Introduction
Gallbladder carcinoma is the most common malignant
tumor of the biliary system (1). Its
incidence ranks seventh among digestive tract tumors (2). Gallbladder cancer cells can spread in
the early stage through direct infiltration, lymphatic metastasis
and blood transfer. The majority of patients are diagnosed at an
advanced stage, which is not suitable for surgery, leading to poor
postoperative survival (3,4). Therefore, in-depth study of key
molecules in the development of gallbladder cancer, exploring the
molecular mechanism of gallbladder cancer proliferation, and
screening for effective diagnostic and therapeutic targets is
critical for the future treatment of gallbladder cancer.
The B-cell specific Moloney murine leukemia virus
integration site 1 (Bmi-1) is a proto-oncogene in the
polycomb group gene family and is a transcriptional repressor.
Human Bmi-1 gene is located in the short arm 13 region of
chromosome 10 and encodes a protein of 326 amino acids expressed in
the cytoplasm and chromatin. Bmi-1 gene plays an important
role in cell cycle, cell immortalization and senescence, and
self-renewal and differentiation of stem cells. The role of Bmi-1
in tumor formation has become a research hotspot in different types
of cancer, such as ovarian (5),
esophageal (6) and cervical cancer
(7). However, whether Bmi-1
is involved in the development of gallbladder cancer and its role
in gallbladder cancer is unclear. Based on a series of molecular
and biochemical experiments, the aim of the study was to explore
the mechanism of the action of Bmi-1 in the occurrence and
development of gallbladder carcinoma, and provide a theoretical
basis for understanding the molecular mechanism and clinical
treatment of gallbladder cancer.
Patients and methods
Patients and cell culture
Fifty patients with gallbladder cancer (20 males, 30
females, aged 35–78 years, mean: 58 years) who underwent surgical
excision were selected from The Second Affiliated Hospital of
Qiqihar Medical University (Qiqihar, China) during the period
January 2011 to August 2017. The patients included 19 cases with
high differentiation, 13 cases with moderate differentiation, and
18 cases with low differentiation. According to TNM staging of
gallbladder carcinoma, there were 18 cases in stage I–II and 32
cases in stage III–IV. None of the patients received radiotherapy
and/or chemotherapy prior to surgery. There were 29 cases with
gallstone invasion and 17 cases with other organ invasion. Another
15 cases of normal gallbladder tissue were selected as the control
group, all from patients with intrahepatic bile duct stones or
liver tumors who underwent right hepatectomy (no stones and tumor
invasion in the gallbladder), including 6 males and 9 females, aged
32–73 years, with a mean age of 52.5 years.
The study was approved by the Ethics Committee of
The Second Affiliated Hospital of Qiqihar Medical University.
Patients who participated in this research had complete clinical
data. Signed informed consents were obtained from the patients or
the guardians.
Pathological specimens were obtained from patients
undergoing cholecystectomy during the same period. There was no
significant difference in sex and age between the gallbladder
cancer group and the normal gallbladder tissue group. The specimens
were fixed with 10% formaldehyde, dehydrated and dipped in wax to
make 4 µm paraffin sections. Human gallbladder cancer cell line
GBC-SD (preserved in the Laboratory of The Second Affiliated
Hospital of Qiqihar Medical University) was cultured in RPMI-1640
medium (1559231, Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) containing 10% fetal calf serum (MB5175, Dalian Meilun
Biological Technology Co., Ltd., Dalian, China) at 37°C and 5%
CO2 under constant temperature. When cells grew to
70–80% confluency, they were digested with 0.25% trypsin and
passaged.
Bmi1-siRNA and Bmi1-NC (negative control) vectors
(vector pSUPER) were constructed by Shanghai Biotech, Shanghai,
China and sequence of Bmi1-si RNA and Bmi1-NC are shown in Table I.
 | Table I.miRNA oligomeric single-stranded DNA
sequences. |
Table I.
miRNA oligomeric single-stranded DNA
sequences.
| Items | Primer sequence
5′-3′ |
|---|
| Bmi1-si RNA
shRNA |
Forward:GATCCGGTATTCCCTCCACCTCTTCTTTCAAGAGAAGA
AGAGGTGGAGGGAATACCTTTTTTGGAAG |
|
|
Reverse:AATTCTTCCAAAAAAGGTATTCCCTCCACCTCTTCTTCT
CTTGAAAGAAGAGGTGGAGGGAATACCG |
| Bmi1-NC shRNA |
Forward:GATCCATACAACTCGCATCTGACATTCAAGAGAATACA
TGACATCAATCTGGTTTTTTGGAAG |
|
|
Reverse:AATTCTTCCAAAAAAATACAACTCGCATCTGACATCTC
TTGAAAGAAGAGGTGGAGGGAATACCG |
Immunohistochemical staining for
detection of Bmi-1 expression in gallbladder carcinoma
After dewaxing with xylene for 10 min × 3 and
dehydration with gradient alcohol (85% ethanol, 95% ethanol and
absolute ethanol), tissue sections were incubated with 3%
H2O2 for 8 min, followed by PBS washing (5
min × 3). After non-immune calf serum was blocked for 20 min, the
tissue sections were washed with PBS (5 min × 3). Following removal
of excess PBS solution, tissue sections were incubated with
anti-mouse anti-human Bim-1 (cat. no. MAB33342, R&D Systems,
Inc.; 1:500 dilution) overnight in a refrigerator at 4°C. After PBS
washing (5 min × 3), the tissue sections were incubated with
biotinylated secondary antibody (1:500 dilution, cat. no.
515-065-003, Jackson ImmunoResearch Laboratories, Inc.) for 20 min
at room temperature. After PBS washing (5 min ×3), the tissue
sections were incubated with freshly prepared DAB solution and
observed under a microscope (Eclipse Ni-E/Ni-U, Nikon).
