Introduction
Lung cancer is the most common malignant tumor
worldwide and is the leading cause of cancer-associated mortality
each year (1–3). Non-small cell lung cancer (NSCLC)
accounts for ~85% of all lung cancer cases, which primarily consist
of adenocarcinomas and squamous cell carcinomas (1–3). Due to
high recurrence and metastasis, the outcomes of the currently
available treatment strategies are unsatisfactory, and the 5-year
survival rate is <16% (1–3). A thorough understanding of the
molecular mechanisms underlying NSCLC progression will enable the
development of novel diagnostic and therapeutic targets (4,5).
LIM domain-containing proteins shuttle between the
cytoplasm and the nucleus and bind to partners in both
compartments, often coupling changes in gene expression with
extracellular cues (6). LIM
domain-containing 2 (LIMD2) is an important member of the LIM
domain-containing protein family (7). Initially, LIMD2 was reported to be a
biomarker of the lymph node metastasis of papillary thyroid
carcinoma (7). In addition, LIMD2
has been demonstrated to be overexpressed in metastatic lesions and
regulates cell motility and tumor progression by directly binding
to and activating integrin-linked kinases (6). However, the expression pattern and
function of LIMD2 in NSCLC has, to the best of our knowledge, not
been reported.
MicroRNAs (miRs), are a class of small non-coding
RNAs containing 22–25 nucleotides, and are important regulators of
gene expression that function by directly binding to the
complementary sequences of 3′unstranslated regions (UTR) in target
mRNAs, causing RNA degradation or translational repression
(8–10). By altering the expression of their
target genes, miRs participate in a variety of biological
processes, including cell proliferation, apoptosis, differentiation
and tumorigenesis (8,11). Furthermore, numerous miRs have been
reported to be deregulated and exert pivotal functions in NSCLC,
including miR-186 (12), miR-148
(13), miR-92 (14), miR-599 (15), as well as miR-124 (16). For instance, a previous study has
demonstrated that miR-124 is significantly downregulated in
patients with gefitinib-resistant NSCLC and cell lines (16). The depletion of miR-124 induces
gefitinib resistance, and the overexpression of miR-124 sensitizes
gefitinib-resistant cells to gefitinib (16). However, the relationship between
miR-124 and LIMD2 in NSCLC has not previously been elucidated.
The aim of the present study was to explore the
clinical significance of LIMD2 expression in NSCLC and to explore
the functions of LIMD2 in regulating the malignant phenotypes of
NSCLC cells. Additionally, the regulatory mechanism underlying
LIMD2 expression in NSCLC cells were examined.
Materials and methods
Tissues samples
The present study was approved by the Ethics
Committee of Nanfang Hospital of Southern Medical University
(Guangzhou, China). A total of 52 NSCLC tissues and their matched
adjacent non-tumor tissues were collected at our hospital between
September 2011 and March 2013. These 52 patients with NSCLC
included 41 males and 11 females, aged 38–74 years with mean age of
62.7 years. Written informed consent was obtained from each
patient. The inclusion/exclusion criteria were that no patient
received any chemotherapy or radiotherapy prior to surgical
resection. The NSCLC tissues and the adjacent non-tumor tissues
were confirmed by the pathologists at Nanfang Hospital, and the
uniform distance between NSCLC tissues and adjacent tissues was 5
cm. All tissues were immediately snap-frozen in liquid nitrogen and
stored at −80°C. The clinical information of the patients is
summarized in Table I.
 | Table I.Correlation of LIMD2 expression with
clinicopathologic characteristics in non-small cell lung
cancer. |
Table I.
Correlation of LIMD2 expression with
clinicopathologic characteristics in non-small cell lung
cancer.
| Variable | Cases (n=52) | Low expression
(n=26) | High expression
(n=26) | P-value |
|---|
| Age (years) |
|
|
| 0.41416 |
|
<60 | 15 | 6 | 9 |
|
| ≥60 | 37 | 20 | 17 |
|
| Sex |
|
|
| 0.499 |
| Male | 41 | 22 | 19 |
|
|
Female | 11 | 4 | 7 |
|
| Smoking |
|
|
| 0.258 |
| Yes | 31 | 18 | 13 |
|
| No | 21 | 8 | 13 |
|
| TNM stage |
|
|
| 0.011a |
| I–II | 24 | 17 | 7 |
|
|
III–IV | 28 | 9 | 19 |
|
| Differentiation |
|
|
| 0.093 |
| Well and
moderate | 23 | 15 | 8 |
|
|
Poor | 29 | 11 | 18 |
|
| Lymph node
metastasis |
|
|
| 0.001a |
|
Yes | 27 | 7 | 20 |
|
| No | 25 | 19 | 6 |
|
Cell culture
The normal human bronchial epithelial cell line
BEAS-2B, and human NSCLC cell lines A549, H522, H1650 and H1975
were purchased from the Chinese Academy of Sciences Cell Bank.
These cells were cultured in DMEM supplemented with 10% FBS (both
from Thermo Fisher Scientific, Inc.) and incubated in a humidified
atmosphere containing 5% CO2 at 37°C.
Cell transfection
For cell transfections, A549 and H1975 cells
(5×105 cells per well) were seeded into 6-well plates.
