Introduction
Colorectal cancer (CRC) is one of the most common
malignant tumors and the fourth leading cause of cancer-associated
mortality worldwide (1). In recent
years, despite improvements in precancerous screening (2–4),
surgical resection (1), chemotherapy
(5), radiotherapy (6) and target therapy (5), patients with CRC still exhibit poor
prognosis, particularly patients at advanced stages of the disease
(7). Therefore, it is crucial to
identify novel therapeutic targets to improve the prognosis of CRC
patients.
Ras-related protein Rab23, a member of the
Ras-related small GTPase family, was first isolated from the brain
in 1994 (8). Studies from the past
three decades have demonstrated that Rab23 serves an important role
in endosomal membrane trafficking (9,10). A
further molecular mechanism study revealed that it may negatively
regulate Sonic hedgehog (Shh) signaling pathway in an indirect
manner (11). In recent years, the
role of Rab23 in tumors has attracted increasing attention. In
2016, Jian et al (12)
reported that Rab23 promoted cutaneous squamous cell carcinoma
migration and invasion via the integrin β1/Ras-related protein Rac1
pathway. In 2018, Zhang et al (13) demonstrated that Rab23 promoted the
cisplatin resistance of ovarian cancer via the Shh-Gli-ATP-binding
cassette sub-family G member 2 signaling pathway. However, the role
of Rab23 in CRC currently remains unknown.
In the present study, it was initially confirmed
that there is a high expression of Rab23 in CRC. Subsequently, it
was observed that high expression of Rab23 was positively
associated with tumor progression and poor prognosis. The role of
Rab23 in the proliferation of CRC cells and the potential molecular
mechanism were then identified.
Materials and methods
Cell culture
Human colon epithelial FHC cells, and the human CRC
cell lines SW1116 and HT29 were all purchased from the American
Type Culture Collection (Manassas, VA, USA). SW1116 and HT29 cell
lines were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS; both from
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
(11,12). The FHC cell line was cultured in
mixed medium including DMEM and Ham's F12 nutrient mixture,
supplemented with 10% FBS. All cells were cultured at 37°C in 5%
CO2.
Tissue specimen collection
Paraffin-embedded tumor tissues and
clinicopathological features were obtained from 90 CRC patients who
had undergone surgery at Shanxian Central Hospital (Heze, China)
between January 2008 and January 2010. Clinical stage was
determined according to the American Joint Committee on Cancer
system (14). The survival data were
collected, and the survival time ranged between 19 and 90 months,
with the median survival time of 90 months. The study was approved
by the Review Board of Shanxian Central Hospital. The specimens
from patients who were diagnosed with CRC by pathologists were
included in the present study; patients who had received
preoperative chemotherapy or irradiation were excluded.
Immunohistochemical (IHC)
analysis
Tissue samples were sectioned into 4-µm slices,
deparaffinized in xylene, rehydrated in graded ethanol and boiled
in 10 mmol/l citrate buffer (pH 6.0) for 3 min at 100°C for antigen
retrieval. The expression of Rab23 and Ki-67 in CRC tissue sections
were determined using IHC staining with the following primary
antibodies: Rab23 (1:400; cat. no. ab230200; Abcam, Cambridge, MA,
USA) and Ki-67 (1:600; cat. no. ab833; Abcam). Subsequent to
staining overnight at 4°C using primary antibodies, the sections
were stained for 30 min at room temperature with secondary antibody
(cat. no. 9902; Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China).
Assessment of IHC staining was then performed by two independent
pathologists simultaneously. The estimated fraction of positively
stained tumor cells was represented by the proportion score, as
follows: 0, ≤25% staining; 1, 26–50% staining; 2, 51–75% staining;
and 3, >75% staining. The estimated average staining intensity
of positive tumor cells was represented by the intensity score,
which was assigned as follows: 0, negative; 1, weak; 2, moderate;
and 3, strong. The expression level of Ki-67 was evaluated using
the proportion score directly, while the expression level of Rab23
was evaluated using the sum of the proportion and intensity scores,
with a score of <4 indicating low expression and ≥4 indicating
high expression.
