Introduction
The diagnosis of gastric metastasis is challenging
in a clinical setting due to a lack of typical symptoms, with the
majority of cases being identified following an autopsy (1). Previous studies investigating
metastatic tumors in the stomach revealed that lung, breast and
esophagus are common primary tumor sites, and renal cell carcinoma
and malignant melanoma were also identified (2,3). A
retrospective analysis of 54 cases of metastatic tumors in the
stomach reported that 16 cases (25%) originated from different
types of lung cancer, including non-small cell lung cancer (NSCLC)
and small cell lung cancer (SCLC) (4). In contrast to primary NSCLC, SCLC is a
less common type of lung cancer that presents with gastric
metastases (5–8). SCLC is characterized by a rapid
progression and a poor prognosis in patients (9). Chemotherapy and supportive care are
routine therapeutic choices for the treatment of late-stage SCLC
(10). The current study presents
the case of a female patient diagnosed with SCLC with gastric
metastasis, who benefited from a 10 month survival time following
chemotherapy. A review of the current literature was also provided
to contextualize the findings of the present study.
Case report
A 77 year old female with no history of smoking or
drinking was admitted to the China-Japan Friendship Hospital
(Beijing, China) on May 23, 2016 after exhibiting a poor appetite,
abdominal distension after meals and occasional epigastralgia for
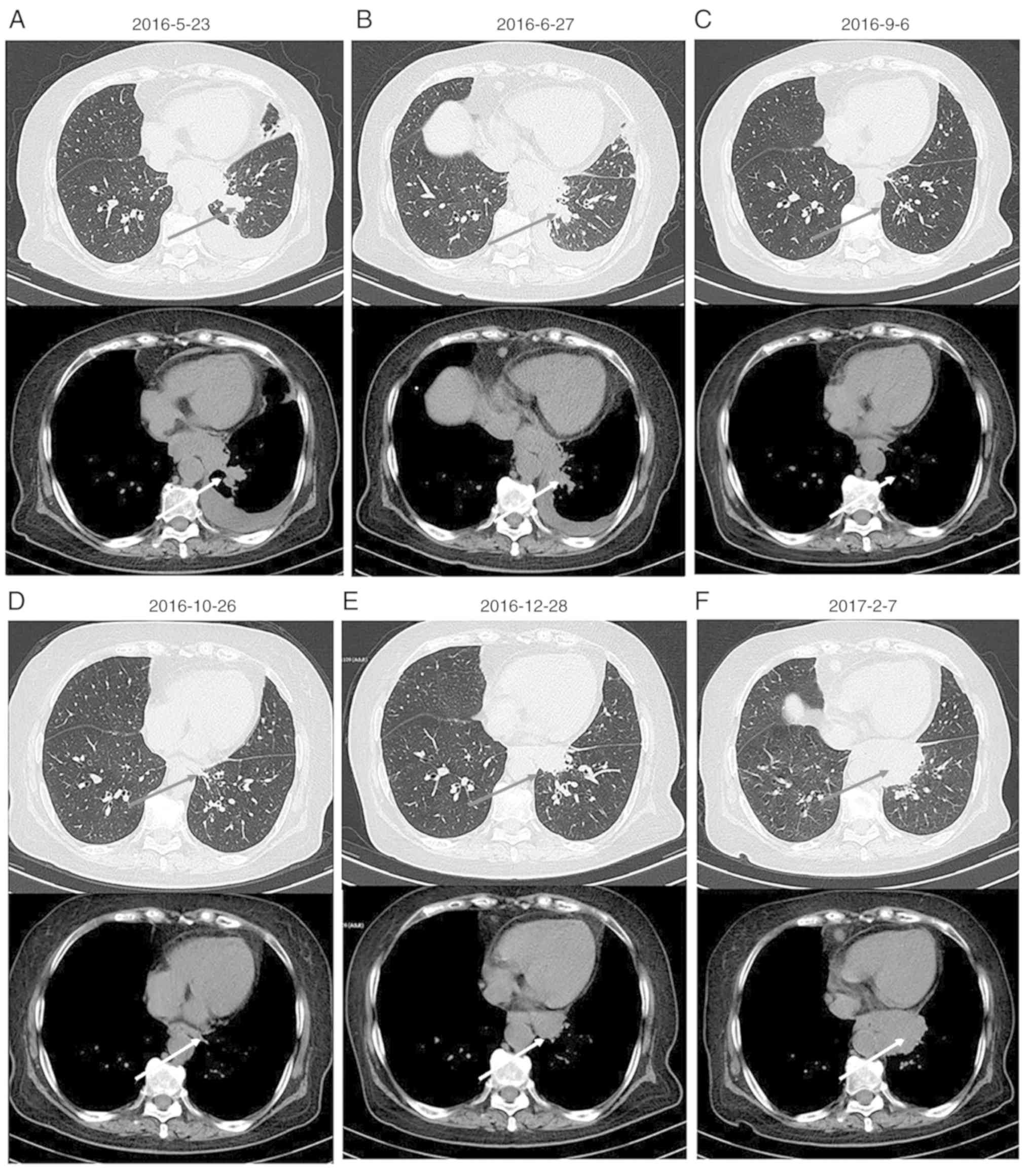
one month. A chest computed tomography (CT) scan revealed masses in
