Introduction
Hepatocellular carcinoma (HCC) accounts for up to
90% of all primary liver cancer worldwide, and has been reported to
cause >662,000 mortality cases worldwide annually, particularly
in developing countries (1,2). Despite improvements in the treatment of
liver cancer, the survival rate after 5 years is <30% due to its
high recurrence and metastasis rate (3,4).
Although the treatment efficacy for HCC is improving, the lack of
biomarkers for early diagnosis and effective therapeutic targets
results in consistently poor curative rates. Consequently, a better
understanding of the mechanisms and potential biomarkers are
warranted to improve HCC patient treatment.
MicroRNAs (miRNAs) are small, single-stranded,
non-coding functional RNA molecules of ~22 nucleotides that are
widely present in eukaryotic cells (5,6). MiRNAs
have been identified in various cancer types and are involved in
cancer development and progression, acting as oncogenes or tumor
suppressors (7,8). Previous studies show that miRNAs are
involved in liver disease and progression of liver cancer. For
example, miR-324-3p promotes HCC growth, however, miR-148b serves
as a tumor suppressor in HCC by inhibiting proliferation and
invasion (9). In addition, several
miRNA signatures are related to chronic hepatic infection,
cirrhosis, and steatosis (10,11). The
specific expression of certain miRNAs has been found to have
biological behavior in terms of tumor aggressiveness, metastatic
potential and even responsiveness to treatment. Therefore, any
abnormal expression of miRNA molecules is thought to be associated
with hepatocarcinogenesis. Accumulating evidence suggests that
multiple miRNAs are aberrantly expressed in and associated with
various processes of cellular carcinoma development. miR-2053 is a
miRNA molecule located at 8q23.3 in the human genome. It has been
reported that miR-2053 may induce proliferation of adult
cardiomyocytes (12). However, to
the best of our knowledge, the function of miR-2053 in HCC has not
been explored.
Hepatocarcinogenesis is a complex, multistep process
in which several signaling cascades are altered, leading to the
development of a heterogeneous biological tumor. The
phosphoinositide 3-kinase (PI3K)/serine/threonine protein kinase
(AKT)/Mammalian target of rapamycin (mTOR) and the Wnt/β-catenin
pathways serve important roles in HCC proliferation and cell cycle
progression (13,14). Studies have shown that PI3K signaling
is activated in 30–50% of HCC cases, and the downstream ribosomal
protein S6 (RPS6) is also activated in 50% of HCC patients
(15). Previous studies have
demonstrated that the Wnt/β-catenin signaling pathway plays a
critical role in proliferation and cell cycle progression of HCC
cells (16,17). miR-1247-5p functions as a tumor
suppressor by activating Wnt3 (18).
It was also demonstrated that miR-138 modulates prostate cancer
cell invasion and migration via the Wnt/β-catenin pathway (19). In the present study, the association
of miR-2053 with the PI3K/AKT/mTOR and Wnt/β-catenin pathways in
HCC cells was explored.
The effects and mechanisms of miR-2053 on the
progression of HCC were investigated. The data demonstrated that
overexpression of miR-2053 inhibited the proliferation, migration
and invasion of HCC cells and induced cell apoptosis, which may be
through inhibiting the activation of the AKT/mTOR and Wnt/β-catenin
pathways. These findings reveal the function and regulatory
mechanisms of miR-2053 in human HCC.
Materials and methods
Cell lines and cell culture
The human HCC cell line Huh7 was obtained from Cell
Bank of Chinese Academy of Sciences (Shanghai, China) and routinely
maintained in high-glucose Dulbecco's Modified Eagle's Media (DMEM)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin, and 100
mg/ml streptomycin. All cell lines were cultured at 37°C in a
humidified atmosphere containing 5% CO2.
Plasmid transfection
Cells were seeded in a 24-well plate and after the
cells reached 80% confluence, transient transfection of miRNA
plasmids was carried out using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Briefly, the miR-2053 or siRNA-miR-2053
sequences were cloned into the pCMV-MIR vectors (Guangzhou Ribobio
Co., Ltd., Guangzhou, China) (20).
Lipofectamine 2000 (10 µl) was added to 250 µl DMEM without serum
and incubated for 5 min at room temperature. The plasmids
[pCMV-MIR-miR-2053 (miR-2053) or pCMV-MIR-si-miR-2053
(si-miR-2053), 50 nM] were added to 250 µl DMEM without serum.