Determination of immunohistochemical results: 5 high-power visual
fields were randomly selected for cancer cell counting, and
comprehensive scoring was performed based on positive expression
cells and positive staining intensity. Scoring rules: total score =
A × B, where ‘A’ represents the percentage of positively expressed
cells (A <10%, 0 point; 10–25%, 1 point; 26–50%, 2 points;
51–75%, 3 points; A >75%, 4 points), ‘B’ is the staining
intensity of positive cells (colorless, 0 point; light yellow, 1
point; yellow, 2 points; brown, 3 points). Total score was: <2
is negative (−), 2–4 is weak positive (+), 5–8 is moderately
positive (++) and 9–12 is strongly positive (+++), and >2 points
are collectively referred to as positive expression.
qPCR detection of Bmi-1 expression in
gallbladder carcinoma
Total RNA was extracted from gallbladder cancer and
normal gallbladder tissues using TRIzol RNA extraction kit
(WLA088a, Wanleibio Co., Ltd.). After measuring the total RNA
concentration, samples with A260/A280 of 1.8–2.0 were selected for
subsequent experiments. GAPDH was used as an internal reference.
Reverse transcription and PCR amplification were carried out using
the RT-PCR kit (RR037A, Takara Bio, Inc.). Primer sequences are
shown in Table II (synthesized by
Sangon Biotech Co., Ltd.). RT-qPCR products were checked using 1%
agarose gel electrophoresis.
 | Table II.Primer sequences for RT-PCR and
qRT-PCR. |
Table II.
Primer sequences for RT-PCR and
qRT-PCR.
| Gene name | Primer sequence
5′-3′ | Product length
(bp) |
|---|
| Bmi-1 | Forward:
GGATCCTCATCCTTCTGCTGATGCTG | 232 |
|
| Reverse:
GAATTCGCATCACAGTCATTGCTGCT |
|
| GAPDH | Forward:
CATATGCAAGGTCATCCATGACAACTTTG | 508 |
|
| Reverse:
AAGCTTGTCCACCACCCTGTTGCTGTAG |
|
Bmi1-si RNA transfection and RT-PCR
for detection of infection efficiency
GBC-SD cell suspension (2×105 cells/ml)
was prepared and seeded in a 6-well plate. When 50% confluency was
reached, Bmi1-si RNA recombinant plasmid (miRNA oligo
single-stranded DNA sequence is shown in Table II), Bmi1-NC (mismatched sequence)
and Lipofectamine 3000 were mixed and incubated for 30 min and then
added to the 6-well plate to transfect GBC-SD cell lines. The plate
was incubated in 5% CO2 and at 37°C in an incubator.
Cells were divided into the GBC-SD-Bmi1-si RNA, GBC-SD-Bmi1-NC and
GBC-SD groups. Transfection efficiency was detected by RT-PCR after
96 h. RT-PCR was performed as follows: frozen tissues were taken
out from the liquid nitrogen tank, and cDNA was reverse transcribed
according to the instructions of reverse transcription kit (primers
shown in Table II, produced by
Sangon) following conditions of 65°C for 5 min, 42°C for 60 min and
70°C for 5 min and then placed on ice to cool after termination
reaction and preserved. Using GAPDH as an internal reference, the
expression of Bmi-1 in each cell line was detected as per the
protocol of the RT-qPCR kit (RR037A, Takara Bio, Inc.). Reaction
conditions were 40 cycles of 95°C for 10 sec, 55°C for 20 sec, 72°C
for 20 sec and 79°C for 20 sec. At the same time, the chain
dissolution curve of the amplified product was detected with the
conditions of 95°C for 2 min, 60°C for 20 sec, 72°C for 20 sec and
99°C for 15 sec. Data normalizations were performed based on the
2−∆∆Cq method (8).
CCK-8 assay detection of cell
proliferation
The transfected cells of each group were
trypsinized, and seeded in a 96-well plate (3 replicate wells per
cell) at 2×103 cells/well. After 24 h of culture, 10
µl/well of CCK-8 reagent was added (40203ES60, Shanghai Yu Sheng).
After incubation for an additional 2 h in the incubator, the
corresponding OD values were measured using a microplate reader
(measuring wavelength: 450 nm, reference wavelength: 650 nm).
Subsequently, measurement was performed every 24 h for 4 days, and
the corresponding cell proliferation curve was plotted.
Flow cytometry detection of
apoptosis
Transfected cells were cultured in the logarithmic
growth phase (after 72 h). Cells (2×106) were treated
with trypsin and transferred to a 10 ml centrifuge tube. Pre-cooled
PBS buffer without calcium and magnesium was added. After
centrifugation at 157 × g for 5 min at 4°C, the cells were washed 3
times and 100 µl of binding buffer was added and incubated with the
cells in the dark for 10 min. Annexin V-FITC and 10 µl of PI stain
(MA0220, Dalian Meilun Biological Technology Co., Ltd.) were added
and incubated in the dark for 25 min. Detection of apoptotic cells
was performed by flow cytometry.
Total protein extraction and western
blot analysis
After the transfected cells were cultured in a
6-well plate, the cells were washed well with pre-cooled PBS for 1
min × 3. After PBS was discarded, cell lysate was added, mixed, and
the mixture was transferred to a 1.5 ml centrifuge tube, followed
by centrifugation at 22,600 × g for 5 min at 4°C. After
centrifugation, the supernatant was removed from the total protein
of the cells. Total protein was stored at −80°C. BCA protein
quantification kit was used to quantify the protein extracted from
each group of cells. Protein (10 µg) was subjected to 10% SDS-PAGE
electrophoresis. Following gel transfer to PVDF membrane, the
membranes were blocked in 1X PBS containing 5% skim milk powder for
3 h at room temperature. Subsequently, the corresponding antibodies
were diluted (1:1,500) in blocking solution (rabbit anti-human Bax:
cat. no. LS-C210507, LSBio; rabbit anti-human caspase 3: cat. no.