Reverse transcription-polymerase chain
reaction (RT-PCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from the samples,
according to the manufacturer's protocol. The RNA concentration was
measured using a 2000/2000C Ultraviolet spectrophotometer (Thermo
Fisher Scientific, Inc.) cDNA was synthesized using the PrimeScript
RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian, China)
according to the manufacturer's protocol. The PCR conditions were
as follows: 25°C for 10 min, 42°C for 60 min and then 70°C for 5
min. The primers used in this study were as follows: Rab23,
5′-AGGCACTGGCAAAAAGGTYA-3′ (forward) and 5′-CGGAGTGACTTCCACCAGAT-3′
(reverse); GAPDH, 5′-AGAAGGCTGGGGCTCATTTG-3′ (forward) and
5′-AGGGGCCATCCACAGTCTTC-3′ (reverse). GAPDH was used as an internal
control.
Western blot analysis
Protein was extracted from the cells using
radioimmunoprecipitation assay lysis buffer containing 1%
phenylmethane sulfonyl fluoride (Beyotime Institute of
Biotechnology, Haimen, China), and protein concentration was
measured using the BCA kit (Pierce; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Next, 200 µg protein was
loaded in each well of 5% acrylamide gel, separated by 10% SDS-PAGE
and transferred to a nitrocellulose membrane. Subsequent to
blocking in 5% non-fat milk (cat. no. 232100; Becton, Dickinson and
Company, New Jersey, USA) at room temperature for 1 h, the membrane
was probed at 4°C overnight using the following primary antibodies:
ERK (cat. no. AF1576; 1:1,000), phosphorylated (p)-ERK (cat. no.
AF1018; 1:1,000), AKT (cat. no. AF2055; 1:1,000) and p-AKT (cat.
no. AF887; 1:1,000), purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA); Rab23 (cat. no. ab230200; 1:300), Ki-67 (cat.
no. ab833; 1:300) and GAPDH (cat. no. ab9485; 1:4,000), all
purchased from Abcam (Cambridge, MA, USA). This was followed by
incubation with peroxidase-linked goat anti-rabbit-IgG antibody
(cat. no. ab150077; 1:4,000; Abcam) at room temperature for 1.5 h.
Signals on the membrane were detected using ECL reagents (Pierce;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The results were
quantified using Image-Pro software (version 5.1; Media
Cybernetics, Inc., Rockville, MA, USA) (15).
Plasmid transfection
pcDNA3.1/Rab23 or control empty vector plasmid
(Shanghai GenePharma Co., Ltd., Shanghai, China) were transfected
into SW1116 and HT29 cells to increase the expression of Rab23. The
transfection was conducted using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. The transfection
efficiency was verified using RT-PCR and western blot analyses.
MTT assay
CRC cells were plated in a 96-well plate at a
density of 3×103 cells/well. After 24, 48, 72, 96 and
120 h of incubation, the cell viability was measured by MTT assay
at a wavelength of 490 nm, according to the manufacturer's protocol
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China). Growth curves were then constructed.
AKT and ERK signaling pathway
inhibition
ERK inhibitor U0126 (cat. no. S1102; Selleck
Chemicals, Houston, Texas, USA) or AKT inhibitor LY294002 (cat. no.
S1105; Selleck Chemicals) was added to the medium of CRC cells
overexpressing Rab23 at a final concentration of 10 µM/l or 50 µM/l
respectively. Cell were collected at 72 h, and western blotting and
MTT assay were performed as described above.
Statistical analysis
SPSS software version 13.0 (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. All data are expressed as
the mean ± standard deviation. Differences between three groups
were analyzed using one-way analysis of variance and Dunnett's
post-hoc test, while differences between two groups were analyzed
using t-test. The correlation of Rab23 with clinical parameters was
analyzed using χ2 analysis. Survival curves were
constructed using the Kaplan-Meier method and compared using the
log-rank test. P<0.05 was considered to denote differences that
were statistically significant.
