Introduction
Ovarian cancer (OC) is the leading cause of
mortality among all gynecological cancer types in developed
countries and the fifth leading global cause of mortality from
cancer in women (1). Due to the lack
of clinical signs and the absence of effective screening tests,
70–80% of patients are diagnosed at the advanced stages, when
peritoneal carcinomatosis (PC) is established, and numerous cases
are complicated with ascites (1,2).
Malignant ascites frequently occur in patients OC when PC is
present, in which fluid containing cancer cells accumulate in the
abdominal cavity (3–7). With the increasing amount of ascites,
discomforts and decreased quality of life (QOL) associated with
symptomatic malignant ascites often exceed that of the cancer
itself, resulting in detrimental physiological, and psychological
states leading to poor prognosis (4–9).
The standard of care for the treatment of advanced
OC includes optimal cytoreductive surgery (CRS) with adjuvant
taxanes- and platinum-based chemotherapy (1,2,10,11).
However, patients with primary OC and massive ascites present
highly variable health conditions, and certain patients with OC and
massive ascites are not eligible for CRS due to poor health
conditions and unstable vital signs (4). The authors of the present study
previously reported that the efficacy of CRS decreased with
increasing amounts of ascites in patients with extensive PC from
gastric and colon cancer (4–7). In addition, a retrospective study
suggested that the presence of malignant ascites significantly
decreases the chances of achieving a complete CRS (3).
Hyperthermic intraperitoneal chemotherapy (HIPEC)
has been demonstrated to be a promising treatment for PC and
malignant ascites (3–9). Maximum CRS according to the Sugarbaker
criteria (12) combined with HIPEC
have achieved good clinical efficacy for improving QOL (13,14) and
prolonging the survival of carcinomatosis of non-ovarian origins,
including the stomach (15–18), colon (19,20), and
appendix (21,22). CRS may be used to remove bulky tumor
tissue (10,11,23–29), and
HIPEC may then be used to eradicate residual microscopic tumors in
the peritoneal cavity (30–38), thereby improving the QOL (13,14).
Unlike peritoneal diffuse metastases from advanced
digestive tract carcinomatosis, whereby CRS result in few changes,
PC in the majority of patients with OC is due to local infiltrative
metastases or limited regional peritoneal metastases (39–41).
Therefore, the complete CRS success rate for these patients should
be considerably higher compared with the rates reported for
patients with digestive tract tumors and malignant ascites
(3,6).
Despite favorable response rates to this combined
approach, numerous patients are not eligible due to poor health
conditions (3,4,42).
Consequently, novel approaches to HIPEC should be considered if
oncologists are to achieve better prognoses in their patients.
HIPEC by minimally invasive or non-invasive surgical approaches,
including B-mode ultrasound-guided placement of perfusion
catheters, have recently been proposed for patients who are not
eligible for CRS (4–6).
Although CRS with HIPEC has been demonstrated to
exhibit more benefits in patients with OC carcinomatosis compared
with non-ovarian carcinomatosis (4–7), it has
not yet been determined if massive ascites, particularly in
patients with poor health conditions, affect the complete CRS rate
and prognosis of patients with primary OC under HIPEC. Therefore,
the aim of the present study study was to evaluate the possibility
of complete CRS and prognosis for primary OC complicated with
massive ascites using the prospective database of patients with
primary OC treated with HIPEC at the Intracelom Hyperthermic
Perfusion Therapy Center of the Cancer Hospital of Guangzhou
Medical University (Guangzhou, China).
Patients and methods
Study design
The present study was a retrospective study
performed using a prospective cohort registered at the Intracelom
Hyperthermic Perfusion Therapy Center of the Cancer Hospital of
Guangzhou Medical University. Patients were treated between
December 2006 and December 2014. Patients with primary OC were
included. The presence of massive ascites was determined by
ultrasound or other imaging modalities. According to their general
health condition prior to treatments, patients with massive ascites
were divided into the good or the poor condition groups. The
patients in a good condition were treated with CRS followed by
HIPEC (CRS+HIPEC). The patients in a poor condition were treated
using B-mode ultrasound-guided HIPEC first followed by delayed CRS
upon general condition improvement (dCRS+HIPEC). Patients were
considered to be in poor condition if the following applied: i)
Heart rate, >100 bpm at rest; ii) respiration rate, >20
breaths/min at rest; and iii) blood oxygen saturation, <95% at
rest. Otherwise, the patients were included in the good condition
group. All patients with primary OC were attempted to be treated
with CRS combined with HIPEC first. Patients with small ascites and
those without ascites were treated in the same way as patients in
the good condition group (immediate CRS followed by HIPEC during
CRS).
The inclusion criteria were: i) ≥18 years old; ii)
diagnosis of primary OC; iii) no radiation therapy in the previous
4 weeks; iv) no chemotherapy in the previous 2 weeks; and v) the
tumor board at the Cancer Hospital of Guangzhou Medical University
determined that the prognosis was >2 months. The exclusion
criteria were as follows: i) Recurrence of OC with ascites; ii)
known or possible ovarian metastases from other organs; iii) known
or possible primary malignant tumor in other internal organs; iv)
known or potential pregnancy; or v) extensive abdominal adhesions
due to multiple surgeries. The present study was approved by the
Medical Ethics Committee of the Cancer Hospital of Guangzhou
Medical College (approval no. GZYY2006-8-20). Written informed
consent was obtained from all patients.
CRS and placement of perfusion
catheters
For CRS, all patients were treated with the first
intention of achieving complete CRS. Patients with massive ascites
in the good condition group and patients without/small ascites were
treated with immediate CRS followed by HIPEC. Patients in the poor
condition group were treated with dCRS following the improvement of
their general health condition after HIPEC.
Surgery was performed under general anesthesia and
endotracheal intubation. For patients with massive ascites, after
opening the abdominal wall, 200 ml of ascites were sent for
cytological examination; then, all remaining abdominal fluid was
suctioned. CRS consisted of the removal of all gross tumors and
involved organs, peritoneum or tissue, as deemed technically
feasible, and safe for the patient. Any tumors adhering to or
invading vital structures that could not be removed were
cytoreduced using a cavitational ultrasonic surgical aspirator
(Valleylab™; Medtronic, Minneapolis, MN, USA).
