deviation. Paired and unpaired Student's t-tests
were used to determine the differences between two groups to
compare FLOT-1 expression in adjacent normal tissues and colon
cancer tissues or left and right colon cancer tissues). Receiver
operating characteristic (ROC) curve analysis was performed to
assess the diagnostic value of FLOT1 in CRC. The survival rates
were calculated by the Kaplan-Meier method and the differences
between the subgroups were determined using the log-rank test.
Univariate and multivariate survival analyses were performed using
Cox proportional hazards models to identify independent prognostic
factors. All statistical analyses were performed using SPSS
software (version 21.0; IBM Corp., Armonk, NY, USA), and P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of FLOT1 in CRC
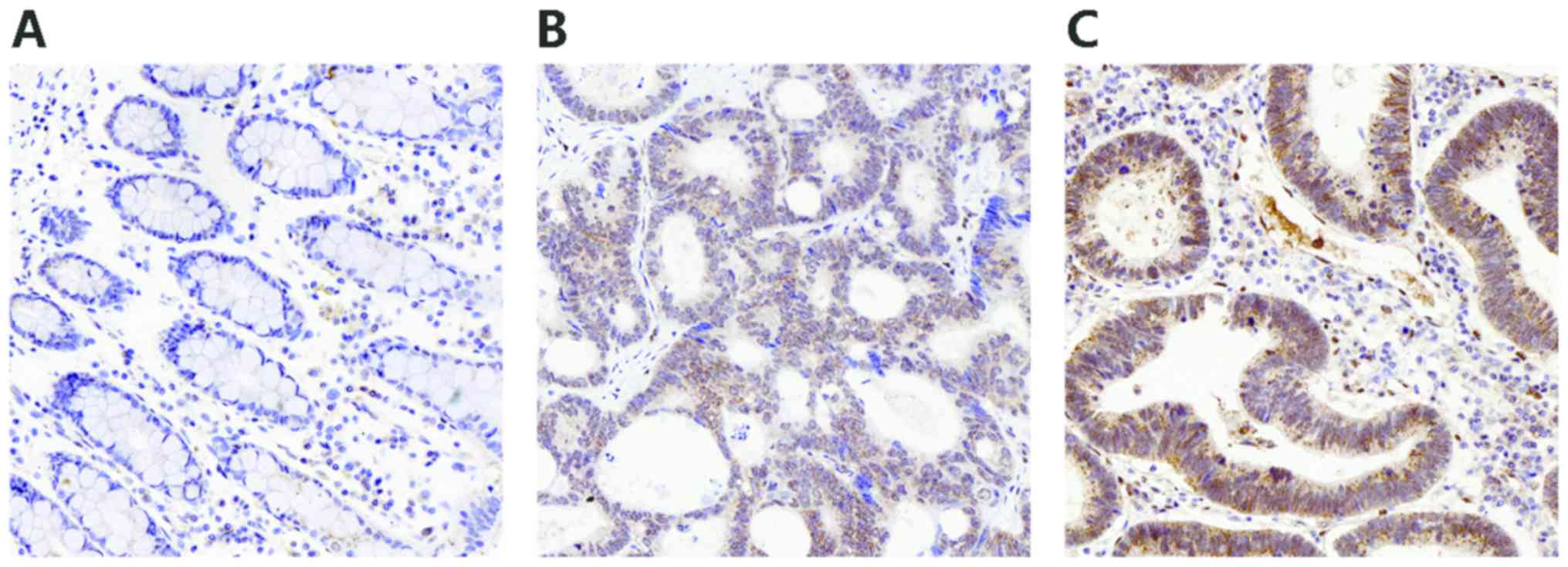
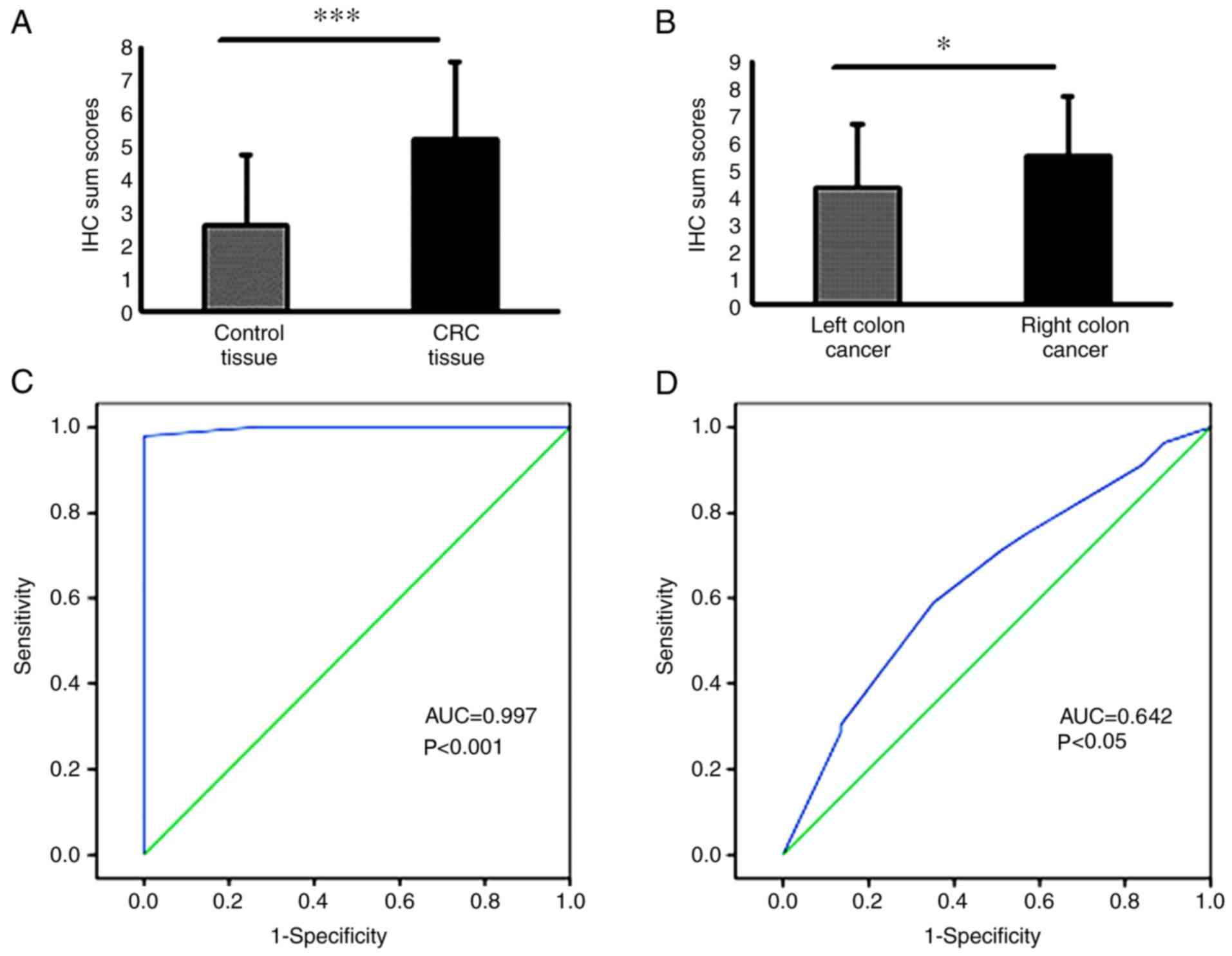
FLOT1 staining was markedly increased in the CRC
tissues compared with their corresponding adjacent non-cancer
tissues, suggesting that FLOT1 is upregulated in CRC (P<0.001;
Figs. 1 and 2A). Of the 81 CRC tissues, 45 (56%)
demonstrated strong positive staining, whereas 36 (44%) revealed