Results
Rab23 is highly expressed in CRC
tissues and cells
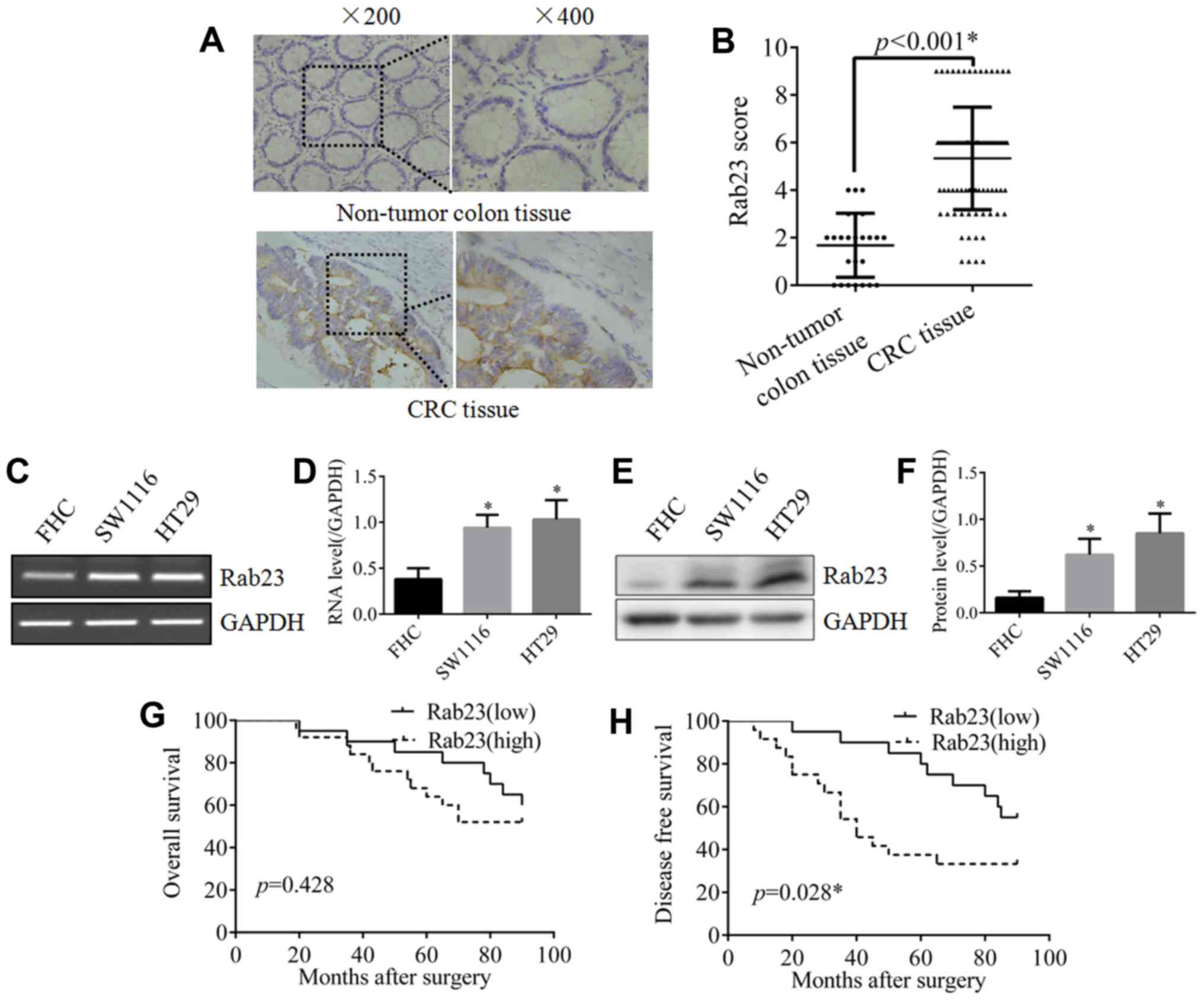
As shown in Fig. 1A,
strong staining of Rab23 was detected in CRC tissues, whereas
almost no staining of Rab23 was observed in the non-tumor colon
tissues. The difference between CRC and non-tumor tissues was
statistically significant (Fig. 1B).
To further verify the expression of Rab23 in CRC, RT-PCR and
western blot analyses were performed in the CRC cell lines SW1116
and HT29. The results revealed that Rab23 expression in the two CRC
cell lines was significantly higher compared with that in the human
colon epithelial cell line FHC, both at the mRNA (Fig. 1C and D) and protein (Fig. 1E and F) levels. These data
demonstrated that there was a high expression of Rab23 in CRC.
Rab23 is positively associated with
tumor size, advanced clinical stage and poor disease-free survival
(DFS)
As shown in Table I,
a tumor diameter of >4 cm was only observed in 16 out of 36
cases in the low Rab23 expression group, which was significantly
reduced in comparison with the number of cases with this diameter
in the high Rab23 expression group (38 out of 54 cases). In
addition, in the low Rab23 expression group, there were only 10 out
of 36 cases at stages III/IV of the disease, which was
significantly less than the cases at this stage in the high Rab23
expression group (29 out of 54 cases). However, there was no
significant difference in terms of age, sex, differentiation degree
and lymph node metastasis between the two groups.
 | Table I.Association of Rab23 expression with
the clinicopathological parameters of patients with colorectal
cancer. |
Table I.