LS-C746939, LSBio; rabbit anti-human Bcl-2: cat. no. LS-B6548,
LSBio; rabbit anti-human cyclin D1: cat. no. LS-B3452, LSBio;
rabbit anti-human CDK2: cat. no. ab32147, Abcam; rabbit anti-human
β-actin antibody: cat. no. ab8227, Abcam) and the membranes were
incubated in first anti-diluent overnight at 4°C. After washing
with TBST 8 min × 3, membranes were incubated with secondary
antibody (1:2,000 diluted, mouse anti-rabbit IgG, cat. no.
LS-C60914, LSBio) for 2 h at room temperature. Then the membrane
was washed with TBST for 7 min × 3, ECL (Amersham Pharmacia
Biotech) was used to develop signals.
Statistical analysis
The data were analyzed by SPSS23.0 professional
statistical software. Measurement data were expressed as mean ±
standard deviation. The countable data were compared by
χ2 test. Comparisons between groups were analyzed by
one-way ANOVA and Scheffe post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Immunohistochemical staining for
detection of Bmi-1 expression
Positive expression rate of Bmi-1 protein in 50
cases of gallbladder carcinoma was 84% (42/50), and the positive
expression rate in normal gallbladder tissues was 40% (6/15). Data
analysis showed that the positive expression rate of Bmi-1 protein
in gallbladder carcinoma tissues was significantly higher than that
in normal gallbladder tissues (control group, P<0.05, Table III). In addition, Bmi-1 protein is
weakly positive or not expressed in normal gallbladder tissues
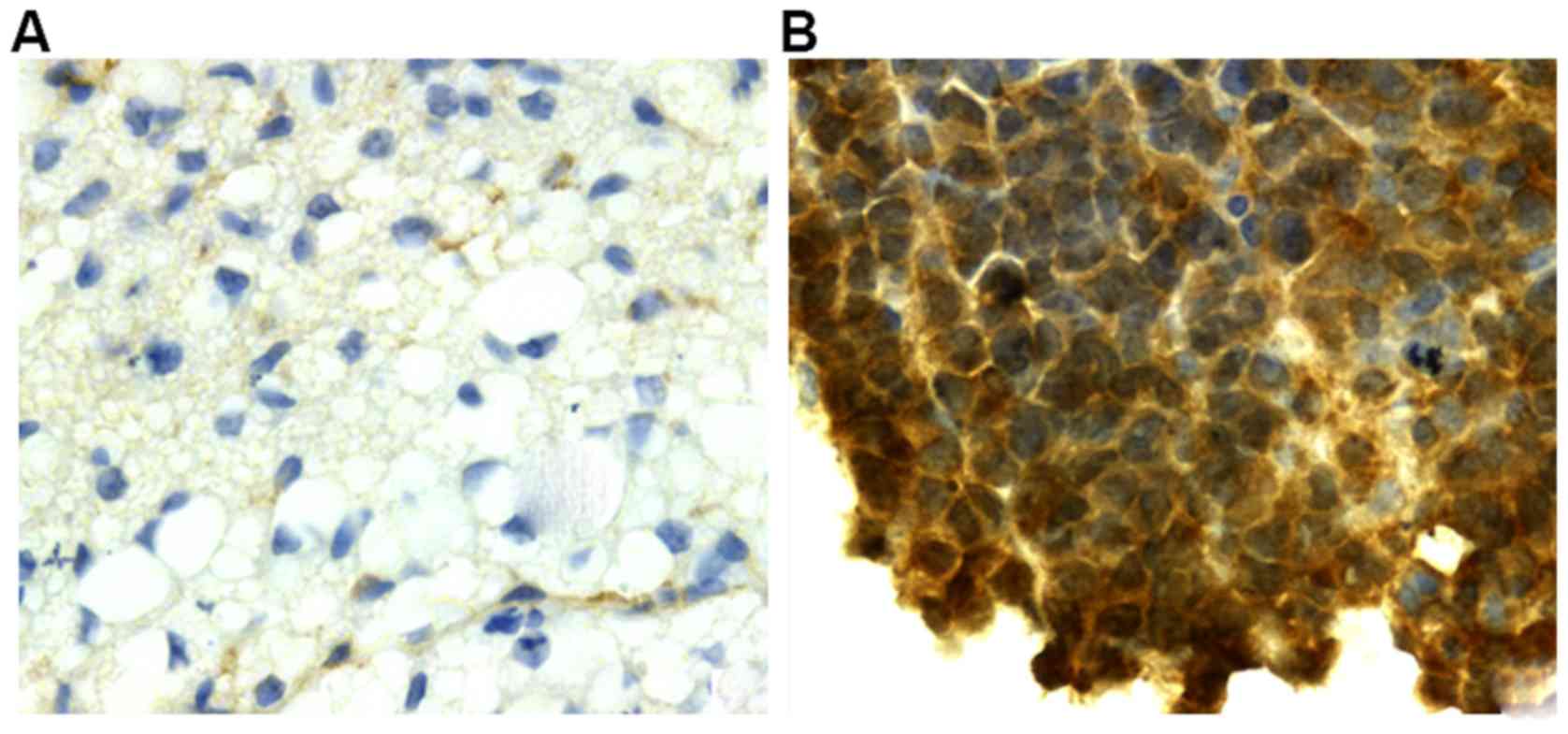
(Fig. 1A), while in gallbladder
carcinoma tissues, the color was brownish yellow (or tan) in the
nucleus and a small amount was expressed in the cytoplasm (Fig. 1B).
 | Table III.Immunohistochemical staining for the
detection of Bmi-1 expression. |
Table III.