either negative or weak staining. In the adjacent healthy tissues,
81 (100%) samples demonstrated either negative or weak staining and
none revealed strong positive staining. Among the CRC samples, the
FLOT1 levels were significantly higher in samples from the right
side of the colon compared with those from the left side,
indicating a differential expression of FLOT1 in CRC location
(P<0.05; Fig. 2B).
To determine the diagnostic value of FLOT1
expression in CRC and in different CRC sites, ROC curves were
constructed. To assess the potential of the FLOT1 expression (IHC
sum scores) for diagnosing CRC and its location, the area under the
curve (AUC) was calculated. The AUC of the ROC curve for FLOT1 as a
predictor of CRC reached 0.997 [95% confidence interval (CI),
0.99–1.00; P<0.001; Fig. 2C],
with an estimated sensitivity and specificity of 100 and 87.0%,
respectively. Furthermore, the AUC for FLOT1 being able to predict
the CRC location was 0.642 (95% CI, 0.53–0.76; P<0.05; Fig. 2D) with an estimated sensitivity and
specificity of 96.4 and 89.2%, respectively. However, the FLOT1
expression was comparable between the tumor tissues with and
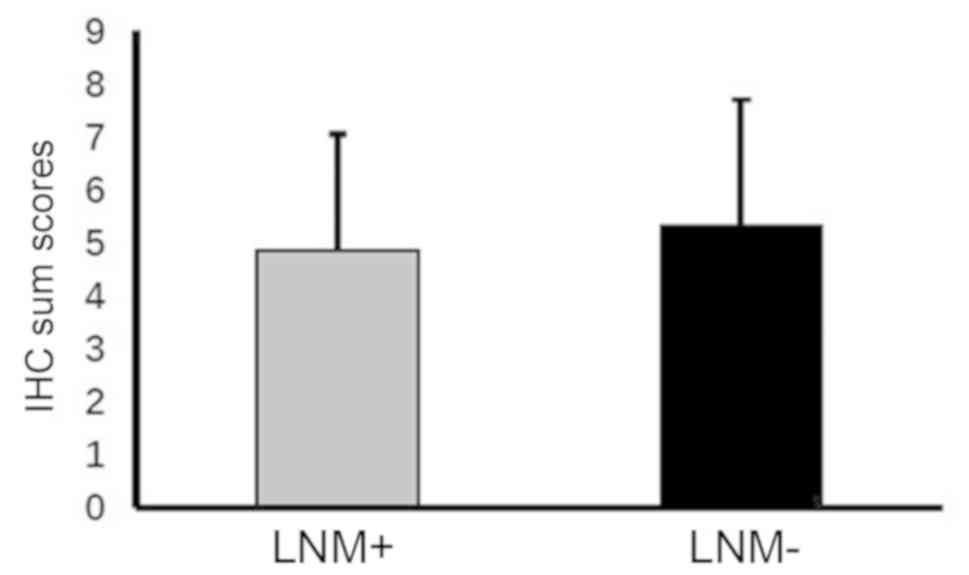
without lymph node metastasis (P=0.473; Fig. 3).