Association of Rab23 expression with
the clinicopathological parameters of patients with colorectal
cancer.
|
|
| Rab23 expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinical
parameters | Cases (n=90) | Low (n=36) | High (n=54) | χ2 | P-value |
|---|
| Age (years) |
|
|
|
|
|
| ≤65 | 40 | 17 | 23 | 0.188 | 0.665 |
|
>65 | 50 | 19 | 31 |
|
|
| Sex |
|
|
|
|
|
| Male | 54 | 25 | 29 | 2.23 | 0.135 |
|
Female | 36 | 11 | 25 |
|
|
| Tumor size |
|
|
|
|
|
| ≤4
cm | 36 | 20 | 16 | 6.049 | 0.014a |
| >4
cm | 54 | 16 | 38 |
|
|
| Differentiation |
|
|
|
|
|
| Low | 36 | 14 | 22 | 0.031 | 0.861 |
|
High/moderate | 54 | 22 | 32 |
|
|
| Clinical stage |
|
|
|
|
|
|
I/II | 51 | 26 | 25 | 5.913 | 0.015a |
|
III/IV | 39 | 10 | 29 |
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
Negative | 46 | 20 | 26 | 0.474 | 0.491 |
|
Positive | 44 | 16 | 28 |
|
|
Subsequently, in order to investigate the effect of
Rab23 on prognosis, survival analysis was performed. Although the
difference in overall survival (OS) between the two groups was not
statistically significant, the OS of the high Rab23 expression
group was poorer compared with that in the low Rab23 expression
group (Fig. 1G). In addition, the
DFS of the high Rab23 expression group was significantly poorer
compared with that of the low Rab23 expression group (Fig. 1H). These results imply that Rab23
serves a role in CRC progression and prognosis.
Rab23 increases the expression of
Ki-67 and the proliferative ability of CRC cells
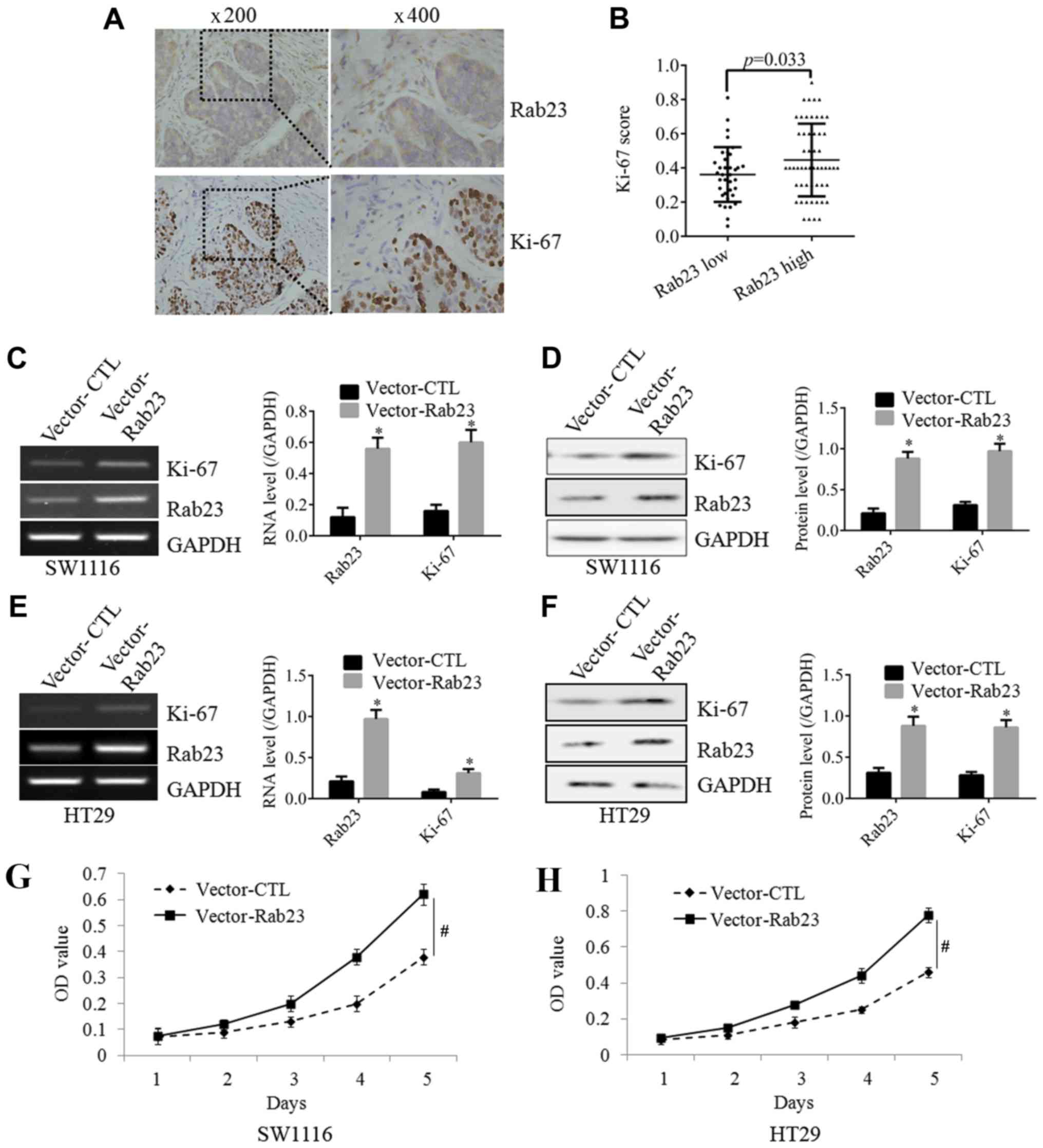
As shown in Fig. 2A,
IHC staining demonstrated that tissues with high expression of