After 5 min incubation, complexes were formed by the total of the
two dilutions as aforementioned, gently mixing and incubating for
30 min at room temperature. The cells were prepared by removing
medium and washing twice with PBS followed by addition of 0.5 ml of
medium without serum. The complexes (500 µl) were added to each
well, and the plate was mixed gently by rocking back and forth, and
incubated at 37°C in a humidified atmosphere containing 5%
CO2 for 24 h prior to evaluating gene expression. The
medium was changed 6 h after transfection. Cells transfected with
an empty pCMV-MIR vector were used as a negative control group
(pNC) for the miR-2053 upregulation group, cells transfected with
pCMV-MIR-siRNA (non-coding siRNA) vector was performed as a
negative control (siNC) for si-miR-2053, and cells without any
treatment was used as blank control group (Con). The mature
sequence of miR-2053 is 5′-GUGUUAAUUAAACCUCUAUUUAC-3′; the siRNA
targeting miR-2053 is 5′-GUAAAUAGAGGUUUAAUUAACAC-3′.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using an RNA extraction kit
(CoWin Bioscience Co., Ltd., Beijing, China) and total RNA was
converted to cDNA using a miRNA cDNA Synthesis Kit (CoWin
Bioscience Co., Ltd.). miRNA levels were examined using a miRNA
qPCR detection kit (CoWin Bioscience Co., Ltd.). The thermocycling
conditions were set at 95°C for 5 min, followed by 40 cycles of
95°C for 10 sec, 60°C for 30 sec, and 72°C for 1 sec. Each reaction
was performed in triplicate. The expression of miR-2053 was
calculated using the 2−ΔΔCq method (21). Three independent experiments were
performed to analyze the relative gene expression. The primers were
as follows: MiR-2053, forward primer,
5′-GUGUUAAUUAAACCUCUAUUUAC-3′; reverse primer was obtained from the
miRNA qPCR detection kit; and U6, forward primer,
5′-CTCGCTTCGGCAGCACA-3′; reverse primer,
5′-AACGCTTCACGAATTTGCGT-3′.
Western blot analysis
After transfection for 48 h, total cellular protein
was extracted using radioimmunoprecipitation (RIPA) lysis buffer
(CoWin Biosciences Co., Ltd.). The lysates containing equal amounts
of protein (20 µg) were loaded onto 10% SDS-PAGE gels (Beijing
Solarbio Science and Technology Co., Ltd., Beijing, China) and
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membrane was blocked with 5% skimmed milk
for 1 h and incubated with primary antibodies overnight at 4°C,
followed by 1 h of incubation with horseradish
peroxidase-conjugated secondary antibodies (anti-rabbit IgG, cat.
no. SA00001-2; anti-mouse IgG, cat. no. SA00001-1; both 1:5,000;
both ProteinTech Group, Inc., Chicago, IL, USA). Finally, the
proteins were detected by enhanced chemiluminescence using the ECL
Plus kit (ProteinTech Group, Inc.). Band densities were analyzed
using the QUANTITY ONE 1-D software (version 4.6.7; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The following primary
antibodies were used for analysis: Anti-caspase-9 (cat. no.
ab219590; 1:1,000), anti-AKT (cat. no. ab32505, 1:1,000),
anti-phosphorylated AKT (cat. no. ab81283, 1:1,000), anti-mTOR
(cat. no. ab2732, 1:1,000), anti-phosphorylated mTOR (cat. no.
ab131538, 1:100), anti-Wnt3 (cat. no. ab32249, 1:10),
anti-β-catenin (cat. no. ab32572, 1:1,000, all Abcam, Cambridge,
UK), anti-E-cadherin (cat. no. 3195; 1:1,000; Cell Signaling
Technology, Danvers, MA, USA), anti-Cyclin D1 (cat. no. 60186-1-Ig,
1:1,000), anti-p70 (cat. no. 14485-1-AP, 1:1,000), anti-B-cell
lymphoma 2 (BCL2; cat. no. 60178-1-Ig, 1:1,000),
anti-BCL-2-associated X protein (BAX; cat. no. 60267-1-Ig, 1:1,000)
and anti-cleaved caspase-3 (cat. no. 25546-1-AP, 1:1,000) and GAPDH
(cat. no. 60004-1-Ig, 1:5,000; all Proteintech Group, Inc.), which
served as the loading control.