Association between FLOT1 expression
and clinicopathological factors
To estimate the clinical importance of FLOT1
expression in CRC, the association between FLOT1 levels and various
clinicopathological factors was analyzed (Table I). FLOT1 expression was significantly
associated with tumor invasiveness (P=0.025), grade (P=0.047) and
differentiation (P=0.023), and the association with tumor location
was confirmed (P=0.021). However, no association was observed
between FLOT1 expression and patient age (P=0.335), sex (P=1.00),
lymph node metastasis (P=0.446) or distant metastasis (P=0.246).
Furthermore, the association between the FLOT1 levels and
clinicopathological factors of the left- compared with the
right-side CRC tissue samples was examined (Table II). The analysis revealed an
association between the FLOT1 expression and tumor differentiation
(P=0.025) only. No tumor location-dependent association was
observed between the other parameters and FLOT1 expression
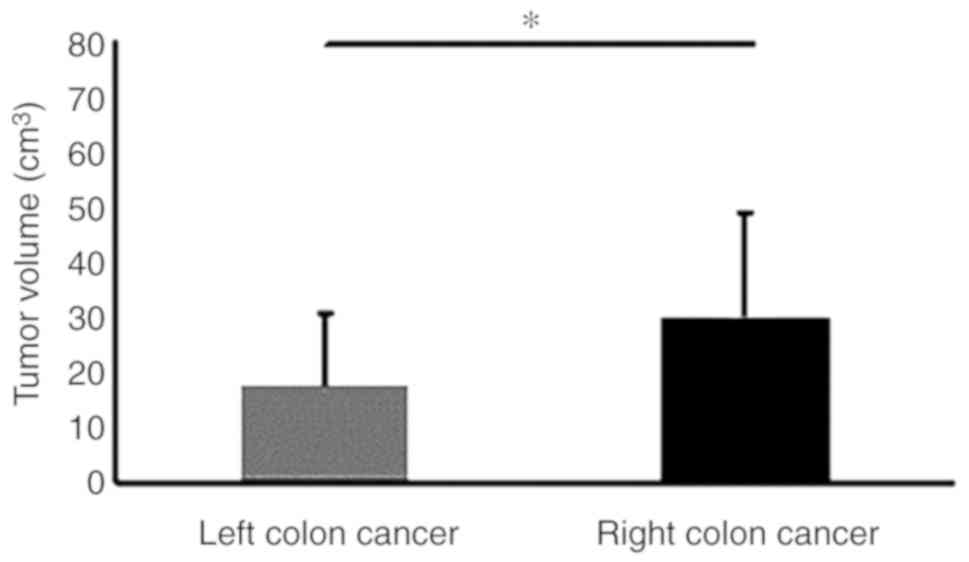
(Table II). Furthermore, although
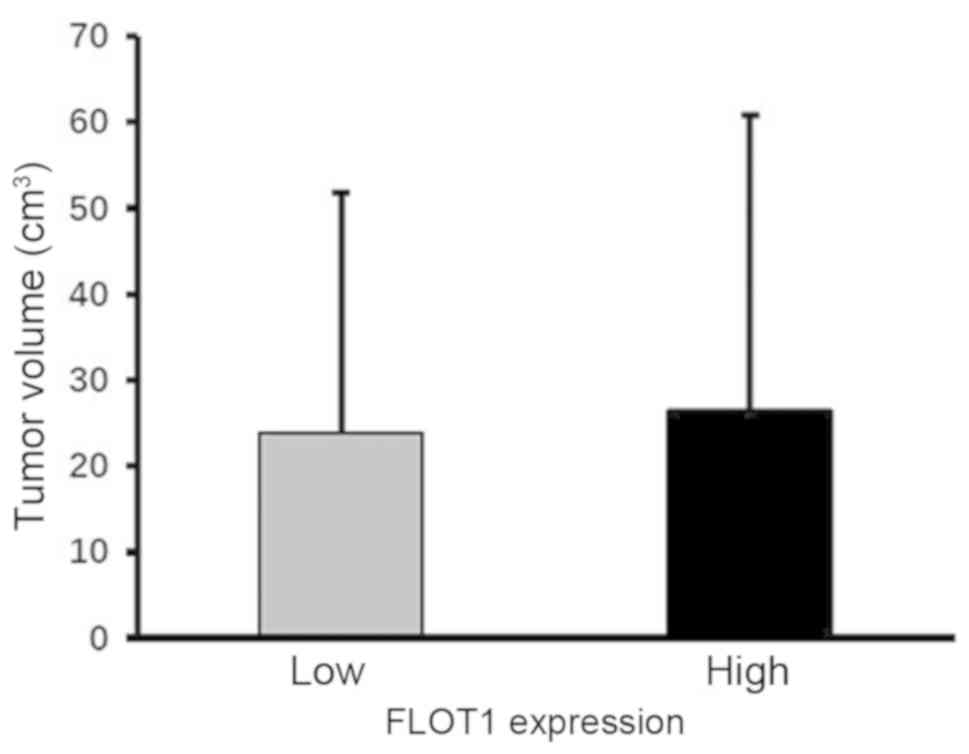
the tumor volume was comparable between CRC tissue samples with low
and high FLOT1 levels, it was markedly increased in right- compared
with left-side colon cancer tissues (Figs. 4 and 5). These data imply that increased FLOT1
expression leads to tumor progression in CRC in general, whereas it
specifically increases proliferation in CRC of the right side of
the colon.