Rab23 also exhibited a strong expression of Ki-67, which is known
as a classical proliferation marker. The positive correlation of
Rab23 and Ki-67 was found to be statistically significant (Fig. 2B). To further confirm the effect of
Rab23 on the expression of Ki-67, the expression of Rab23 in CRC
cells was upregulated by plasmid transfection. Following the
induction of Rab23 overexpression in SW1116 cells, the expression
of Ki-67 was significantly increased at the mRNA (Fig. 2C) and protein (Fig. 2D) levels. In the CRC cell line HT29,
overexpression of Rab23 also significantly increased the expression
of Ki-67, both at the mRNA (Fig. 2E)
and protein (Fig. 2F) levels. These
findings suggested that Rab23 may have an effect on CRC
proliferation. To further test this hypothesis, an MTT assay was
performed, and the result revealed that overexpression of Rab23
significantly increased the proliferative ability of SW1116
(Fig. 2G) and HT29 (Fig. 2H) cells. These results demonstrated
that Rab23 can significantly increase the proliferation of CRC
cells.
ERK and AKT signaling pathways are
required for Rab23 to regulate the proliferation of CRC cells
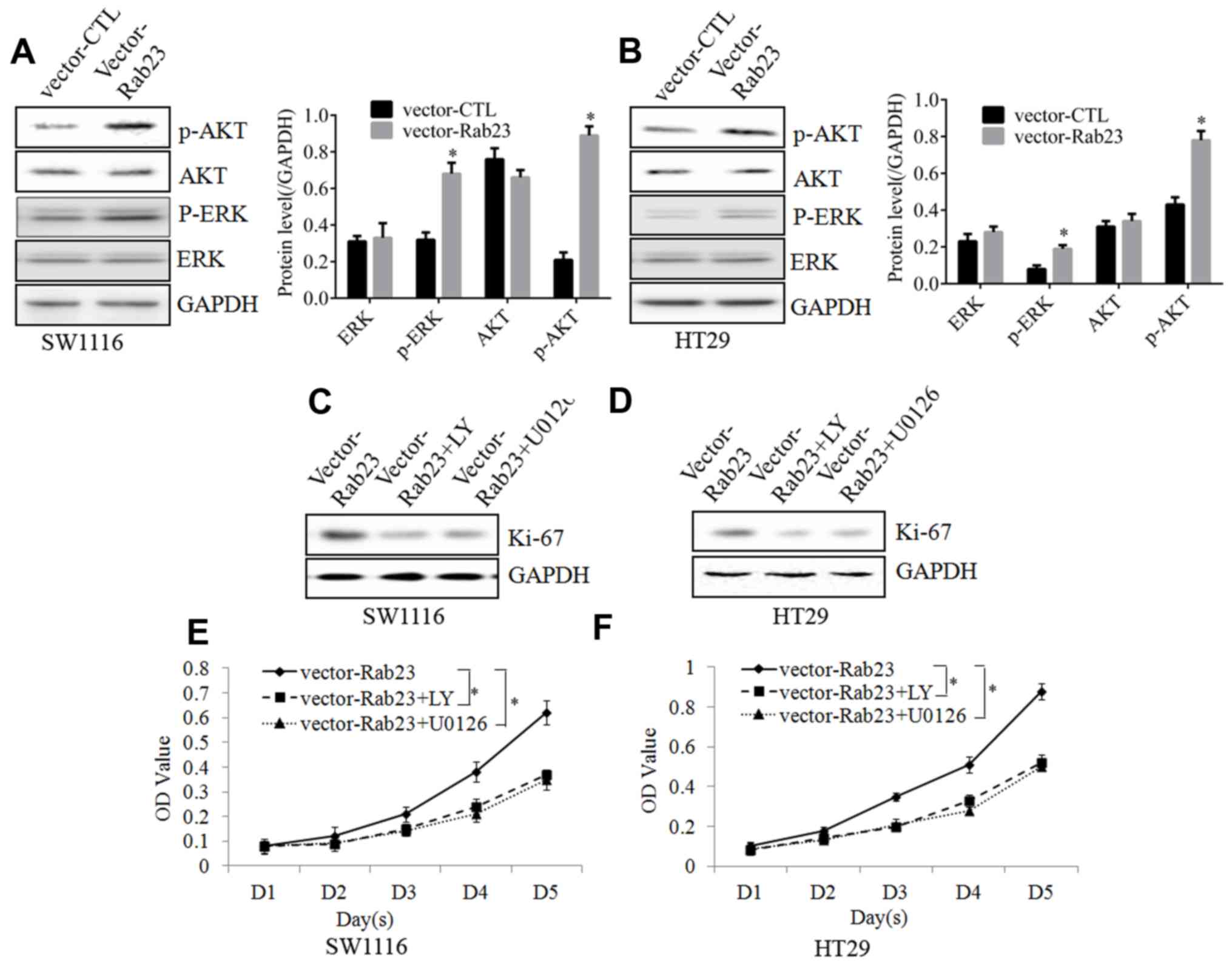
To investigate the potential molecular mechanism,
possible molecular targets were detected. Subsequent to Rab23
overexpression in SW1116 (Fig. 3A)
and HT29 (Fig. 3B) cells, the
phosphorylation of ERK and AKT was activated, whereas no
significant changes were detected in the total ERK and AKT levels.
This implied that the ERK and AKT signaling pathways could be
activated by Rab23 in CRC cells.
When SW1116 (Fig. 3C)
and HT29 (Fig. 3D) cells were