Immunohistochemical staining for the
detection of Bmi-1 expression.
|
|
| Bmi-1 expression
level |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
features | Cases | Negative (%) | Positive (%) | χ2
value | P-value |
|---|
| Gallbladder cancer
tissue | 50 | 8 (16) | 42 (84) | 14.927 | <0.05 |
| Normal gallbladder
tissue | 15 | 9 (60) | 6
(40) |
|
|
Analysis of the relationship between the expression
of Bmi-1 in gallbladder carcinoma and clinicopathological factors.
As shown in Table IV, positive
expression of Bmi-1 protein in gallbladder carcinoma was correlated
with the degree and stage of tumor differentiation. Positive
expression of Bmi-1 in poorly differentiated gallbladder carcinoma
was significantly higher than that in high/medium differentiated
carcinoma, while positive expression of Bmi-1 in stage III and IV
was significantly higher than that in I and II (P<0.05).
Positive expression of Bmi-1 protein in gallbladder carcinoma was
not associated with sex, age, presence of gallstones and other
organ invasion (P>0.05).
 | Table IV.Relationship between Bmi-1 protein
expression and clinicopathological factors of gallbladder
carcinoma. |
Table IV.
Relationship between Bmi-1 protein
expression and clinicopathological factors of gallbladder
carcinoma.
|
|
| Bmi-1 expression
level |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
factors | Cases | Negative (%) | Positive (%) | χ2
value | P-value |
|---|
| Sex |
| Male | 20 | 6 (30) | 14 (70) | 2.681 | >0.05 |
|
Female | 30 | 3 (10) | 27 (90) |
|
|
| Age |
| <60
years | 28 | 5 (17.9) | 23 (82.1) | 0.931 | >0.05 |
| ≥60
years | 22 | 6 (27.2) | 16 (72.8) |
|
|
| Differentiation |
|
High/medium | 32 | 8 (25) | 24 (75) | 9.182 | <0.05 |
| Low | 18 | 2 (11.1) | 16 (88.9) |
|
|
| TNM staging |
| I–II | 18 | 5 (27.8) | 13 (72.2) | 6.521 | <0.05 |
|
III–IV | 32 | 4 (12.5) | 28 (87.5) |
|
|
| Gallstones |
|
Yes | 29 | 4 (13.8) | 25 (86.2) | 0.107 | >0.05 |
| No | 21 | 4 (19.0) | 17 (81.0) |
|
|
| Other organ
invasion |
|
Yes | 17 | 2 (11.8) | 15 (88.2) | 0.427 | >0.05 |
| No | 33 | 6 (18.2) | 27 (81.8) |
|
|
qPCR detection of Bmi-1 expression in
gallbladder carcinoma
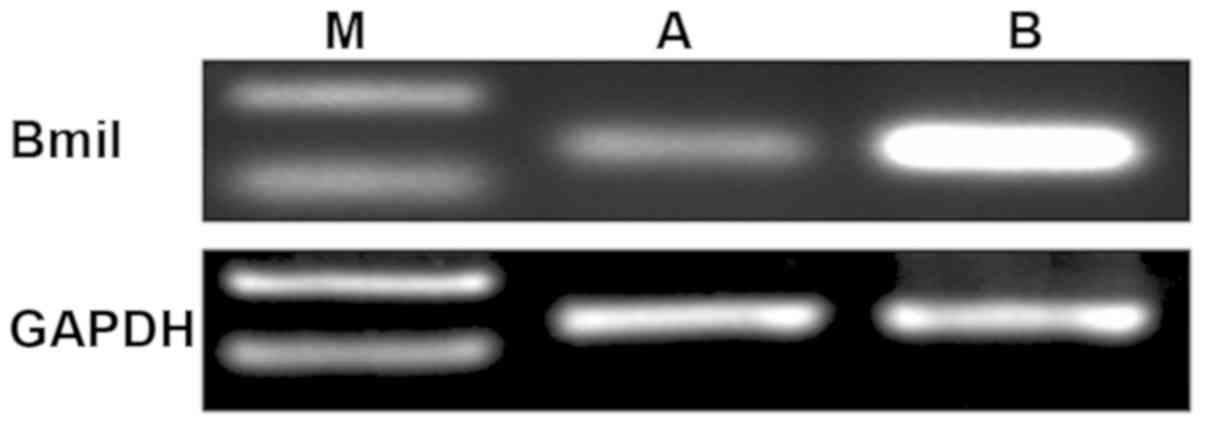
Results showed that the mRNA expression level of
Bmi-1 in gallbladder carcinoma tissues was significantly higher
than that in normal gallbladder tissues (Fig. 2, P<0.05).