 | Table I.Association between FLOT1 expression
and clinicopathological factors in colorectal cancer. |
Table I.
Association between FLOT1 expression
and clinicopathological factors in colorectal cancer.
|
|
| FLOT1 expression |
|
|---|
|
|
|
|
|
|---|
| Variable | Cases, n | Low | High | P-value |
|---|
| Age, years |
|
|
| 0.335 |
|
<60 | 25 | 9 | 16 |
|
| ≥60 | 56 | 28 | 28 |
|
| Sex |
|
|
| 1.000 |
| Male | 45 | 21 | 24 |
|
|
Female | 36 | 16 | 20 |
|
| Depth of invasion
(T) |
|
|
| 0.025 |
| T2 | 5 | 4 | 1 |
|
| T3 | 56 | 25 | 31 |
|
| T4 | 12 | 2 | 10 |
|
| Missing
data | 8 | 6 | 2 |
|
| Lymph node
metastasis (N) |
|
|
| 0.149 |
|
Negative (N0) | 45 | 24 | 21 |
|
|
Positive (N1-N2) | 33 | 13 | 20 |
|
| Missing
data | 3 | 0 | 3 |
|
| Distant metastasis
(M) |
|
|
| 0.246 |
|
Negative (M0) | 78 | 37 | 41 |
|
|
Positive (M1) | 3 | 0 | 3 |
|
| Tumor stage |
|
|
| 0.047 |
| I | 4 | 0 | 4 |
|
| II | 36 | 16 | 20 |
|
|
III | 29 | 11 | 18 |
|
| IV | 3 | 0 | 3 |
|
| Missing
data | 9 | 6 | 3 |
|
| Tumor
differentiation |
|
|
| 0.023 |
|
Well | 1 | 1 | 0 |
|
|
Moderate | 67 | 34 | 33 |
|
|
Poor | 13 | 2 | 11 |
|
| Tumor location |
|
|
| 0.021 |
|
Right | 51 | 18 | 33 |
|
|
Left | 30 | 19 | 11 |
|
 | Table II.Association between FLOT1 proteins
levels and clinicopathological factors in right- compared with
left-side colon cancer tissue samples. |
Table II.
Association between FLOT1 proteins
levels and clinicopathological factors in right- compared with
left-side colon cancer tissue samples.
|
|
| FLOT1
expression |
|
|---|
|
|
|
|
|
|---|
| Variable | Cases, n | Left | Right | P-value |
|---|
| Age, years |
|
|
| 0.805 |
|
<60 | 25 | 10 | 15 |
|
|
≥60 | 56 | 20 | 36 |
|
| Sex |
|
|
| 0.645 |
|
Male | 45 | 18 | 27 |
|
|
Female | 36 | 12 | 24 |
|
| Depth of invasion
(T) |
|
|
| 1.000 |
| T2 | 5 | 2 | 3 |
|
| T3 | 56 | 21 | 35 |
|
| T4 | 12 | 4 | 8 |
|
| Missing
data | 8 | 3 | 5 |
|
| Lymph node
metastasis (N) |
|
|
| 0.326 |
|
Negative (N0) | 45 | 19 | 26 |
|
|
Positive (N1-N2) | 33 | 11 | 22 |
|
| Missing
data | 3 | 0 | 3 |
|
| Distant metastasis
(M) |
|
|
| 1.000 |
|
