Introduction
Pancreatic cancer is a highly malignant tumor type
of the digestive tract that is ranked as the fourth leading cause
of cancer-associated mortality (1),
with an estimated 55,440 new cases and an estimated 44,330
mortalities in the USA in 2018 according to statistics from
Surveillance, Epidemiology, and End Results (2). Its aggressive biological properties,
lack of early symptoms and rapid spread to surrounding organs lead
are responsible for the high mortality rate (3). Furthermore, the treatment of pancreatic
cancer is limited due to difficulties associated with surgical
removal, and poor sensitivity to radiotherapy and chemotherapy
(4–6). Therefore, identification of therapeutic
targets is urgently required to improve patient outcome (7).
It has been reported that β-catenin, a versatile
protein that mediates intercellular adhesion and gene expression,
is abnormally expressed in pancreatic cancer (8). As the transcriptional cofactor of
β-catenin, transcription factor 7 like 1 (TCF7L1), also termed
transcription factor 3, is a member of the mammalian TCF/LEF
family. Nuclear DNA-binding TCF/LEF proteins and β-catenin
represent key components of the canonical branch of the Wnt
signaling pathway, which serves a key role in pancreatic cancer
carcinogenesis (9,10).
Once the Wnt pathway is activated, β-catenin
accumulates in the cytoplasm and enters the nucleus, where it
engages DNA-bound TCF transcription factors and subsequently
regulates the transcription of downstream target genes (11). It is understood that β-catenin and
TCF7L1 are pivotal proteins in the Wnt/β-catenin pathway;
therefore, the genes they regulate may be drug targets for
pancreatic cancer (12).
In recent years, microarray and high throughput
sequencing technologies have widely been used to explore the
genetic characteristics of tumorigenesis, which may promote the
development of diagnostic and treatment strategies (13). Bioinformatics research methods are
required to handle large sample data; therefore, different
databases have been established to provide convenience for research
(14,15). In the present study, the gene
expression profiles GSE57728, an array focused on β-catenin, and
GSE90926, an array developed by high throughput sequencing
regarding TCF7L1, were downloaded from the Gene Expression Omnibus
(GEO) and analyzed to obtain the differentially expressed genes
(DEGs) between pancreatic control groups and experimental groups.
Clustering analysis, and functional and pathways enrichment
analysis were performed to identify the associations and functions
of the DEGs. In addition, a protein-protein interaction (PPI)
network was constructed, and overall survival (OS) and promoter
analyses was performed, to identify the associated key genes and
pathways downstream of the β-catenin-TCF7L1 complex in pancreatic
cancer cells.
Materials and methods
Collection and inclusion criteria of
the studies
The GEO database (www.ncbi.nlm.nih.gov/geo/) was searched for the
following keywords: ‘pancreatic cancer’ (study keyword),
‘β-catenin’ (study keyword), ‘Homo sapiens’ (organism) and
‘Expression profiling by array or sequencing’ (study type). This
search revealed seven studies. The inclusion criteria for the
studies were as follows: i) Samples were required to be in two
groups, including the control group and the experimental group, ii)
the sample count needed to be >10, iii) β-catenin or TCF7L1 in
the experimental group should be overexpressed or inhibited, and
iv) sufficient information had to be present to perform the
analysis. Consequently, GSE57728 (16) was downloaded for analysis regarding
β-catenin and GSE90926, which was contributed by Dr David Dawson
(Dawson Laboratory, Department of Pathology and Laboratory
Medicine, David Geffen School of Medicine, University of
California, Los Angeles, CA, USA), was downloaded for analysis
regarding TCF7L1.
Microarray data and validation
Two gene expression profiles (GSE57728 and GSE90926)
were downloaded from the GEO database. The array data regarding
β-catenin knockdown in GSE57728 included 16 samples, from this the
present study selected two control samples with control small
interfering RNA (siRNA) transfection and two experimental samples
with β-catenin siRNA transfection for analysis. Similarly, the
sequencing data regarding TCF7L1 knockdown in GSE90926 included 12
samples and the current study selected three control samples with
control siRNA transfection and three experimental samples with
TCF7L1 siRNA transfection for further analysis. Subsequently, a
microarray assay regarding β-catenin knockdown was conducted to
confirm the results from the microarray data downloaded from the
GEO database. This was performed based on previous studies in which
relevant results regarding the Wnt pathway in pancreatic cancer
were revealed, including the identification of FH535 as a
small-molecule inhibitor of the Wnt/β-catenin signaling pathway
(10,17). FH535, as a classic inhibitor of the
β-catenin pathway which could repress pancreatic cancer cell growth
and metastasis, played the same role as siRNA in the inhibition of
the β-catenin pathway. Sample preparation and processing were
performed as described in the GeneChip Expression Analysis Manual
(Agilent Technologies, Inc., Santa Clara, CA, USA). Differentially
expressed genes were screened using Agilent 44K human whole-genome
oligonucleotide microarrays (Agilent Technologies, Inc.). After
obtaining the two completed microarrays with different gene
expressions, 10 shared genes were selected randomly and the gene
expression levels of the control and experimental groups were
compared to confirm that the data downloaded from the GEO database
was reliable.
Data processing
R (version 3.3.3 for Windows; https://www.r-project.org/) is a software system used
for data processing, computing and mapping based on the different R
packages. The limma package was used to identify the DEGs by linear
modeling of the genes. P<0.05 and a fold change >1.5 or
<0.667 were set as the cut-off criteria. Subsequently, a heat
map of DEGs was generated using R and P<0.05 was set as the
cut-off criterion.
Functional and pathway enrichment
analysis, and PPI network construction
Database for Annotation, Visualization and
Integrated Discovery (DAVID) provides a comprehensive set of
functional annotation tools for investigators to understand the
biological meaning behind a large list of genes. FunRich is a
stand-alone software tool used predominantly for functional
enrichment and interaction network analysis of genes and proteins.
The results of the analysis can be depicted graphically in the form
of Venn, bar, column, pie and doughnut charts. In the present
study, gene ontology (GO) enrichment analysis was performed for the
identified DEGs using the FunRich (version 3.1.3 for Windows;
http://www.funrich.org/) and DAVID databases
(version 6.8; http://david.ncifcrf.gov/). P<0.05 was set as the
cut-off criterion, however, for the sake of symmetry and sharp
contrast, the P-value of several terms was >0.05. In every
figure, eight columns were sorted using Funrich. Pathway enrichment
analysis was performed for the identified DEGs using KOBAS
(http://kobas.cbi.pku.edu.cn/), which is
a web server for gene/protein functional annotation and functional
gene set enrichment. In addition, the Kyoto Encyclopedia of Genes
and Genomes (KEGG; http://www.kegg.jp/) database was used, which is an
integrated database resource for biological interpretation of
genome sequences and other high-throughput data (18). P<0.05 was set as the cut-off
criterion. In addition, a PPI network of the DEGs was constructed
using the STRING database (http://string-db.org/) and Cytoscape (version 3.7.1
for Windows; http://cytoscape.org/), which is a
commonly used software to generate integrated models of
biomolecular interaction networks. A combined score >0.15 was
set as the cut-off criterion. To screen the hub genes, a node
degree ≥8 was set as the cut-off criterion.
Survival analysis of DEGs
Gene expression datasets were downloaded from The
Cancer Genome Atlas (TCGA; http://tcga-data.nci.nih.gov/tcga) to analyze the
prognosis of target DEGs. Data from a total of 178 patients with
complete clinicopathological and RNASeq data were collected from
the TCGA pancreatic cancer cohort. Clinical characteristics of the
178 patients are presented in Table
I, including case ID, sex, year of birth, year of mortality,
tumor stage, age at diagnosis measured in days, vital status and
time from diagnosis to the last follow-up date or mortality. The
patients were divided into two groups according to the expression
of a particular gene, including a high expression group and a low
expression group. The OS of patients with pancreatic cancer was
analyzed using R software and the results were compared using
Kaplan-Meier curves on which the P-value was presented. A log-rank
test was conducted as the post hoc test.
 | Table I.Clinical characteristics of 178
patients used for overall survival analysis. |
Table I.
Clinical characteristics of 178
patients used for overall survival analysis.