the left hilar region and left lower lobe of the lung, indicating
the presence of malignant tumors. In addition, a dispersed
distribution of nodules was identified in left and right lung lobes
with lymph node tumefaction in the left cervical, cardiophrenic
angle and retroperitoneal regions. The left adrenal gland presented
with thickening due to the presence of nodules, suggesting the
possibility of metastases. In addition, pericardial effusion was
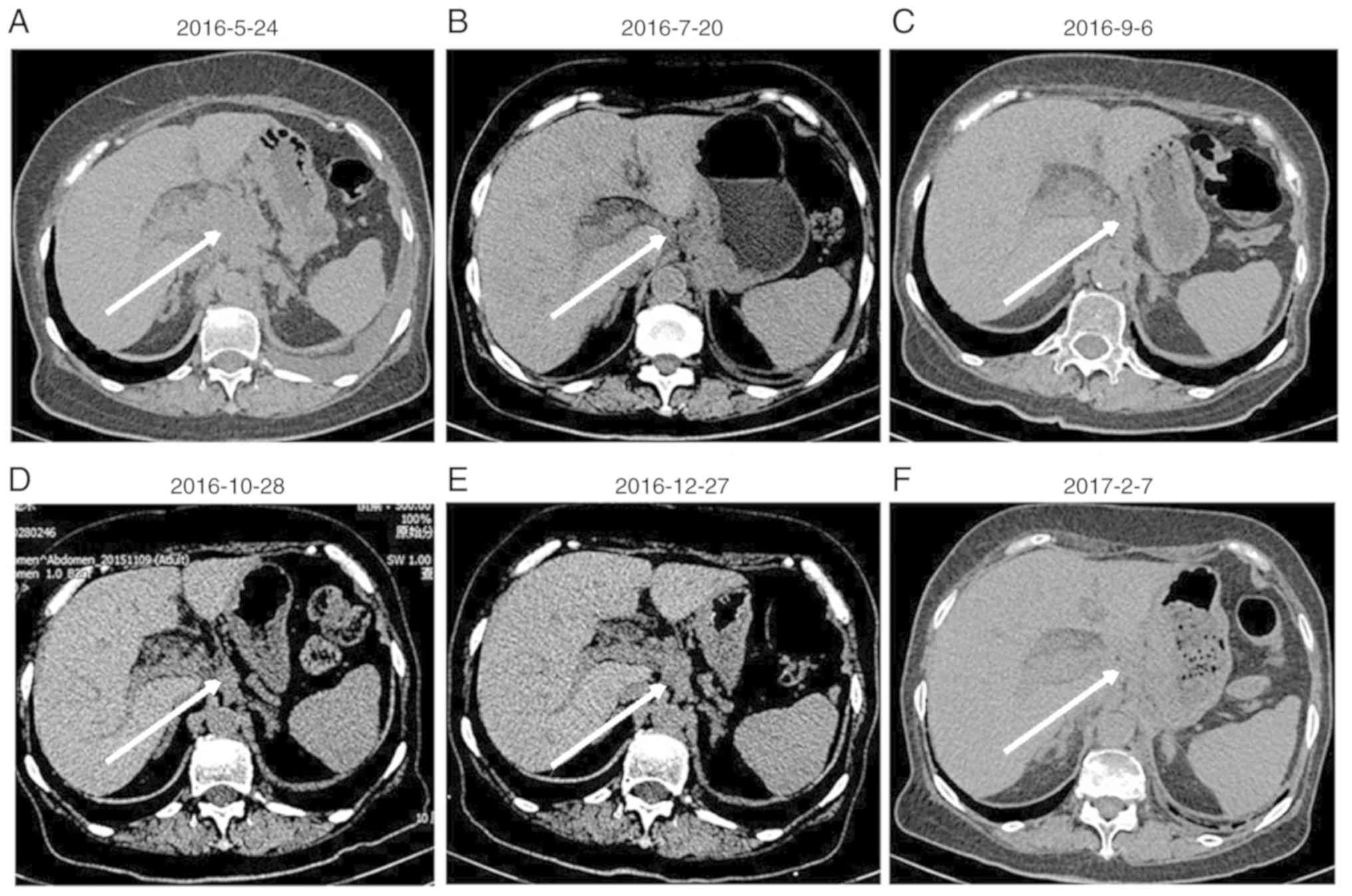
observed (Fig. 1A). An abdominal and
pelvic CT scan revealed that in the hepatic hilar and pancreatic
peripheral regions, mesenteric roots and retro-peritoneum, lymph
node tumefaction and partial integration were present, indicating
extensive metastasis (Fig. 2A). A
head CT and radionuclide bone imaging identified no abnormalities.
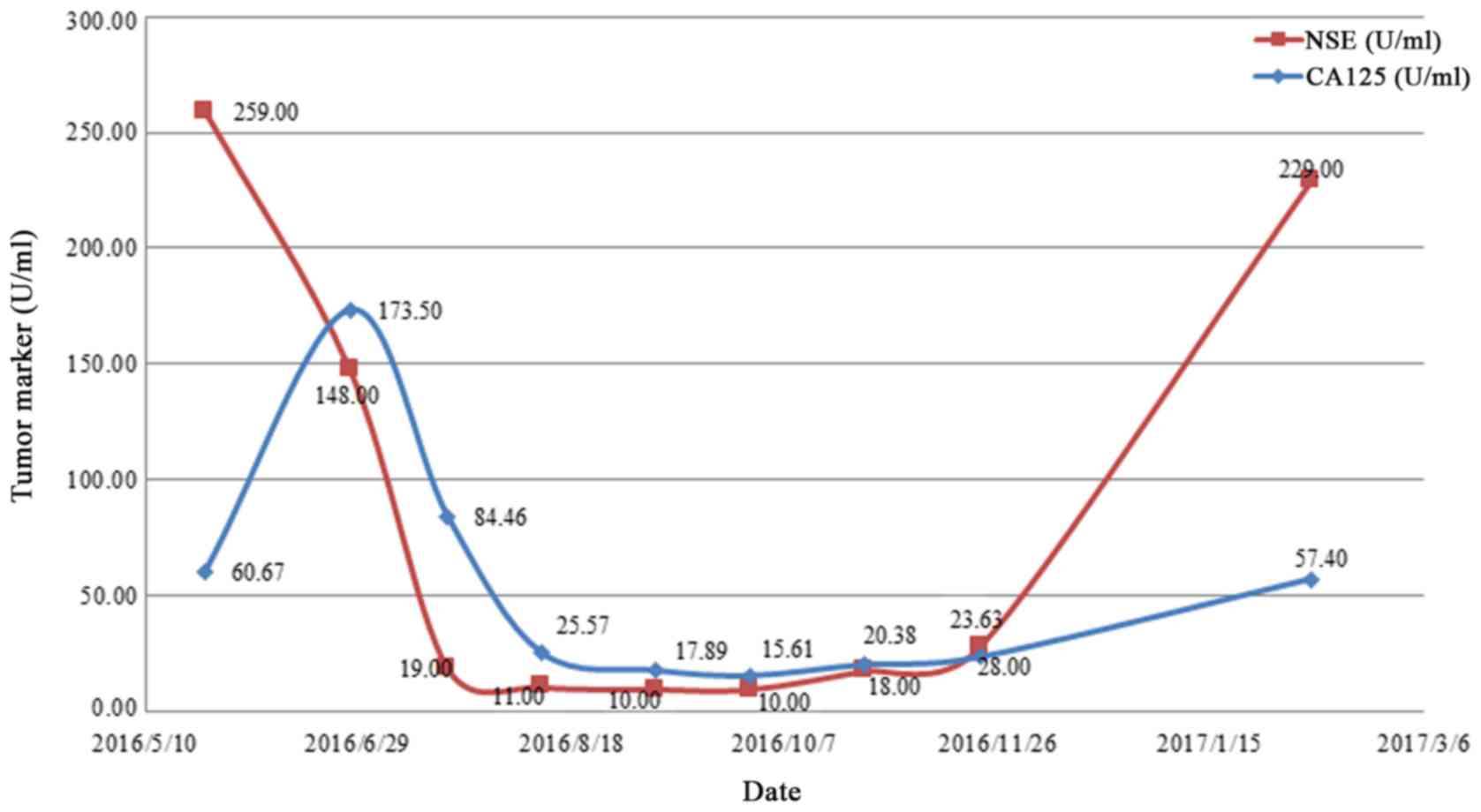
Venous blood of the patient was collected to detect tumor markers
by electrochemiluminescence following centrifugation. The normal
range of the tumor markers carbohydrate antigen 125 (CA125) and
neuron specific enolase (NSE) are <35.00 U/ml and <16.3
ng/ml, respectively (11); the
levels of CA125 and NSE in the patient's blood were above the
normal range (Fig. 3). A circulating
tumor cell count of 14.15 FU/3 ml was detected in the serum sample
collected at the first visit, a level which was considerably
elevated compared with the normal range (<8.7 FU/3 ml). A
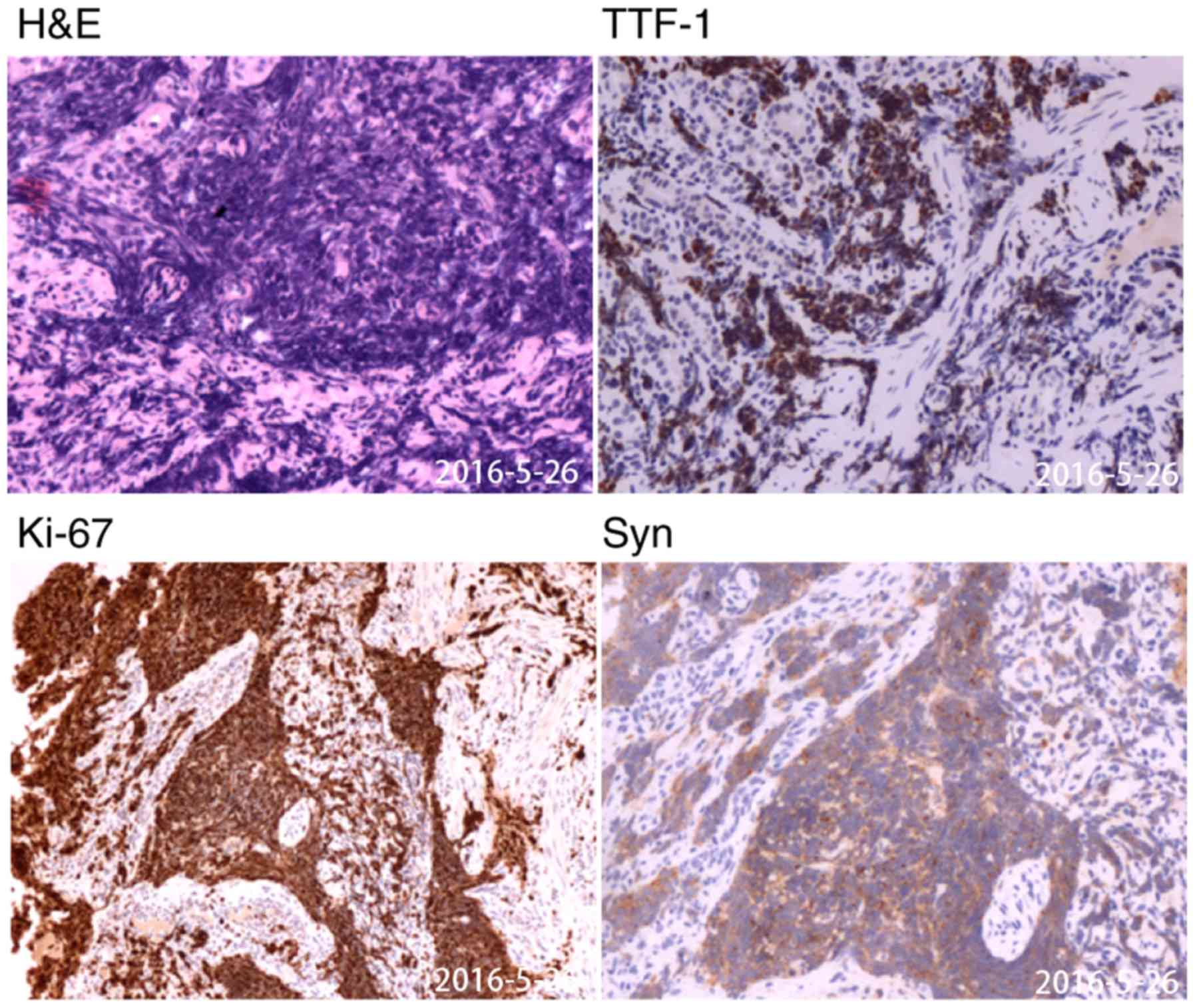
gastroscopy revealed a solitary lesion in the fundus of the stomach
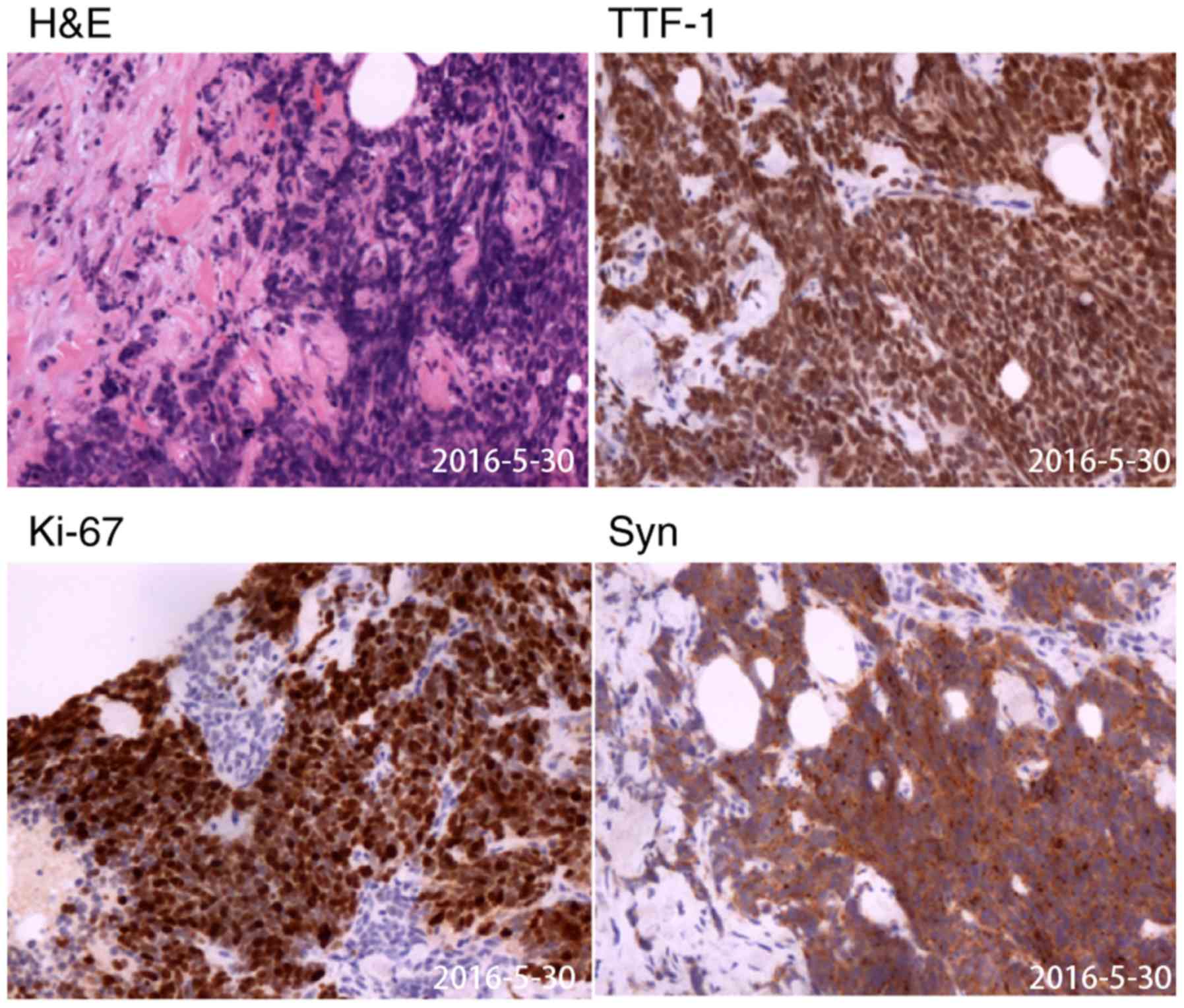