RT-PCR detection of Bmi-1 expression
level
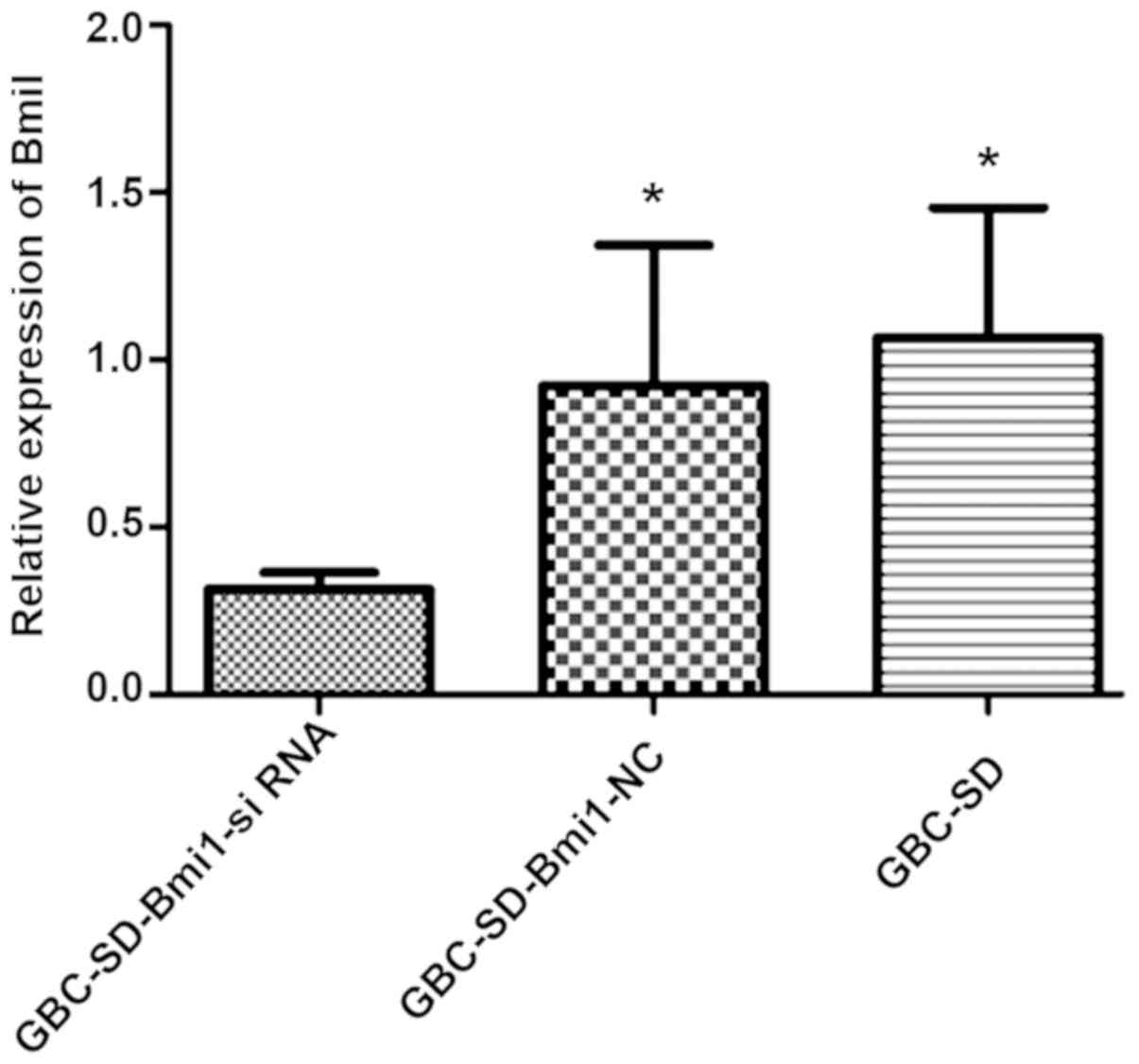
Compared with human normal gallbladder cancer cell
line GBC-SD and negative control GBC-SD-Bmi1-NC cells, the
expression level of Bmi-1 in GBC-SD-Bmi1-si RNA transfected with
Bmi1-siRNA was significantly lower (Fig.
3, P<0.05). This result demonstrates that Bmi1-si RNA can
induce the degradation of Bmi-1 mRNA in gallbladder cancer cell
lines.
CCK-8 assay detection of cell
proliferation
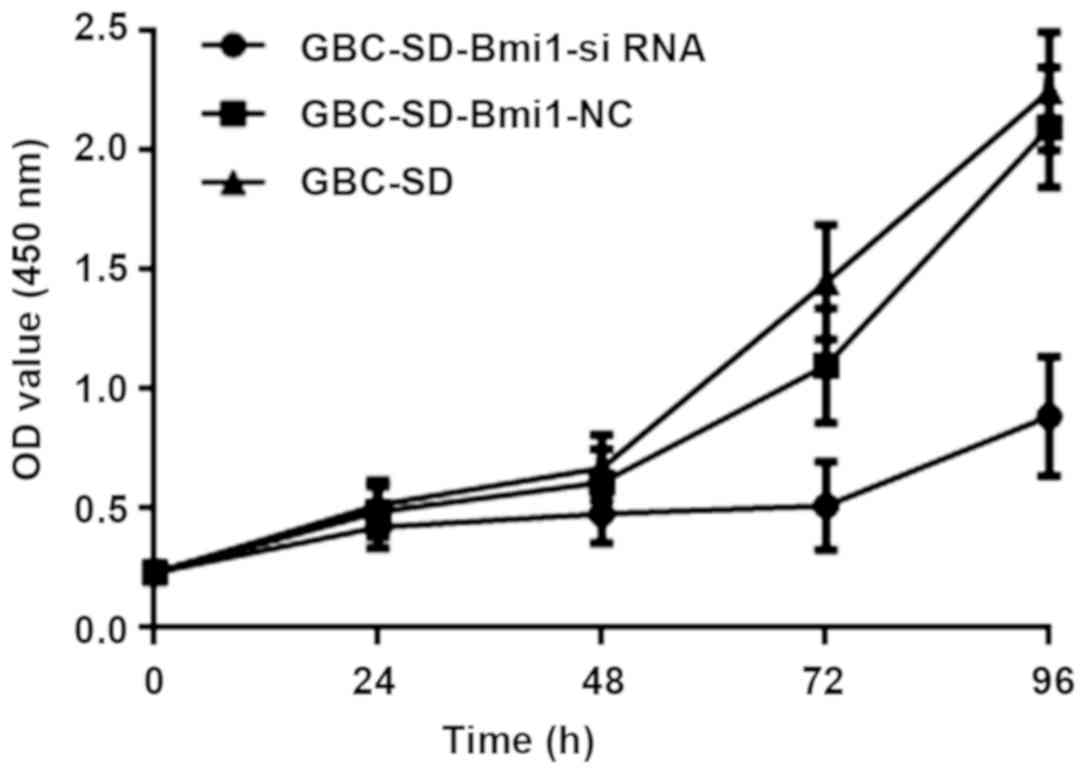
As shown in Fig. 4,
at 72 and 96 h, the absorbance of GBC-SD-Bmi1-siRNA cells was
significantly lower than that of control human normal gallbladder
cancer cells GBC-SD and the negative control GBC-SD-Bmi1-NC cells.