The International Federation of Gynecology and
Obstetrics classification for OC staging was used (42). The anatomical extension of the OC in
the peritoneal cavity was best evaluated by the PC index (PCI), as
described by Sugarbaker (43). The
resection status was determined after CRS using the following
classification: R0, complete removal of all visible tumor and
negative cytological findings or microscopic margins; R1, complete
removal of all visible tumor and positive post perfusion cytologic
findings or microscopic margins; R2a, minimal residual tumor,
nodule(s) measuring ≤5 mm; R2b, gross residual tumor, nodule(s)
measuring >5 mm, but ≤2 cm; and R2c, extensive disease
remaining, nodule(s) measuring >2 cm. According to these, R0/R1
resections were considered as complete CRS, while R2a-R2c
resections were considered as incomplete CRS (43).
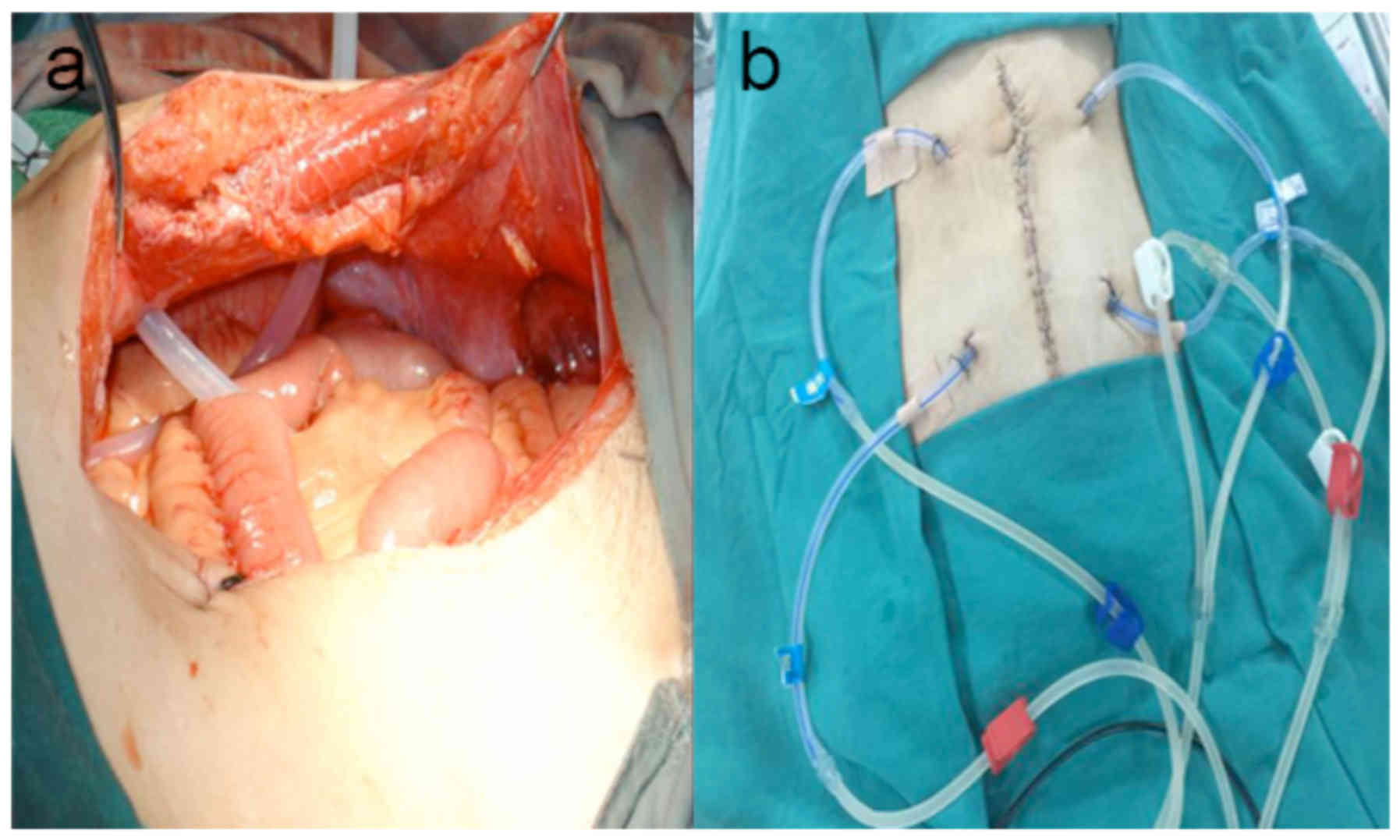
Following CRS, an infusion catheter with multiple
side holes (inner diameter of 0.8 cm, outer diameter of 1.0 and 100
cm in length) was placed into the peritoneal cavity in the upper
left and right quadrant with 40–60 cm of catheter inside the body
(Fig. 1). Similarly, an outflow
catheter with the same dimensions was placed in the lower left and
right quadrant of the pelvic cavity. The abdominal wall was sutured
and the perfusion catheter was fixed to the abdominal wall by
cutaneous sutures, as previously described (4).
B-mode ultrasound-guided placement of
catheters for chemotherapy (Fig
2). For patients in the poor condition group, those
who could not tolerate endotracheal general anesthesia and CRS,
B-mode ultrasound-guided HIPEC was performed first, and dCRS was
performed after improvement of their general condition.
In a standard operating room, patients were placed
in the supine position. Pethidine hydrochloride (75 mg; Qinghai
Pharmaceutical Factory Co., Xining, China) and promethazine
hydrochloride (25 mg; Shuntong Pharmaceuticals (Shuntong, China)
were administered by intramuscular injection. Propofol (Astra
Zeneca, London, UK) was given intravenously in a continuous manner
at a dosage rate of 3–8 ml/h; this was adjusted according to the
patient's status. B-ultrasound examination of all four abdominal
quadrants was performed to select the best puncture location. The
region with the largest amount of ascites that were not adhering to
the abdominal wall or to the tissues of the peritoneal cavity was
selected. Additionally, the region had to be without previous
abdominal incision or tumor. A 1.2-cm incision was made after
locally administering 0.5% lidocaine and a Hasson trocar (1.2 cm in
diameter) was placed into the peritoneal cavity. Then, the infusion
and outflow catheters with multiple side holes (inner diameter of
0.8 cm, outer diameter of 1.0 and 100 cm in length) were placed
into the intraperitoneal cavity. The infusion catheters were
positioned in the left and right upper quadrants of the
intraperitoneal cavity with an inside length of 40–80 cm. The
outflow catheters were placed in the pelvic cavity of the left and
right lower quadrants with the same length as the infusion
catheters. The ascites were extracted as completely as possible.
All perfusion catheters were fixed to the abdominal wall by
cutaneous sutures, as previously described (4).