Negative (M0) | 78 | 29 | 49 |
|
|
Positive (M1) | 3 | 1 | 2 |
|
| Tumor stage |
|
|
| 0.664 |
| I | 4 | 2 | 2 |
|
| II | 36 | 16 | 20 |
|
|
III | 29 | 9 | 20 |
|
| IV | 3 | 1 | 2 |
|
| Missing
data | 9 | 2 | 7 |
|
| Tumor
differentiation |
|
|
| 0.025 |
|
Well | 1 | 1 | 0 |
|
|
Moderate | 67 | 29 | 38 |
|
|
Poor | 13 | 1 | 12 |
|
FLOT1 as a marker for prognosis
The overall median survival time of the patients in
the present study was 48 months (range, 1–68 months). The
prognostic value of high FLOT1 expression in samples of patients
with CRC was assessed using Kaplan-Meier analysis. The survival
time analysis revealed that patients with low FLOT1 expression in
their biopsy samples exhibited longer overall survival times
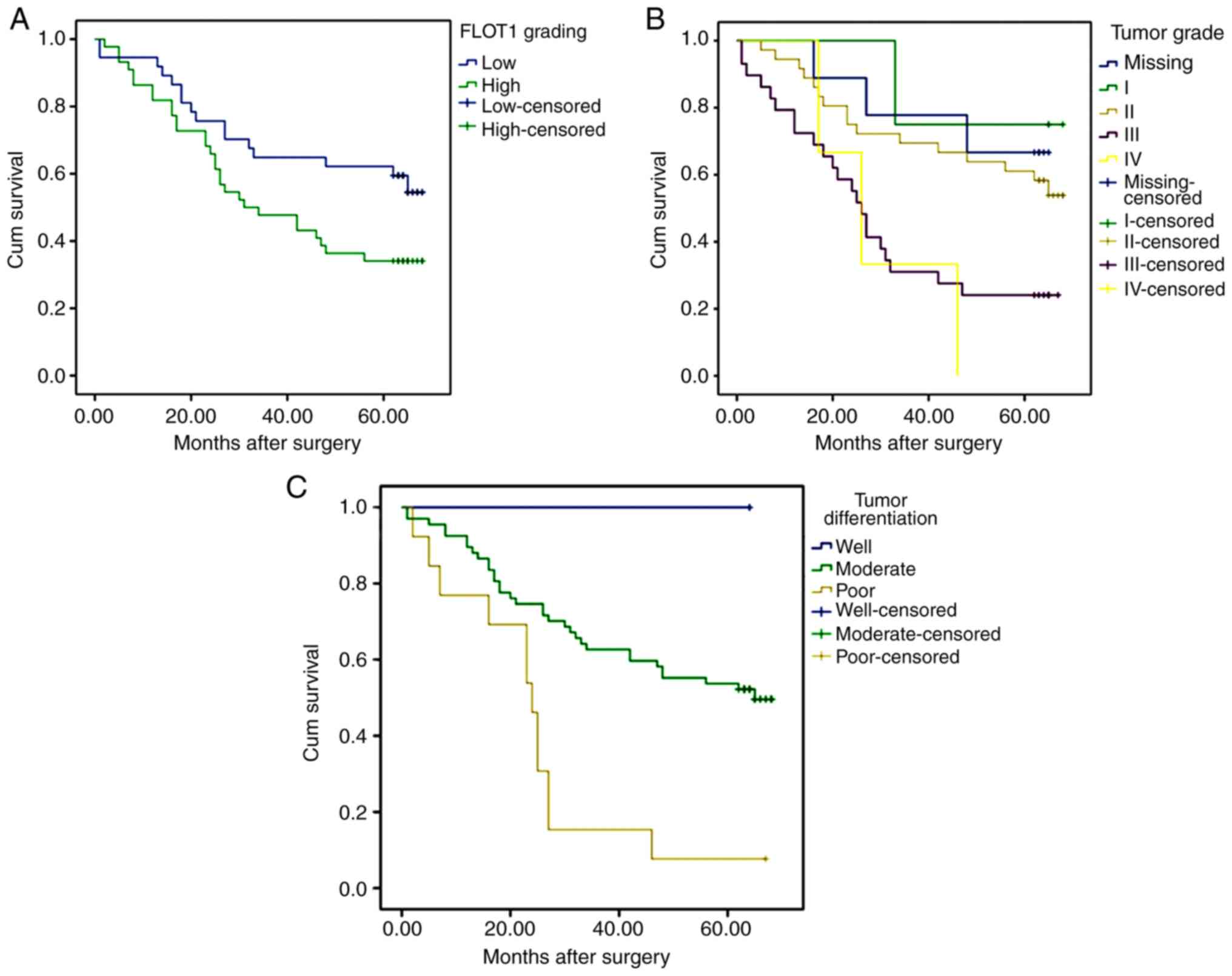
compared with those with high FLOT levels (P=0.043; Fig. 6A). Furthermore, patients with higher
tumor grade and poorer tumor differentiation exhibited decreased
overall survival times compared with those with lower tumor grade
(P=0.004; Fig. 6B) and
better-differentiated tumors (P=0.001; Fig. 6C), respectively. No significant
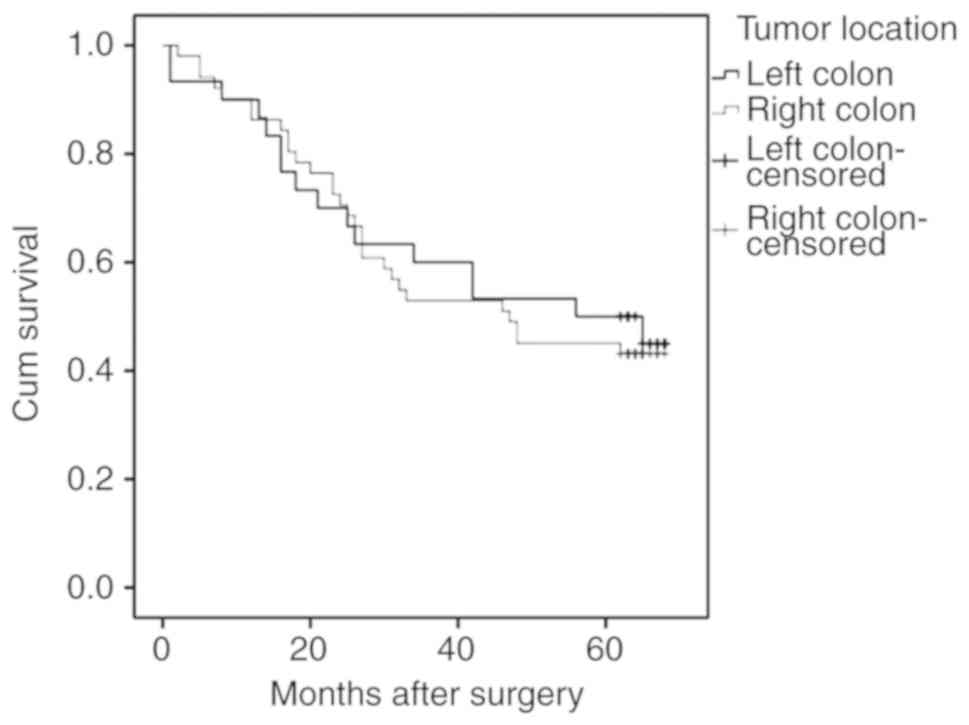
association was revealed between the tumor location (right or left
side of the colon) and the overall survival time (Fig. 7). However, the survival estimates
demonstrated a lower median survival duration of 44 months for
patients with CRC tumors on the right side of the colon (95% CI,
16.01–77.98) compared with a median survival time of 56 months for
patients with left-side tumors (95% CI, 19.02–92.97).
Univariate and multivariate analyses using the Cox
proportional hazards regression model were performed to further
investigate factors associated with patient outcome. The univariate
analysis indicated that tumor stage, differentiation and volume
were significantly associated with the overall survival time of
patients with CRC (Table III).
However, FLOT1 expression, tumor location, lymph node metastasis,
distant metastasis and depth of invasion indicated no prognostic
effect in the patients. The multivariate analysis confirmed tumor
