Introduction
Malignant effusion is the accumulation of cavity
fluid due to the spread of malignant cells. It is a late-stage
manifestation of cancer and is associated with poor prognosis.
Since the most frequent cancer in effusions is adenocarcinoma,
epithelial cell markers are among those used for the detection of
cancer (1,2).
Epithelial cell adhesion molecule (EpCAM) is a 40
kDa transmembrane cell surface glycoprotein that is highly
expressed in epithelial cancers and at lower levels in normal
epithelia (3). Although it promotes
homophilic cell-cell interactions, EpCAM modulates negatively
cadherin-mediated cell adhesion resulting in an anti-adhesive
effect during neoplasm development (4,5). Besides
this, EpCAM was shown to play a role in cell proliferation. EpCAM's
signaling mechanism suggests that EpCAM is subject to regulated
intramembrane proteolysis and the cleaved intracellular domain is
responsible for the induction of EpCAM's target genes (6,7). In
gastric cancer, overexpression of EpCAM disrupts cell-cell contact,
enabling the cellular migration that is required for metastasis
(8).
Due to its frequent overexpression in carcinomas,
EpCAM has been used as diagnostic/prognostic marker and therapeutic
target (9). Liquid biopsy, for
instance, a modern technology for cancer prognosis based on markers
found in the peripheral blood, may be performed on EpCAM detection.
Thus, a large number of antibodies against EpCAM have been used for
detection of carcinomas in effusion, blood and in biopsy and
surgical specimens.
EpCAM is a polypeptide of 314 amino acids (aa) and
contains a large extracellular domain (ectodomain) of 242 aa, a
transmembrane domain of 23 aa, and an intracellular domain of 26 aa
(10). EpCAM's extracellular domain
contains a first motif with epidermal growth factor (EGF)-like
repeats, a second motif that resembles a thyroglobulin (TY) type 1A
repeat and a third motif that is cysteine free/poor and unrelated
to any known molecule (3).
Most commercially available antibodies for carcinoma
detection in blood and cavity fluids bind to the small N-terminal
EF-like (EGF) domain (11). The
clones Moc-31 and Ber-EP4 are the antibodies used for routine
diagnosis of carcinomas in effusion. Both monoclonal antibodies
recognize specific epitopes in the EGF-like domain.
By using monoclonal antibodies against different
epitopes on the EpCAM ectodomain, different patterns of EpCAM
expression would be expected. Parts of the protein might be absent,
since EpCAM can be cleaved at multiple positions within its
ectodomain (12).
To our knowledge, there are no published reports in
which antibodies that recognize epitopes in the cysteine-poor
region of EpCAM have been studied for detection of carcinoma in
effusion and peritoneal wash. The aim of the present study was to
compare immunoreactivity of antibodies with distinct epitopes in
the ectodomain of EpCAM for detection of carcinoma from different
primary sites and of different histological types in effusions and
peritoneal wash.
Patients and methods
Patients and samples
Samples (n=55) of effusions (pleural, n=33;
peritoneal, n=15; pericardial, n=5 and peritoneal wash n=2) were
enrolled in the study. The samples were obtained at Department of
Pathology of Brasilia University Hospital, Brazil, between 2015 to
2018. The study protocol was approved by the Human Ethics Review
Committee of Brasilia University.
Diagnoses of carcinoma were established from
clinical information, results of previous biopsy and results of
cytology and immunocytochemistry.
All samples used here were fresh, and free of
fixative or preservative solution. For cell block preparations, the
method plasma/thromboplastin was used. The effusion/wash was
centrifuged at 2,000 rpm for 2 min. Cell pellets were homogenized
with 100 µl of plasma and 100 µl of tromboplastin
(Stago®, Asnières sur Seine, France). After 2 min, the
clots were fixed in formalin and subjected to usual histological
processing. Sections of cell block on silanized microscope slides
were stained with hematoxylin-eosin and used for
immunocytochemistry.