HIPEC
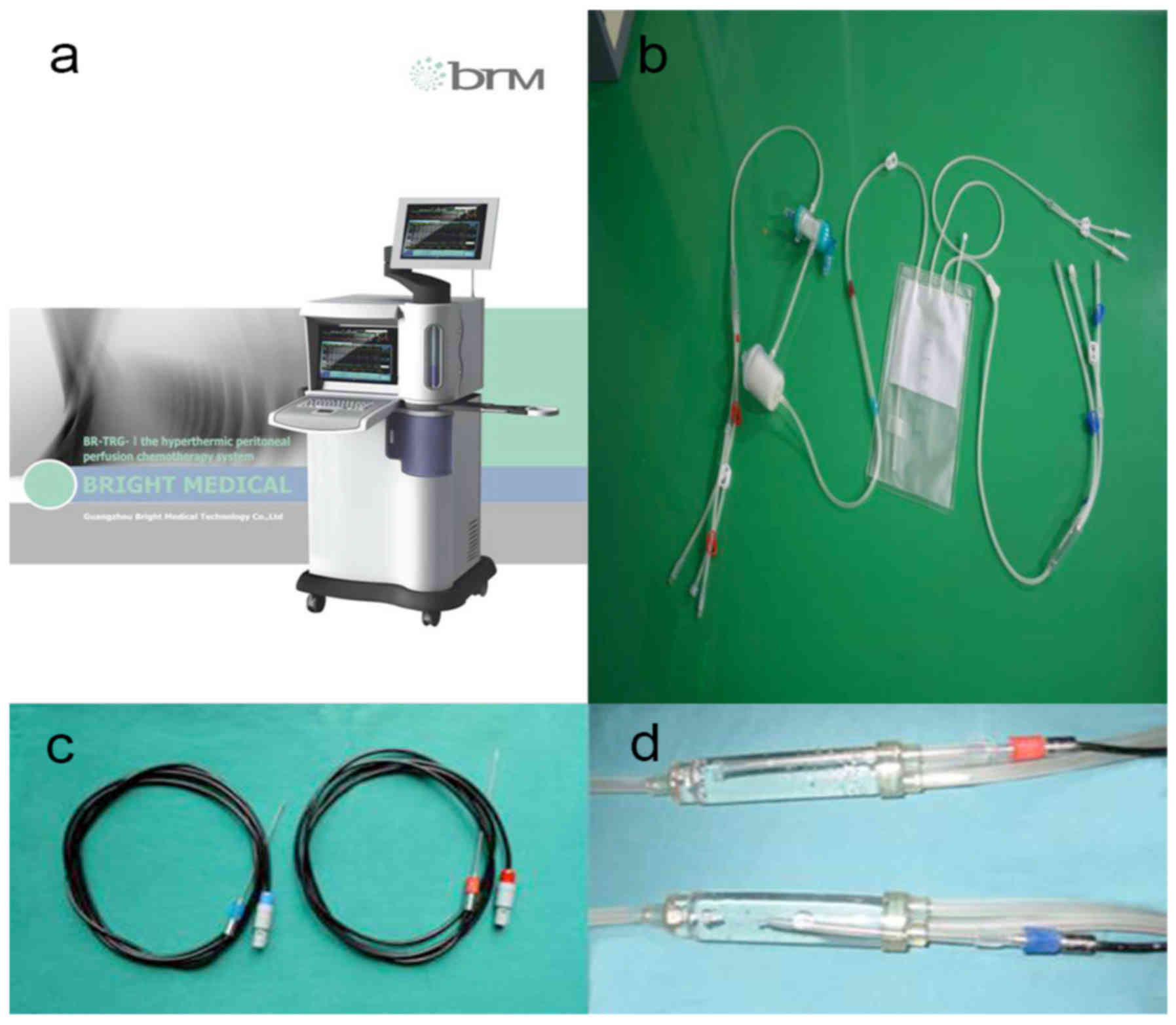
HIPEC was performed using our self-developed
‘BR-TRG-I type high-precision hyperthermic intraperitoneal
perfusion treatment system’ (BR-TRG-I; Guangzhou Baorui Medical
Devices Ltd. Company, Guangzhou, China; www.gzbrm.com), as previously described (4–7)
(Fig. 3). This device, the only one
of its kind, was approved for use by the State Food Drug
Administration Firearms of China (approval no. 2009-3260924).
The treatment temperature during HIPEC was measured
using the BR-TRG-I treatment system with temperature-monitoring
probes at several locations: In the infusion and outflow catheters;
and at the peritoneal surface of pelvic cavity. The vital signs of
patients (blood pressure, heart rate, respiratory rate and blood
oxygen saturation) were assessed using the ‘multi-parameter
patient-monitoring machine’ (G3HJ20025; Shenzen Mindray Bio Medical
Electronics Co., Ltd., Shenzhen, China).
HIPEC therapy consisted of three sessions. First,
0.9% saline solution or 5% glucose, with a volume 500 ml greater
than the cavity (3,000-7, 000 ml) was added to the custom infusion
bag and delivered via the infusion tubes over 90 min with a
velocity of 450–600 ml/min, and an in-flow temperature of 43°C to
achieve an interior abdominal temperature of 41.5–42.5°C. The
hyperthermic intraperitoneal chemotherapeutic agents added into the
perfusion fluid were docetaxel 75 mg/m2 (Jiangsu
Aosaikang Pharmaceutical Co., Ltd., Nanjing, China) for the first
session and cisplatin 80 mg/m2 (Jiangsu Haosen
Pharmaceutical Ltd., Nanjing, China, http://www.hspharm.cn/en/) for the second and third
sessions. After the third HIPEC session, all ascites was drained
out and the infusion catheter was removed. The outflow catheter was
kept for 3–5 days as a closed drainage catheter.
Follow-up
All patients were followed up till July 2015 or
mortality. Follow-up was performed at 1 and 3 months, and then
every 3–6 months thereafter for up to 1 year. After 1 year,
follow-up was performed at 6-month intervals or less frequently if
the patient continued to remain without evidence of disease.
Abdominal and pelvic computed tomography were obtained at 3, 6 and
12 months following treatment or when clinically indicated. All
patients received systemic chemotherapy using paclitaxel and
cisplatin at the discretion of their oncologist.
Remission from ascites and tumor progression was
classified into three grades according to our previously modified
World Health Organization criteria on efficacy assessment in
malignant tumors: i) Complete remission (CR): Ascites are
completely absorbed after treatment, which is sustained for at
least 4 weeks; ii) partial remission (PR): Ascites are reduced by
50%, which is sustained for at least 4 weeks; iii) no consequence
(NC): Ascites are not reduced obviously or increased within 4 weeks
after treatment (4–7). The Eastern Cooperative Oncology Group
performance status and Karnofsky performance score (KPS score) were
used to evaluate ascite remission, and patient QOL (5,6).