and a histopathological examination of a gastric specimen revealed
that the patient had SCLC, which had infiltrated the mucosa and
submucosa (Fig. 4 and S1).
The aforementioned gastric specimens were fixed with
10% formalin for 24 h and embedded in paraffin. Four to six
paraffin embedded samples (~5 µm) including the complete tumor
tissue were selected and stained as follows. Paraffin-embedded
sections were deparaffinized and immersed in distilled water. The
sections were rinsed three times for 5 min in PBS-T (0.01 M PBS pH
7.4: KH2PO4 0.02%,
N2HPO4 0.29%, KCl 0.02%, 0.8% NaCl, 0.05%
BSA, 0.05% Tween-20 and 0.0015% Triton X-100) prior to staining.
Slides were placed in a wet chamber and sections were blocked with
3% peroxide-methanol blocking buffer for >30 min at room
temperature. The slides were incubated with primary antibodies
against thyroid transcription factor-1 (TTF-1; cat. no. MAB-0677;
1:200; AXIM® Biotechnologies, Inc.), synaptophysin (Syn;
cat. no. kit-0022; 1:200; AXIM® Biotechnologies, Inc.),
marker of proliferation Ki-67 (cat. no. ZM-0166; 1:200; OriGene
Technologies, Inc.), neural cell adhesion molecule 1 (NCAM1; cat.
no. kit-0028; 1:200; AXIM® Biotechnologies, Inc.),
chromogranin A (CgA; cat. no. MAB-0707; 1:200; AXIM®
Biotechnologies, Inc.), cytokeratin 7 (CK7; cat. no. ZM-0069;
1:200; OriGene Technologies, Inc.), CK20 (cat. no. kit-0025; 1:200;
AXIM® Biotechnologies, Inc.) and caudal type homeobox 2
(CDX-2; cat. no. RMA-0631; 1:200; AXIM® Biotechnologies,
Inc.) overnight at 4°C. Slides were washed with PBS (3×3 min) and
incubated with horseradish peroxidase-labeled secondary antibodies