Cells were transfected with 100 nM negative control (NC) small
interfering RNA (siRNA; Santa Cruz Biotechnology, Inc.), 100 nM
LIMD2-specific siRNA (Santa Cruz Biotechnology, Inc.), 100 nM
miR-NC mimics, 100 nM miR-124 mimics, 100 nM NC inhibitor or 100 nM
miR-124 inhibitor (all from Guangzhou FulenGen, Co., Ltd.,
Guangzhou, China), using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the cultured cells or
tissues using TRIzol (Thermo Fisher Scientific, Inc.). Total RNA
was reverse transcribed to cDNA by using a PrimeScript®
RT reagent kit (Takara Biotechnology Co., Ltd.), according to the
manufacturer's protocol. PCR performed with the SYBR premix
real-time PCR reagent (Takara Biotechnology Co., Ltd.) using the
ABI7500 real-time PCR system (Thermo Fisher Scientific, Inc.). The
thermocycling conditions were 95°C for 5 min, followed by 35 cycles
of 95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec. GAPDH and
U6 were used as the internal controls for LIMD2 and miR-124,
respectively. Relative quantification was performed using the
comparative 2−ΔΔCq method (17). The primers for LIMD2 were, forward,
5′-TGCCAGAAGACCGTGTACC-3′ and reverse, 5′-TTTGCAGTAGAACTCCCCGTG-3′.
The primers for GAPDH were, forward, 5′-CTGGGCTACACTGAGCACC-3′ and
reverse, 5′-AAGTGGTCGTTGAGGGCAATG-3′. The primers for U6 (cat. no.
HmiRQP9001) and miR-124 (cat. no. HmiRQP0074) were purchased from
Guangzhou FulenGen, Co., Ltd., and these sequences were not
supplied by the manufacturer.
Western blot analysis
Total protein was isolated from tissues or cell
lines using radioimmunoprecipitation assay lysis buffer (Beyotime
Biotechnology of Biotechnology, Haimen, China). The lysates were
centrifuged at 12,000 × g for 5 min at 4°C, and the supernatant was
collected. The protein concentration was determined using a BCA kit
(Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. The proteins (50 µg per lane) were
separated by SDS-PAGE on 10% gels and then transferred onto
polyvinylidene fluoride membranes (EMD Millipore). Following
blocking with 5% skim milk in TBS-Tween at room temperature for 3
h, the membrane was incubated with rabbit anti-human LIMD2 antibody
(1:500; cat. no. ab205375; Abcam) or rabbit anti-human GAPDH
antibody (1:500; cat. no. ab9485; Abcam) at room temperature for 3
h. Following three washes with TBS-Tween, the membrane was
incubated with horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (1:5,000; ab6721; Abcam) at room temperature for
40 min. The protein bands were detected using Pierce ECL Western
Blotting Substrate kit (Thermo Fisher Scientific, Inc.).
Densitometric analysis was performed using ImageJ software (version
1.48; National Institutes of Health).
Cell proliferation assay
Cell Counting Kit-8 (CCK-8) assays were conducted to
assess cell proliferation. Transfected cells were seeded into
96-well plates (5,000 cells per well). Following incubation at 37°C
in 5% CO2 for 0, 24, 48 and 72 h, 10 µl CCK-8 (Beyotime
Institute of Biotechnology) was added into each well. Following
incubation at 37°C for 30 min, the absorbance at a wavelength of
450 nm was determined.
Cell migration assay
Transfected cells in DMEM supplemented with 10% FBS
were seeded in 6-well plates (100,000 cells per well). Following
incubation at 37°C in 5% CO2 for 48 h, wounds were
created by scratching the cell surface with a 10 µl pipette tip.
Following two washes with PBS, the cells were cultured at 37°C in a
humidified incubator containing 5% CO2. At 0 and 24 h,
the scratches were images under an inverted light microscope
(CKX41; Olympus Corporation).
Cell invasion assay
The cell invasion assay was performed in a Transwell
chamber (24-well, 8-mm pore size; Corning, Inc.) that was precoated
with Matrigel (BD Biosciences). The transfected cells (100,000
cells) in DMEM were seeded in the upper chambers, and DMEM
supplemented with 10% FBS was added to the bottom chambers.
Following incubation at 37°C for 24 h, the non-invaded cells on the
upper surface of the membrane were removed with cotton swabs. The
invaded cells were stained with 0.5% crystal violet at room
temperature for 5 min, and counted under an inverted light
microscope.
Bioinformatics analysis and luciferase
reporter gene assay
TargetScan online software (version 7.2; http://www.targetscan.org/) was used to analyze the
targeting relationship between miR-124 and LIMD2. The wild-type
(WT) or mutant (MT) LIMD2 3′UTR containing the miR-124 targeting
sequence was inserted into the pMIR-REPORT™ miRNA Expression
Reporter Vector system (Ambion; Thermo Fisher Scientific, Inc.).
Subsequently, the WT or MT luciferase reporter gene plasmid was
then co-transfected with miR-NC or miR-124 mimics into H1975 and
A549 cells using Lipofectamine 2000 (Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Following
transfection for 48 h, the luciferase activities were measured
using a Dual-Luciferase® Reporter assay kit (Promega
Corporation, Madison, WI, USA) on Multiskan™ GO Microplate
Spectrophotometer (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. The ratio of firefly luciferase
activity to Renilla luciferase activity was determined. The
vector used to express Renilla luciferase was the
pMIR-REPORT™ miRNA Expression Reporter Vector system (Thermo Fisher
Scientific, Inc.).
Statistical analysis
All data are presented as the mean ± standard
deviation. All statistical calculations were performed using SPSS
20.0 (IBM Corp.). Statistical analysis between two groups was
performed using Student's t-test. Statistical analysis between more
than two groups was performed using one-way ANOVA followed by
Tukey's post hoc test. Survival analysis was conducted using the
Kaplan-Meier method with the log-rank test. Spearman correlation
analysis was used to analyze the correlation between miR-124 and
LIMD2 mRNA expression in NSCLC tissues. P<0.05 was considered to
indicate a statistically significant difference.