Cell proliferation assay
After 24 h of transfection, the cells
(1×104 cells/ml) were plated onto 96-well plates in 100
µl of complete medium. Cell Counting Kit-8 (CCK-8; Solarbio Science
& Technology Co., Ltd., Beijing, China) was used according to
the manufacturer's protocol. The plates were incubated at 37°C for
1.5 h, and the absorbance at 450 nm was measured. The proliferation
rates were determined at 0, 24, 48 and 72 h post-transfection.
Clone formation assay
After 24 h of transfection, cells (~500) were
planted in a 6-cm dish in 5 ml of medium and cultured until visible
clones formed. After staining with 0.1% crystal violet for 30 min
at room temperature, the colonies were counted under a microscope
(magnification ×4; Nikon Corporation, Tokyo, Japan) and imaged with
a camera.
Cell migration and invasion assay
Cell invasion and migration assays were performed 24
h after transfection using a Transwell system (EMD Millipore,
Billerica, MA, USA). Migration was assessed using uncoated
transwells, and invasion was investigated using transwells coated
with Matrigel (BD Biosciences, San Jose, CA, USA). A total of
1×105 cells transfected for 24 h in serum-free medium
were added to the top chamber of the transwell. The bottom chamber
was filled with medium containing 10% FBS. After 24 h incubating at
37°C, the cells on the upper surface of the membrane were gently
removed with a cotton swab, and the membranes were washed three
times with PBS, fixed and stained with 0.1% crystal violet for 5
min at room temperature. The cells were imaged and counted in in 5
random fields using a light microscope (Nikon Corporation;
magnification ×100).
Flow cytometry analysis
Cell apoptosis was measured using the fluorescein
isothiocyanate (FITC) Annexin V Apoptosis Detection Kit (4A Biotech
Co., Ltd., Beijing, China), according to the manufacturer's
protocol. Briefly, after transfection of the cells for 24 h, the
medium was removed and the cells were incubated with serum-free
medium for 24 h. The cells were digested using trypsin, washed with
ice-cold PBS, centrifuged at 200 × g for 5 min, washed twice with
ice-cold PBS and then stained with FITC-Annexin V and propidium
iodide (PI). Apoptotic cells were detected using a flow cytometer