(cat. no. 18G48D10, 1:200; OriGene Technologies, Inc.) for 30–60
min at room temperature. Slides were washed with PBS (3×3 min) and
stained with 3,3′-diaminobenzidine in the dark at room temperature
for 10 min. Hematoxylin (0.7% for 2 min) was used as a counterstain
for the nuclei. Images (5 fields/slice) were captured using a light
microscope at ×200 magnification. Immunohistochemical staining for
TTF-1, NCAM1, CgA, Syn and Ki67 was positive (Fig. 3), CK7 staining was positive in a
limited area, whereas CK20 and CDX-2 staining was negative
(Fig. S1).
On May 30, 2016, a percutaneous biopsy of the
cervical lymph node suggested the presence of SCLC, as it exhibited
immunohistochemical staining features similar to those of the
gastric specimen (Figs. 5 and
S2). The patient was subsequently
diagnosed with extensive-stage SCLC with gastric metastases and
extensive lymph node metastases according to the National
Comprehensive Cancer Network (NCCN) Guidelines (12).
Upon admission to the Department of Oncology, the
patient had nutrition deficiency. The patient had an Eastern
Cooperative Oncology Group (13)
performance status of 2. Taking into consideration patient
condition and age, etoposide monotherapy was started on June 7,
2016 (14). Following the first
course of chemotherapy, the patient's general condition and
appetite were markedly improved without significant adverse
effects, such as gastrointestinal reaction. Between June 29 and
November 23, 2016, the patient was treated with an additional seven
courses of systemic chemotherapy with etoposide and oxaliplatin.