| Case ID | Sex | Year of birth | Year of
mortality | Tumor stage | Age at diagnosis,
days | Alive at last
follow-up | Days from diagnosis
to mortality | Days from diagnosis
to last follow-up |
|---|
| 1 | Male | 1929 | 2011 | iib | 30,092 | No | 292 | – |
| 2 | Female | 1942 | – | iIb | 26,179 | No | 375 | 1 |
| 3 | Male | 1970 | – | iib | 15,807 | Yes | – | 286 |
| 4 | Male | 1938 | – | ib | 27,362 | No | 498 | 449 |
| 5 | Female | 1953 | – | iia | 22,131 | Yes | – | 438 |
| 6 | Male | 1947 | 2012 | iib | 23,962 | No | 66 | – |
| 7 | Male | 1938 | 2013 | iib | 27,082 | No | 652 | – |
| 8 | Female | 1938 | 2014 | iib | 27,662 | No | 532 | – |
| 9 | Male | 1972 | – | ia | 14,729 | Yes | – | 1,037 |
| 10 | Male | 1932 | – | iib | 29,792 | Yes | – | 483 |
| 11 | Male | 1932 | – | ib | 29,631 | Yes | – | 7 |
| 12 | Female | 1938 | – | iib | 27,645 | Yes | – | 525 |
| 13 | Female | 1962 | – | iib | 18,202 | No | 913 | 648 |
| 14 | Male | 1962 | – | iib | 18,357 | Yes | – | 920 |
| 15 | Male | 1949 | – | iib | 23,152 | Yes | – | 666 |
| 16 | Male | 1926 | 2010 | iia | 29,633 | No | 1,101 | – |
| 17 | Female | 1957 | 2012 | iib | 20,051 | No | 511 | – |
| 18 | Male | 1936 | 2009 | iib | 26,085 | No | 1,059 | – |
| 19 | Female | 1946 | – | ib | 23,406 | Yes | – | 1,542 |
| 20 | Male | 1957 | 2013 | iib | 20,133 | No | 607 | – |
| 21 | Male | 1941 | – | iib | 24,760 | Yes | – | 2,285 |
| 22 | Female | 1940 | – | iib | 26,635 | No | 732 | 385 |
| 23 | Male | 1943 | – | ib | 24,621 | Yes | – | 998 |
| 24 | Male | 1933 | – | iib | 28,174 | No | 661 | 240 |
| 25 | Female | 1936 | – | iib | 24,025 | No | 2,036 | 1,953 |
| 26 | Male | 1937 | – | iib | 27,453 | Yes | – | 743 |
| 27 | Male | 1965 | 2012 | iib | 17,294 | No | 308 | – |
| 28 | Female | 1955 | – | iib | 20,741 | Yes | – | 392 |
| 29 | Female | 1930 | 2011 | iib | 29,585 | No | 153 | – |
| 30 | Male | 1964 | – | iib | 17,794 | Yes | – | 729 |
| 31 | Female | 1947 | – | iv | 24,291 | Yes | – | 420 |
| 32 | Male | 1925 | 2009 | iia | 30,571 | No | 480 | – |
| 33 | Female | 1932 | – | iii | 29,213 | Yes | – | 462 |
| 34 | Female | 1948 | – | iib | 23,672 | Yes | – | 635 |
| 35 | Male | 1964 | – | iib | 18,059 | Yes | – | 404 |
| 36 | Male | 1938 | – | iia | 27,684 | No | 267 | 110 |
| 37 | Female | 1936 | – | iib | 27,929 | No | 517 | 0 |
| 38 | Female | 1952 | – | ib | 21,732 | Yes | – | 1,103 |
| 39 | Male | 1941 | – | iib | 26,028 | Yes | – | 80 |
| 40 | Female | 1939 | – | iia | 27,152 | Yes | – | 467 |
| 41 | Female | 1946 | – | iib | 22,981 | Yes | – | 228 |
| 42 | Female | 1942 | 2013 | iib | 25,920 | No | 627 | – |
| 43 | Male | 1946 | 2012 | iib | 23,998 | No | 458 | – |
| 44 | Female | 1929 | 2011 | iib | 29,904 | No | 568 | – |
| 45 | Female | 1959 | – | iib | 19,064 | No | 593 | 20 |
| 46 | Female | 1928 | – | ia | 30,821 | No | 151 | 91 |
| 47 | Male | 1958 | – | iib | 19,904 | Yes | – | 767 |
| 48 | Female | 1946 | – | iib | 23,868 | No | 596 | 21 |
| 49 | Male | 1952 | – | iib | 21,676 | Yes | – | 522 |
| 50 | Female | 1947 | 2009 | iib | 22,990 | No | 110 | – |
| 51 | Female | 1958 | – | iia | 19,839 | No | 299 | 28 |
| 52 | Male | 1936 | – | iib | 27,637 | Yes | – | 194 |
| 53 | Female | 1945 | 2010 | iib | 23,953 | No | 31 | – |
| 54 | Male | 1939 | 2013 | iib | 26,936 | No | 691 | – |
| 55 | Female | 1948 | – | iib | 22,376 | Yes | – | 2,016 |
| 56 | Male | 1939 | – | ia | 26,947 | Yes | – | 454 |
| 57 | Male | 1943 | 2011 | iib | 24,078 | No | 1,130 | – |
| 58 | Female | 1951 | – | iia | 22,090 | Yes | – | 840 |
| 59 | Female | 1965 | – | iib | 17,821 | No | 278 | 164 |
| 60 | Female | 1936 | – | iib | 28,434 | No | 160 | 11 |
| 61 | Female | 1945 | 2010 | iib | 23,580 | No | 603 | – |
| 62 | Male | 1926 | 2011 | ia | 31,319 | No | 244 | – |
| 63 | Female | 1968 | – | i | 14,599 | Yes | – | 2,741 |
| 64 | Male | 1954 | – | iib | 19,847 | Yes | – | 716 |
| 65 | Female | 1953 | – | ib | 22,126 | Yes | – | 9 |
| 66 | Male | 1978 | – | iib | 13,127 | Yes | – | 245 |
| 67 | Male | 1947 | – | iia | 24,007 | Yes | – | 586 |
| 68 | Male | 1944 | 2012 | iia | 24,731 | No | 634 | – |
| 69 | Male | 1959 | – | iia | 19,677 | Yes | – | 671 |
| 70 | Male | 1943 | – | iv | 25,849 | Yes | – | 603 |
| 71 | Male | 1937 | – | iib | 27,850 | Yes | – | 0 |
| 72 | Female | 1939 | 2013 | ib | 27,128 | No | 144 | – |
| 73 | Male | 1938 | 2010 | iib | 26,239 | No | 485 | – |
| 74 | Female | 1934 | 2008 | iib | 26,773 | No | 467 | – |
| 75 | Male | 1934 | 2010 | iib | 28,074 | No | 143 | – |
| 76 | Male | 1963 | 2013 | iib | 18,315 | No | 183 | – |
| 77 | Male | 1935 | 2009 | ib | 26,747 | No | 598 | – |
| 78 | Male | 1956 | 2012 | iib | 20,641 | No | 277 | – |
| 79 | Male | 1940 | – | iib | 26,503 | Yes | – | 657 |
| 80 | Male | 1937 | – | iia | 28,047 | Yes | – | 517 |
| 81 | Female | 1968 | – | iib | 16,255 | No | 470 | 247 |
| 82 | Female | 1933 | – | iib | 29,150 | No | 233 | 153 |
| 83 | Male | 1957 | – | iib | 20,071 | No | 592 | 360 |
| 84 | Male | 1945 | – | iib | 24,150 | No | 614 | 361 |