For antigen retrieval, the slides were incubated for
45 min in a waterbath at 95–99°C with citrate buffer pH 6.0. For
blockade of endogenous tissue peroxide, the slides were immersed in
3% H2O2 solution at room temperature for 30
min. After washing with phosphate buffered saline (PBS), the slides
were incubated with primary antibody overnight at 4°C. The primary
antibodies used are shown in Table
I. A commercially available antibody against claudin 4 was used
as additional positive control. After washing with PBS, the slides
were incubated with a secondary antibody for 30 min at room
temperature and subsequently with the streptavidin-peroxidase
complex (Kit REVEAL-Biotin-Free Polyvalent DAB; Spring Bioscience,
Inc., Pleasanton, CA, USA) for 30 min at room temperature. All
reactions were developed using a diaminobenzidine chromogen
solution (kit REVEAL-Biotin-Free Polyvalent DAB; Spring Bioscience,
Inc.). The counterstaining was performed with Harris hematoxylin.
The slides were dehydrated, cleared and mounted. Positive and
negative control were used for each primary antibody according to
the manufacturer recommendation. For all antibodies, positive
staining was defined as a brown stain in the cell membrane.
Expression was evaluated by calculating a total immunostaining
score (TIS) as the product of a proportion score (PS) and an
intensity score (IS). The PS describes the estimated fraction of
positively stained tumor cells (0, none; 1, <10%; 2, 10–50%; 3,
51–80%; 4, >80%). The IS represents the estimated staining
intensity as compared with control (0, no staining; 1, weak; 2,
moderate; 3, strong). The TIS (TIS=PSxIS) ranges from 0 to 12 with
only nine possible values (that is, 0, 1, 2, 3, 4, 6, 8, 9 and 12).
Four subgroups were defined: No expression, TIS 0; weak expression,
TIS 1–4; moderate expression, TIS 6, 8; intense expression, TIS
9,12. EpCAM ‘overexpression’ has been defined previously as a
TIS>4 (13).