Monthly imaging examinations revealed the effectiveness of
chemotherapy (Figs. 1B-D and
2B-D), which was corroborated by a
change in the expression of tumor markers. The expression of CA125
and NSE decreased with disease palliation and increased with
disease aggravation (Fig. 5).
However, on December 28, 2016, thoracic and abdominal CT images
revealed enlarged masses, indicating disease progression (Figs. 1E and 2E). The second- and third-line chemotherapy
agents, irinotecan with carboplatin (15) and gemcitabine (16), respectively, were ineffective against
these masses (Figs. 1F and 2F), suggesting chemotherapy resistance
developed.
The patient was referred to the Oncology Department
on March 8, 2017 as the condition rapidly deteriorated, and liver
dysfunction and anemia were identified. The liver function test
revealed 200 IU/l aspartate aminotransferase, 83 IU/l alanine
aminotransferase, 69.37 µmol/l direct bilirubin and 124.75 µmol/l
total bilirubin. Routine blood examination revealed 86 g/l
hemoglobin; the patient exhibited severe jaundice and anorexia. On
March 9, 2017, the patient succumbed to liver failure and an
autopsy was refused.
Literature review
Only a limited number of cases of SCLC gastric
metastasis have previously been reported. Databases including
PubMed (https://www.ncbi.nlm.nih.gov/pubmed), WanFang Data
(http://www.wanfangdata.com.cn/index.html) and China
National Knowledge Infrastructure (CNKI; http://kns.cnki.net/kns/brief/default_result.aspx)
were investigated between October 2017 and March 2018 to analyze
the clinicopathological features and outcomes of patients with SCLC
and gastric metastases. Search terms included ‘small cell lung
cancer’, ‘gastric/gastrointestinal/stomach’ and
‘metastasis/metastases’. A total of 11 case reports (17–27)
(Table I) and 6 retrospective
studies (5,28–32)
including 20 cases were reviewed (Table
II). As presented in Table II,
gastric metastases account for only 0.6–16.4% of the total cases of
metastasis in patients with SCLC. As observed in Table I, a total of 7 of the 11 cases of
SCLC with gastric metastases presented in patients >65 years and
9 cases were male patients. SCLC was frequently identified in
patients >65 years with a long history of smoking. Among the 11
cases, 6 patients were smokers, 1 patient did not smoke and the
information for the other 4 patients was not available. The female
patient reported in the current study did not have a history of
smoking or consuming alcohol and thus differed from the majority of
previously reported cases.
 | Table I.Case reports of lung small cell
carcinoma with gastric metastasis. |
Table I.
Case reports of lung small cell
carcinoma with gastric metastasis.
| Author, year | Age/sex | History of
smoking | DP (L/S) | PLL | CPs | TPM | GML | OMS | ATM | Treatment | TTH | (Refs.) |
|---|
| Maeda et al,
1992 | 60/F | ND | Bronchial lung
biopsy gastroscopy | Right lower
lobe | Nausea,
vomiting | MC | Multiple
tumors | Skin | ND | Chemotherapy | ND | (17) |
| Kim et al,
1993 | 66/M | ND | Biopsy of
subcutaneous nodule/gastroscopy | Left hilar
region | Epigastric pain,
general weakness | SC | Upper body and
fundus | Skin | ND | ND | ND | (18) |
| Chen et al,
2004 | 74/M | ND |
Bronchoscopy/gastroscopy | Right hilar
region | Melena | MC | Gastric corpus | Right hilar lymph
node | ND | None | ND | (19) |
| Oh et al,
2004 | 87/M | No | Chest
CT/gastroscopy | Right upper
lobe | Epigastrium
pain | SC | Upper body | Bone | CEA | None | 2 months | (20) |
| Casella et
al, 2006 | 63/M | Yes | Bronchoscopy and
percutaneous biopsy of the left supraclavicular lymph
node/gastroscopy | ND | Weight loss,
epigastric pain, constipation | SC | Gastric corpus | Liver, brain,
bone | ND | Supportive
care | 1 month | (21) |
| Kim et al,
2009 | 66/M | ND | ND | ND | Hematemesis | SC | ND | Adrenal gland | ND | Electrocautery for
bleeding | ND | (22) |
| Koch et al,
2009 | 65/M | Yes | Biopsy of
mediastinal lymph nodes/gastroscopy | Right middle
lobe | None | MC | Multiple
tumors | Mediastinal lymph
nodes, pleural | NSE | Chemotherapy | 3 months | (23) |
| Xu et al,
2013 | 48/M | Yes |
PET-CT/gastroscopy | Left hilar
region | ND | MC | Gastric corpus | Mediastinal lymph
nodes | NSE | Chemotherapy | 2 months | (24) |
| Zhang et al,
2015 | 63/M | Yes |
Bronchoscopy/gastroscopy | Left upper
lobe | Abdominal
satiety | MC | ND | Lung, mediastinal
lymph nodes | pro-GRP | Chemotherapy,
radiotherapy | ND | (25) |
| Gao et al,
2015 | 66/M | Yes | Sputum
cytology/gastroscopy | Right hilar
region | Epigastrium
pain | MC | Posterior wall of
the stomach | ND | ND | Chemotherapy,
radiotherapy | 3 months | (26) |
| Zhu et al,
2017 | 73/M | Yes |
Bronchoscopy/gastroscopy | Right hilar
region | Epigastrium pain,
melena | SC | Multiple
tumors | Bone, pelvic,
submaxillary lymph nodes | NSE | Chemotherapy | 8.5 months | (27) |
 | Table II.Retrospective studies describing
small cell lung carcinoma with gastric metastasis. |
Table II.
Retrospective studies describing
small cell lung carcinoma with gastric metastasis.
| Author, year | Cases (n) | Gastric metastasis
(n) | Sex | Clinical
presentation | Treatment | Overall
survival | (Refs.) |
|---|
| Green 1990 | 67 | 11 | ND | ND | ND | ND | (28) |
| Ryo et al,
1996 | 30 | 1 | ND | ND | ND | ND | (29) |
| Yoshimoto et
al, 2006 | 470 | 3 | ND | ND | ND | ND | (5) |
| Kim et al,
2009 | 28 | 3 | ND | ND | ND | ND | (30) |
| Lee et al,
2011 | 21 | 1 | M | Abdominal pain | None | 3 months | (31) |
| Liu et al,
2012 | 12 | 1 | M | Epigastric
discomfort | Chemotherapy | 1 year | (32) |
Discussion
The treatment of SCLC is often complicated by the
presence of distant metastases, which typically occur in the head,
bone and adrenal gland; however, gastrointestinal metastases are
less common (30). A previous
analysis of 18 consecutive cases of lung cancer with
gastrointestinal tract involvement reported that only 4 cases
presented with stomach metastasis (33). In previous studies, the clinical
prevalence of stomach metastases in patients with lung cancer was
0.035–3.4% and metastases were diagnosed by endoscopy or autopsy
(30,34).