Results
Upregulation of LIMD2 is associated
with NSCLC progression
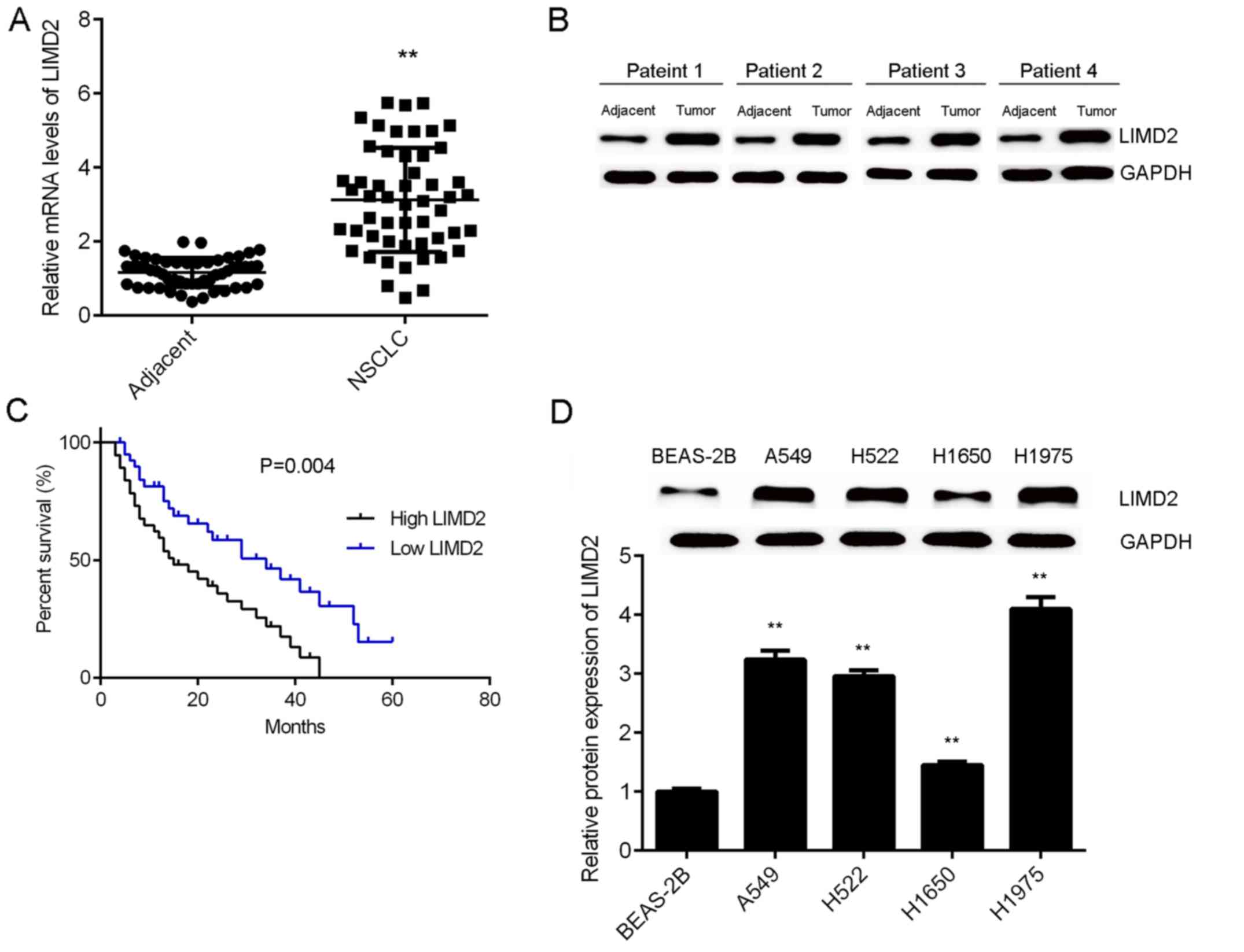
To reveal the role of LIMD2 in NSCLC, the present
study initially examined the mRNA and protein expression of LIMD2
in NSCLC tissues and adjacent non-tumor tissues via RT-qPCR and
western blotting. As illustrated in Fig.
1A and B, the expression levels of LIMD2 were significantly
higher in NSCLC tissues (only epithelial tissues), than in their
matched adjacent non-tumor tissues. Based on the mean expression
value of LIMD2, these patients were divided into a high LIMD2
expression group and low LIMD2 expression group. Further
investigation revealed that the high expression of LIMD2 was
significantly associated with lymph node metastasis, distant
metastasis and advanced clinical stage in NSCLC (Table I). In addition, it was observed that
patients with NSCLC that had high LIMD2 expression exhibited worse
prognoses (Fig. 1C). Furthermore,
the expression levels of LIMD2 were significantly increased in the
NSCLC cell lines (A549, H522, H1650 and H1975) compared with the
normal human lung epithelial cells (BEAS-2B; Fig. 1D). Taken together, these findings
suggest that the upregulation of LIMD2 may participate in the
malignant progression of NSCLC.