| 85 | Female | 1954 | – | iib | 21,491 | Yes | – | 660 |
| 86 | Male | 1947 | 2011 | iib | 23,713 | No | 216 | – |
| 87 | Female | 1944 | – | iib | 24,891 | Yes | – | 491 |
| 88 | Male | 1962 | 2011 | iib | 18,172 | No | 123 | – |
| 89 | Female | 1946 | – | iv | 24,043 | No | 394 | 347 |
| 90 | Female | 1947 | 2012 | iib | 23,431 | No | 460 | – |
| 91 | Male | 1936 | – | iib | 28,403 | Yes | – | 330 |
| 92 | Female | 1963 | – | iib | 18,607 | No | 366 | 202 |
| 93 | Female | 1956 | – | iia | 20,316 | Yes | – | 969 |
| 94 | Female | 1929 | – | iib | 30,684 | Yes | – | 225 |
| 95 | Female | 1940 | – | iib | 26,379 | Yes | – | 319 |
| 96 | Female | 1939 | – | iib | 27,295 | No | 393 | 127 |
| 97 | Male | 1945 | – | ib | 24,810 | Yes | – | 951 |
| 98 | Female | 1950 | – | iib | 23,218 | No | 313 | 155 |
| 99 | Female | 1950 | – | iib | 22,413 | Yes | – | 4 |
| 100 | Female | 1942 | 2011 | iib | 25,312 | No | 224 | – |
| 101 | Female | 1948 | 2009 | iib | 21,611 | No | 741 | – |
| 102 | Male | 1955 | 2007 | iib | 19,287 | No | 61 | – |
| 103 | Female | 1955 | 2009 | iib | 19,718 | No | 486 | – |
| 104 | Male | 1945 | – | iib | 24,864 | Yes | – | 431 |
| 105 | Male | 1939 | – | iib | 25,809 | Yes | – | 289 |
| 106 | Male | 1950 | – | iib | 22,433 | No | 366 | 24 |
| 107 | Male | 1936 | 2013 | iib | 28,239 | No | 95 | – |
| 108 | Female | 1943 | – | iib | 25,412 | No | 179 | 4 |
| 109 | Female | 1926 | 2012 | iib | 31,393 | No | 481 | – |
| 110 | Male | 1946 | – | iib | 24,589 | Yes | – | 737 |
| 111 | Female | 1933 | 2011 | iib | 28,353 | No | 702 | – |
| 112 | Female | 1958 | – | iib | 20,366 | Yes | – | 33 |
| 113 | Female | 1950 | – | iib | 23,306 | No | 230 | 179 |
| 114 | Male | 1954 | – | iib | 21,024 | No | 518 | 8 |
| 115 | Male | 1945 | 2009 | iia | 23,703 | No | 117 | – |
| 116 | Female | 1922 | 2007 | iib | 31,074 | No | 155 | – |
| 117 | Male | 1950 | – | iia | 22,283 | Yes | – | 1,216 |
| 118 | Female | 1954 | – | iv | 21,501 | No | 545 | 5 |
| 119 | Male | 1931 | 2012 | iib | 29,674 | No | 120 | – |
| 120 | Male | 1957 | – | iia | 20,607 | Yes | – | 498 |
| 121 | Female | 1935 | 2012 | iib | 27,957 | No | 695 | – |
| 122 | Female | 1956 | – | iib | 20,858 | Yes | – | 395 |
| 123 | Female | – | – | iia | 17,628 | Yes | – | 584 |
| 124 | Female | 1949 | 2013 | iib | 23,622 | No | 239 | – |
| 125 | Male | 1934 | – | iia | 28,317 | Yes | – | 482 |
| 126 | Male | 1946 | – | iia | 23,760 | Yes | – | 314 |
| 127 | Male | 1946 | 2010 | iib | 23,443 | No | 12 | – |
| 128 | Male | 1937 | 2009 | iv | 26,216 | No | 619 | – |
| 129 | Male | 1930 | 2010 | iib | 29,319 | No | 123 | – |
| 130 | Female | 1946 | – | ia | 24,174 | Yes | – | 1,021 |
| 131 | Female | 1924 | – | iib | 32,475 | No | 421 | 233 |
| 132 | Male | 1944 | – | ib | 23,791 | Yes | – | 1,854 |
| 133 | Male | 1952 | 2009 | iib | 20,984 | No | 334 | – |
| 134 | Male | 1950 | – | iia | 22,425 | Yes | – | 1,287 |
| 135 | Female | 1951 | – | iib | 22,329 | Yes | – | 289 |
| 136 | Female | 1949 | – | ib | 23,685 | Yes | – | 95 |
| 137 | Male | 1935 | – | iib | 28,454 | No | 308 | 0 |
| 138 | Male | 1946 | – | iib | 24,576 | Yes | – | 338 |
| 139 | Male | 1952 | – | Not reported | 21,175 | Yes | – | 1,794 |
| 140 | Female | 1956 | 2012 | ib | 20,760 | No | 219 | – |
| 141 | Male | 1965 | – | iib | 16,766 | Yes | – | 1,323 |
| 142 | Male | 1970 | – | iib | 15,869 | Yes | – | 440 |
| 143 | Female | 1932 | – | iib | 28,554 | Yes | – | 1,257 |
| 144 | Female | 1943 | – | iib | 25,214 | No | 378 | 16 |
| 145 | Male | 1939 | – | iib | 26,573 | Yes | – | 969 |
| 146 | Male | 1964 | – | iia | 17,649 | No | 353 | 166 |
| 147 | Female | 1955 | – | iib | 21,484 | Yes | – | 463 |
| 148 | Female | 1963 | 2011 | iib | 16,126 | No | 1,502 | – |
| 149 | Male | 1941 | – | iib | 26,188 | Yes | – | 484 |
| 150 | Male | 1955 | 2012 | iib | 20,618 | No | 684 | – |
| 151 | Male | 1937 | 2012 | iia | 27,600 | No | 293 | – |
| 152 | Male | 1942 | – | iia | 25,768 | Yes | – | 252 |
| 153 | Female | 1946 | – | ib | 22,799 | Yes | – | 2,084 |
| 154 | Female | 1940 | – | iia | 26,311 | Yes | – | 232 |
| 155 | Male | 1948 | – | iib | 23,801 | Yes | – | 287 |
| 156 | Male | 1942 | – | iii | 25,227 | Yes | – | 706 |
| 157 | Male | 1967 | 2009 | iib | 15,188 | No | 666 | – |
| 158 | Female | 1938 | – | Not reported | 26,859 | Yes | – | 388 |
| 159 | Male | 1947 | 2007 | iib | 22,148 | No | 145 | – |
| 160 | Male | 1939 | 2013 | iib | 26,745 | No | 430 | – |
| 161 | Male | 1954 | – | Not reported | 20,451 | Yes | – | 1,942 |
| 162 | Male | 1954 | – | iib | 21,792 | Yes | – | 350 |
| 163 | Female | 1928 | 2002 | iii | 26,881 | No | 541 | – |
| 164 | Male | 1962 | 2012 | iia | 18,475 | No | 128 | – |
| 165 | Female | 1942 | 2011 | iia | 24,117 | No | 1,332 | – |
| 166 | Female | 1950 | 2013 | iib | 22,400 | No | 738 | – |
| 167 | Female | 1932 | – | iib | 29,585 | No | 466 | 36 |
| 168 | Male | 1937 | – | iib | 28,013 | Yes | – | 8 |
| 169 | Female | 1949 | – | iia | 23,624 | Yes | – | 379 |