Lung cancer is typically diagnosed by bronchoscopy,
biopsy of the lymph nodes, positron emission tomography (PET)-CT
and sputum cytology, while stomach metastases are identified via a
gastroscopy (35). A systematic
review and meta-analysis reported that PET/CT is a valuable tool
for the diagnosis of SCLC (36).
However, gastric metastasis should be distinguished from primary
gastric carcinoma, as this influences the therapeutic approach.
Criteria for the diagnosis of gastric metastases include
morphologic and immunohistochemical features consistent with a
primary pulmonary tumor, as well as the clinicoradiologic
demonstration of a primary lung tumor and exclusion of tumors
elsewhere (37). Xu et al
(24) and Zhang et al
(25) reported Ki-67 values of 95
and 60%, respectively (Table I). The
Ki-67 value of the patient described in the current study was 80%.
Ki-67 is of particular importance in SCLC, as it is indicative of
high proliferation (38). TTF-1 is a
tissue-specific transcription factor essential for the normal
development of the lung and its expression has been detected in
various types of lung carcinomas, including SCLC (39). Therefore, TTF-1 is used to
distinguish metastatic carcinomas of pulmonary origin from other
carcinomas (40). CDX2 expression is
maintained in the adult small and large intestinal epithelia, and
is upregulated in gastrointestinal pathological states (41). Furthermore, the co-expression of
TTF-1 and neuroendocrine markers, including NCAM1, CgA and Syn,
have contributed to the diagnosis of SCLC metastasis in the stomach
(42).
The literature review revealed that SCLC with
gastric metastasis commonly occurred in the hilar region lymph
nodes, while the metastatic sites of the stomach were either
scattered, single or multiple (8).
It was reported that among the 54 cases diagnosed via an endoscopy,
solitary lesions (65%) presented more frequently than multiple
lesions (35%) with common metastatic sites in the middle or upper
third of the stomach (3). In a
clinicopathological study, the sites of metastasis in the stomach
were solitary for 94.4% of patients, with 5.6% developing multiple
lesions in the stomach; and the body of the stomach was the most
common site of metastasis (43).
Four different processes have been hypothesized to
be involved in the metastatic spread of primary cancers to the
stomach, including peritoneal and hematogenous dissemination,
lymphatic spread and direct tumor invasion (2). The metastasis of lung cancer to the
stomach is principally caused by hematogenous metastasis. The
direct invasion of cancer cells often occurs through the pulmonary
vein and the left side of the heart, resulting in transfer to
organs and tissues throughout the body, including the stomach
(26). SCLC has previously exhibited
a high incidence of vascular invasion (6). Therefore, when gastric metastases are
identified, other metastases, including skin, bone, liver, brain,
adrenal gland and visceral pleura also occur (44). In addition, a previous study reported
the possibility of cancer cells in phlegm being swallowed and
entering the stomach, thereby causing implantation metastasis
(37).
The majority of patients with gastrointestinal
metastases are asymptomatic, resulting in the majority of cases
being diagnosed by an autopsy. However, epigastric pain, chronic
bleeding, nausea and vomiting, melena and weight loss are commonly
reported and constipation, abdominal satiety and hematemesis also
occur. Common complications include perforation, obstruction and
ulceration due to disease progression (45,46). In
patients at extensive-stages of the disease, chemotherapy is the
preferred therapeutic option to attenuate symptoms and prolong
survival in patients; however, long-term survival is rare (9). The recommended first-line regimens are
cisplatin and etoposide according to the NCCN Guidelines (47). However, a systematic review comparing
cispatin- and carboplatin-based chemotherapy in the first-line
treatment of SCLC suggested that there is no difference in efficacy
between the two treatments (48).
Second-line chemotherapy treatment typically includes irinotecan
and gemcitabine (49). The patient
in the current study received oxaliplatin to decrease the risk of
severe myelosuppression and gastrointestinal reaction, and
benefited from first-line chemotherapy with only mild adverse
chemotherapy-associated effects. Chemotherapeutic treatment results
in rapid tumor necrosis with perforation resulting in mortality and
should therefore be used with caution (50). A report of 13 patients with
metastatic lung cancer receiving exploratory celiotomy revealed
that surgical intervention in combination with chemotherapy is
effective, especially when obstruction, bleeding or perforation
occur, with 8/13 patients surviving and discharged from hospital
after a mean stay of 17 days (51).
Radiotherapy is occasionally performed in the treatment of regional
tumors in the lungs, brain or lymph nodes (53).
For patients with metastasis in the upper
gastrointestinal tract, prognosis is poor, with an average of 5.5
months from diagnosis to mortality (53). A previous study reported the median
survival time of patients with metastasis in the upper
gastrointestinal tract was 4.75 months and none survived for >2
years (54). The patient described
in the current case report had a survival period <1 year.
The low prevalence and poor prognosis of patients
with SCLC and gastric metastasis cannot be ignored in clinical
practice. Identifying metastatic or primary gastric carcinoma may
be clinically challenging; however, immunohistochemical staining
aids to detect the primary origin site, which presents with an
identical phenotype to the metastatic site. The pathology of tissue
also contributes to the diagnosis and may impact the selection of
the therapeutic regimen. SCLC is sensitive to chemotherapy; yet
treatment-induced bleeding may trigger perforation and the rapid
onset of mortality (50). Surgery
may be possible in certain cases when obstruction, bleeding or
perforation occurs; however, further studies are required in order
to identify the optimal treatment for patients with SCLC and
gastric metastasis.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Qing Wu
(English Department, School of Humanities, Beijing University of
Chinese Medicine, Beijing, China) for the English language
editing.
Funding
This project was funded by the National Natural
Science Foundation of China (grant no. 8187151262).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YMP, QL and HJC collected the patient's data. YW,
APS and HD analyzed the data and performed reference search. YMP,
YQQ and QL drafted and revised the manuscript. All authors
contributed toward data analysis, drafting and revision of the
manuscript, and read and approved the final manuscript.