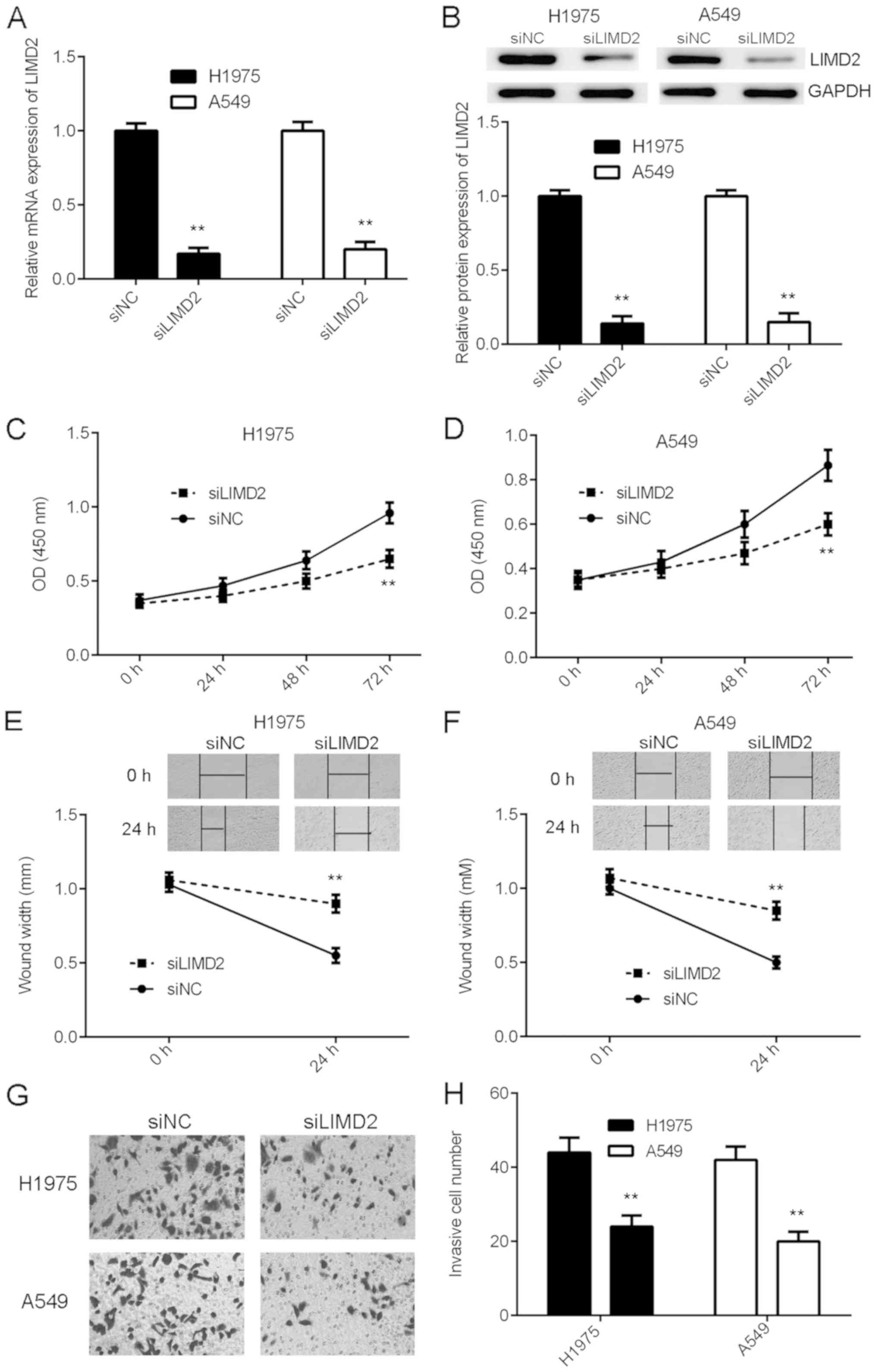
Knockdown of LIMD2 inhibits the
malignant phenotypes of NSCLC cells
As LIMD2 was significantly upregulated in NSCLC,
H1975 and A549 cells were transfected with LIMD2 siRNA to knockdown
its expression. Transfection with NC siRNA was used as the control
group. Following transfection, the mRNA and protein expression of
LIMD2 were significantly reduced in the siLIMD2 group, compared
with the siNC group (Fig. 2A and B).
The present study then examined the effects of LIMD2 downregulation
on NSCLC cell proliferation, migration and invasion. As indicated
in Fig. 2C-H, knockdown of LIMD2
markedly inhibited the proliferation, migration and invasion of
H1975 and A549 cells, compared with siNC. Therefore, the knockdown
of LIMD2 inhibited the malignant phenotype of NSCLC cells in
vitro.