 | Table I.Primary antibodies. |
Table I.
Primary antibodies.
| Target molecule | Manufacturer | Clone | Dilution | Control |
|---|
| EpCAM | R&D Systems | 158210 | 1:1,200 | Gastric cancer |
| Epithelial related
antigen | DAKO | Moc-31 | 1:200 | Gastric cancer |
| Epithelial related
antigen | DAKO | Ber-EP4 | 1:300 | Breast |
| Claudin-4 | NOVEX | 3E2C1 | 1:200 | Gastric cancer |
Results
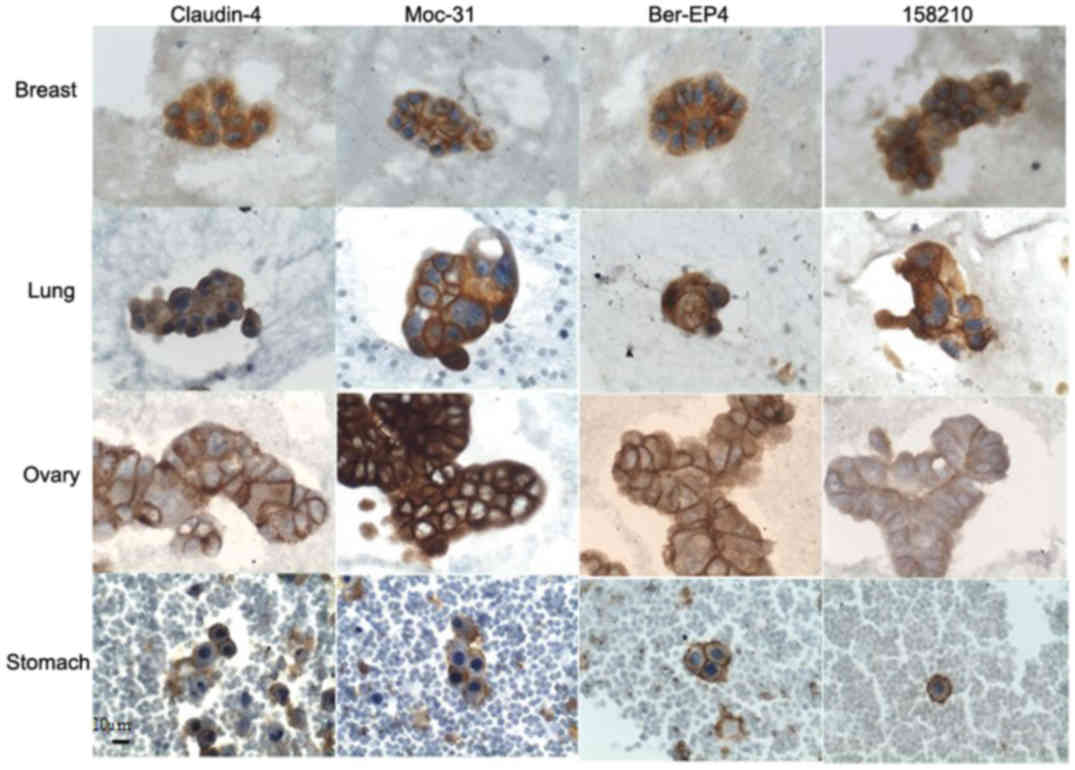
Claudin 4
Overexpression of Claudin 4 was observed in all
samples, including adenocarcinoma samples from different primary
sites and carcinomas of different histological types such as
squamous cell carcinoma (cervical), small cell carcinoma (lung),
and urothelial carcinoma (bladder). The TIS values ranged from 6–12
corresponding to moderate and intense expression (Table II; Figs.
1 and 2).
 | Table II.Overexpression of Claudin-4 and EpCAM
(as detected by Moc-31, Ber-EP4 and 158210) according to primary
sites of carcinoma and histological types. |
Table II.
Overexpression of Claudin-4 and EpCAM
(as detected by Moc-31, Ber-EP4 and 158210) according to primary
sites of carcinoma and histological types.
|
| Claudin-4
Overexpression n (TIS range) | Moc-31 Overexpression
n (TIS range) | Ber-EP4
Overexpression n (TIS range) | 158210 Overexpression
n (TIS range) |
|---|
|
|
|
|
|
|
|---|
| Carcinoma | Absence | Presence | Absence | Presence | Absence | Presence | Absence | Presence |
|---|
|
Adenocarcinomas |
| Lung
(n=15) | 0 | 15 (6–12) | 0 | 15 (6–12) | 0 | 15 (6–12) | 0 | 15 (6–12) |
| Ovary
(n=12) | 0 | 12 (6,12) | 0 | 12 (6,12) | 0 | 12 (12) | 2 (4) | 10 (6,12) |
| Breast
(n=10) | 0 | 10 (6–12) | 0 | 10 (6–12) | 2 (4) | 8 (6–12) | 1 (4) | 9 (6–12) |
| Stomach
(n=4) | 0 | 4 (6–12) | 0 | 4 (6–12) | 0 | 4 (6–12) | 1 (4) | 3 (6–12) |
| Colon
(n=2) | 0 | 2 (8,12) | 0 | 2 (8,12) | 0 | 2 (8,12) | 0 | 2 (6,12) |
| Cervix
(n=2) | 0 | 2 (8,12) | 0 | 2 (8,12) | 0 | 2 (8) | 0 | 2 (8) |
| Biliary