| 170 | Male | 1954 | – | iib | 21,277 | Yes | – | 416 |
| 171 | Female | 1962 | – | iib | 18,129 | Yes | – | 1,116 |
| 172 | Male | 1940 | – | ib | 26,167 | No | 236 | 0 |
| 173 | Female | 1959 | – | ib | 19,707 | Yes | – | 720 |
| 174 | Male | 1958 | – | iib | 19,315 | Yes | – | 1,383 |
| 175 | Male | 1939 | – | iia | 26,943 | Yes | – | 676 |
| 176 | Male | 1941 | – | iib | 26,129 | No | 365 | 329 |
| 177 | Male | 1937 | 2013 | iia | 26,234 | No | 2,182 | – |
| 178 | Male | 1940 | – | iib | 26,322 | Yes | – | 978 |
Promoter analysis of DEGs
Ensemble (http://www.ensembl.org/index.html) is an online
website that was used to perform promoter analysis of the DEGs. The
eligible transcript of every DEG associated with prognosis was
selected and then the 3,000 base pairs 5′ upstream were selected as
the promoter. Subsequently, the transcription factors (TFs) site
analysis function of Genomatix (http://www.genomatix.de/solutions/genomatix-software-suite.html)
was used to predict potential TF families and TF binding sites by
analyzing the sequence of promoter obtained from Ensemble. Core
similarity was set as 1 for an accurate prediction.
Results
Microarray data and validation
As demonstrated in the Fig. 1, ten genes that were shared between
the original microarray data downloaded from the GEO database and
our own microarray data regarding β-catenin knockdown, including
CGN, CNN3, DZIP1, EGR1, FBXL17, MDM2, SRXN1, HMOX1 and TMEM2, were
selected to confirm the results from microarray data downloaded
from the GEO database. The results obtained for samples with
β-catenin siRNA transfection and samples treated with FH535
exhibited consistent trends, with the exception of the results for
IL32, HMOX1 and TMEM2.
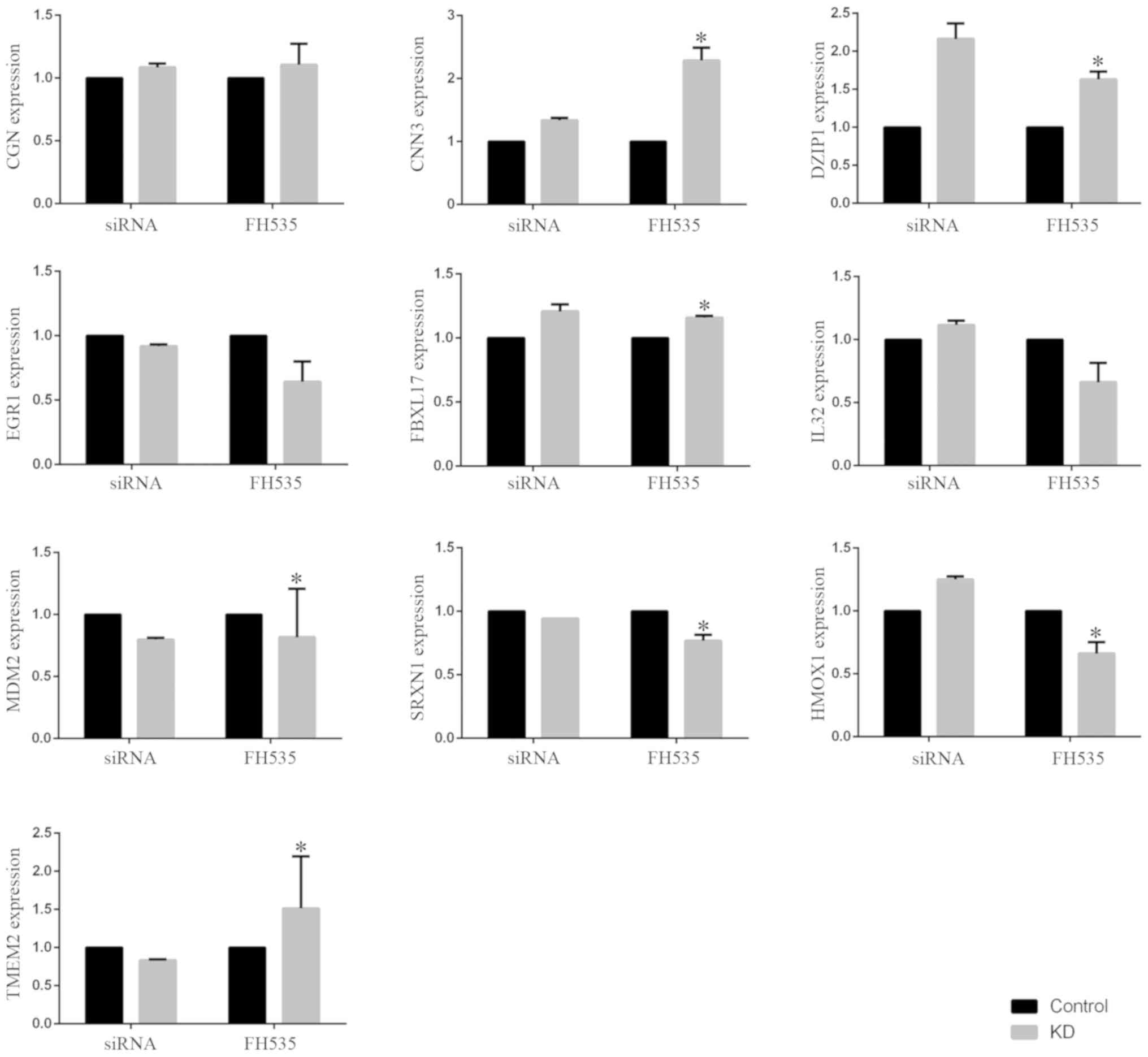 | Figure 1.Microarray data and validation. Ten
shared genes, including CGN, CNN3, DZIP1, EGR1, FBXL17, MDM2,
SRXN1, HMOX1 and TMEM2, were selected to confirm the results from
microarray data downloaded from the Gene Expression Omnibus
database. Microarray analysis was performed to detect the
expression of genes of samples transfected with 20 nM control siRNA
or β-catenin siRNA in the original microarray data downloaded from
GEO database. Microarray analysis was also performed to measure the
expression of genes in samples treated with 20 µM FH535 in our own
microarray data. The data are presented as the means ± standard
deviation. *P<0.05 vs. respective control. siRNA, small
interfering RNA; KD, knockdown. |
Identification of DEGs and clustering
analysis
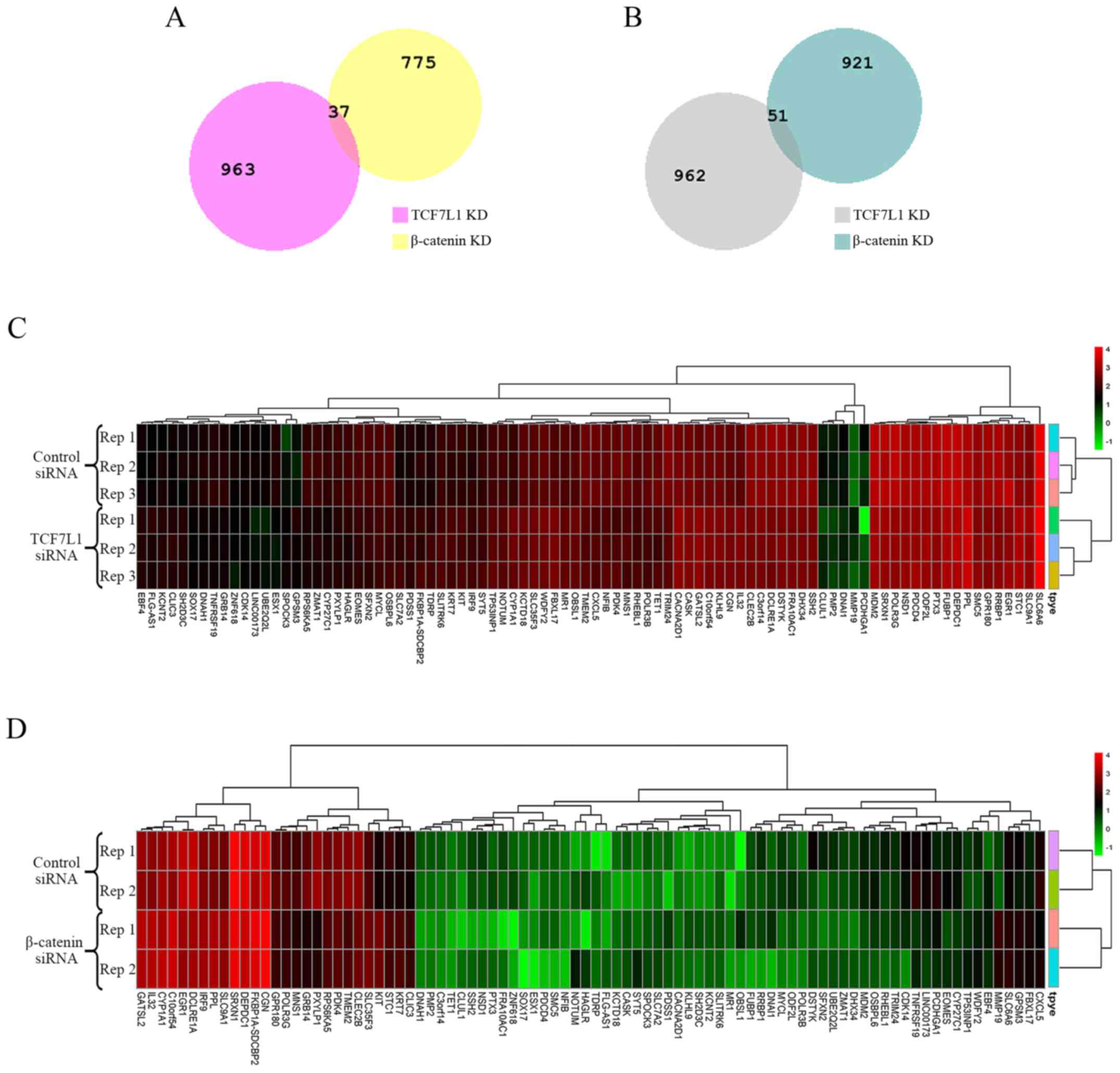
A total of 1,784 DEGs, including, 812 upregulated
and 972 downregulated genes, were identified from GSE90926
regarding TCF7L1 knockdown. A total of 2,013 DEGs, including 1,000
upregulated and 1,013 downregulated genes, were identified from
GSE57728 regarding β-catenin knockdown. Among these DEGs, 88 DEGs
were screened out as shared by the two datasets. The upregulated
and downregulated DEGs were considered separately when selecting
the shared genes. As a result, 37 upregulated and 51 downregulated
DEGs were identified (Fig. 2A and B;
Table II). The respective heatmaps
of the 88 DEGs were generated by R software (Fig. 2C and D).
 | Table II.Identification of differentially
expressed genes. |
Table II.
Identification of differentially
expressed genes.
| Regulation | Genes name |
|---|
| Upregulated | MMP19, OBSL1, KIT,
PDSS1, SYT5, KLHL9, KCNT2, PPL, KRT7, FBXL17, SH2D3C, MR1,
C10orf54, IL32, FLG-AS1, SLC9A1, TDRP, GPSM3, CGN, FKBP1A-SDCBP2,
CASK, WDFY2, SLC35F3, SLC7A2, EBF4, KCTD18, SLITRK6, IRF9, STC1,
CLIC3, SLC6A6, CYP1A1, GATSL2, NOTUM, TP53INP1, CACNA2D1,
SPOCK3. |
| Downregulated | POLR3G, MNS1,
ZMAT1, CXCL5, PMP2, DEPDC1, TRIM24, SRXN1, CYP27C1, GPR180, OSBPL6,
DNAI1, DCLRE1A, POLR3B, PCDHGA1, CLUL1, C3orf14, SMC5, EGR1, PDK4,
RPS6KA5, CLEC2B, SFXN2, HAGLR, PDCD4, RHEBL1, RRBP1, NFIB, DHX34,
UBE2Q2L, EOMES, MDM2, FUBP1, DNAH1, DSTYK, ESX1, TET1, ODF2L, NSD1,
SSH2, PTX3, LINC00173, MYCL, TMEM2, GRB14, TNFRSF19, CDK14,
FRA10AC1, SOX17, PXYLP1, ZNF618. |
Functional and pathway enrichment
analysis, and PPI network construction
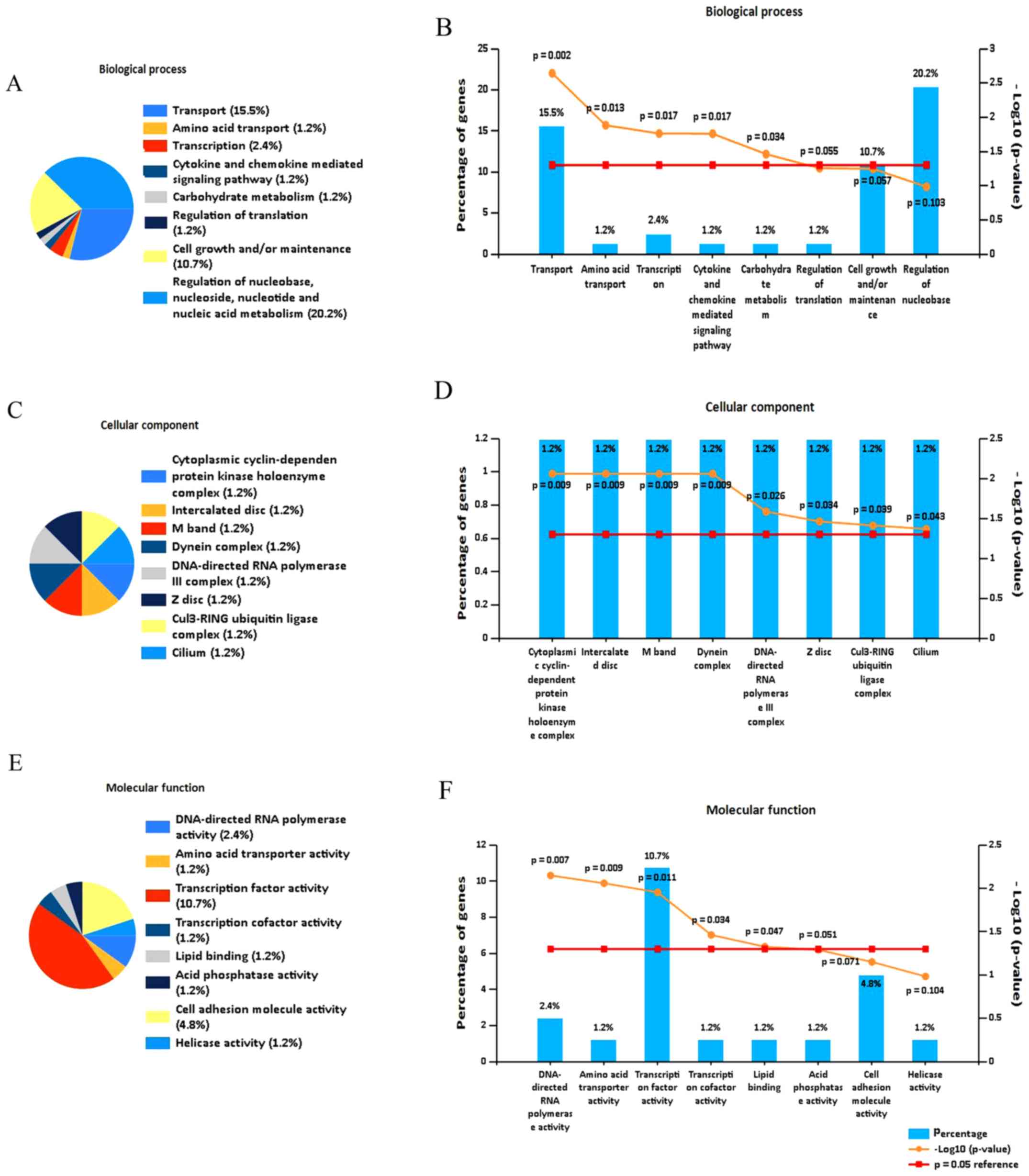
To investigate the function of the DEGs, functional
enrichment analysis was performed. Analysis using FunRich software
indicated that the DEGs were predominantly enriched in the
following biological process terms: Transport, amino acid
transport, transcription, cytokine and chemokine mediated signaling
pathway, and carbohydrate metabolism (Fig. 3A and B). In addition, the DEGs were
predominantly enriched in following cell component terms:
Cytoplasmic cyclin-dependent protein kinase holoenzyme complex,
interacted disc, M band and DNA-directed RNA polymerase III complex
(Fig. 3C and D). Furthermore, for
molecular function, the DEGs were enriched in the following terms:
Transcription factor activity, DNA-directed RNA polymerase
activity, amino acid transporter activity, transcription and lipid
binding (Fig. 3E and F).