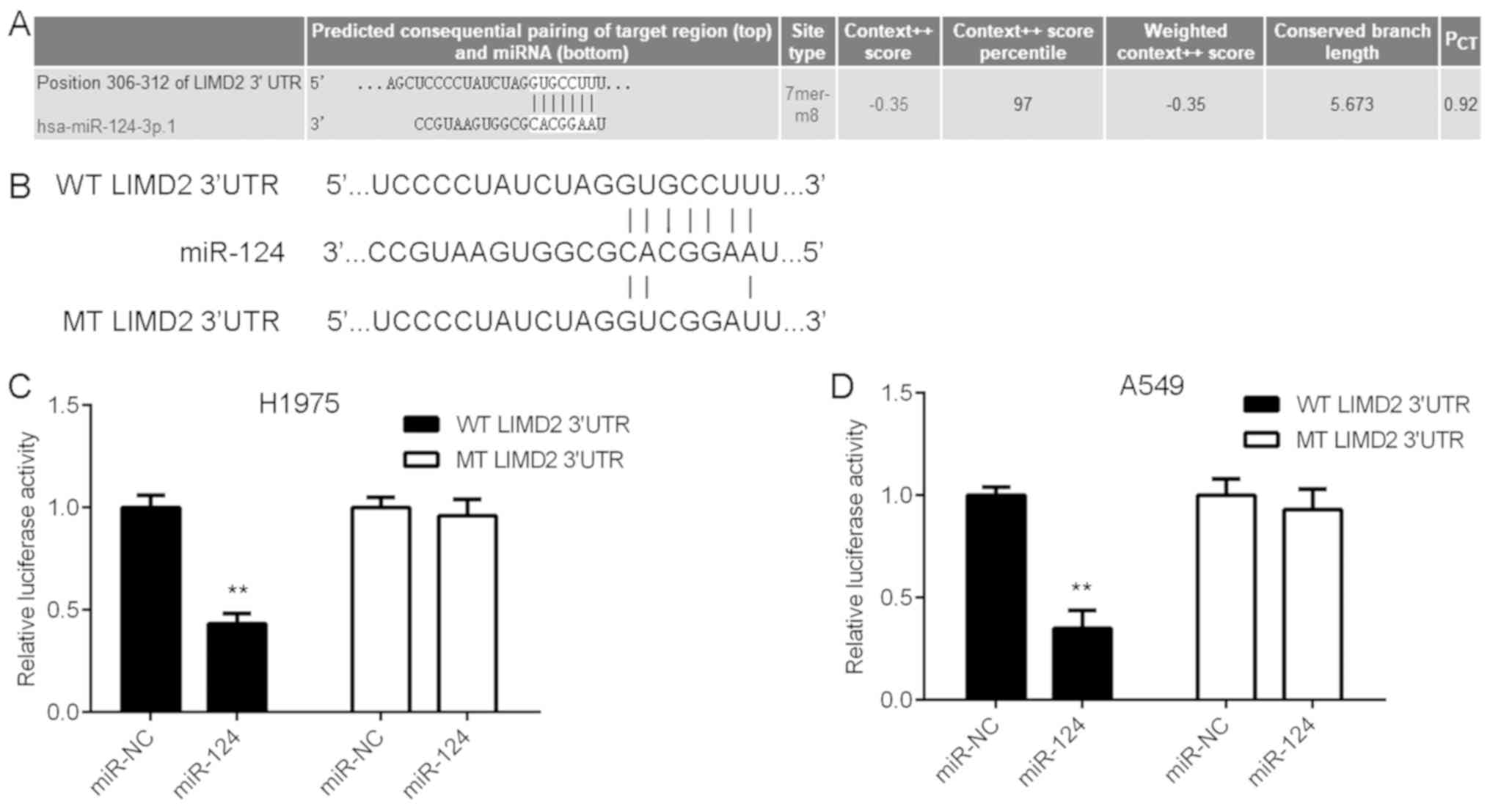
LIMD2 is a target gene of miR-124
The present study subsequently attempted to examine
the regulatory mechanism underlying the expression of LIMD2 in
NSCLC using bioinformatics analysis. The TargetScan online software
demonstrated that LIMD2 was a potential target of miR-124 (Fig. 3A). To verify this bioinformatics
prediction, WT and MT LIMD2 luciferase reporter plasmids were
generated (Fig. 3B) and then
luciferase reporter gene assays were performed using H1975 and A549
cells. As shown in Fig. 3C and D,
the overexpression of miR-124 led to a significant reduction in the
luciferase activity of the cells transfected with the WT LIMD2
3′UTR reporter plasmid, yet did not affect the luciferase activity
of the cells transfected with the MT LIMD2 3′UTR reporter plasmid.
These findings suggest that LIMD2 is a target gene of miR-124 in
NSCLC cells.
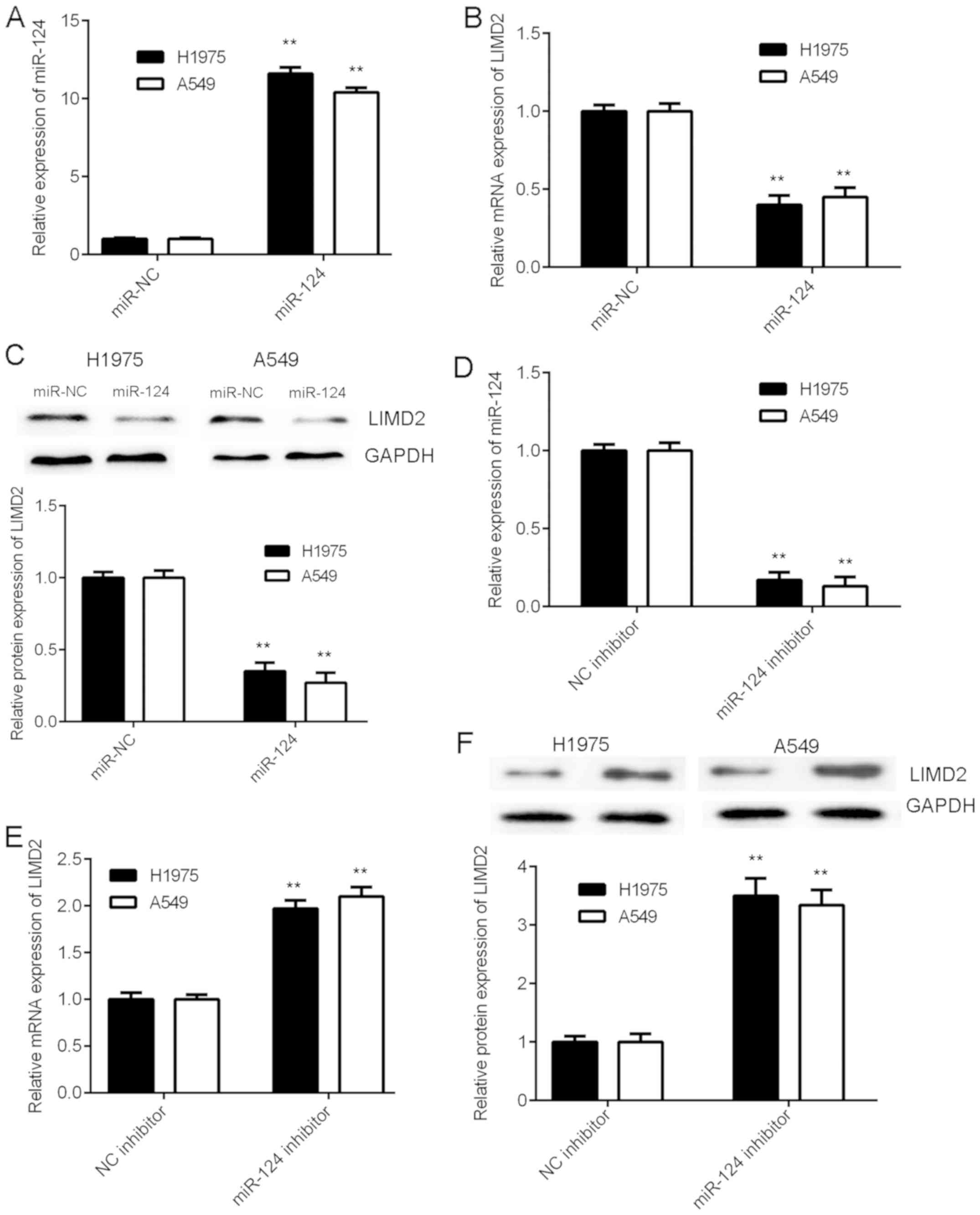
LIMD2 is negatively regulated by
miR-124 in NSCLC cells
As miRs generally negatively regulate the expression
of their target genes, the present study examined the effects of
miR-124 on the expression of LIMD2 in NSCLC cells. H1975 and A549
cells were initially transfected with an miR-124 mimic or miR-NC.
The RT-qPCR data revealed that the expression levels of miR-124
were significantly increased in the miR-124 group compared with the
miR-NC group (Fig. 4A). As
illustrated in Fig. 4B and C, the
mRNA and protein levels of LIMD2 were significantly reduced in the
miR-124 group compared with the miR-NC group. Therefore, the
overexpression of miR-124 resulted in the marked downregulation of
LIMD2 expression in NSCLC cells. To further confirm these findings,
H1975 and A549 cells were transfected with miR-124 inhibitor or NC
inhibitor. Following transfection, the expression levels of miR-124