incubated with the ERK inhibitor U0126 or the AKT inhibitor
LY294002, the increase in Ki-67 expression induced by Rab23
overexpression was reduced. Furthermore, the MTT assay revealed
that incubation with U0126 or LY294002 inhibited the proliferative
abilities induced by Rab23 overexpression in the SW1116 (Fig. 3E) and HT29 (Fig. 3F) cells. Taken together, these
results proved that Rab23 was able to increase the proliferation of
CRC cells via the ERK and AKT signaling pathways.
Discussion
Rab23 is a member of the Ras-related small GTPase
family, which serves a key role in the regulation of the Shh
signaling pathway. High expression of Rab23 has been reported in
several types of tumors. For instance, Wang et al (16) reported that Rab23 was overexpressed
in human astrocytoma, and promoted cell migration and invasion
through the regulation of Rac1. Liu et al (17) also reported that Rab23 was
overexpressed and activated in hepatocellular carcinoma. In the
present study, high expression of Rab23 was detected in CRC tissues
using IHC analysis, and this was further confirmed in CRC cells,
both at the mRNA and protein levels. These results are consistent
with the previously reported data by Wang et al (16) and Liu et al (17). Thus, it is suggested that Rab23 may
serve as a diagnostic marker in CRC and may function as an
oncogenic protein in CRC.
Thus far, the role of Rab23 has been reported in
several types of tumors, but this remains under debate. Jiang et
al (18) demonstrated that Rab23
promoted the proliferation and invasion of bladder cancer cells. In
addition, Chang et al (19)
found that Rab23 was required for tumor growth in prostate cancer.