Objective remission rate (ORR) for assessment remission of ascites,
and the KPS score for measure of functional impairment was
performed on day 1 at the beginning of CSR and HIPEC treatment, and
at 4 weeks post-completion of all CSR and HIPEC treatment. Overall
survival (OS) was defined as the time between CRS+HIPEC or
dCRS+HIPEC completion and mortality. Disease-free survival (DFS)
was defined as the time between HIPEC or dCRS+HIPEC completion and
the recurrence of PC. Data from the 4-week follow up after
HIPEC+CRS were recorded and used in statistical analyses. All
patients were monitored until mortality or December 2015.
Statistical analysis
Data were analyzed using SPSS 17.0 (IBM Corp.,
Armonk, NY, USA). All continuous data are presented as mean ±
standard deviation and were analyzed using the Student's t test
(between groups) or the paired t test (before/after therapy).
Categorical data are presented as frequencies and were analyzed
using the chi-square test for categorical variables and the
independent two-way analysis of variance (ANOVA) for continuous
variables. Multiple group comparison was conducted by one-way ANOVA
followed by the Bonferroni correction. OS and DFS were analyzed,
and compared using the Kaplan-Meier estimator method. Differences
in survival between groups were determined by the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characteristics of patients
A total of 1,293 female patients with OC treated
with CRS+HIPEC were included in the current study, which included
1,225 patients (mean age, 54.3±2.6 years; range, 33–77 years)
without/small ascites, and 68 patients (mean age, 54.0±2.9 years;
range, 30–79 years) with massive ascites. Age was comparable
between patients without/small ascites and patients with massive
ascites (P=0.100). The frequency of massive ascites was 5.3% in
patients with OC in the present study.
CRS and HIPEC during CRS (CRS+HIPEC) were performed
in 1,225 female patients without/small ascites and in 46 patients
with massive ascites, but a good general condition. B-mode
ultrasound-guided HIPEC was performed in 22 patients with massive
ascites and a poor general condition, and delayed CRS was performed
once their general health condition improved (Table I).
 | Table I.Analyses of the general condition of
OC patients with malignant ascites treated with CRS + HIPEC. |
Table I.
Analyses of the general condition of
OC patients with malignant ascites treated with CRS + HIPEC.
|
|
| Patients with
ascites (n=68) |
|
|---|
|
|
|
|
|
|---|
| Category | Patients without
ascites (n=1,225) | CRS + HIPEC
(n=46) | dCRS + HIPEC
(n=22) | Total (n=68) | P-value |
|---|
| Ages, years
old | 54.29±2.63 | 54.47±2.28 | 53.87±3.41 | 54.16±2.87 | 0.100 |
| Pretreatment KPS
score, % | 73.98±4.8 | 52.8±3.1 | 36.2±3.7 | 48.9±2. 3 | 0.001 |
| Ascites, ml | – | 2650±332 | 3953±360 | 3532±349 | 0.010 |
| FCC, % | – | 56.52 (26/46) | 59.09 (13/22) | 57.35 (39/68) | 0.080 |
| PCI | 18±1.4 (6–29) | 21±1.3 (13–29) | 13±1.1 (5–23) | 17±1.6 (5–27) | 0.002 |
Characteristics of ascites
Among the 68 patients with massive ascites, the
amount of ascites ranged between 2,000 and 6,500 ml, with a mean
amount of 3,532±379 ml, as confirmed by exploratory laparotomy
drainage or by B-mode ultrasound-guided paracentesis drainage. Free
cancer cells were observed in 57.4% (39/68) of massive ascites
(Table I).
In the good condition group, the amount of ascites
ranged between 2,000 and 4,600 ml, with a mean amount of 2,650±232
ml, as confirmed by exploratory laparotomy drainage. Free cancer
cells were observed in 56.5% (26/46) of massive ascites. In the
poor condition group, the amount of ascites ranged between 3,200
and 6,500 ml, with a mean amount of 3,953±360 ml, as confirmed by
paracentesis drainage guided by B-mode ultrasound. Free cancer
cells were observed in 59.1% (13/22) of massive ascites. The amount
of ascites between the two groups was significantly different
(P=0.010), whereas, no significant differences in the rate of free
cancer cells positivity was observed between the two groups
(P=0.080; Table I).
Remission for malignant ascites
For 68 patients with massive ascites, all symptoms
exhibited ascites regression after HIPEC, for a total ORR of 100%,
even for patients in the poor condition group. There were no cases
of complete eradication of following HIPEC. There were no cases of
unfeasible CRS among patients in both groups. No significant
differences in ascites ORR rates were observed between the good and
poor condition groups (P=0.100; Table
I). Imaging and clinical examinations confirmed complete CRS in
58 patients. The 10 patients who underwent incomplete CRS exhibited
no recurrence of malignant ascites during follow-up.
Changes in KPS score
In the present study, there were significant
differences in KPS scores (P<0.01) between patients
without/small malignant ascites and patients with massive malignant
ascites prior to treatments. However, there were no significant
differences in KPS scores between before treatment and after
treatments (P=0.080).
Among the 1,125 patients with OC, but without/small
ascites, KPS scores increased from 73.9±4.8 before treatment to
74.1±5.3 after treatment. There was a significant difference in KPS
scores before treatments in patients without ascites and patients
with massive ascites (P=0.001; Table
II). Among the 68 patients with OC and massive ascites, KPS
scores significantly increased from 48.9±2.8 before treatment to
74.1±4.3 after treatment (P=0.001). In the good condition group,
KPS scores significantly increased from 52.8±3.1 before treatment
to 73.9±4.7 after treatment (P=0.001). In the poor condition group,
KPS scores significantly increased from 36.2±3.7 before treatment
to 74.6±3.9 after treatment (P=0.001). There were significant
differences in KPS scores between the good and the poor condition
groups at admission (P=0.001). However, no significant differences
were noted in KPS scores following treatments (P=0.080; Table II).
 | Table II.Analyses of the clinical
effectiveness and side effects in OC patients with malignant
ascites treated with HIPEC. |
Table II.