were significantly increased in the miR-124 inhibitor group
compared with the NC inhibitor group (Fig. 4D). In addition, a significant
increase was observed in the mRNA and protein expression of LIMD2
in the miR-124 inhibitor group, when compared with the NC inhibitor
group (Fig. 4E and F). Taken
together, the above data indicate that LIMD2 is negatively
regulated by miR-124 in NSCLC cells.
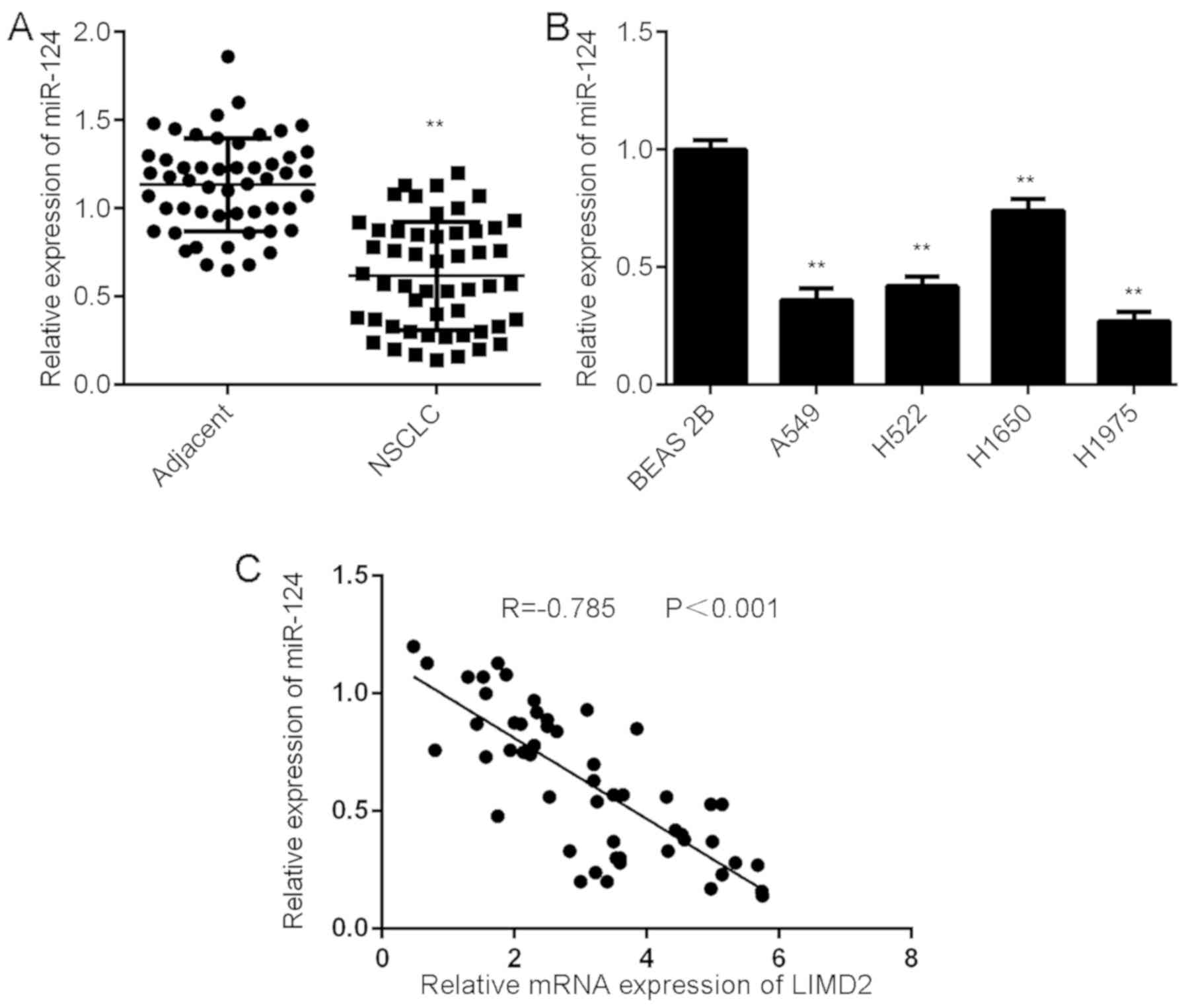
Downregulation of miR-124 is inversely
correlated with the upregulation of LIMD2 in NSCLC tissues
Further investigation revealed that the expression
levels of miR-124 were significantly lower in NSCLC tissues than in
their matched adjacent non-tumor tissues (Fig. 5A). Consistently, miR-124 was also
downregulated in the NSCLC cell lines compared with BEAS-2B cells
(Fig. 5B). Notably, an inverse
correlation was observed between miR-124 and LIMD2 expression in
the NSCLC tissues (Fig. 5C). These
findings suggest that the reduced expression of miR-124 may
contribute to the increased expression of LIMD2 in NSCLC
tissues.
Discussion
The results of the present study revealed that the
expression levels of LIMD2 were significantly increased in NSCLC
tissues and cell lines, compared with adjacent non-tumor tissues
and normal lung epithelial cells. In addition, the high expression
of LIMD2 was demonstrated to be significantly associated with lymph
node metastasis, distant metastasis and advanced clinical stage in
NSCLC. The patients with NSCLC that had a high expression of LIMD2
expression exhibited shorter survival times than those with low
LIMD2 expression. The knockdown of LIMD2 resulted in marked
decreases in the proliferation, migration and invasion of NSCLC
cells. Bioinformatics analysis and luciferase reporter gene assay
data further confirmed that LIMD2 was a direct target gene of