stage and differentiation as independent prognostic factors for CRC
survival time (Table III).
 | Table III.Univariate and multivariate Cox
proportional hazard analyses of survival times of patients with
colorectal cancer. |
Table III.
Univariate and multivariate Cox
proportional hazard analyses of survival times of patients with
colorectal cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| FLOT1
expression | 1.084 | 0.588–1.996 | 0.797 | 1.333 | 0.666–2.669 | 0.416 |
| Depth of
invasion | 1.383 | 0.947–2.020 | 0.093 | 1.070 | 0.696–1.646 | 0.757 |
| Lymph node
metastasis | 1.518 | 0.977–2.359 | 0.063 | 1.320 | 0.692–2.333 | 0.400 |
| Distant
metastasis | 2.430 | 0.746–7.914 | 0.141 | 0.410 | 0.095–1.768 | 0.232 |
| Tumor stage | 1.799 | 1.259–2.572 | 0.001 | 1.587 | 1.004–2.508 | 0.048 |
| Tumor
differentiation | 3.438 | 1.760–6.716 | 0.000 | 2.520 | 1.042–6.093 | 0.040 |
| Tumor volume | 1.008 | 1.001–1.015 | 0.032 | 1.004 | 0.995–1.014 | 0.345 |
| Tumor location | 1.084 | 0.588–1.996 | 0.797 | 0.724 | 0.36–1.452 | 0.363 |
Discussion
The lipid raft marker protein FLOT1 is upregulated
in various types of cancer. Previously, Thorn et al
(24) performed expression profiling
in the marginal edges of CRC tumors and demonstrated that FLOT1
expression is increased in the more invasive cancer tissues. In
accordance with this study, the results of the present study
demonstrated that FLOT1 expression was significantly increased in
CRC tissues, with increased expression specifically in the
right-side tumors, and was associated with tumor differentiation,
tumor grade and depth of tumor invasion. Another landmark study by
Niu et al (25) suggested the
active involvement of FLOT1 and histone H1 as downstream factors in
the cytoplasmic and nuclear pathway of the S100 calcium-binding
protein A11 (S100A11), and that they are required for LIM and SH3
protein 1 (LASP1)-S100A11 axis-mediated epithelial-mesenchymal
transition and CRC progression. This previous study suggested that
S100A11, in combination with LASP1, serves an important function in
CRC metastasis through its subcellular effectors FLOT1 and histone
H1.