tract (n=3) | 0 | 3 (8,12) | 0 | 3 (8,12) | 0 | 3 (6–12) | 0 | 3 (8,12) |
|
Pancreas (n=1) | 0 | 1 (12) | 0 | 1 (12) | 0 | 1 (6) | 0 | 1 (6) |
|
Endometrium (n=1) | 0 | 1 (12) | 0 | 1 (12) | 0 | 1 (6) | 0 | 1 (6) |
| Unknown
site (n=2) | 0 | 2 (8,12) | 0 | 2 (12) | 0 | 2 (12) | 0 | 2 (6,12) |
|
Subtotal Adenocarcinomas
(n=52) | 0 | 52 (6–12) | 0 | 52 (6–12) | 2 (4) | 50 (6–12) | 4 (4) | 48 (6–12) |
| Other histological
types |
|
|
|
|
|
|
|
|
|
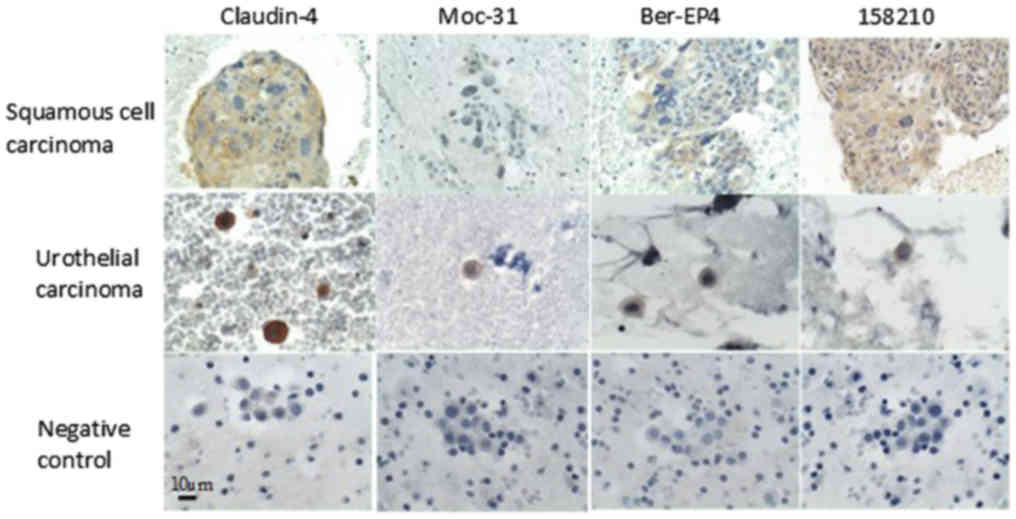
Squamous carcinoma (cervix)
(n=1) | 0 | 1 (9) | 1 (1) | 0 | 1 (2) | 0 | 0 | 1 (9) |
|
Urothelial carcinoma (bladder)
(n=1) | 0 | 1 (12) | 1 (4) | 0 | 1 (2) | 0 | 1(2) | 0 |
| Small
cell carcinoma (lung) (n=1) | 0 | 1 (8) | 0 | 1 (12) | 1 (4) | 0 | 0 | 1 (6) |
| Total (n=55) | 0 | 55 | 2 | 53 | 5 | 50 | 5 | 50 |
Moc-31
EpCAM overexpression as detected by Moc-31 antibody
was observed in 96.36% (53/55) of all carcinoma samples.
Overexpression was found in all adenocarcinoma samples, from all
primary sites with TIS values ranging from 6–12 corresponding to
moderate and intense expression. Among other histological types,
overexpression was observed in small cell carcinoma (lung). The
expression was weak in squamous cell carcinoma (cervical) and
urothelial carcinoma (bladder). (Table
II; Figs. 1 and 2).
Ber-EP4
EpCAM overexpression as detected by Ber-EP4 was
observed in 90.90% (50/55) of all carcinoma samples. Overexpression
was found in almost all adenocarcinoma samples from all primary
sites, except in two samples of breast. The overexpression in
adenocarcinomas of the breast was observed in 80% of the samples.
The TIS values in samples with overexpression ranged from 6–12,
corresponding to moderate and intense expression. No overexpression
was observed in all non-adenocarcinoma histological types of
carcinoma analyzed: Small cell carcinoma (lung), squamous cell
carcinoma (cervical) and urothelial carcinoma (bladder). (Table II; Figs.
1 and 2).