Using the DAVID database, GO analysis identified
that the DEGs were enriched in the following terms: Negative
regulation of myofibroblast differentiation, stem cell population
maintenance and cellular response to antibiotic (Fig. 3G; Table
III).
 | Table III.GO analysis of differentially
expressed genes in pancreatic cancer. |
Table III.
GO analysis of differentially
expressed genes in pancreatic cancer.
| Category | Term | Count | % |
P-valuea |
|---|
|
GOTERM_BP_DIRECT | GO:1904761~negative
regulation of myofibroblast differentiation | 2 | 2.272727273 | 0.009268837 |
|
GOTERM_BP_DIRECT | GO:0019827~stem
cell population maintenance | 3 | 3.409090909 | 0.02259656 |
|
GOTERM_MF_DIRECT | GO:0002039~p53
binding | 3 | 3.409090909 | 0.033191281 |
|
GOTERM_BP_DIRECT | GO:0071236~cellular
response to antibiotic | 2 | 2.272727273 | 0.036569485 |
|
GOTERM_BP_DIRECT | GO:0001706~endoderm
formation | 2 | 2.272727273 | 0.054355943 |
|
GOTERM_BP_DIRECT |
GO:0003351~epithelial cilium movement | 2 | 2.272727273 | 0.058751666 |
|
GOTERM_BP_DIRECT | GO:0071391~cellular
response to estrogen stimulus | 2 | 2.272727273 | 0.058751666 |
|
GOTERM_MF_DIRECT | GO:0000977~RNA
polymerase II regulatory region sequence-specific DNA binding | 4 | 4.545454545 | 0.059249025 |
|
GOTERM_BP_DIRECT |
GO:0070498~interleukin-1-mediated
signaling pathway | 2 | 2.272727273 | 0.063127217 |
|
GOTERM_BP_DIRECT |
GO:0006885~regulation of pH | 2 | 2.272727273 | 0.071818168 |
|
GOTERM_BP_DIRECT | GO:0090280~positive
regulation of calcium ion import | 2 | 2.272727273 | 0.071818168 |
|
GOTERM_BP_DIRECT | GO:0071456~cellular
response to hypoxia | 3 | 3.409090909 | 0.07344514 |
|
GOTERM_MF_DIRECT | GO:0001056~RNA
polymerase III activity | 2 | 2.272727273 | 0.074087687 |
|
GOTERM_CC_DIRECT |
GO:0005666~DNA-directed RNA polymerase III
complex | 2 | 2.272727273 | 0.079265755 |
|
GOTERM_BP_DIRECT | GO:0045089~positive
regulation of innate immune response | 2 | 2.272727273 | 0.08042952 |
|
GOTERM_BP_DIRECT | GO:0002690~positive
regulation of leukocyte chemotaxis | 2 | 2.272727273 | 0.08042952 |
|
GOTERM_BP_DIRECT | GO:0045892~negative
regulation of transcription, DNA-templated | 6 | 6.818181818 | 0.082369804 |
|
GOTERM_BP_DIRECT | GO:0045944~positive
regulation of transcription from RNA polymerase II promoter | 9 | 10.22727273 | 0.084587325 |
|
GOTERM_CC_DIRECT | GO:0036126~sperm
flagellum | 2 | 2.272727273 | 0.087239628 |
|
GOTERM_BP_DIRECT |
GO:0006366~transcription from RNA
polymerase II promoter | 6 | 6.818181818 | 0.090139483 |
KEGG pathway analysis using KOBAS revealed that the
DEGs were significantly enriched in the following terms: RNA
polymerase, Wnt signaling pathway and cytokine-cytokine receptor
interaction (Fig. 3H; Table IV).
 | Table IV.Kyoto Encyclopedia of Genes and
Genomes signaling pathway enrichment analysis of differentially
expressed genes in pancreatic cancer. |
Table IV.
Kyoto Encyclopedia of Genes and
Genomes signaling pathway enrichment analysis of differentially
expressed genes in pancreatic cancer.
| Pathway ID | Term | Count |
P-valuea |
|---|
| hsa03020 | RNA polymerase | 2 | 0.002583461 |
| hsa05219 | Bladder cancer | 2 | 0.004105174 |
| hsa04261 | Adrenergic
signaling in cardiomyocytes | 3 | 0.004711319 |
| hsa04623 | Cytosolic
DNA-sensing pathway | 2 | 0.009436485 |
| hsa05169 | Epstein-Barr virus
infection | 3 | 0.010969532 |
| hsa05205 | Proteoglycans in
cancer | 3 | 0.011112587 |
| hsa04260 | Cardiac muscle
contraction | 2 | 0.013627503 |
| hsa04060 | Cytokine-cytokine
receptor interaction | 3 | 0.021708158 |
| hsa00240 | Pyrimidine
metabolism | 2 | 0.023537332 |
| hsa04668 | TNF signaling
pathway | 2 | 0.025617518 |
| hsa04919 | Thyroid hormone
signaling pathway | 2 | 0.029094794 |
| hsa05206 | MicroRNAs in
cancer | 3 | 0.029496436 |
| hsa04120 | Ubiquitin mediated
proteolysis | 2 | 0.038049532 |
| hsa04530 | Tight junction | 2 | 0.039046349 |
| hsa04310 | Wnt signaling
pathway | 2 | 0.041069627 |
| hsa00900 | Terpenoid backbone
biosynthesis | 1 | 0.049591361 |
The PPI network of DEGs consisted of 58 nodes and
171 edges, including 24 upregulated genes and 34 downregulated
genes (Fig. 3I). As aforementioned,
the shared 88 DEGs sorted from the two GSE datasets included 37
upregulated and 51 downregulated genes; however, all shared DEGs
were not included in the PPI network as certain genes that were
isolated at the edge were removed. Therefore, as presented in
Fig. 3I, 58 shared DEGs were
included in the PPI network, in which the red nodes represent the
upregulated genes and the green nodes represent the downregulated
genes. Furthermore, the most significant hub genes were selected as
those with the highest numbers of edges. A total of 15 genes were
selected as hub genes, including WDFY2, KIT, EGR1, NSD1, DSTYK,
CDK14, MDM2, RPS6KA5, CYP1A1, POLR3B, SMC5, DNAI1, SSH2, TRIM24 and
CASK.
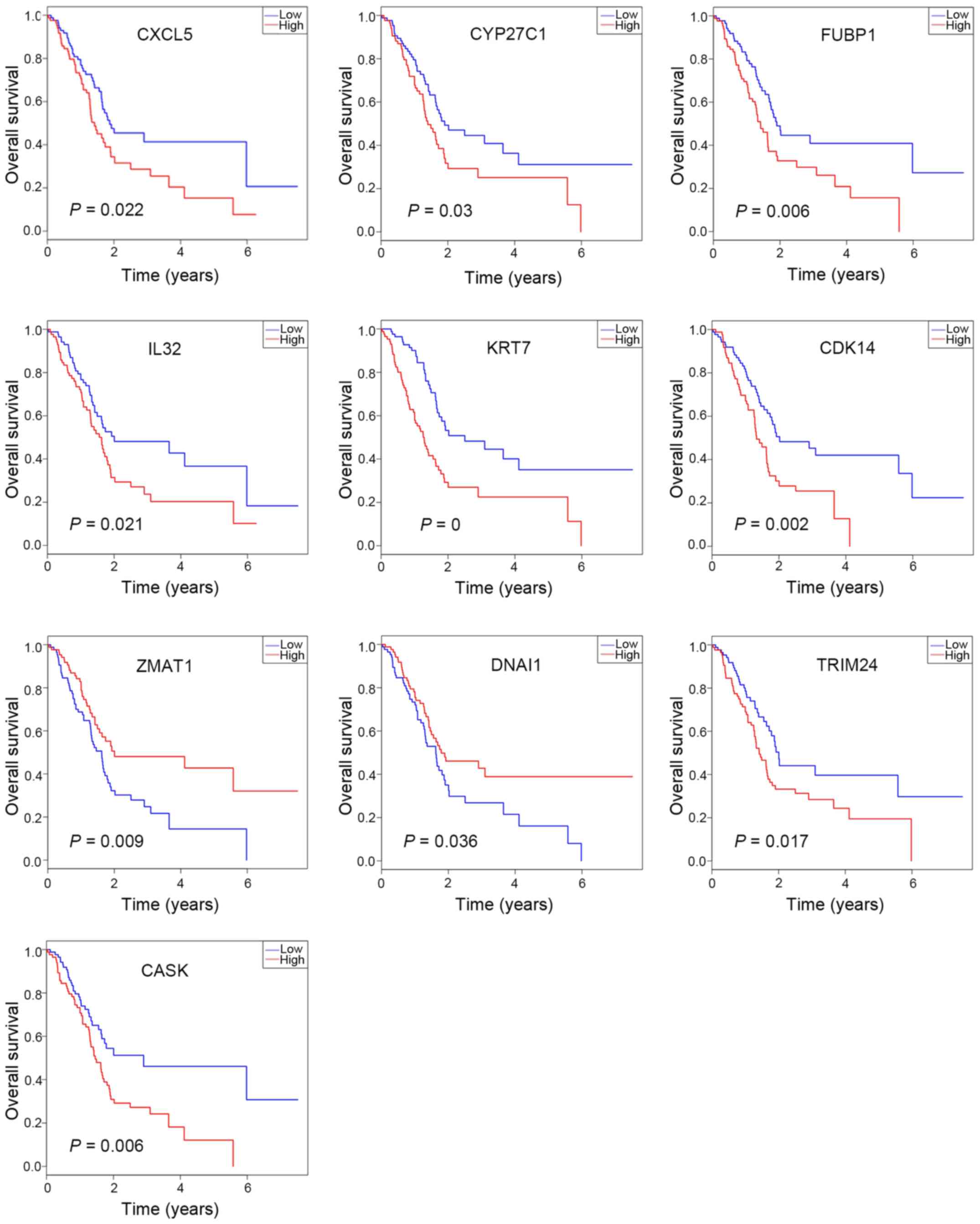
OS analysis
OS analysis was performed using R software to
investigate the prognostic value of the 88 DEGs and the results
were presented as Kaplan-Meier curves. Among the 37 upregulated
DEGs, CASK, IL32, and KRT7 were significantly associated with
prognosis. In addition, among the 51 downregulated DEGs, the
expression levels of CDK14, CXCL5, CYP27C1, DNAI1, FUBP1, TRIM24
and ZMAT1 were identified to be significantly associated with
prognosis (Fig. 4). Furthermore,
among the downregulated DEGs, high expression levels of CXCL5,
CYP27C1, FUBP1, CDK14 and TRIM24 were associated with significantly
worse overall survival (Fig. 4),
which suggests inhibition of the β-catenin-TCF7L1 complex may
result in the downregulation of these five potential oncogenic
genes. Notably, CDK14 and TRIM24 were identified as hub genes in
the PPI network, which indicates these genes may be the key
downstream regulators of the β-catenin-TCF7L1 complex.