miR-124, which is a well-established tumor suppressor in NSCLC
(16). The expression of LIMD2 was
negatively regulated by miR-124 in NSCLC cells. Furthermore,
miR-124 was downregulated in NSCLC tissues when compared with
adjacent non-tumor tissues, and an inverse correlation was observed
between the expression of LIMD2 and miR-124 in NSCLC tissues.
LIM domain-containing proteins have been
demonstrated to exert different functions, including the regulation
of gene expression, cell adhesion and motility, and these proteins
have also been revealed to participate in the development and
progression of human cancers (18).
LIMD2, a member of the LIM domain-containing protein family, has
previously been demonstrated to promote tumor cell metastasis
(6). Inhibition of the expression of
LIMD2 can inhibit tumor cell motility and invasiveness in thyroid
cancer (7). However, whether LIMD2
exerts a promoting function in NSCLC remains unknown. In the
present study, the expression of LIMD2 in NSCLC tissues and matched
adjacent non-tumor tissues was measured using RT-qPCR and western
blot analysis, and it was observed that LIMD2 was significantly
upregulated in NSCLC tissues compared with adjacent non-tumor
tissues. Further investigation demonstrated that the increased
expression of LIMD2 was significantly associated with NSCLC
progression as well as the poor prognosis of patients. These
findings suggest that LIMD2 may function as an oncogene in NSCLC.
To further clarify the exact function of LIMD2 in NSCLC, the
present study used two common NSCLC cell lines H1975 and A549 to
perform in vitro experiments. The findings of these
experiments revealed that the knockdown of LIMD2 significantly
reduced the proliferation, migration and invasion of H1975 and A549
cells. These findings suggest that targeting LIMD2 may be a
promising strategy for the treatment of NSCLC.