Therefore, the results indicate that the cell line GBC-SD-Bmi1-si
RNA constructed by the RNA interference technique grows slowly,
that is, its growth is inhibited, and the growth cycle is
prolonged.
Flow cytometry detection of
apoptosis
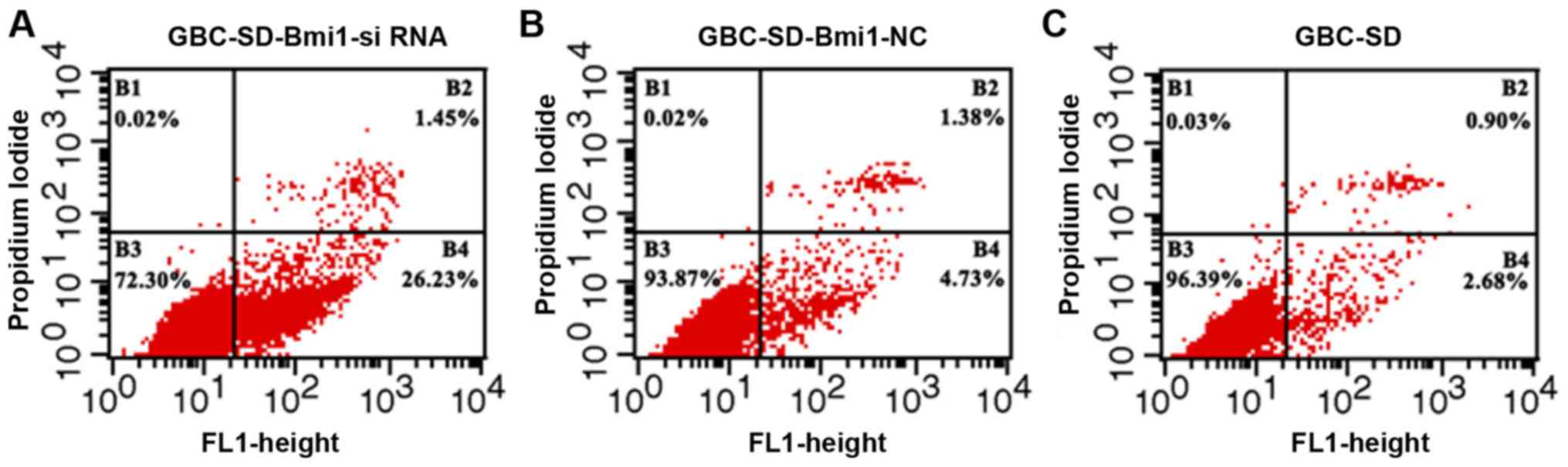
As shown in Fig. 5,
compared with GBC-SD and negative control GBC-SD-Bmi1-NC cells, the
apoptosis rate of GBC-SD-Bmi1-si RNA cells was significantly higher
than those of the other two groups (P<0.05, B4 quadrant as
statistical object), i.e., the inhibited expression of Bmi-1
gene led to promoted apoptosis of gallbladder cancer cell line
GBC-SD. This result is consistent with the CCK-8 experimental
results.
Effect of Bim1-siRNA transfection on
the expression of related proteins
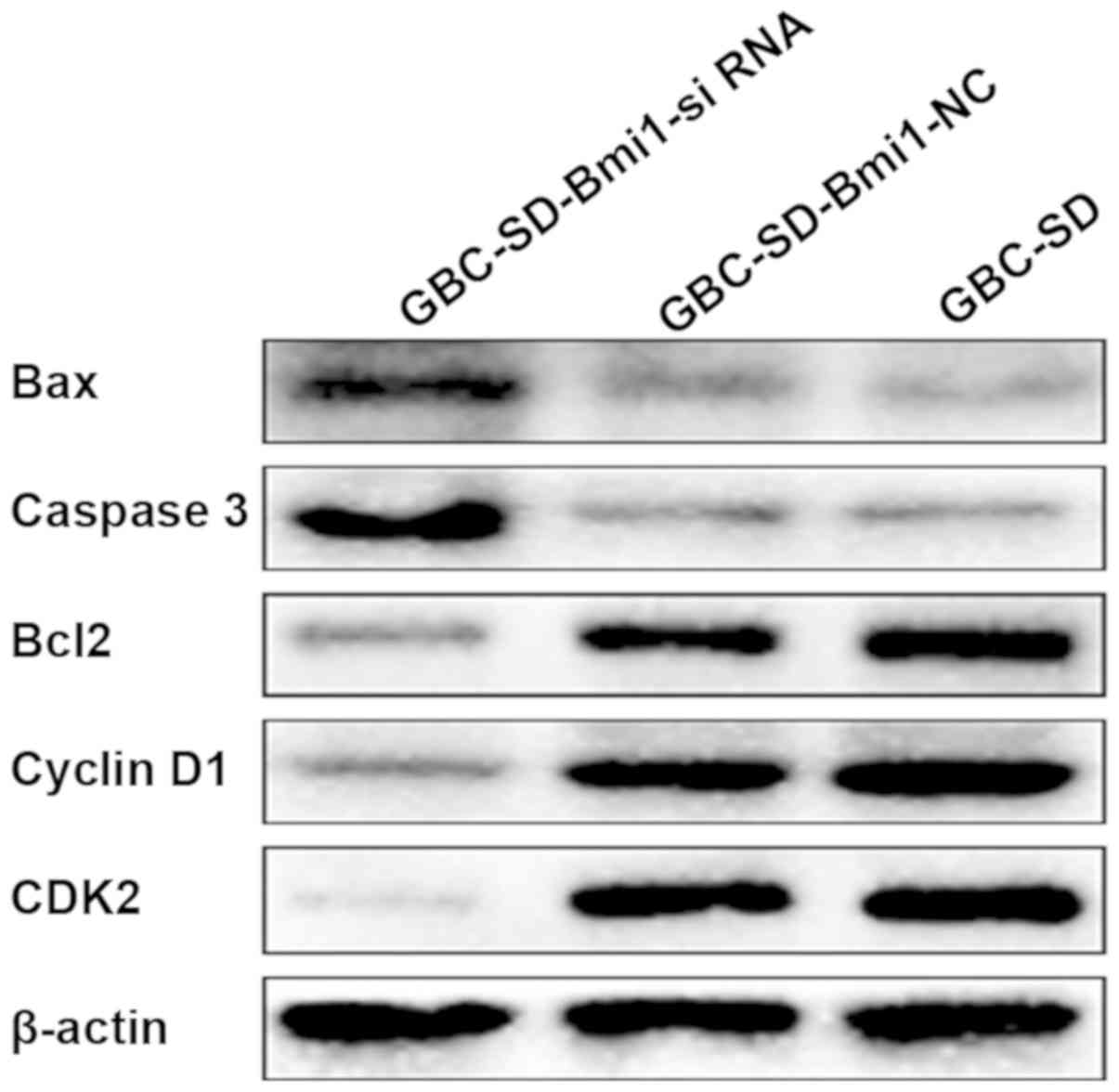
As shown in Fig. 6,
the expression level of the anti-apoptotic protein Bcl-2 in
GBC-SD-Bmi1-siRNA cells was decreased compared with GBC-SD and
negative control GBC-SD-Bmi1-NC cells. Expression level of Bax was
increased, and the expression level of the apoptotic kinase caspase
3 was increased, which was consistent with the increase in the
apoptotic rate of GBC-SD-Bmi1-si RNA cells. In addition, the
expression levels of the cyclins, cyclin D1 and CDK2, in
GBC-SD-Bmi1-si RNA cells were decreased compared with the two
control groups, which was consistent with the prolonged
GBC-SD-Bmi1-NC cell proliferation cycle.
Discussion
Gallbladder cancer is the most common malignant
tumor in the biliary system worldwide, and its geographical
distribution is uneven, it is relatively rare in most countries,
more common in countries such as India, Japan and Chile (9–12).
In China, the incidence of gallbladder cancer has
also increased in recent years (13). Although recent data show that
treatment efficacy of early gallbladder cancer is greatly improved
(4), the prognosis of advanced
patients is still not optimistic (14). Thus, worldwide scholars are committed
to the in-depth research of the incidence and progression of
gallbladder cancer in many aspects in order to achieve better
prevention and treatment. Long-term in-depth research has found and
identified a large number of related genes involved in gallbladder
cancer. This study aimed to investigate the expression of
Bmi-1 in gallbladder carcinoma and its clinicopathology and
mechanisms of regulating human gallbladder carcinoma cell
proliferation.
In this study, immunohistochemical staining showed