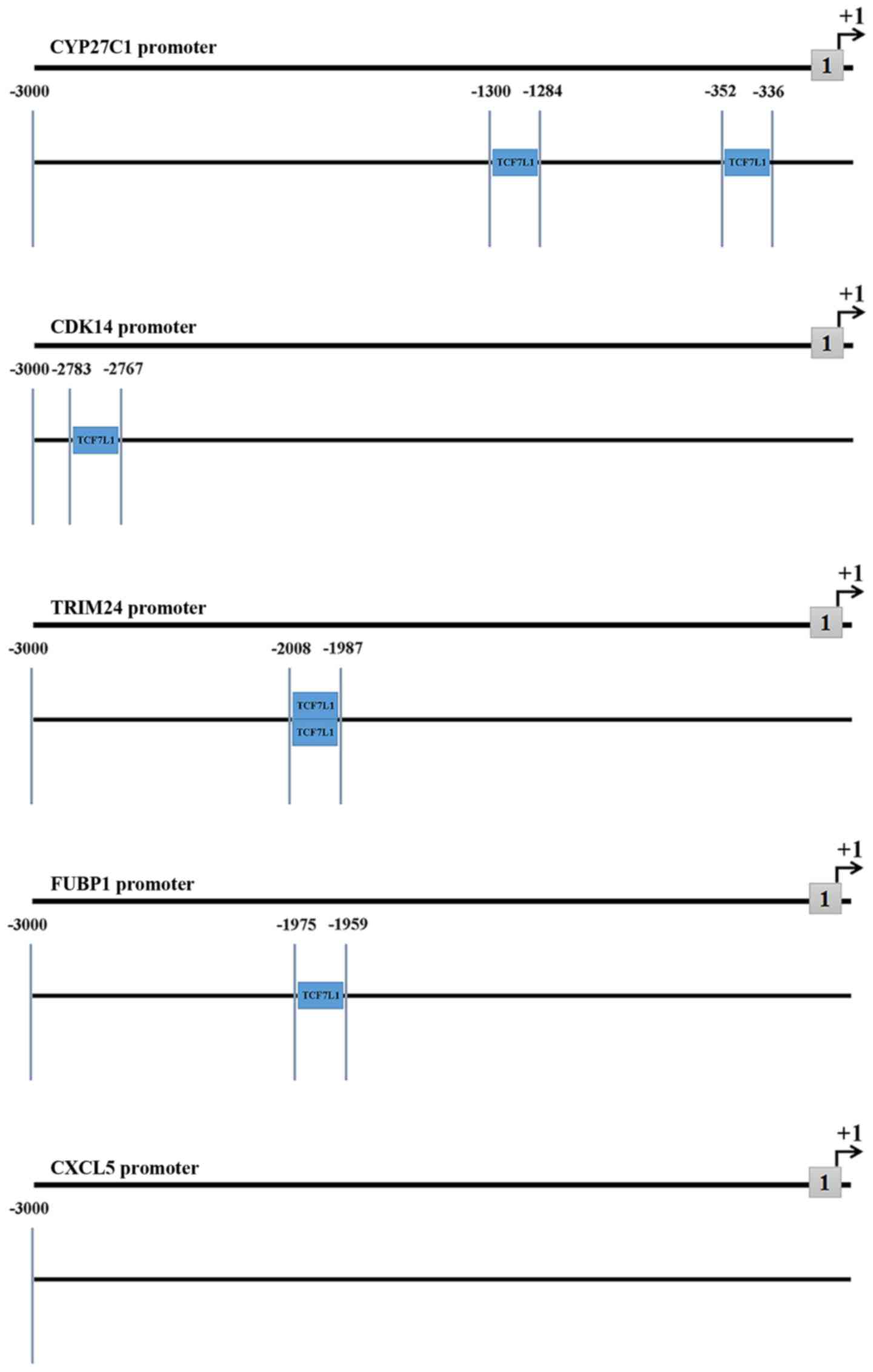
Promoter analysis of DEGs
Promoter analysis of DEGs performed using the
Ensemble and Genomatix databases revealed that the predicted TFs of
the five DEGs associated with poor OS, including CXCL5, CYP27C1,
FUBP1, CDK14 and TRIM24, covered different TF families. Only TFs
associated with TCF7L1 were selected to obtain a precise result. As
presented in the Fig. 5, TCF7L1 was
identified as a TF of four of the DEGs but not CXCL5. This result
suggests that TCF7L1 may not be a TF of CXCL5, however, certain
unavoidable errors of the prediction may have occurred.
Furthermore, the locations of predicted TF sites of each promoter
are demonstrated distinctly in Fig.
5. Two DEGs, including CDK14 and FUBP1, exhibited only one TF
site, whereas, TRIM24 and CYP27C1 possessed two different sites. In
addition, the locations of the two TF sites of TRIM24 were
separated by <5 base pairs (Fig.
5).
Discussion
Pancreatic cancer is a highly lethal type of tumor
of the digestive tract as its mortality rate is closely associated
with the incidence rate (19). The
majority of patients with pancreatic cancer exhibit no clinical
signs until the disease reaches an advanced stage (20). Despite rapid developments in
treatment strategies, effective early detective tests and drug
targets for pancreatic cancer remain limited (21). Therefore, further understanding of
the mechanisms underlying pancreatic cancer carcinogenesis is
essential to improve prognosis and reduce the mortality rate. With
developments in microarray technology, it can be useful to
determine the general genetic alterations associated with disease
progression, which may provide beneficial insight into the
diagnosis, treatment and prognosis of the disease (22).
The present study selected two datasets of
pancreatic cancer in which β-catenin and TCF7L1 knockdown had been
performed separately to identify DEGs. A total of 88 shared DEGs
were screened out consisting of 37 upregulated and 51 downregulated
DEGs. According to functional and pathway enrichment analysis, the
shared DEGs were predominantly involved in transport,
transcription, and the cytokine and chemokine mediated signaling
pathway process. Furthermore, a PPI network was constructed and 15
genes were selected as hub genes, including WDFY2, KIT, EGR1, NSD1,
DSTYK, CDK14, MDM2, RPS6KA5, CYP1A1, POLR3B, SMC5, DNAI1, SSH2,
TRIM24 and CASK. According to OS analysis, high expression levels
of CXCL5, CYP27C1, FUBP1, CDK14 and TRIM24, which were
downregulated by inhibition of the β-catenin-TCF7L1 complex, were
associated with worse prognosis. Notably, both CDK14 and TRIM24
were identified as hub genes in the PPI network and were negatively
associated with OS, which suggests these two genes may serve key
roles downstream of β-catenin-TCF7L1 complex.
CDK14, a member of the cyclin-dependent kinases, is
a cdc2-associated serine/threonine protein kinase, which serves a
vital role in normal cell cycle progression (23). It has been reported that CDK14 may
interact with cyclin D3 and human cyclin Y to regulate cell cycle
and cell proliferation (24,25). Furthermore, certain reports have
suggested that CDK14 also regulates a number of pathways, including
the Wnt/β-catenin signaling pathway and phosphoinositide 3-kinase
(PI3K)/Akt signaling pathway, and cellular mechanisms to act as an
oncogene (26,27). It is understood that the
Wnt/β-catenin signaling pathway is a conserved signaling pathway
associated with cell proliferation, migration, apoptosis,
differentiation and normal stem cell self-renewal (28). In the absence of Wnt signaling, the
mitosis-specific CDK14-Cyclin Y kinase complex phosphorylates
Ser-1490 of LRP5/6, which are co-receptors for Wnt ligands at the
G2/M stage, thereby triggering the receptor for Wnt-induced
phosphorylation (29,30). Furthermore, a previous study has
identified that CDK14 is highly expressed in pancreatic cancer,
which promotes the proliferation, migration and invasion of cancer
cells (31). In addition, this high
expression has been observed in a number of other types of
malignant tumor, including hepatocellular carcinoma, gastric cancer
and breast cancer (26,32,33). By
contrast, knockout or inhibition of CDK14 has been demonstrated to
exhibit a benefit on the prognosis of cancer types, including
ovarian cancer and breast cancer (32,34).