Analyses of the clinical
effectiveness and side effects in OC patients with malignant
ascites treated with HIPEC.
|
|
| Patients with
ascites (n=68) |
|
|---|
|
|
|
|
|
|---|
| Category | Patients without
ascites (n=1,225) | CRS + HIPEC
(n=46) | HIPEC + dCRS
(n=22) | Total (n=68) | P-value |
|---|
| SR, months |
|
|
|
|
|
| OS | 59±1.6 | 59±1.4 | 56±1.6 | 52±1.5 | 0.060 |
|
DFS | 25±1.1 | 25±1.2 | 27±1.3 | 15.6±1.2 | 0.070 |
| CRS, % |
|
|
|
|
|
|
C-CRS | 86.31
(971/1,125) | 84.78 (39/46) | 86.36 (19/22) | 85.29 (58/68) | 0.080 |
|
I-CRS | 13.68
(154/1,125) | 15.22 (4/46) | 13.64 (3/22) | 14.71 (10/68) | 0.090 |
| KPS score | 75.9±4.7 | 73.9±4.7 | 74.6±3.9 | 74.1±4.3 | 0.080 |
| Ascites CR, % | 100 | 100 | 100 | 100 | 0.100 |
| Side effects,
% |
|
|
|
|
|
|
BMS | 8.71
(98/1,125) | 8.70 (4/46) | 9.09 (2/22) | 8.82 (6/68) | 0.050 |
|
SRF | 0.26 (3/1,125) | 4.34 (2/46) | 4.54 (1/22) | 4.41 (3/68) | 0.010 |
CRS outcomes and PCI
Among the 1,225 patients with OC without/small
ascites, complete CRS was achieved in 1,063 patients with a
complete CRS success rate of 86.8% (1,063/1,225); incomplete CRS
was achieved in 162 (13.2%; 162/1,225) patients without/small
ascites (Table II). Among patients
with ascites, complete CRS was achieved in 58 (85.3%, 58/68)
patients with primary OC and massive ascites, while incomplete CRS
was achieved in 10 patients (14.71%). There were no significant
difference in the success of CRS between patients with ascites and
those without/small ascites (P=0.100).
In the good condition group, complete CRS was
achieved in 84.8% (39/46) of patients, and incomplete CRS was
achieved in 15.2% (7/46). In the poor condition group, complete CRS
was achieved in 86.4% (19/22) of patients and incomplete CRS
achieved in 13.6% (3/22). There were no significant differences in
the success of CRS between the two groups (P=0.080; Table II).
Among the 1,225 patients without/small ascites, the
mean PCI was 18.0±1.4 (range, 6–29). Among the 68 patients with
massive ascites, the mean PCI was 17.0±1.4 (range, 5–29). There
were no significant differences in the mean PCI between patients
without/small ascites and those with massive ascites (P=0.080;
Table I).
In the good condition group, the mean PCI was
21.0±1.3 (range, 13–29) before HIPEC. In the poor condition group,
the mean PCI was 13.0±1.1 (range, 5–23) as determined by CRS 2–4
weeks after HIPEC. There were significant differences in the mean
PCI between the two groups (P=0.002; Table I).
Adverse events due to HIPEC
Among the 1,225 patients with OC without/small
ascites, adverse events due to HIPEC were observed in 101 (8.9%,
101/1,225) patients, including 98 (8.7%, 98/1,225) patients with
grade IV (CTCAE) bone marrow suppression (BMS) and three (0.3%,
3/1,125) cases of severe renal failure (SRF) that required
hemodialysis. Among the 68 patients with massive ascites, adverse
events due to HIPEC were observed in nine (13.2%, 9/68) patients,
including six (8.8%, 6/68) patients with grade IV (CTCAE) BMS and
three (4.4%, 3/68) cases of SRF that required hemodialysis. There
were no significant differences in adverse events between patients
with ascites and those without/small ascites (P=0.050; Table II).
Grade IV BMS was observed in four (8.7%, 4/46)
patients in the good condition group, and in two (9.1%, 2/22)
patients in the poor condition group. SRF was observed in two
(4.4%, 2/46) patients in the good condition group, and in one
(4.5%, 2/22) patient in the poor condition group. There were no
significant differences in Grade IV BMS rates and SRF mobility
between the two groups (P=0.050). The grade IV BMS were resolved
after 1–3 weeks of granulocyte colony stimulating factor treatment
and SRF cases were resolved by hemodialysis for >1 month. No
other severe complications, including visceral injury, abdominal
incision infection, or adhesive bowel obstruction, were observed
following HIPEC (Table II).
Follow-up
The mean follow-up was 48 months (range, 7–98
months). The mean OS and DFS of patients without/small ascites who
had complete CRS were 58.0±1.6 months (range, 7–98 months) and
26.0±1.1 months (range, 8–52 months), respectively. The mean OS of
patients without/small ascites was 15.0±0.8 months (range, 4–48
months) for those with incomplete CRS. The median OS and DFS of
patients with massive ascites who had complete CRS was 56.0±1.5
months (range, 7–96 months), and 28.0±1.2 months (range, 8–49
months), respectively. The mean OS of patients with ascites was
15.0±1.0 months (range, 6–42 months) for those with incomplete CRS.
There were no significant differences in mean OS and DFS between
patients with ascites, and those without/small ascites (P=0.060),
but there were significant differences in mean OS between patients
with complete and incomplete CRS (P=0.001; data not shown).
In the good condition group, the mean OS and DFS of
patients with massive ascites were 59.0±1.4 months (range, 8–89
months) and 25.0±1.2 months (range, 6–48 months), respectively. The
mean OS of patients with massive ascites was 15.4 months (range,
7–49 months) for those with incomplete CRS. For patients with
massive ascites in the poor condition group, the OS and DFS were
56.0±1.6 months (range, 6–83 months) and 27.0±1.3 months (range,
8–46 months), respectively. The median OS of patients with massive
ascites was 15.0±1.1 months (range, 6–46 months) for those with
incomplete CRS. There were no significant differences in median OS
and median DFS between the two groups (all P>0.05), but there
was a significant difference in OS between patients with complete
and incomplete CRS patients (P=0.001; Table II).
Discussion
Ascites are frequently associated with PC
originating from advanced OC, and PC is a typical feature of cancer
spread in patients with primary advanced or recurrent OC, while
distant metastases are rare (1,2). CRS is
the current standard therapy for patients with OC and peritoneal
metastases. However, whether massive ascites affect the rate of
complete CRS and prognosis for patients with primary OC under HIPEC
treatment is not well defined. In the present study, optimal
outcomes were achieved for patients with primary OC using HIPEC
combined with CSR, as evidenced by the prolonged OS and DFS
observed in the subgroup of patients treated with this combined
therapy. These results suggest that massive ascites does not
significantly affect the rate of complete CRS and prognosis. HIPEC
is effective in increasing the rate of complete CRS. CRS is
suitable for the majority of patients with primary OC and massive
ascites, including those with poor conditions.