that the positive expression rate of Bmi-1 protein in gallbladder
carcinoma tissues was significantly higher than that in normal
gallbladder tissues, and data analysis showed that the expression
of Bmi-1 protein in gallbladder carcinoma tissues was associated
with tumor differentiation degree and stage. There was no
association with sex, age, presence of gallstones and other organ
invasions. After transfecting Bmi1-si RNA and Bmi1-NC vector into
gallbladder cancer cell line GBC-SD, RT-qPCR showed that Bmi-1
expression level in GBC-SD-Bmi1-s RNA was significantly lower than
that in GBC-SD-Bmi1-NC and GBC-SD cells, which proved that Bmi1-si
RNA played an inhibitory role. Absorbance of GBC-SD-Bmi1-si RNA
cells in CCK-8 assay was significantly lower than that in the two
control groups. That is, after inhibiting the expression of Bmi-1,
the growth cycle of gallbladder cancer cells is prolonged, thereby
leading to decreased proliferation. Qin et al (15) and Becker et al (16) found that the cell cycle was arrested
after downregulating Bmi-1 expression in a mouse lung cancer model,
which was consistent with the results of this study. Flow cytometry
showed that the apoptosis rate of GBC-SD-Bmi1-si RNA cells was
significantly higher than that of the two control groups. The
results were consistent with the CCK-8 experiment, in which the
apoptosis rate of the cells was higher than that of the control
group. It was again demonstrated that inhibition of Bmi-1
expression in gallbladder cancer cells can promote apoptosis of
gallbladder cancer cell line GBC-SD. This was consistent with the
results reported by Xiao and Deng (17) that after the expression of
Bmi-1 gene was downregulated by gene knockout technology,
the proliferation and invasion ability of gastric cancer cells were
weakened, which again proved that Bmi-1 can be a potential target
for targeted treatment of gallbladder cancer. In addition, the cell
cycle arrest of GBC-SD-Bmi1-si RNA cells affects the proliferation
of gallbladder cancer cells, suggesting that Bmi-1 may regulate
cell cycle and affect cell proliferation by acting on cell cycle
factors. It can also act on proapoptotic and anti-apoptotic
proteins to affect the apoptosis process of gallbladder cancer
cells. Therefore, we extracted the total protein of GBC-SD-Bmi1-si
RNA cells and detected the expression of a series of cell cycle
factors, pro-apoptotic proteins and anti-apoptotic proteins.