158210
EpCAM overexpression as detected by clone 158210 was
observed in 90.90% (50/55) of all carcinoma samples. Among
adenocarcinoma samples, overexpression was detected in almost all
samples, except in some from ovary, breast, and colon. The
overexpression in adenocarcinomas of the ovary, breast and stomach
was observed in 83.33, 90 and 75% of the samples, respectively. The
TIS values in samples with overexpression ranged from 6–12,
corresponding to moderate and intense expression. Among different
histological types analyzed, overexpression was observed in small
cell carcinoma (lung) and squamous cell carcinoma (cervical). The
expression was weak in urothelial carcinoma (bladder). (Table II; Figs.
1 and 2).
Discussion
The absence of EpCAM expression in normal cells
found in the cavities lining and fluids (mesothelial cells,
leukocyte and macrophages) would indicate that EpCAM is a highly
specific marker for diagnosis and target therapy for carcinomas in
effusions. There are several immunocytochemical markers for
identification of non-neoplastic cells found in the effusions;
calretinin and HBME, for instance, are routinely used for
mesothelial cells and CD68 for macrophages, respectively (14).
In the present study, the expression of EpCAM was
evaluated in metastatic carcinomas of effusions originated from
different primary sites and of distinct histological types. We
compared the immunoreactivity of antibodies that react to different
epitopes of the extracellular domain of EpCAM. We used two
antibodies against epitopes in the EGF-like domain I (clones Moc-31
and Ber-EP4) and one antibody against the epitope in the
cysteine-poor region (158210) of EpCAM, all of them commercially
available. EpCAM expression was evaluated by calculating the total
immunostaining score (TIS), which is the product of the proportion
score and the intensity score. This score has been used to evaluate
the expression of EpCAM in surgical histological specimens with
primary carcinoma. Here, for the first time, we applied this score
in cytological effusion samples to make the expression in effusions
and that in primary sites, previously established by other studies,
comparable.
All immunocytochemistry reactions were performed on
cell block sections which provide a better morphologic
interpretation with less background staining when compared to
Cytospin and ThinPrep samples (15).
The results most closely approximated those reported in the
surgical pathology specimens. The cell block preparation method
used was plasma/tromboplastin, which in comparison with other
methods, is easily prepared and produce the best cell block results
in regards to cellularity, cell distribution and background on
immunocytochemistry (16).
Claudin-4 has been described as the most sensitive
marker to distinguish adenocarcinomas from reactive and malignant
mesothelial cells in cytology of effusions, so the results of
reactions with anti-claudin-4 were used as a reference for
comparison with the results with anti-EpCAM antibodies (17,18). In
the present study, Claudin-4 overexpression was observed in all
adenocarcinoma samples and its TIS values was higher than those
obtained with anti-EpCAM antibodies. Anti-claudin-4 was also
superior to anti-EpCAM antibodies for the detection of other types
of carcinomas, such as squamous cell and urothelial carcinoma.
Independently of the clone used, EpCAM
overexpression was observed in almost all adenocarcinoma samples.
However, different degrees of EpCAM expression were observed
depending on the site of origin and histological type of carcinoma
and depending on the antibody used. Heterogeneous detection of
EpCAM was mainly observed in types of carcinoma other than
adenocarcinoma.
Given that both (clones Moc-31 and Ber-EP4)
antibodies react with epitopes in the same extracellular domain of
EpCAM, one would expect similar reactivity with these antibodies.
However, Balzar et al (19)
suggested that different conformational states of the cell surface
EpCAM protein might hide some epitopes leading to subpopulations of
EpCAM and thus heterogeneous affinity. In present study, Moc-31
presented higher TIS values for adenocarcinomas but a lower TIS
value for squamous cell carcinoma in comparison with Ber-EP4. For
adenocarcinoma of origin in breast, EpCAM overexpression was
observed in 80% of samples by using Ber-Ep4 in comparison to 100%
EpCAM overexpression with Moc-31.