At the Intracelom Hyperthermic Perfusion Therapy
Center, the first intention of CRS in patients with OC is always to
achieve complete CRS. Eisenkop et al (38) suggested that a PCI of <15 is a
determinant factor in achieving optimal cytoreduction and that a
PCI of >15 would invariably preclude satisfactory surgical
results. However, in the present study, the mean PCI was 17, and
complete CRS was achieved in >85% of patients with OC and
malignant ascites, which is considerably higher compared with
results previously published (38).
Di Giorgio et al (44),
demonstrated that in patients with ovarian carcinomatosis, a PCI of
>15 does not invariably preclude satisfactory surgical results,
which is supported by the results in the present study. Randle
et al (3), suggested that the
presence of malignant ascites significantly decreased the chances
of achieving a complete CRS. In the present study, the success rate
of complete CRS was 86.8% in patients without/small ascites, and
85.3% in patients with primary OC and malignant ascites. These
results suggest that massive ascites does not affect the rate of
complete CRS. In fact, a retrospective analysis of the study by
Randle et al (3), revealed
that there were numerous patients with digestive tract tumors, and
that the rate of complete CRS in patients with OC and malignant
ascites remained 100% (2).
Therefore, the results presented by Randle et al (3), remain inconclusive for patients with
OC.
Large ascites may affect respiratory and circulatory
functions, making the tolerance of these patients to CRS and
general anesthesia poor (4). B-mode
ultrasound-guided HIPEC only requires local anesthesia, has limited
effects on respiration and circulation compared with more invasive
surgeries, and may offer palliative improvement by controlling
ascites (4–7). In the present study, complete CRS was
achieved in 86.4% of patients with poor health condition after
HIPEC and there was no significant difference in the success rate
of complete CRS compared with patients with a good health condition
(84.8%). This study suggests that with the improvement of the
general condition after B-mode ultrasound-guided HIPEC, treatment
with dCRS may allow outcomes that are similar to those of patients
eligible for HIPEC during CRS. These results revealed that HIPEC
may provide a possibility for complete CRS in patients with OC and
ascites who are initially ineligible for CRS.
Previous studies have suggested that killing
microscopic tumors in the peritoneal cavity may be the major factor
for HIPEC controlling malignant ascites from PC (34,35,37).
However, studies with laparoscopic HIPEC and B-mode
ultrasound-guided HIPEC for the palliative control of malignant
ascites, leaving the majority of tumor burden unaddressed following
HIPEC, do not support this conclusion (3–9). In the
present study, all patients with massive ascites exhibited ascites
regression. The total objective remission rate was 100% following
CRS and HIPEC in all groups, which included 22 patients in poor
general condition in whom there were no cases of PC being
completely eradicated after HIPEC confirmed by CRS, and 10 patients
with incomplete CRS. Malignant ascites rarely relapse until PC
progression and eventual mortality (4–7).
Remission of ascites was completely independent of resection status
of PC suggesting that it is more likely a function of HIPEC than
CRS, or that HIPEC affects the ascites by an unidentified mechanism
(3). Further studies are required to
examine this issue.
Although CRS combined with adjuvant chemotherapy
initially appears effective for advanced OC, insofar as high rates
of patients achieve a good response, ~50% of patients relapse
within 5 years and DFS times rarely exceed 18 months (44). The present study reported that a
median DFS of 28 months and a median OS of 53.4 months is possible
in patients with primary OC treated by HIPEC combined with CSR,
even for patients with a poor general condition who are initially
ineligible for CRS. In the present study, the median OS of patients
with massive ascites from primary OC who underwent complete CRS was
56 months, while incomplete CRS resection led to an OS of 15
months. These results suggest that CRS combined with HIPEC may
prolong the median DFS and OS of patients with primary OC and
massive ascites, but only in cases in which a successful complete
CRS is achieved. In addition, patients with OC and malignant
ascites with incomplete CRS exhibit a poor prognosis, as do
patients without/small malignant ascites treated by incomplete
CRS.
The procedures presented in the current study have
no unacceptable toxic effects, except for grade IV BMS and SRF
(4–7). In the present study, no significant
difference was identified between patients with ascites and
without/small ascites in grade IV BMS (8.7 vs. 8.8%), but the SRF
of patients with massive ascites was significant higher compared
with that in patients without/small ascites (0.3 vs. 4.4%). At
present, there is a lack of understanding of the adverse effects of
heat stress combined with chemotherapeutic drugs.
The present study is not without limitations. The
number of patients with massive ascites was relatively small,
limiting the conclusions and in-depth analyses. Certain patients
may have been lost to follow-up, introducing a bias. Finally, the
patients were selected according to a criterion, introducing
another bias. Multicenter randomized controlled trials are
necessary to assess adequately the effect of HIPEC in patients with
OC and ascites.
In conclusion, ascites does not appear to affect the
rate of complete CRS and prognosis of patients with massive ascites
following HIPEC. Patients with OC and massive ascites still have
relatively high rates of complete CRS with a good prognosis.
CRS+HIPEC may prolong the median DFS and OS of patients with
primary OC, and massive ascites.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Guangzhou Key
Medical Discipline Construction Project (grant no. 2017), Guangdong
Science and Technology Plan Project (grant no. 20160918), Guangzhou
Science Technology and Innovation Commission (grant no.
2014Y2-00152), and Guangzhou Science Technology and Innovation
Commission (grant no. 2014Y2-00548).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MB conceived and coordinated the study, designed,
performed and analyzed the experiments, wrote the paper. MB and HL
carried out the data collection, data analysis, and revised the
paper. MB, HL, XZ, ZY, SW, YW, YG and SC carried out the data
collection, data analysis and reviewed the results. All authors
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the Cancer Hospital of Guangzhou Medical College
(approval no. GZYY2006-8-20). Written informed consent was obtained
from all patients.
Patient consent for publication
Patients provide consent for the publication of
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coleman RL, Monk BJ, Sood AK and Herzog
TJ: Latest research and treatment of advanced-stage epithelial
ovarian cancer. Nat Rev Clin Oncol. 10:211–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sato S and Itamochi H: Neoadjuvant
chemotherapy in advanced ovarian cancer: Latest results and place
in therapy. Ther Adv Med Oncol. 6:293–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Randle RW, Swett KR, Swords DS, Shen P,
Stewart JH, Levine EA and Votanopoulos KI: Efficacy of
cytoreductive surgery with hyperthermic intraperitoneal
chemotherapy in the management of malignant ascites. Ann Surg
Oncol. 21:1474–1479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ba M, Long H, Zhang X, Tang Y, Wu Y, Yu F,
Wang S and Cui S: Different sequential approaches of cytoreductive
surgery and hyperthermic intraperitoneal chemotherapy in treating
ovarian cancer with malignant ascites. J Cancer Res Clin Oncol.