In various types of cancer, FLOT1 expression is
associated with different clinicopathological parameters, including
tumor size and tumor stage. Pust et al (26) identified that FLOT1 activates ErbB2
receptor tyrosine kinase 2 expression in vitro and in
vivo, which in turn increases the proliferation of breast
cancer cells, whereas decreasing FLOT1 expression significantly
suppresses breast cancer cell proliferation. In another study,
Zhang et al demonstrated an association between FLOT1 and
tumorigenesis and progression of hepatocellular carcinoma. In
addition, the study also suggested that FLOT1 may be used as a
prognostic marker in patients with hepatocellular carcinoma
(20). Winship et al revealed
upregulation of FLOT1 in endometrial cancer tissue and its
association with increasing tumor grade (12). In tongue squamous cell cancer, the
FLOT1 protein is correlated with pathological stage, depth of
invasiveness, lymph node metastasis, recurrence following the
surgical removal of the cancerous tissue, and with shorter survival
time (15). In the present study,
the association of increased FLOT1 expression with higher tumor
grade, poorer differentiation state and increased tumor volume
indicates that FLOT1 expression may contribute to the progression
of the tumor from early to advanced phenotype, and to the
proliferation of CRC cells. Decreased overall survival time was
also associated with increased FLOT1 expression. Multivariate
analysis confirmed tumor grade and differentiation as independent
predictors of overall survival time in patients with CRC.
The notion that patients with tumors located on the
right side of the colon have more advanced tumors with poorer
prognosis and decreased overall survival time, remains unclear.
Although a number of studies have supported this hypothesis
(27,28), others failed to demonstrate any
site-specific differences (5,29). In
the present study, FLOT1 protein levels were significantly
increased in right-side colon CRC tissue samples compared with
those from the left side. In concordance, the tumor volume was also
higher in the right-side colon CRC tissue samples. No other
examined parameters differed between the CRC tumors from different
locations. This suggests that FLOT1 may affect the proliferation of
cancer cells on the right side of the colon. However, in the
multivariate analysis, the tumor location failed to predict the
overall survival time outcome. This could be due to the small
sample size in the present study. Although this is a notable
result, further study of this phenomenon is required.
In conclusion, the FLOT1 protein level is increased
in CRC tissue compared with in adjacent healthy colon tissue, with
tissue from tumors of the right side of the colon specifically
exhibiting higher FLOT1 expression. FLOT1 in CRC tissues is
associated with the tumor proliferation, differentiation, tumor
grade and overall survival time. In right-side colon tumors, FLOT1
specifically affects tumor proliferation. Since, to the best of our
knowledge, the present study is the first of its type, the factors
investigated should be taken into consideration by the physician
when screening patients for CRC. Further studies with larger study
sample numbers are warranted, to elucidate the pathological
molecular mechanism of this event.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation (grant no. 81572414).
Availability of data and materials
The datasets used and analyzed for the present study
are available from the corresponding author upon reasonable
request.
Authors' contributions
JN and ZL designed the study. NB and JL performed
the experiments and wrote the initial draft of the manuscript. HC,
SY and TL contributed to the analysis and interpretation of data.
ZN revised the paper, assisted with the experiments and supervised
the experimental work. All authors approved the final
manuscript.
Ethics approval and consent to
participate
The present study has been approved by the Ethics
Committee of Shandong University, Jinan, China (KYLL-2005(KS)-114).
All the research works have been conducted in accordance with the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
ROC
|
receiver operating characteristic
|
|
AUC
|
area under the curve
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clini. 68:7–30. 2018. View Article : Google Scholar
|
|
3
|
Tuan J and Chen YX: Dietary and lifestyle
factors associated with colorectal cancer risk and interactions
with microbiota: Fiber, red or processed meat and alcoholic drinks.
Gastrointest Tumors. 3:17–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laohavinij S, Maneechavakajorn J and
Techatanol P: Prognostic factors for survival in colorectal cancer
patients. J Med Assoc Thai. 93:1156–1166. 2010.PubMed/NCBI
|
|
5
|
Hansen IO and Jess P: Possible better
long-term survival in left versus right-sided colon cancer - A
systematic review. Dan Med J. 59:A44442012.PubMed/NCBI
|
|
6
|
Wray CM, Ziogas A, Hinojosa MW, Le H,
Stamos MJ and Zell JA: Tumor subsite location within the colon is
prognostic for survival after colon cancer diagnosis. Dis Colon
Rectum. 52:1359–1366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Benedix F, Kube R, Meyer F, Schmid U,
Gastinger I and Lippert H; Colon/Rectum Carcinomas and (Primary
Tumor) Study Group, : Comparison of 17,641 patients with right- and
left-sided colon cancer: Differences in epidemiology, perioperative
course, histology, and survival. Dis Colon Rectum. 53:57–64. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hutchins G, Southward K, Handley K, Magill
L, Beaumont C, Stahlschmidt J, Richman S, Chambers P, Seymour M,
Kerr D, et al: Value of mismatch repair, KRAS, and
BRAF mutations in predicting recurrence and benefits from
chemotherapy in colorectal cancer. J Clini Oncol. 29:1261–1270.
2011. View Article : Google Scholar
|
|
9
|
Elnatan J, Goh HS and Smith DR:
C-KI-RAS activation and the biological behaviour of proximal
and distal colonic adenocarcinomas. Eur J Cancer 32A. 491–497.
1996. View Article : Google Scholar
|
|
10
|
Babuke T and Tikkanen R: Dissecting the
molecular function of reggie/flotillin proteins. Eur J Cell Biol.