Similarly to present results on metastatic
carcinoma, in surgical specimens with primary carcinomas, different
degrees of EpCAM expression has also been observed according to
site of origin and histological type of carcinoma (20–22).
Overall, the percentage of positive samples and TIS values for
EpCAM were higher in our metastatic carcinoma samples than in the
primary carcinoma samples analyzed in previous studies (20–22). In
the case of breast cancer, our TIS values for EpCAM were higher
than those obtained in previous studies in primary and metastatic
carcinomas for lymph node and CNS (20).
The weak EpCAM expression in urothelial and squamous
cell carcinoma observed in present study is in accordance with the
results of studies in primary carcinoma samples (21). This result indicates that if EpCAM
specific antibodies are intended to be used for treatment in
patients with these histological types of cancer, prior
immunohistochemical evaluation of EpCAM expression should be
recommended.
To our knowledge, for the first time, EpCAM
expression was evaluated in metastatic carcinoma from effusion by
using an antibody directed against an epitope in the cysteine poor
region of the ectodomain of the EpCAM molecule. By using 158210,
overexpression was observed in 90.90% of all carcinoma samples.
With regard to adenocarcinoma samples, almost all primary sites
showed overexpression in all samples, except some samples of ovary,
breast, and colon. Among the antibodies, it was the only one that
detects overexpression in the sample of squamous cell
carcinoma.
In healthy adult tissue, EpCAM is expressed in cell
membrane of simple, pseudo-stratified, and transitional epithelia,
but no expression can be detected in the differentiated cells of
normal squamous stratified epithelia. In primary squamous cell
carcinoma (SCC) of the uterine cervix, EpCAM expression have showed
heterogeneity depending on the antibody clone (20–22).
Similarly, in metastatic samples of effusion, other authors showed
that EpCAM expression in SCC was lower than in adenocarcinoma
samples, 67% vs. 100%, respectively (23). In a previous study, anti-EpCAM
monoclonal antibodies that recognize the 6 kDa fragment (located
distant from the cell membrane and removed after cleavage at the
position Arginine80/Arginine81) and the 32 kDa fragment (located
proximal to the membrane) of EpCAM extracellular domain were
generated and used to compare detection of EpCAM expression in
cervical SCC (24). These authors
showed that EpCAM expression is consistently detected on SCC of
cervix by using anti-EpCAM that recognizes the membrane-proximal
part (24). The clone 158210 used in
present study detects an epitope found in the extracellular domain
between amino acids 136 and 265 and, therefore, located at the
membrane-proximal part of the extracellular domain. Thus, the high
TIS value of EpCAM expression in squamous cell carcinoma by using
the clone 158210 is in agreement with the results of this previous
study and suggest a potential use of antibodies directed against an
epitope in the cysteine poor region of the ectodomain of the EpCAM
molecule for detection of this type of carcinoma in effusion.
Another commercially available cysteine-poor
region-specific EpCAM antibody is 311-1K1. In a previous study,
this antibody and Ber-Ep4 were used to evaluate EpCAM expression in
tissue sections of colorectal carcinoma (25). These authors showed that in contrast
to the tumor mass, budding cells of colorectal carcinoma displayed
lack of membranous but highly increased cytoplasmic EpCAM staining.
Significant cytoplasmic EpCAM staining was also observed in the
present study by using all three EpCAM antibodies and
anti-Claudin-4.
EpCAM expression defined by IHC predicts whether
patients may benefit with a specific EpCAM targeting agent and its
possible therapy response. First studies targeting EpCAM lacked
patient randomization according to the actual EpCAM status on tumor
cells and this can explain the disparate results sometimes obtained
(26).
The main limitation of the present study was the
small number of samples from some sites of origin of adenocarcinoma
and of different histological types of carcinoma. However, even
with this small sampling, it was possible to demonstrate
heterogeneity in the EpCAM expression by using antibodies against
different epitopes of its ectodomain.