Ethics approval and consent to
participate
This case report was approved by the China-Japan
Friendship Hospital.
Patient consent for publication
Consent for publication was signed by the patient's
daughter.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nitipir C, Ginghina O, Popa L, Andrei F,
Tudor N, Radu I, Iaciu C, Orlov C, Vasilescu F, Balalau C, et al: A
rare case of advanced lung cancer presenting as a symptomatic
gastric tumor. Mol Clin Oncol. 8:600–602. 2018.PubMed/NCBI
|
|
2
|
Namikawa T and Hanazaki K:
Clinicopathological features and treatment outcomes of metastatic
tumors in the stomach. Surg Today. 44:1392–1399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oda I, Kondo H, Yamao T, Saito D, Ono H,
Gotoda T, Yamaguchi H, Yoshida S and Shimoda T: Metastases tumors
to the stomach: Analysis of 54 patients diagnosed at endoscope and
347 autopsy cases. Endoscopy. 33:507–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Palma GD, Masone S, Rega M, Simeoli I,
Donisi M, Addeo P, Iannone L, Pilone V and Persico G: Metastatic
tumors to the stomach: Clinical and endoscopic features. World J
Gastroenterol. 12:7326–7328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshimotoa A, Kasaharab K and Kawashima A:
Gastrointestinal metastases from primary lung cancer. Eur J Cancer.
42:3157–3160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Antler AS, Ough Y, Pitchumoni CS, Davidian
M and Thelmo W: Gastrointestinal metastases from malignant tumors
of the lung. Cancer. 49:170–172. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang CJ, Hwang JJ, Kang WY, Chong IW, Wang
TH, Sheu CC, Tsai JR and Huang MS: Gastro-intestinal metastasis of
primary lung carcinoma: Clinical presentations and outcome. Lung
Cancer. 54:319–323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang Q, Su X, Bella AE, Luo K, Jin J,
Zhang S, Luo G, Rong T and Fu J: Clinicopathological features and
outcome of gastric metastases from primary lung cancer: A case
report and systematic review. Oncol Lett. 9:1373–1379. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Demedts IK, Vermaelen KY and van Meerbeeck
JP: Treatment of extensive-stage small cell lung carcinoma: Current
status and future prospects. Eur Respir J. 35:202–215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng S, Evans WK, Stys-Norman D and
Shepherd FA: Chemotherapy for relapsed small cell lung cancer: A
systematic review and practice guideline. J Thorac Oncol.
2:348–354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou M, Wang Z, Yao Y, Zhou H, Liu M and
Sun J: Neuron-specific enolase and response to initial therapy are
important prognostic factors in patients with small cell lung
cancer. Clin Transl Oncol. 19:865–873. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stahel R, Ginsberg R and Havemann K:
Staging and prognostic factors in small cell lung cancer: A
consensus report. Lung Cancer. 5:119–126. 1989. View Article : Google Scholar
|
|
13
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperation oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mascaux C, Paesmans M, Berghmans T, Branle
F, Lafitte JJ, Lemaitre F, Meert AP, Vermylen P and Sculier JP;
European Lung Cancer Working Party (ELCWP), : A systematic review
of the role of etoposide and cisplatin in the chemotherapy of small
cell lung cancer with methodology assessment and meta-analysis.
Lung Cancer. 30:23–36. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmittel A, Fischer von Weikersthal L,
Sebastian M, Martus P, Schulze K, Hortig P, Reeb M, Thiel E and
Keilholz U: A randomized phase II trial of irinotecan plus
carboplatin versus etoposide plus carboplatin treatment in patients
with extended disease small-cell lung cancer. Ann Oncol.
17:663–667. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Van der Lee I, Smit EF, van Putten JW,
Groen HJ, Schlösser NJ, Postmus PE and Schramel FM: Single-agent
gemcitabine in patients with resistant small-cell lung cancer. Ann
Oncol. 12:557–561. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maeda J, Miyake M, Tokita K, Iwahashi N,
Nakano T, Tamura S, Hada T and Higashino K: Small cell lung cancer
with extensive cutaneous and gastric metastases. Intern Med.
31:1325–1328. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim HS, Jang WI, Hong HS, Lee CI, Lee DK,
Yong SJ, Shin KC and Shim YH: Metastases involvement of the stomach
secondary to lung carcinoma. J Korean Med Sci. 8:24–29. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen H, Lin YG and Liu Y: Older lung
cancer patient with gastric metastasis: A case report. J Guangxi
Med Univ. 21:7662004.(In Chinese).
|
|
20
|
Oh JC, Lee GS, Kim JS, Park Y, Lee SH, Kim
A, Lee JM and Kim KS: A case of gastric metastasis from small cell
lung carcinoma. Korean J Gastroenterol. 44:168–171. 2004.PubMed/NCBI
|
|
21
|
Casella G, Di Bella C, Cambareri AR, Buda
CA, Corti G, Magri F, Crippa S and Baldini V: Gastric metastasis by
lung small cell carcinoma. World J Gastroenterol. 12:4096–4097.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim MS, Kook EH, Ahn SH, Jeon SY, Yoon JH,
Han MS, Kim CH and Lee JC: Gastrointestinal metastasis of lung
cancer with special emphasis on a long-term survivor after
operation. J Cancer Res Clin Oncol. 135:297–301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koch B, Tannapfel A, Vieth M and Grün R:
Gastric metastasis from small cell lung cancer. Pneumologie.
63:585–587. 2009.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu EW and Xui LT: Gastric metastasis from
small cell lung cancer: A case report. Chin J Clin. 7:7260–7262.
2013.
|
|
25
|
Zhang QJ, Zhao HB and Han CH: Gastric
metastasis by lung small cell carcinoma. J Int Oncol. 42:877–878.
2015.
|
|
26
|
Gao S, Hu XD, Wang SZ, Liu N, Zhao W, Yu
QX, Hou WH and Yuan SH: Gastric metastasis from small cell lung
cancer: A case report. World J Gastroenterol. 21:1684–1688. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu YP, Lv WB and Wang Bo: Gastric
metastasis by lung small cell carcinoma. J Mod Med Health.