86:525–532. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Langhorst MF, Reuter A and Stuermer CA:
Scaffolding microdomains and beyond: The function of
reggie/flotillin proteins. Cell Mol Life Sci. 62:2228–2240. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Winship AL, Rainczuk K and Dimitriadis E:
Flotillin-1 protein is upregulated in human endometrial cancer and
localization shifts from epithelial to stromal with increasing
tumor grade. Cancer Investigat. 34:26–31. 2016. View Article : Google Scholar
|
|
13
|
Yang FQ, Zhang HM, Chen SJ, Yan Y and
Zheng JH: MiR-506 is down-regulated in clear cell renal cell
carcinoma and inhibits cell growth and metastasis via targeting
FLOT1. PLoS One. 10:e01202582015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guan Y, Song H, Zhang G and Ai X:
Overexpression of flotillin-1 is involved in proliferation and
recurrence of bladder transitional cell carcinoma. Oncol Rep.
32:748–754. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Zhang Y, Chen SW, Li FJ, Zhuang SM,
Wang LP, Zhang J and Song M: Prognostic significance of Flotillin1
expression in clinically N0 tongue squamous cell cancer. Int J
Clini Exp Pathol. 7:996–1003. 2014.
|
|
16
|
Gao W, Xu J, Wang F, Zhang L, Peng R, Shu
Y, Wu J, Tang Q and Zhu Y: Plasma membrane proteomic analysis of
human gastric cancer tissues: Revealing flotillin 1 as a marker for
gastric cancer. BMC Cancer. 15:3672015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Luo J, Wang B, Wang D, Xie X, Yuan
L, Guo J, Xi S, Gao J, Lin X, et al: Microrna-124 targets
flotillin-1 to regulate proliferation and migration in breast
cancer. Mol Cancer. 12:1632013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arkhipova KA, Sheyderman AN, Laktionov KK,
Mochalnikova VV and Zborovskaya IB: Simultaneous expression of
flotillin-1, flotillin-2, stomatin and caveolin-1 in non-small cell
lung cancer and soft tissue sarcomas. BMC Cancer. 14:1002014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z, Yang Y, Gao Y, Wu X, Yang X, Zhu Y,
Yang H, Wu L, Yang C and Song L: Elevated expression of flotillin-1
is associated with lymph node metastasis and poor prognosis in
early-stage cervical cancer. Ameri J Cancer Res. 6:38–50. 2015.
|
|
20
|
Zhang SH, Wang CJ, Shi L, Li XH, Zhou J,
Song LB and Liao WT: High expression of FLOT1 is associated with
progression and poor prognosis in hepatocellular carcinoma. PLoS
One. 8:e647092013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gomez V, Sese M, Santamaria A, Martínez
JD, Castellanos E, Soler M, Thomson TM and Paciucci R: Regulation
of aurora B kinase by the lipid raft protein flotillin-1. J
Biological Chem. 285:20683–20690. 2010. View Article : Google Scholar
|
|
22
|
Ou YX, Liu FT, Chen FY and Zhu ZM:
Prognostic value of Flotillin-1 expression in patients with solid
tumors. Oncotarget. 8:52665–52677. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SH, Ha TK and Kwon SJ: Evaluation of
the 7th AJCC TNM staging system in point of lymph node
classification. J Gastric Cancer. 11:94–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thorn CC, Freeman TC, Scott N, Guillou PJ
and Jayne DG: Laser microdissection expression profiling of
marginal edges of colorectal tumours reveals evidence of increased
lactate metabolism in the aggressive phenotype. Gut. 58:404–412.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Niu Y, Shao Z, Wang H, Yang J, Zhang F,
Luo Y, Xu L, Ding Y and Zhao L: LASP1-S100A11 axis promotes
colorectal cancer aggressiveness by modulating TGFβ/Smad signaling.
Sci Rep. 6:261122016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pust S, Klokk TI, Musa N, Jenstad M,
Risberg B, Erikstein B, Tcatchoff L, Liestøl K, Danielsen HE, van
Deurs B, et al: Flotillins as regulators of ErbB2 levels in breast
cancer. Oncogene. 32:3443–3451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nawa T, Kato J, Kawamoto H, Okada H,
Yamamoto H, Kohno H, Endo H and Shiratori Y: Differences between
right- and left-sided colon cancer in patient characteristics,
cancer morphology and histology. J Gastroenterol Hepatol.
23:418–423. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rothberg PG, Spandorfer JM, Erisman MD,
Staroscik RN, Sears HF, Petersen RO and Astrin SM: Evidence that
c-myc expression defines two genetically distinct forms of
colorectal adenocarcinoma. Br J Cancer. 52:629–632. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gervaz P, Bouzourene H, Cerottini JP,
Chaubert P, Benhattar J, Secic M, Wexner S, Givel JC and Belin B:
Dukes B colorectal cancer: Distinct genetic categories and clinical
outcome based on proximal or distal tumor location. Dis Colon
Rectum. 44:364–372. 2001. View Article : Google Scholar : PubMed/NCBI
|