However, Liu et al (20)
reported that ectopic expression of Rab23 inhibited the growth and
proliferation, as well as induced cell apoptosis in breast cancer
cells. In the present study, it was observed that high expression
of Rab23 was positively associated with tumor size and advanced
clinical stage. This implied an oncogenic role of Rab23 in CRC.
Further cell experiments revealed that Rab23 promoted the
expression of Ki-67 and the proliferative ability of CRC cells. The
present study results are consistent with the findings of Jiang
et al (18) and Chang et
al (19), although they differ
from the results of the study conducted by Liu et al
(20), and provide further evidence
on the roles of Rab23 in the progression of CRC. Furthermore, the
survival analysis performed in the current study demonstrated that
Rab23 was positively associated with poor DFS. This is in agreement
with the study by Zhang et al (13), which reported that Rab23 was
positively associated with the poor prognosis of patients with
ovarian cancer, implying that Rab23 may be used for predicting the
prognosis of patients with CRC.
The ERK and phosphoinositide 3-kinase/AKT signaling
pathways serve an important role in a multitude of cellular
functions, including cell proliferation (21), survival (22), migration (23), apoptosis (24,25) and
angiogenesis (15). However, whether
ERK or AKT can function as downstream targets of Rab23 remains
unknown. In the present study, it was observed that overexpression
of Rab23 activated the ERK and AKT signaling pathways. In addition,
blocking the ERK or AKT signaling pathways eliminated the effects
of Rab23 on the proliferation of CRC cells. This proved the
important role of the ERK and AKT signaling pathways in the effect
of Rab23 in CRC cell proliferation.
In conclusion, the present study confirmed the
important role of Rab23 in CRC progression and poor prognosis,
suggesting the potential use of Rab23 in diagnosis and prognosis
prediction in this disease. The current study data provided a
preliminary experimental basis for further research on Rab23 in
CRC, and also suggested that Rab23 may serve as a novel treatment
target for CRC.
Acknowledgements
The authors would like to thank all the members of
the Pathology Department for their help in collecting tissue
specimens, IHC staining and scoring.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HM designed this study and wrote the manuscript. TZ
and DH collected the tissue specimens, performed IHC staining and
analyzed the data.
Ethics approval and consent to
participate
This retrospective study involving tissues from
human patients was approved by the Ethics Committee of Shanxian
Central Hospital.
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schreckenbach T, Zeller MV, El Youzouri H,
Bechstein WO and Woeste G: Identification of factors predictive of
postoperative morbidity and short-term mortality in older patients
after colorectal carcinoma resection: A single-center retrospective
study. J Geriatr Oncol. 9:649–658. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Issa AM, Hutchinson JF, Tufail W, Fletcher
E, Ajike R and Tenorio J: Provision of personalized genomic
diagnostic technologies for breast and colorectal cancer: An
analysis of patient needs, expectations and priorities. Per Med.
8:401–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhen Y, Luo C and Zhang H: Early detection
of ulcerative colitis-associated colorectal cancer. Gastroenterol
Rep (Oxf). 6:83–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mansfield C, Ekwueme DU, Tangka FKL, Brown
DS, Smith JL, Guy GP Jr, Li C and Hauber B: Colorectal cancer
screening: Preferences, past behavior, and future intentions.
Patient. 11:599–611. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Satake H, Sunakawa Y, Miyamoto Y, Nakamura
M, Nakayama H, Shiozawa M, Makiyama A, Kobayashi K, Kubota Y, Mori
M, et al: A phase II trial of 1st-line modified-FOLFOXIRI plus
bevacizumab treatment for metastatic colorectal cancer harboring
RAS mutation: JACCRO CC-11. Oncotarget. 9:18811–18820. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ihnát P, Vávra P, Slívová I, Tulinský L
and Penka I: Radiotherapy in the treatment of rectal cancer is it
time to move on? Rozhl Chir. 97:156–160. 2018.(In Czech).
PubMed/NCBI
|
|
7
|
Hathout L, Maloney-Patel N, Malhotra U,
Wang SJ, Chokhavatia S, Dalal I, Poplin E and Jabbour SK:
Management of locally advanced rectal cancer in the elderly: A
critical review and algorithm. J Gastrointest Oncol. 9:363–376.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olkkonen VM, Peterson JR, Dupree P, Lütcke
A, Zerial M and Simons K: Isolation of a mouse cDNA encoding Rab23,
a small novel GTPase expressed predominantly in the brain. Gene.