Furthermore, the PI3K/Akt signaling pathway also serves a vital
role in cell proliferation, migration, apoptosis and
differentiation, and dysregulation of this pathway is common in
pancreatic cancer. A previous study demonstrated that knockdown of
CDK14 inhibited the proliferation and invasion of pancreatic cancer
cells, in addition to the epithelial-to-mesenchymal transition, by
suppressing the PI3K/Akt signaling pathway (31).
TRIM24, also termed transcription intermediary
factor 1-α, is a member of the transcription intermediary factor
family and has been confirmed to serve a key role in tumor
development and progression (35,36).
Furthermore, previous studies have demonstrated that TRIM24 is
upregulated in several types of cancer and involved in numerous
pathways. For example, certain studies have identified that TRIM24
is overexpressed, and promotes cancer cell growth and invasion in
bladder cancer and cervical cancer, possibly via the nuclear
factor-κB and PI3K/Akt signaling pathways (36,37).
Similarly, it has been reported that TRIM24 can accelerate cell
growth and facilitate gastric cancer progression by activation of
the Akt pathway (37) and the
Wnt/β-catenin signaling pathway (38). Notably, in contrast to the
aforementioned studies that suggest TRIM24 is an important oncogene
in tumor development, TRIM24 has been identified to suppress the
progression of murine hepatocellular carcinoma (39). Therefore, the contradictory role or
TRIM24 requires further investigation.
In addition to CDK14 and TRIM24, three other genes
downstream of β-catenin-TCF7L1 were revealed to be negatively
associated with prognosis including, CXCL5, CYP27C1 and FUBP1.
CXCL5, CYP27C1 and FUBP1 were not identified as hub genes in the
PPI network; however, these genes may also be target genes that
affect OS and respond to the β-catenin-TCF7L1 complex.
FUBP1 encodes far upstream element-binding protein
1; a single stranded DNA-binding protein containing three domains
that contribute to c-myc transcriptional regulation by binding to
the far upstream element (40,41). As
a member of the myc oncoprotein family, c-myc has been confirmed to
be associated with oncogenesis (42,43).
Therefore, it is not surprising that FUBP1 has also been revealed
to be expressed in many types of malignant tissue and promote tumor
proliferation and migration, and led to poor prognosis (44,45),
which is consistent with the previous study. In addition, FUBP1 has
been identified to function as an oncogene by regulating c-myc
transcription in tumor progression (46). By contrast, the role of FUBP1
tumorigenesis may be c-myc independent, as a previous report
demonstrated that knockdown of FUBP1 had no effect on the level of
c-myc in hepatocellular carcinoma (44). In summary, FUBP1 may serve as a
potential drug target due to its significant role in tumorigenesis.
A recent study revealed that camptothecin and its analog SN-38, the
active metabolite of irinotecan, may serve as a novel therapy for
hepatocellular carcinoma by targeting FUBP1 (47). In addition, a previous study
suggested that miR-16 may suppress FUBP1, both of which were
associated with the trastuzumab response in ErbB-2-positive primary
breast cancer (48).
CXCL5 is a member of the CXC subfamily of
chemokines, which are produced locally in tissues. These chemokines
function by interacting with specific G protein-coupled receptors,
which are mainly expressed on leukocytes (49). It is well understood that chemokines
serve a key role in infection and inflammation. Similarly, a number
of reports have suggested that CXCL5 may contribute to pathogen-
and autoimmune-induced inflammatory reactions, and angiogenesis by
driving neutrophil recruitment (50,51).
Furthermore, CXCL5 has also been confirmed to participate in cancer
progression. Previous studies have demonstrated that overexpression
of CXCL5 mediates neutrophil infiltration, and promotes cell
proliferation and invasion in different types of tumor, including
hepatocellular carcinoma and colorectal cancer, which suggests a
poor prognosis (52,53). Knockdown of CXCL5 has been revealed
to inhibit the proliferation and migration of human bladder cancer
T24 cells (54). Furthermore, CXCL5
is associated with the PI3K/Akt/glycogen synthase kinase-3β/Snail
signaling pathway (55,56) and epidermal growth factor (EGF)-EGF
receptor signaling pathway (57),
which have been demonstrated to serve significant roles in
tumorigenesis.
CYP27C1 belongs to the cytochrome P450 superfamily
of enzymes, which is understood to catalyze a number of reactions
associated with drug metabolism (58). However, the number of studies
regarding CYP27C1 is very limited. Certain studies have revealed
that CYP27C1 can convert vitamin A1 into A2, which could be a
switch for visual sensitivity (59,60).
However, the other functions of this gene require further
investigation.
In conclusion, the genes identified in the current
study may serve as potential targets in pancreatic cancer.
Furthermore, the associated functions and pathways may also provide
information that can assist with the diagnosis and treatment of
patients with pancreatic cancer. However, it is undeniable that
there is a limitation of the present study due to the lack of
experimental validation. In the future, the results predicted by
bioinformatics analysis may be verified by advanced research and
technology to provide benefits for the clinical outcome of patients
with pancreatic cancer. In summary, the genes identified in the
present study may provide potential targets for the diagnosis and
treatment of pancreatic cancer, and they need to be validated prior
to clinical use.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YHY, JZ and YZ participated in the design of this
study and performed the statistical analysis. JZ collected
important background information and contributed to the data
acquisition. YZ carried out the study and contributed to figure
preparation. YHY drafted the manuscript. MDX, JW and WL contributed
to data acquisition, data analysis and statistical analysis. MYW
and DML made great contributions to the original conception of the
study and perfomed part of the data analysis. In addition, MYW and
DML also performed manuscript review and critically revised the
manuscript for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oberstein PE and Olive KP: Pancreatic
cancer: Why is it so hard to treat? Therap Adv Gastroenterol.
6:321–337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Long J, Luo GP, Xiao ZW, Liu ZQ, Guo M,
Liu L, Liu C, Xu J, Gao YT, Zheng Y, et al: Cancer statistics:
Current diagnosis and treatment of pancreatic cancer in Shanghai,
China. Cancer Lett. 346:273–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Provenzano PP, Cuevas C, Chang AE, Goel
VK, Von Hoff DD and Hingorani SR: Enzymatic targeting of the stroma
ablates physical barriers to treatment of pancreatic ductal
adenocarcinoma. Cancer Cell. 21:418–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: Current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z, Ma Q, Li P, Sha H, Li X and Xu J:
Aberrant expression of CXCR4 and β-catenin in pancreatic cancer.
Anticancer Res. 33:4103–4110. 2013.PubMed/NCBI
|
|
9
|
Hrckulak D, Kolar M, Strnad H and Korinek
V: TCF/LEF transcription factors: An update from the internet
resources. Cancers. 8(pii): E702016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu L, Zhi Q, Shen M, Gong FR, Zhou BP,
Lian L, Shen B, Chen K, Duan W, Wu MY, et al: FH535, a β-catenin
pathway inhibitor, represses pancreatic cancer xenograft growth and
angiogenesis. Oncotarget. 7:47145–47162. 2016.PubMed/NCBI
|
|
11
|
Behrens J, von Kries JP, Kühl M, Bruhn L,
Wedlich D, Grosschedl R and Birchmeier W: Functional interaction of
beta-catenin with the transcription factor LEF-1. Nature.
382:638–642. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shang S, Hua F and Hu ZW: The regulation
of β-catenin activity and function in cancer: Therapeutic
opportunities. Oncotarget. 8:33972–33989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duarte JG and Blackburn JM: Advances in
the development of human protein microarrays. Expert Rev
Proteomics. 14:627–641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sato Y, Miya M, Fukunaga T, Sado T and
Iwasaki W: MitoFish and MiFish pipeline: A mitochondrial genome
database of fish with an analysis pipeline for environmental DNA
metabarcoding. Mol Biol Evol. 35:1553–1555. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Győrffy B, Pongor L, Bottai G, Li X,
Budczies J, Szabó A, Hatzis C, Pusztai L and Santarpia L: An
integrative bioinformatics approach reveals coding and non-coding
gene variants associated with gene expression profiles and outcome
in breast cancer molecular subtypes. Br J Cancer. 118:1107–1114.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arensman MD, Telesca D, Lay AR, Kershaw
KM, Wu N, Donahue TR and Dawson DW: The CREB-binding protein
inhibitor ICG-001 suppresses pancreatic cancer growth. Mol Cancer
Ther. 13:2303–2314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu MY, Liang RR, Chen K, Shen M, Tian YL,
Li DM, Duan WM, Gui Q, Gong FR, Lian L, et al: FH535 inhibited
metastasis and growth of pancreatic cancer cells. Onco Targets
Ther. 8:1651–1670. 2015.PubMed/NCBI
|
|
18
|
Kanehisa M, Sato Y, Kawashima M, Furumichi
M and Tanabe M: KEGG as a reference resource for gene and protein
annotation. Nucleic Acids Res. 44(D1): D457–D462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ilic M and Ilic I: Epidemiology of
pancreatic cancer. World J Gastroenterol. 22:9694–9705. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mohammed S, Van Buren G II and Fisher WE:
Pancreatic cancer: Advances in treatment. World J Gastroenterol.