(EPICS, Xl-4; Beckman Coulter, Inc., Brea, CA, USA) and analyzed
using FlowJo software (v7.6.1; FlowJo LLC, Ashland, OR, USA).
Statistical analysis
All statistical analyses were performed using SPSS
18.0 software. (SPSS, Inc., Chicago, IL, USA) The data were
expressed as mean ± standard deviation. The Student's t-test was
used to determine statistically significant differences between the
two groups, and one-way analysis of variance was used to determine
the statistical significance across multiple groups followed by
Least Significant Difference post hoc comparison. P<0.05 was
considered to indicate a statistically significant difference. All
experiments were performed in triplicate.
Results
miR-2053 expression in
Huh7-transfected cells
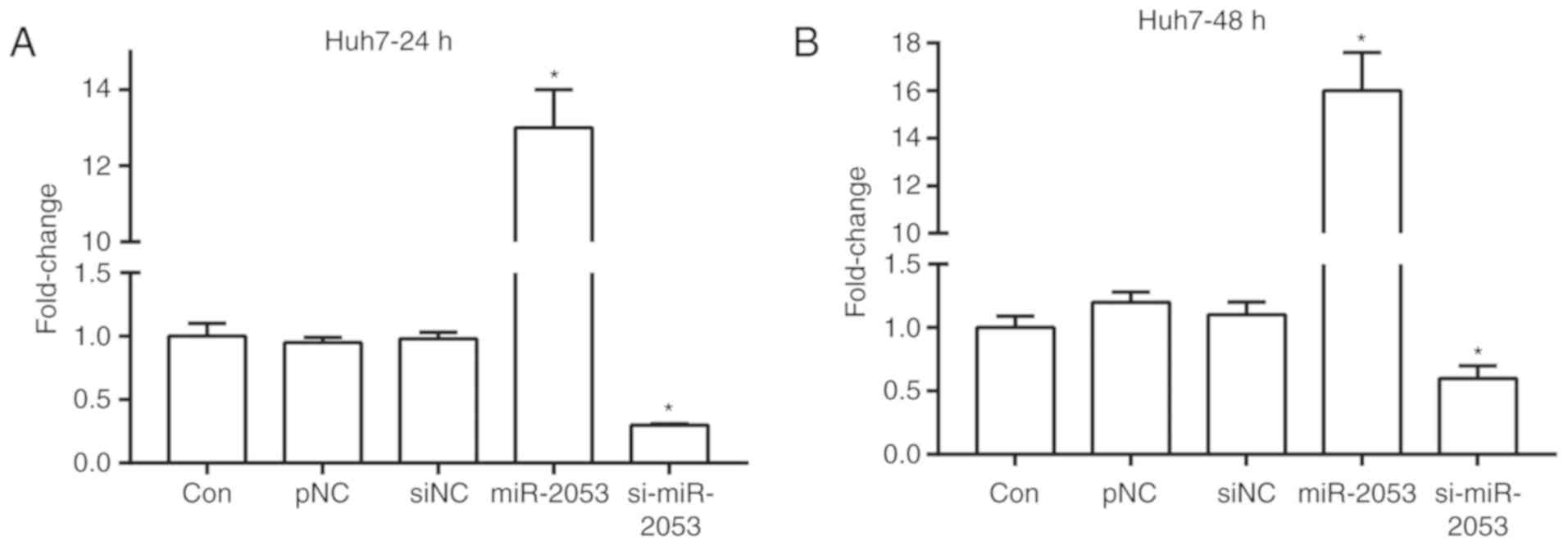
To increase or decrease miR-2053 expression,
pCMV-MIR-miR-2053 and pCMV-MIR-si-miR-2053 plasmids were
transfected into Huh7 cells, a human HCC cell line. RT-qPCR
analysis revealed that miR-2053 expression exhibited no significant
difference in control, pNC and siNC groups. miR-2053 expression was
significantly increased in cells transfected with pCMV-MIR-miR-2053
plasmid (miR-2053 group) compared with that in the pNC group, and
decreased by siRNA-miR-2053 (si-miR-2053 group) at 24 and 48 h
after transfection compared with that in the siNC group (Fig. 1A and B; P<0.05).
miR-2053 inhibits proliferation of
Huh7 cells
Proliferation serves an important role in the
development of a tumor (22). CCK-8
assay was used to study the role of miR-2053 in the proliferation
of Huh7 cells. The proliferation rate was measured at 72 h.
Following transfection for 72 h, proliferation of Huh7 cells was
significantly inhibited by miR-2053 overexpression in the miR-2053
group compared with that in the pNC group, and was promoted in the
si-miR-2053 group compared with that in the siNC group (Fig. 2A). Additionally, a plate colony assay
showed that cells transfected with miR-2053 formed fewer and
smaller colonies compared with that in the pNC group in Huh7 cells,
and si-miR-2053 increased the colony number compared with the siNC
group (Fig. 2B and C). These results
demonstrate that overexpression of miR-2053 inhibited the
proliferative potential, and miR-2053 knockdown promoted cell
proliferation of this HCC cell line.