140:1497–1506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ba MC, Cui SZ, Lin SQ, Tang YQ, Wu YB,
Wang B and Zhang XL: Chemotherapy with laparoscope-assisted
continuous circulatory hyperthermic intraperitoneal perfusion for
malignant ascites. World J Gastroenterol. 16:1901–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ba MC, Long H, Cui SZ, Tang YQ, Wu YB,
Zhang XL, Tang HS and Bai SX: Multivariate comparison of
B-ultrasound guided and laparoscopic continuous circulatory
hyperthermic intraperitoneal perfusion chemotherapy for malignant
ascites. Surg Endosc. 27:2735–2743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui S, Ba M, Tang Y, Liu J, Wu Y, Wang B,
Zhang X, Tang H and Zhong S: B ultrasound-guided hyperthermic
intraperitoneal perfusion chemotherapy for the treatment of
malignant ascites. Oncol Rep. 28:1325–1331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Facchiano E, Scaringi S, Kianmanesh R,
Sabate JM, Castel B, Flamant Y, Coffin B and Msika S: Laparoscopic
hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment
of malignant ascites secondary to unresectable peritoneal
carcinomatosis from advanced gastric cancer. Eur J Surg Oncol.
34:154–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garofalo A, Valle M, Garcia J and
Sugarbaker PH: Laparoscopic intraperitoneal hyperthermic
chemotherapy for palliation of debilitating malignant ascites. Eur
J Surg Oncol. 32:682–685. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parson EN, Lentz S, Russell G, Shen P,
Levine EA and Stewart JH 4th: Outcomes after cytoreductive surgery
and hyperthermic intraperitoneal chemotherapy for peritoneal
surface dissemination from ovarian neoplasms. Am J Surg.
202:481–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Endo Y, Fujita T, Ishibashi H,
Nishioka T, Canbay E, Li Y, Ogura S and Yonemura Y: Cytoreductive
surgery under aminolevulinic acid-mediated photodynamic diagnosis
plus hyperthermic intraperitoneal chemotherapy in patients with
peritoneal carcinomatosis from ovarian cancer and primary
peritoneal carcinoma: Results of a phase I trial. Ann Surg Oncol.
21:4256–4262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chia CS, Tan WJ, Wong JF, Tan GH, Lim C,
Wang W, Sin EI, Tham CK, Soo KC and Teo MC: Quality of life in
patients with peritoneal surface malignancies after cytoreductive
surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg
Oncol. 40:909–916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan WJ, Wong JF, Chia CS, Tan GH, Soo KC
and Teo MC: Quality of life after cytoreductive surgery and
hyperthermic intraperitoneal chemotherapy: An Asian perspective.
Ann Surg Oncol. 20:4219–4223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Glehen O, Gilly FN, Arvieux C, Cotte E,
Boutitie F, Mansvelt B, Bereder JM, Lorimier G, Quenet F and Elias
D; Association Française de Chirurgie, : Peritoneal carcinomatosis
from gastric cancer: A multi-institutional study of 159 patients
treated by cytoreductive surgery combined with perioperative
intraperitoneal chemotherapy. Ann Surg Oncol. 17:2370–2377. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tabrizian P, Shrager B, Jibara G, Yang MJ,
Romanoff A, Hiotis S, Sarpel U and Labow DM: Cytoreductive surgery
and hyperthermic intraperitoneal chemotherapy for peritoneal
carcinomatosis: Outcomes from a single tertiary institution. J
Gastrointest Surg. 18:1024–1031. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Graziosi L, Marino E and Donini A: Reply
to ‘Peritoneal carcinomatosis in patients with gastric cancer, and
the role for surgical resection, cytoreductive surgery, and
hyperthermic intraperitoneal chemotherapy’. Am J Surg. 208:158–159.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scaringi S, Kianmanesh R, Sabate JM,
Facchiano E, Jouet P, Coffin B, Parmentier G, Hay JM, Flamant Y and
Msika S: Advanced gastric cancer with or without peritoneal
carcinomatosis treated with hyperthermic intraperitoneal
chemotherapy: A single western center experience. Eur J Surg Oncol.
34:1246–1252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zanon C, Bortolini M, Chiappino I, Simone
P, Bruno F, Gaglia P, Airoldi M, Deriu L and Mashiah A:
Cytoreductive surgery combined with intraperitoneal
chemohyperthermia for the treatment of advanced colon cancer. World
J Surg. 30:2025–2032. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Virzi S, Iusco D, Baratti D, Bonomi S,
Grassi A, Kusamura S and Deraco M: Pilot study of adjuvant
hyperthermic intraperitoneal chemotherapy in patients with
colorectal cancer at high risk for the development of peritoneal
metastases. Tumori. 99:589–595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deraco M, Baratti D, Inglese MG, Allaria
B, Andreola S, Gavazzi C and Kusamura S: Peritonectomy and
intraperitoneal hyperthermic perfusion (IPHP): A strategy that has
confirmed its efficacy in patients with pseudomyxoma peritonei. Ann
Surg Oncol. 11:393–398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuijpers AM, Mehta AM, Aalbers AG, van
Driel WJ, Boot H and Verwaal VJ: Treatment of ovarian metastases of
colorectal and appendiceal carcinoma in the era of cytoreductive
surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg
Oncol. 40:937–942. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ansaloni L, Agnoletti V, Amadori A, Catena
F, Cavaliere D, Coccolini F, De Iaco P, Di Battista M, Framarini M,
Gazzotti F, et al: Evaluation of extensive cytoreductive surgery
and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients
with advanced epithelial ovarian cancer. Int J Gynecol Cancer.
22:778–785. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pavlov MJ, Kovacevic PA, Ceranic MS,
Stamenkovic AB, Ivanovic AM and Kecmanovic DM: Cytoreductive
surgery and modified heated intraoperative intraperitoneal
chemotherapy (HIPEC) for advanced and recurrent ovarian
cancer-12-year single center experience. Eur J Surg Oncol.