20:9354–9360. 2014.PubMed/NCBI
|
|
22
|
López-Casas PP and López-Fernández LA:
Gene-expression profiling in pancreatic cancer. Expert Rev Mol
Diagn. 10:591–601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duan C, Liu Y, Lu L, Cai R, Xue H, Mao X,
Chen C, Qian R, Zhang D and Shen A: CDK14 contributes to reactive
gliosis via interaction with cyclin Y in rat model of spinal cord
injury. J Mol Neurosci. 57:571–579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hamilton T and Schifrin BS: Delayed
cesarean section in preeclampsia with placental abruption and fetal
distress. J Perinatol. 11:182–185. 1991.PubMed/NCBI
|
|
25
|
Li S, Song W, Jiang M, Zeng L, Zhu X and
Chen J: Phosphorylation of cyclin Y by CDK14 induces its
ubiquitination and degradation. FEBS Lett. 588:1989–1996. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang L, Zhu J, Huang H, Yang Q, Cai J,
Wang Q, Zhu J, Shao M, Xiao J, Cao J, et al: PFTK1 promotes gastric
cancer progression by regulating proliferation, migration and
invasion. PLoS One. 10:e01404512015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang B, Zou A, Ma L, Chen X, Wang L, Zeng
X and Tan T: miR-455 inhibits breast cancer cell proliferation
through targeting CDK14. Eur J Pharmacol. 807:138–143. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prakash N and Wurst W: A Wnt signal
regulates stem cell fate and differentiation in vivo. Neurodegener
Dis. 4:333–338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Davidson G and Niehrs C: Emerging links
between CDK cell cycle regulators and Wnt signaling. Trends Cell
Biol. 20:453–460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Jia Y, Fei C, Song X and Li L:
Activation/proliferation-associated protein 2 (Caprin-2) positively
regulates CDK14/Cyclin Y-mediated lipoprotein receptor-related
protein 5 and 6 (LRP5/6) constitutive phosphorylation. J Biol Chem.
291:26427–26434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng L, Zhou Z and He Z: Knockdown of
PFTK1 inhibits tumor cell proliferation, invasion and
epithelial-to-mesenchymal transition in pancreatic cancer. Int J
Clin Exp Pathol. 8:14005–14012. 2015.PubMed/NCBI
|
|
32
|
Gu X, Wang Y, Wang H, Ni Q, Zhang C, Zhu
J, Huang W, Xu P, Mao G and Yang S: Upregulated PFTK1 promotes
tumor cell proliferation, migration, and invasion in breast cancer.
Med Oncol. 32:1952015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Du B, Zhang P, Tan Z and Xu J: MiR-1202
suppresses hepatocellular carcinoma cells migration and invasion by
targeting cyclin dependent kinase 14. Biomed Pharmacother.
96:1246–1252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang W, Liu R, Tang C, Xi Q, Lu S, Chen
W, Zhu L, Cheng J, Chen Y, Wang W, et al: PFTK1 regulates cell
proliferation, migration and invasion in epithelial ovarian cancer.
Int J Biol Macromol. 85:405–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang LH, Yin AA, Cheng JX, Huang HY, Li
XM, Zhang YQ, Han N and Zhang X: TRIM24 promotes glioma progression
and enhances chemoresistance through activation of the PI3K/Akt
signaling pathway. Oncogene. 34:600–610. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xue D, Zhang X, Zhang X, Liu J, Li N, Liu
C, Liu Y and Wang P: Clinical significance and biological roles of
TRIM24 in human bladder carcinoma. Tumour Biol. 36:6849–6855. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miao ZF, Wang ZN, Zhao TT, Xu YY, Wu JH,
Liu XY, Xu H, You Y and Xu HM: TRIM24 is upregulated in human
gastric cancer and promotes gastric cancer cell growth and
chemoresistance. Virchows Arch. 466:525–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fang Z, Deng J, Zhang L, Xiang X, Yu F,
Chen J, Feng M and Xiong J: TRIM24 promotes the aggression of
gastric cancer via the Wnt/β-catenin signaling pathway. Oncol Lett.
13:1797–1806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang S, Minter LC, Stratton SA, Yang P,
Abbas HA, Akdemir ZC, Pant V, Post S, Gagea M, Lee RG, et al:
TRIM24 suppresses development of spontaneous hepatic lipid
accumulation and hepatocellular carcinoma in mice. J Hepatol.
62:371–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bazar L, Harris V, Sunitha I, Hartmann D
and Avigan M: A transactivator of c-myc is coordinately regulated
with the proto-oncogene during cellular growth. Oncogene.
10:2229–2238. 1995.PubMed/NCBI
|
|
41
|
Zhang J and Chen QM: Far upstream element
binding protein 1: A commander of transcription, translation and
beyond. Oncogene. 32:2907–2916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kozma L, Kiss I, Nagy A, Szakáll S and
Ember I: Investigation of c-myc and K-ras amplification in renal
clear cell adenocarcinoma. Cancer Lett. 111:127–131. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lian Y, Niu X, Cai H, Yang X, Ma H, Ma S,
Zhang Y and Chen Y: Clinicopathological significance of c-MYC in
esophageal squamous cell carcinoma. Tumour Biol. 39:1–7. 2017.
View Article : Google Scholar
|
|
44
|
Rabenhorst U, Beinoraviciute-Kellner R,
Brezniceanu ML, Joos S, Devens F, Lichter P, Rieker RJ, Trojan J,
Chung HJ, Levens DL and Zörnig M: Overexpression of the far
upstream element binding protein 1 in hepatocellular carcinoma is
required for tumor growth. Hepatology. 50:1121–1129. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Malz M, Weber A, Singer S, Riehmer V,
Bissinger M, Riener MO, Longerich T, Soll C, Vogel A, Angel P, et
al: Overexpression of far upstream element binding proteins: A
mechanism regulating proliferation and migration in liver cancer
cells. Hepatology. 50:1130–1139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang L, Zhu JY, Zhang JG, Bao BJ, Guan CQ,
Yang XJ, Liu YH, Huang YJ, Ni RZ and Ji LL: Far upstream
element-binding protein 1 (FUBP1) is a potential c-Myc regulator in
esophageal squamous cell carcinoma (ESCC) and its expression
promotes ESCC progression. Tumour Biol. 37:4115–4126. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Khageh Hosseini S, Kolterer S, Steiner M,
von Manstein V, Gerlach K, Trojan J, Waidmann O, Zeuzem S, Schulze
JO, Hahn S, et al: Camptothecin and its analog SN-38, the active
metabolite of irinotecan, inhibit binding of the transcriptional
regulator and oncoprotein FUBP1 to its DNA target sequence FUSE.
Biochem Pharmacol. 146:53–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Venturutti L, Cordo Russo RI, Rivas MA,
Mercogliano MF, Izzo F, Oakley RH, Pereyra MG, De Martino M,
Proietti CJ, Yankilevich P, et al: MiR-16 mediates trastuzumab and
lapatinib response in ErbB-2-positive breast and gastric cancer via
its novel targets CCNJ and FUBP1. Oncogene. 35:6189–6202. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Disteldorf EM, Krebs CF, Paust HJ, Turner
JE, Nouailles G, Tittel A, Meyer-Schwesinger C, Stege G, Brix S,
Velden J, et al: CXCL5 drives neutrophil recruitment in
TH17-mediated GN. J Am Soc Nephrol. 26:55–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mei J, Liu Y, Dai N, Hoffmann C, Hudock
KM, Zhang P, Guttentag SH, Kolls JK, Oliver PM, Bushman FD and
Worthen GS: Cxcr2 and Cxcl5 regulate the IL-17/G-CSF axis and
neutrophil homeostasis in mice. J Clin Invest. 122:974–986. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nouailles G, Dorhoi A, Koch M, Zerrahn J,
Weiner J III, Faé KC, Arrey F, Kuhlmann S, Bandermann S, Loewe D,
et al: CXCL5-secreting pulmonary epithelial cells drive destructive
neutrophilic inflammation in tuberculosis. J Clin Invest.
124:1268–1282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH,
Wang Z, Huang XW, Fan J and Zhou J: Overexpression of CXCL5
mediates neutrophil infiltration and indicates poor prognosis for
hepatocellular carcinoma. Hepatology. 56:2242–2254. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Speetjens FM, Kuppen PJ, Sandel MH, Menon
AG, Burg D, van de Velde CJ, Tollenaar RA, de Bont HJ and
Nagelkerke JF: Disrupted expression of CXCL5 in colorectal cancer
is associated with rapid tumor formation in rats and poor prognosis
in patients. Clin Cancer Res. 14:2276–2284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zheng J, Zhu X and Zhang J: CXCL5
knockdown expression inhibits human bladder cancer T24 cells
proliferation and migration. Biochem Biophys Res Commun. 446:18–24.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhao J, Ou B, Han D, Wang P, Zong Y, Zhu
C, Liu D, Zheng M, Sun J, Feng H and Lu A: Tumor-derived CXCL5
promotes human colorectal cancer metastasis through activation of
the ERK/Elk-1/Snail and AKT/GSK3β/β-catenin pathways. Mol Cancer.
16:702017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhou SL, Zhou ZJ, Hu ZQ, Li X, Huang XW,
Wang Z, Fan J, Dai Z and Zhou J: CXCR2/CXCL5 axis contributes to
epithelial-mesenchymal transition of HCC cells through activating
PI3K/Akt/GSK-3β/Snail signaling. Cancer Lett. 358:124–135. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Huang P, Xu X, Wang L, Zhu B, Wang X and
Xia J: The role of EGF-EGFR signalling pathway in hepatocellular
carcinoma inflammatory microenvironment. J Cell Mol Med.
18:218–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Johnson KM, Phan TTN, Albertolle ME and
Guengerich FP: Human mitochondrial cytochrome P450 27C1 is
localized in skin and preferentially desaturates trans-retinol to
3,4-dehydroretinol. J Biol Chem. 292:13672–13687. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Enright JM, Toomey MB, Sato SY, Temple SE,
Allen JR, Fujiwara R, Kramlinger VM, Nagy LD, Johnson KM, Xiao Y,
et al: Cyp27c1 red-shifts the spectral sensitivity of
photoreceptors by converting vitamin A1 into A2. Curr Biol.
25:3048–3057. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Morshedian A, Toomey MB, Pollock GE,
Frederiksen R, Enright JM, McCormick SD, Cornwall MC, Fain GL and
Corbo JC: Cambrian origin of the CYP27C1-mediated vitamin
A1-to-A2 switch, a key mechanism of
vertebrate sensory plasticity. R Soc Open Sci. 4:1703622017.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|