33:3734–3735. 2017.(In Chinese).
|
|
28
|
Green LK: Hematogenous metastases to the
stomach: A review of 67 cases. Cancer. 65:168–171. 1990. View Article : Google Scholar
|
|
29
|
Ryo H, Sakai H, Ikeda T, Hibino S, Goto I,
Yoneda S and Noguchi Y: Gastrointestinal metastasis from lung
cancer. Nihon Kyobu Shikkan Gakkai Zasshi. 34:968–972. 1996.(In
Japanese). PubMed/NCBI
|
|
30
|
Kim SY, Ha HK, Park SW, Kang J, Kim KW,
Lee SS, Park SH and Kim AY: Gastrointestinal metastasis from
primary lung cancer: CT findings and clinicopathologic features.
AJR Am J Roentgenol. 193:W197–W201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee PC, Lo C, Lin MT, Liang JT and Lin BR:
Role of surgical intervention in managing gastrointestinal
metastases from lung cancer. World J Gastroenterol. 17:4314–4320.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu YP, Jin B and Wang QS: Metastatic
carcinoma to the stomach from different primary sites: An analysis
of 12 cases. Word Chin J Digestol. 20:2092–2096. 2012.(In Chinese).
View Article : Google Scholar
|
|
33
|
Rossi G, Marchioni A, Romagnani E,
Bertolini F, Longo L, Cavazza A and Barbieri F: Primary lung cancer
presenting with gastrointestinal tract involvement:
Clinicopathologic and immunohistochemical features in a series of
18 consecutive cases. J Thorac Oncol. 2:115–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hasegawa N, Yamasawa F, Kanazawa M,
Kawashiro T, Kikuchi K, Kobayashi K, Ishihara T, Kuramochi S and
Mukai M: Gastric metastasis of primary lung cancer. Nihon Kyobu
Shikkan Gakkai Zasshi. 31:1390–1396. 1993.(In Japanese). PubMed/NCBI
|
|
35
|
Rivera MP, Mehta AC and Wahidi MM:
Establishing the diagnosis of lung cancer: Diagnosis and management
of lung cancer, 3rd ed: American College of Chest Physicians
evidence-based clinical practice guidelines. Chest. 143 (Suppl
5):e142S–e165S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu YY, Chen JH, Liang JA, Chu S, Lin WY
and Kao CH: F-18-FDG PET or PET/CT for detecting extensive disease
in small-cell lung cancer: A systematic review and meta-analysis.
Nucl Med Commun. 35:697–703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guinee DG Jr, Fishback NF, Koss MN,
Abbondanzo SL and Travis WD: The spectrum of immunohistochemical
staining of small-cell lung carcinoma in specimens from
transbronchial and open-lung biopsies. Am J Clin Pathol.
102:406–414. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kyritsis I, Krebs B, Kampe S, Theegarten
D, Aigner C and Welter S: Erroneous diagnosis of small cell lung
cancer based on small biopsies with far-reaching consequences: Case
report of a typical carcinoid tumor. J Thorac Dis. 9:E99–E102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oliveira AM, Tazelaar HD, Myers JL,
Erickson LA and Lloyd RV: Thyroid transcription factor-1
distinguishes metastatic pulmonary from well-differentiated
neuroendocrine tumors of other sites. Am J Surg Pathol. 25:815–819.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stenhouse G, Fyfe N, King G, Chapman A and
Kerr KM: Thyroid transcription factor 1 in pulmonary
adenocarcinoma. J Clin Pathol. 57:383–387. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Saqi A, Alexis D, Remotti F and Bhagat G:
Usefulness of CDX2 and TTF-1 in differentiating gastrointestinal
from pulmonary carcinoids. Am J Clin Pathol. 123:394–404. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miskovic J, Brekalo Z, Vukojevic K,
Miskovic HR, Kraljevic D, Todorovic J and Soljic V: Co-expression
of TTF-1 and neuroendocrine markers in the human fetal lung and
pulmonary neuroendocrine tumors. Acta Histochem. 117:451–459. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu MH, Lin MT and Lee PH:
Clinicopathological study of gastric metastases. World J Surg.
31:132–136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Van Meerbeeck JP, Fennell DA and De
Ruysscher DK: Small-cell lung cancer. Lancet. 378:1741–1755. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Alibazoglu H, Alibazoglu B, Ali A and La
Monica G: False-negative FDG PET imaging in a patient with
metastasis melanoma and ideal intussusception. Clin Nucl Med.
24:1291999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Capasso L, Iarrobino G, D'Ambrosio R,
Carfora E, Ventriglia R and Borsi E: Surgical complications for
gastric and small bowel metastasis due to primary lung carcinoma.
Minerva Chir. 59:397–403. 2004.PubMed/NCBI
|
|
47
|
Evans WK, Shepherd FA, Feld R, Osoba D,
Dang P and Deboer G: VP-16 and cisplatin as frst-line therapy for
small-cell lung cancer. J Clin Oncol. 3:1471–1477. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rossi A, Di Maio M, Chiodini P, Rudd RM,
Okamoto H, Skarlos DV, Früh M, Qian W, Tamura T, Samantas E, et al:
Carboplatin- or cisplatin-based chemotherapy in first-line
treatment of small-cell lung cancer: The COCIS meta-analysis of
individual patient data. J Clin Oncol. 30:1692–1698. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Owonikoko TK, Behera M, Chen Z, Bhimani C,
Curran WJ, Khuri FR and Ramalingam SS: A systematic analysis of
efficacy of second-line chemotherapy in sensitive and refractory
small-cell lung cancer. J Thorac Oncol. 7:866–872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Medell A and Lochman D: An unusual
metastatic manifestation of a primary bronchogenic carcinoma.
Cancer. 30:806–809. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Woods JM IV and Koretz MJ: Emergency
abdominal surgery for complications of metastatic lung carcinoma.
Arch Surg. 125:583–585. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Slotman BJ and Senan S: Radiotherapy in
small-cell lung cancer: Lessons learned and future directions. Int
J Radiat Oncol Biol Phys. 79:998–1003. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Brady LW, O'Neill EA and Farber SH:
Unusual sites of metastases. Semin Oncol. 4:59–64. 1977.PubMed/NCBI
|
|
54
|
Campoli PM, Ejima FH, Cardoso DM, Silva
OQ, Santana Filho JB, Queiroz Barreto PA, Machado MM, Mota ED,
Araujo Filho JA, Alencar Rde C and Mota OM: Metastatic cancer to
the stomach. Gastric Cancer. 9:19–25. 2006. View Article : Google Scholar : PubMed/NCBI
|