Overall, most samples of metastatic carcinoma from
effusions showed overexpression of EpCAM. However, there are
significant variations in its expression according to the primary
site and histological type of the carcinoma and depending on the
antibody used. Thus, the use of more than one type of anti-EpCAM
would increase the chance of its detection in metastatic carcinoma
of effusion.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Federal
District Research Support Foundation (FAP-DF) and the Foundation
for Teaching and Research in Health Sciences (FEPECS), FAHUB,
CAPES, CNPQ.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
TKSB and MIMJ made substantial contributions to the
conception and design of the study. MVC acquired clinical data. VMF
and ABM made substantial contributions to the design of the
experiments and the interpretation of the results obtained with
different antibodies. TMMLC, NVH and IAO performed
immunocytochemistry, including the optimization of the experimental
conditions. LMRB, LLF and RVMS acquired samples and prepared
microscopy slides. DLMV and ACS performed cell blocks. GCC and AMP
analyzed the clinical data. FPC, IP and LMSM performed microscopy
examinations. LMSV and GHST analyzed and interpreted sample data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics in Research
Committee of Brasilia University, Brazil. Informed consent was
obtained from all individual participants included in the study.
CAAE: 37194114.4.0000.5553.
Patient consent for publication
Informed consent was obtained from all individual
participants included in the study. CAAE: 37194114.4.0000.5553.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li D, Wang B, Hu Q, Shen Y, Xu D, Wang T
and Wen F: Diagnostic accuracy of MOC-31 for malignant effusions: A
meta-analysis. Tumour Biol. 35:6003–6009. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang B, Li D, Ou X, Yi Q and Feng Y:
Diagnostic accuracy of Ber-EP4 for metastatic adenocarcinoma in
serous effusions: A meta-analysis. PLoS One. 17:e1077412014.
View Article : Google Scholar
|
|
3
|
Schnell U, Cirulli V and Giepmans BN:
EpCAM: Structure and function in health and disease. Biochim
Biophys Acta. 1828:1989–2001. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Litvinov SV, Velders MP, Bakker HA,
Fleuren GJ and Warnaar SO: Ep-CAM: A human epithelial antigen is a
homophilic cell-cell adhesion molecule. J Cell Biol. 125:437–446.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winter MJ, Nagelkerken B, Mertens AE,
Rees-Bakker HA, Briaire-de Bruijn IH and Litvinov SV: Expression of
EpCAM shifts the state of cadherin-mediated adhesions from strong
to weak. Exp Cell Res. 285:50–58. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maetzel D, Denzel S, Mack B, Canis M, Went
P, Benk M, Kieu C, Papior P, Baeuerle PA, Münz M and Gires O:
Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell
Biol. 11:162–171. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chaves-Pérez A, Mack B, Maetzel D,
Kremling H, Eggert C, Harréus U and Gires O: EpCAM regulates cell
cycle progression via control of cyclin D1 expression. Oncogene.
32:641–650. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du W, Ji H, Cao S, Wang L, Bai F, Liu J
and Fan D: EpCAM: A potential antimetastatic target for gastric
cancer. Dig Dis Sci. 55:2165–2171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baeuerle PA and Gires O: EpCAM (CD326)
finding its role in cancer. Br J Cancer. 96:417–423. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Strand J, Hamilton AE, Beavers LS, Gamboa
GC, Apelgren LD, Taber LD, Sportsman JR, Bumol TF, Sharp JD and
Gadski RA: Molecular cloning and characterization of a human
adenocarcinoma/epithelial cell surface antigen complementary DNA.