35:1186–1191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deraco M, Kusamura S, Virzi S, Puccio F,
Macri A, Famulari C, Solazzo M, Bonomi S, Iusco DR and Baratti D:
Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy
as upfront therapy for advanced epithelial ovarian cancer:
multi-institutional phase-II trial. Gynecol Oncol. 122:215–220.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tentes AA, Kakolyris S, Kyziridis D and
Karamveri C: Cytoreductive surgery combined with hyperthermic
intraperitoneal intraoperative chemotherapy in the treatment of
advanced epithelial ovarian cancer. J Oncol. 2012:3583412012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spiliotis J, Vaxevanidou A, Sergouniotis
F, Lambropoulou E, Datsis A and Christopoulou A: The role of
cytoreductive surgery and hyperthermic intraperitoneal chemotherapy
in the management of recurrent advanced ovarian cancer: A
prospective study. J BUON. 16:74–79. 2011.PubMed/NCBI
|
|
27
|
Safra T, Grisaru D, Inbar M, Abu-Abeid S,
Dayan D, Matceyevsky D, Weizman A and Klausner JM: Cytoreduction
surgery with hyperthermic intraperitoneal chemotherapy in recurrent
ovarian cancer improves progression-free survival, especially in
BRCA-positive patients-a case-control study. J Surg Oncol.
110:661–665. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rettenmaier MA, Mendivil AA, Abaid LN,
Brown Iii JV, Wilcox AM and Goldstein BH: Consolidation
hyperthermic intraperitoneal chemotherapy and maintenance
chemotherapy following laparoscopic cytoreductive surgery in the
treatment of ovarian carcinoma. Int J Hyperthermia. 31:8–14. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chiva LM and Gonzalez-Martin A: A critical
appraisal of hyperthermic intraperitoneal chemotherapy (HIPEC) in
the treatment of advanced and recurrent ovarian cancer. Gynecol
Oncol. 136:130–5. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Munoz-Casares FC, Rufian S, Rubio MJ, Diaz
CJ, Diaz R, Casado A, Arjona A, Muñoz-Villanueva MC and Muntané J:
The role of hyperthermic intraoperative intraperitoneal
chemotherapy (HIPEC) in the treatment of peritoneal carcinomatosis
in recurrent ovarian cancer. Clin Transl Oncol. 11:753–759. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Le Brun JF, Campion L, Berton-Rigaud D,
Lorimier G, Marchal F, Ferron G, Oger AS, Dravet F, Jaffre I and
Classe JM: Survival benefit of hyperthermic intraperitoneal
chemotherapy for recurrent ovarian cancer: A multi-institutional
case control study. Ann Surg Oncol. 21:3621–3627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rettenmaier MA, Mendivil AA, Abaid LN,
Brown JV III, Micha JP, Wilcox AM and Goldstein BH: The feasibility
of administering varying high-dose consolidation hyperthermic
intraperitoneal chemotherapy with carboplatin in the treatment of
ovarian carcinoma. Arch Gynecol Obstet. 291:1381–1386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cascales Campos P, Gil J and Parrilla P:
Morbidity and mortality outcomes of cytoreductive surgery and
hyperthermic intraperitoneal chemotherapy in patients with primary
and recurrent advanced ovarian cancer. Eur J Surg Oncol.
40:970–975. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cascales Campos PA, Gil Martinez J,
Galindo Fernandez PJ, Gil Gomez E, Martinez Frutos IM and Parrilla
Paricio P: Perioperative fast track program in intraoperative
hyperthermic intraperitoneal chemotherapy (HIPEC) after
cytoreductive surgery in advanced ovarian cancer. Eur J Surg Oncol.
37:543–548. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cascales-Campos PA, Gil J, Feliciangeli E,
Gil E, Gonzalez-Gil A, Lopez V, Ruiz-Pardo J, Nieto A, Parrilla JJ
and Parrilla P: The role of hyperthermic intraperitoneal
chemotherapy using paclitaxel in platinum-sensitive recurrent
epithelial ovarian cancer patients with microscopic residual
disease after cytoreduction. Ann Surg Oncol. 22:987–993. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cascales-Campos PA, Gil J, Gil E,
Feliciangeli E, Gonzalez-Gil A, Parrilla JJ and Parrilla P:
Treatment of microscopic disease with hyperthermic intraoperative
intraperitoneal chemotherapy after complete cytoreduction improves
disease-free survival in patients with stage IIIC/IV ovarian
cancer. Ann Surg Oncol. 21:2383–2389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Coccolini F, Campanati L, Catena F, Ceni
V, Ceresoli M, Jimenez Cruz J, Lotti M, Magnone S, Napoli J,
Rossetti D, et al: Hyperthermic intraperitoneal chemotherapy with
cisplatin and paclitaxel in advanced ovarian cancer: A multicenter
prospective observational study. J Gynecol Oncol. 26:54–61. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eisenkop SM, Spirtos NM, Friedman RL, Lin
WC, Pisani AL and Perticucci S: Relative influences of tumor volume
before surgery and the cytoreductive outcome on survival for
patients with advanced ovarian cancer: A prospective study. Gynecol
Oncol. 90:390–396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Coccolini F, Gheza F, Lotti M, Virzi S,
Iusco D, Ghermandi C, Melotti R, Baiocchi G, Giulini SM, Ansaloni L
and Catena F: Peritoneal carcinomatosis. World J Gastroenterol.
19:6979–6994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Munoz-Casares FC, Rufian S, Rubio MJ,
Lizarraga E, Diaz-Iglesias C, Aranda E, Ciria R, Muntané J, Barrios
P, Torres-Melero J, et al: Treatment of peritoneal carcinomatosis
from ovarian cancer. Present, future directions and proposals. Clin
Transl Oncol. 9:652–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fagotti A, Gallotta V, Romano F, Fanfani
F, Rossitto C, Naldini A, Vigliotta M and Scambia G: Peritoneal
carcinosis of ovarian origin. World J Gastrointest Oncol.
2:102–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Prat J; FIGO Committee on Gynecologic
Oncology, : Staging classification for cancer of the ovary,
fallopian tube, and peritoneum. Int J Gynaecol Obstet. 124:1–5.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sugarbaker PH: Peritonectomy procedures.
Ann Surg. 221:29–42. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Di Giorgio A, Naticchioni E, Biacchi D,
Sibio S, Accarpio F, Rocco M, Tarquini S, Di Seri M, Ciardi A,
Montruccoli D and Sammartino P: Cytoreductive surgery
(peritonectomy procedures) combined with hyperthermic
intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse
peritoneal carcinomatosis from ovarian cancer. Cancer. 113:315–325.
2008. View Article : Google Scholar : PubMed/NCBI
|