Results showed that compared with the two control groups, the
expression level of anti-apoptotic protein Bcl-2 was decreased in
GBC-SD-Bmi1-si RNA cells, and the expression level of proapoptotic
protein Bax and caspase 3 was increased, and expression level of
cyclin D1 and CDK2 was decreased. This result indicates that
downregulation of Bmi-1 expression may affect the expression levels
of cyclin D1 and CDK2 in gallbladder cancer cells, leading to a
delay in cell proliferation cycle, and also a decrease in the
expression of anti-apoptotic protein Bcl-2 in gallbladder cancer
cells. Increased expression of the proapoptotic protein Bax and the
apoptotic kinase caspase 3 led to an increase in apoptosis.
Studies have shown that imbalance between cell
proliferation and apoptotic state plays an important role in the
occurrence and development of most malignant tumors. Cell
proliferation imbalance and cell cycle disorder may affect the
occurrence and development of tumors. This study demonstrates that
targeted inhibition of Bmi-1 expression can affect the
proliferation and apoptosis of gallbladder cancer cells. The
molecular mechanism is related to the decreased expression of
cyclin D1 and CDK2, decreased expression of anti-apoptotic protein
Bcl-2, and increased expression of proapoptotic protein Bax and
caspase 3, which confirms that Bmi-1 is a potential target for
clinical treatment of gallbladder cancer. However, more clinical
studies on its mechanism are still needed.
Acknowledgements
Not applicable.
Funding
The study was funded by the Qiqihar City Science and
Technology Project.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KJ conceived the study, wrote the manuscript and was
responsible for immunohistochemical staining for detection of Bmi-1
expression in gallbladder carcinoma. HZ, WJ and CZ were responsible
for western blot analysis and PCR. HZ and DS helped with flow
cytometry and CCK-8 assay. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Second Affiliated Hospital of Qiqihar Medical University
(Qiqihar, China). Patients who participated in this research had
complete clinical data. Signed informed consents were obtained from
the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kapoor VK: Gallbladder cancer: A global
perspective. J Surg Oncol. 93:607–609. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu XS, Shi LB, Li ML, Ding Q, Weng H, Wu
WG, Cao Y, Bao RF, Shu YJ, Ding QC, et al: Evaluation of two
inflammation-based prognostic scores in patients with resectable
gallbladder carcinoma. Ann Surg Oncol. 21:449–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu AX, Hong TS, Hezel AF and Kooby DA:
Current management of gallbladder carcinoma. Oncologist.
15:168–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wakai T, Shirai Y, Yokoyama N, Nagakura S,
Watanabe H and Hatakeyama K: Early gallbladder carcinoma does not
warrant radical resection. Br J Surg. 88:675–678. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang GF, He WP, Cai MY, He LR, Luo JH,
Deng HX, Guan XY, Zeng MS, Zeng YX and Xie D: Intensive expression
of Bmi-1 is a new independent predictor of poor outcome in patients
with ovarian carcinoma. BMC Cancer. 10:1332010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He XT, Cao XF, Ji L, Zhu B, Lv J, Wang DD,
Lu PH and Cui HG: Association between Bmi1 and clinicopathological
status of esophageal squamous cell carcinoma. World J
Gastroenterol. 15:2389–2394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo M, Shen D-X, Guo X-T, Guan T and Chen
X-D: Clinicopathological and prognostic significance of Bmi-1
expression in human cervical cancer. Acta Obstet Gynecol Scand.
90:737–745. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lazcano-Ponce EC, Miquel JF, Muñoz N,
Herrero R, Ferrecio C, Wistuba II, Alonso de Ruiz P, Aristi Urista
G and Nervi F: Epidemiology and molecular pathology of gallbladder
cancer. CA Cancer J Clin. 51:349–364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pandey M: Risk factors for gallbladder
cancer: A reappraisal. Eur J Cancer Prev. 12:15–24. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nandakumar A, Gupta PC, Gangadharan P,
Visweswara RN and Parkin DM: Geographic pathology revisited:
Development of an atlas of cancer in India. Int J Cancer.
116:740–754. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Misra S, Chaturvedi A, Misra NC and Sharma
ID: Carcinoma of the gallbladder. Lancet Oncol. 4:167–176. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsing AW, Gao YT, Devesa SS, Jin F and
Fraumeni JF Jr: Rising incidence of biliary tract cancers in
Shanghai, China. Int J Cancer. 75:368–370. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ito H, Matros E, Brooks DC, Osteen RT,
Zinner MJ, Swanson RS, Ashley SW and Whang EE: Treatment outcomes
associated with surgery for gallbladder cancer: A 20-year
experience. J Gastrointest Surg. 8:183–190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin L, Zhang X, Zhang L, Feng Y, Weng GX,
Li MZ, Kong QL, Qian CN, Zeng YX, Zeng MS, et al: Downregulation of
BMI-1 enhances 5-fluorouracil-induced apoptosis in nasopharyngeal
carcinoma cells. Biochem Biophys Res Commun. 371:531–535. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Becker M, Korn C, Sienerth AR, Voswinckel
R, Luetkenhaus K, Ceteci F and Rapp UR: Polycomb group protein Bmi1
is required for growth of RAF driven non-small-cell lung cancer.
PLoS One. 4:e42302009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao J and Deng C: Knockdown of Bmi-1
impairs growth and invasiveness of human gastric carcinoma cells.
Oncol Res. 17:613–620. 2009. View Article : Google Scholar : PubMed/NCBI
|