The present study further examined the regulatory
mechanism underlying LIMD2 upregulation in NSCLC. As miRs are key
regulators of gene expression (10),
a bioinformatics prediction was performed to analyze the potential
miRs that can directly target LIMD2. Among the predicted miRs,
miR-124 was selected for subsequent investigation, as miR-124 has
been demonstrated to serve a tumor-suppressive role in NSCLC
(19,20). The downregulation of miR-204 in
plasma and tissues is associated with a poor prognosis in patients
with NSCLC (19,20). Additionally, certain target genes of
miR-124 have been identified in NSCLC (20). For example, the study of Li et
al (20) reported that miR-124
inhibited NSCLC cell proliferation by targeting STAT3. Lin et
al (21) demonstrated that
miR-124 served a suppressive role in NSCLC by targeting cadherin 2
and regulating the epithelial-mesenchymal transition. Yang et
al (22) reported that miR-204
suppressed NSCLC cell invasion and migration by targeting Janus
kinase 2. In addition, CD164, LIM homeobox 2, STAT3 and snail
family transcriptional repressor 2 have been identified as target
genes of miR-124 in NSCLC (16,21–23). In
the present study, a luciferase reporter gene assay data confirmed
that LIMD2 was a direct target gene of miR-124, and the expression
of LIMD2 was negatively mediated by miR-124 in NSCLC. In addition,
the increased expression of LIMD2 was inversely correlated with the
decreased expression of miR-124 in NSCLC tissues, suggesting that
the downregulation of miR-124 may contribute to the upregulation of
LIMD2 in NSCLC.
To the best of our knowledge, the present study is
the first to report that the overexpression of LIMD2 promotes the
progression of NSCLC and that the knockdown of LIMD2 may inhibit
the malignant phenotypes of NSCLC cells. These findings suggest
that LIMD2 may be used as a potential therapeutic target for
NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
SQ collected sample tissues and performed clinical
experiments. XX, YT and PH performed cell experiments and analyzed
data. FZ and YX were responsible for study design wrote the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Nanfang Hospital of Southern Medical University,
Guangzhou, China. Written informed consents were obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Landi L and Cappuzzo F: Pharmacotherapy
targeting the EGFR oncogene in NSCLC. Expert Opin Pharmacother.
15:2293–2305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Markou A, Sourvinou I, Vorkas PA, Yousef
GM and Lianidou E: Clinical evaluation of microRNA expression
profiling in non small cell lung cancer. Lung Cancer. 81:388–396.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng H, Talebzadeh-Farrooji M, Osborne MJ,
Prokop JW, McDonald PC, Karar J, Hou Z, He M, Kebebew E, Orntoft T,
et al: LIMD2 is a small LIM-only protein overexpressed in
metastatic lesions that regulates cell motility and tumor
progression by directly binding to and activating the
integrin-linked kinase. Cancer Res. 74:1390–1403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cerutti JM, Oler G, Michaluart P Jr,
Delcelo R, Beaty RM, Shoemaker J and Riggins GJ: Molecular
profiling of matched samples identifies biomarkers of papillary
thyroid carcinoma lymph node metastasis. Cancer Res. 67:7885–7892.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moss EG: MicroRNAs: Hidden in the genome.
Curr Biol. 12:R138–R140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
John B, Enright AJ, Aravin A, Tuschl T,
Sande C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang T, Wang G, Yang L, Peng B, Wen Y,
Ding G and Wang Z: MiR-186 inhibits proliferation, migration, and
invasion of non-small cell lung cancer cells by downregulating Yin
Yang 1. Cancer Biomark. 21:221–228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He M and Xue Y: MicroRNA-148a suppresses
proliferation and invasion potential of non-small cell lung
carcinomas via regulation of STAT3. Onco Targets Ther.
10:1353–1361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren P, Gong F, Zhang Y, Jiang J and Zhang
H: MicroRNA-92a promotes growth, metastasis, and chemoresistance in
non-small cell lung cancer cells by targeting PTEN. Tumour Biol.
37:3215–3225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian W, Wang G, Liu Y, Huang Z, Zhang C,
Ning K, Yu C, Shen Y, Wang M, Li Y, et al: The miR-599 promotes
non-small cell lung cancer cell invasion via SATB2. Biochem Biophys
Res Commun. 485:35–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu FY, Cao XN, Xu QZ, Huang Z, Zhang C,
Ning K, Yu C, Shen Y, Wang M, Li Y, et al: iR-124 modulates
gefitinib resistance through SNAI2 and STAT3 in non-small cell lung
cancer. J Huazhong Univ Sci Technolog Med Sci. 36:839–845. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matthews JM, Lester K, Joseph S and Curtis
DJ: LIM-domain-only proteins in cancer. Nat Rev Cancer. 13:111–122.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo W, Zhang Y, Shi Y, Xi J, Fan H and Xu
S: Decreased expression of miR-204 in plasma is associated with a
poor prognosis in patients with non-small cell lung cancer. Int J
Mol Med. 36:1720–1726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Yu Z, Li Y, Liu S, Gao C, Hou X, Yao
R and Cui L: The tumor suppressor miR-124 inhibits cell
proliferation by targeting STAT3 and functions as a prognostic
marker for postoperative NSCLC patients. Int J Oncol. 46:798–808.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin J, Xu K, Wei J, Heimberger AB, Roth JA
and Ji L: MicroRNA-124 suppresses tumor cell proliferation and
invasion by targeting CD164 signaling pathway in non-small cell
lung cancer. J Gene Ther. 2:62016.PubMed/NCBI
|
|
22
|
Yang Q, Wan L, Xiao C, Hu H, Wang L, Zhao
J, Lei Z and Zhang HT: Inhibition of LHX2 by miR-124 suppresses
cellular migration and invasion in non-small cell lung cancer.
Oncol Lett. 14:3429–3436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang M, Meng B and Liu Y, Yu J, Chen Q and
Liu Y: MiR-124 inhibits growth and enhances radiation-induced
apoptosis in non-small cell lung cancer by inhibiting STAT3. Cell
Physiol Biochem. 44:2017–2028. 2017. View Article : Google Scholar : PubMed/NCBI
|