138:207–211. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Evans TM, Ferguson C, Wainwright BJ,
Parton RG and Wicking C: Rab23, a negative regulator of hedgehog
signaling, localizes to the plasma membrane and the endocytic
pathway. Traffic. 4:869–884. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo A, Wang T, Ng EL, Aulia S, Chong KH,
Teng FY, Wang Y and Tang BL: Open brain gene product Rab23:
Expression pattern in the adult mouse brain and functional
characterization. J Neurosci Res. 83:1118–127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eggenschwiler JT, Espinoza E and Anderson
KV: Rab23 is an essential negative regulator of the mouse Sonic
hedgehog signalling pathway. Nature. 412:194–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jian Q, Miao Y, Tang L, Huang M, Yang Y,
Ba W, Liu Y, Chi S and Li C: Rab23 promotes squamous cell carcinoma
cell migration and invasion via integrin β1/Rac1 pathway.
Oncotarget. 7:5342–5352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Yu F, Wang Y, Zhang Y, Meng L and
Chi Y: Rab23 promotes the cisplatin resistance of ovarian cancer
via the Shh-Gli-ABCG2 signaling pathway. Oncol Lett. 15:5155–5160.
2018.PubMed/NCBI
|
|
14
|
Hari DM, Leung AM, Lee JH, Sim MS, Vuong
B, Chiu CG and Bilchik AJ: AJCC cancer staging manual 7th edition
criteria for colon cancer: Do the complex modifications improve
prognostic assessment? J Am Coll Surg. 217:181–90. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng C, Wen M and Liu X: Fibroblast
activation protein in osteosarcoma cells promotes angiogenesis via
AKT and ERK signaling pathways. Oncol Lett. 15:6029–6035.
2018.PubMed/NCBI
|
|
16
|
Wang M, Dong Q and Wang Y: Rab23 is
overexpressed in human astrocytoma and promotes cell migration and
invasion through regulation of Rac1. Tumour Biol. 37:11049–11055.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu YJ, Wang Q, Li W, Huang XH, Zhen MC,
Huang SH, Chen LZ, Xue L and Zhang HW: Rab23 is a potential
biological target for treating hepatocellular carcinoma. World J
Gastroenterol. 13:1010–1017. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang Y, Han Y, Sun C, Han C, Han N, Zhi W
and Qiao Q: Rab23 is overexpressed in human bladder cancer and
promotes cancer cell proliferation and invasion. Tumour Biol.
37:8131–8138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang J, Xu W, Liu G, Du X and Li X:
Downregulation of Rab23 in prostate cancer inhibits tumor growth in
vitro and in vivo. Oncol Res. 25:241–248. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Zeng C, Bao N, Zhao J, Hu Y, Li C
and Chi S: Effect of Rab23 on the proliferation and apoptosis in
breast cancer. Oncol Rep. 34:1835–1844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu J, Pan X and Hu Z: MiR-502 mediates
esophageal cancer cell TE1 proliferation by promoting AKT
phosphorylation. Biochem Biophys Res Commun. 501:119–123. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zohrap N, Saatci Ö, Ozes B, Coban I, Atay
HM, Battaloglu E, Şahin Ö and Bugra K: SIK2 attenuates
proliferation and survival of breast cancer cells with simultaneous
perturbation of MAPK and PI3K/Akt pathways. Oncotarget.
9:21876–21892. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yao WF, Liu JW and Huang DS: MiR-200a
inhibits cell proliferation and EMT by down-regulating the ASPH
expression levels and affecting ERK and PI3K/Akt pathways in human
hepatoma cells. Am J Transl Res. 10:1117–1130. 2018.PubMed/NCBI
|
|
24
|
Qi Z, Yin L, Xu Y and Wang F: Pegylated
liposomal-paclitaxel induces ovarian cancer cell apoptosis via
TNF-induced ERK/AKT signaling pathway. Mol Med Rep. 17:7497–504.
2018.PubMed/NCBI
|
|
25
|
Park CH, Han SE, Nam-Goong IS, Kim YI and
Kim ES: Combined effects of baicalein and docetaxel on apoptosis in
8505c anaplastic thyroid cancer cells via downregulation of the ERK
and Akt/mTOR pathways. Endocrinol Metab (Seoul). 33:121–132. 2018.
View Article : Google Scholar : PubMed/NCBI
|