Cancer Res. 49:314–317. 1989.PubMed/NCBI
|
|
11
|
Winter MJ, Nagtegaal ID, van Krieken JH
and Litvinov SV: The epithelial cell adhesion molecule (Ep-CAM) as
a morphoregulatory molecule is a tool in surgical pathology. Am J
Pathol. 163:2139–2148. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schnell U, Kuipers J and Giepmans BN:
EpCAM proteolysis: New fragments with distinct functions? Biosci
Rep. 33:e000302013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gastl G, Spizzo G, Obrist P, Dünser M and
Mikuz G: Ep-CAM overexpression in breast cancer as a predictor of
survival. Lancet. 356:1981–1982. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carneiro FP, Muniz-Junqueira MI,
Pittella-Silva F, Carneiro MV, Takano GHS, Vianna LMS, De Andrade
LB, De Castro TMML, Peres I, Dos Santos Borges TK, et al: A panel
of markers for identification of malignant and non-malignant cells
in culture from effusions. Oncol Rep. 38:3538–3544. 2017.PubMed/NCBI
|
|
15
|
Fetsch PA, Simsir A, Brosky K and Abati A:
Comparison of three commonly used cytologic preparations in
effusion immunocytochemistry. Diagn Cytopathol. 26:61–66. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nigro K, Tynski Z, Wasman J, Abdul-Karim F
and Wang N: Comparison of cell block preparation methods for
nongynecologic ThinPrep specimens. Diagn Cytopathol. 35:640–643.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oda T, Ogata S, Kawaguchi S, Minabe S,
Dokyu M, Takahashi H, Kumazawa F, Shimazaki H, Takano M, Hase K, et
al: Immunocytochemical utility of claudin-4 versus those of Ber-EP4
and MOC-31 in effusion cytology. Diagn Cytopathol. 44:499–504.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jo VY, Cibas ES and Pinkus GS: Claudin-4
immunohistochemistry is highly effective in distinguishing
adenocarcinoma from malignant mesothelioma in effusion cytology.
Cancer Cytopathol. 122:299–306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Balzar M, Briaire-de Bruijn IH,
Rees-Bakker HA, Prins FA, Helfrich W, de Leij L, Riethmuller G,
Alberti S, Warnaar SO, Fleuren GJ and Litvinov SV: Epidermal growth
factor-like repeats mediate lateral and reciprocal interactions of
Ep-CAM molecules in homophilic adhesions. Mol Cell Biol.
21:2570–2580. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fong D, Seeber A, Terracciano L, Kasal A,
Mazzoleni G, Lehne F, Gastl G and Spizzo G: Expression of EpCAM(MF)
and EpCAM(MT) variants in human carcinomas. J Clin Pathol.
67:408–414. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spizzo G, Fong D, Wurm M, Ensinger C,
Obrist P, Hofer C, Mazzoleni G, Gastl G and Went P: EpCAM
expression in primary tumour tissues and metastases: An
immunohistochemical analysis. J Clin Pathol. 64:415–420. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Went PT, Lugli A, Meier S, Bundi M,
Mirlacher M, Sauter G and Dirnhofer S: Frequent EpCam protein
expression in human carcinomas. Hum Pathol. 35:122–128. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pu RT, Pang Y and Michael CW: Utility of
WT-1, p63, MOC31, mesothelin, and cytokeratin (K903 and CK5/6)
immunostains in differentiating adenocarcinoma, squamous cell
carcinoma, and malignant mesothelioma in effusions. Diagn
Cytopathol. 36:20–25. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chantima W, Thepthai C, Cheunsuchon P and
Dharakul T: EpCAM expression in squamous cell carcinoma of the
uterine cervix detected by monoclonal antibody to the
membrane-proximal part of EpCAM. BMC Cancer. 17:8112017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gosens MJ, van Kempen LC, van de Velde CJ,
van Krieken JH and Nagtegaal ID: Loss of membranous Ep-CAM in
budding colorectal carcinoma cells. Mod Pathol. 20:221–232. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Herreros-Pomares A, Aguilar-Gallardo C,
Calabuig-Fariñas S, Sirera R, Jantus-Lewintre E and Camps C: EpCAM
duality becomes this molecule in a new Dr. Jekyll and Mr. Hyde
tale. Crit Rev Oncol Hematol. 126:52–63. 2018. View Article : Google Scholar : PubMed/NCBI
|