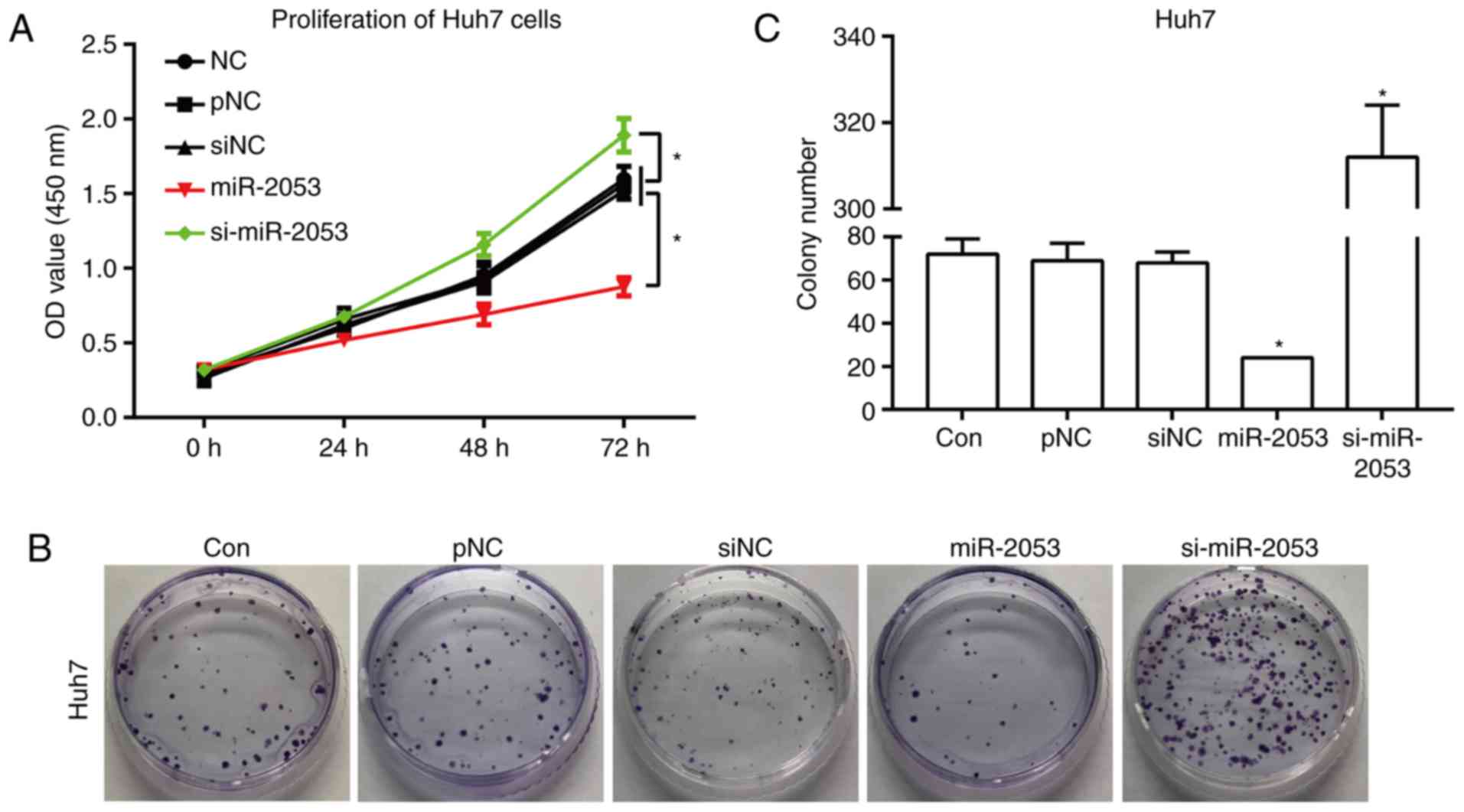 | Figure 2.miR-2053 inhibits the proliferation of
Huh7 cells. (A) Huh7 cell proliferation measured by Cell Counting
Kit-8 assay at 0, 24, 48 and 72 h after transfection. (B)
Representative images and (C) quantification of the number of
colonies resulting from the colony formation assay, 24 h following
transfection. *P<0.05, miR-2053 group vs. pNC group, si-miR-2053
group vs. siNC group; n=3. Con, untransfected group; pNC, cells
transfected with pCMV-MIR empty vector; miR-2053, cells transfected
with pCMV-MIR- miR-2053 vector, miR-2053 overexpression group;
siNC, cells transfected with pCMV-MIR-siRNA (non-coding siRNA)
vector; si-miR-2053, cells transfected with pCMV-MIR-si-miR-2053,
miR-2053 knockdown group. |
miR-2053 inhibits Huh7 cell migration
and invasion
Tumor invasion refers to the process of tumor cells
destroying the surrounding normal tissue structure and detaching
from the primary tumor (23). It was
found that the number of cells successfully invading through a
Matrigel-coated membrane was inhibited significantly in the
miR-2053 group compared with that in the pNC group. Additionally,
the number of invaded cells increased in the si-miR-2053 group
compared with that in the siNC group (Fig. 3A and B; P<0.05). The number of
cells migrating through non-coated membranes also decreased
markedly in miR-2053-overexpresing Huh7 cells, and increased
significantly in si-miR-2053 group (Fig.
3A and C; P<0.05). These results demonstrate that miR-2053
markedly suppresses the migration and invasion of Huh7 cells.
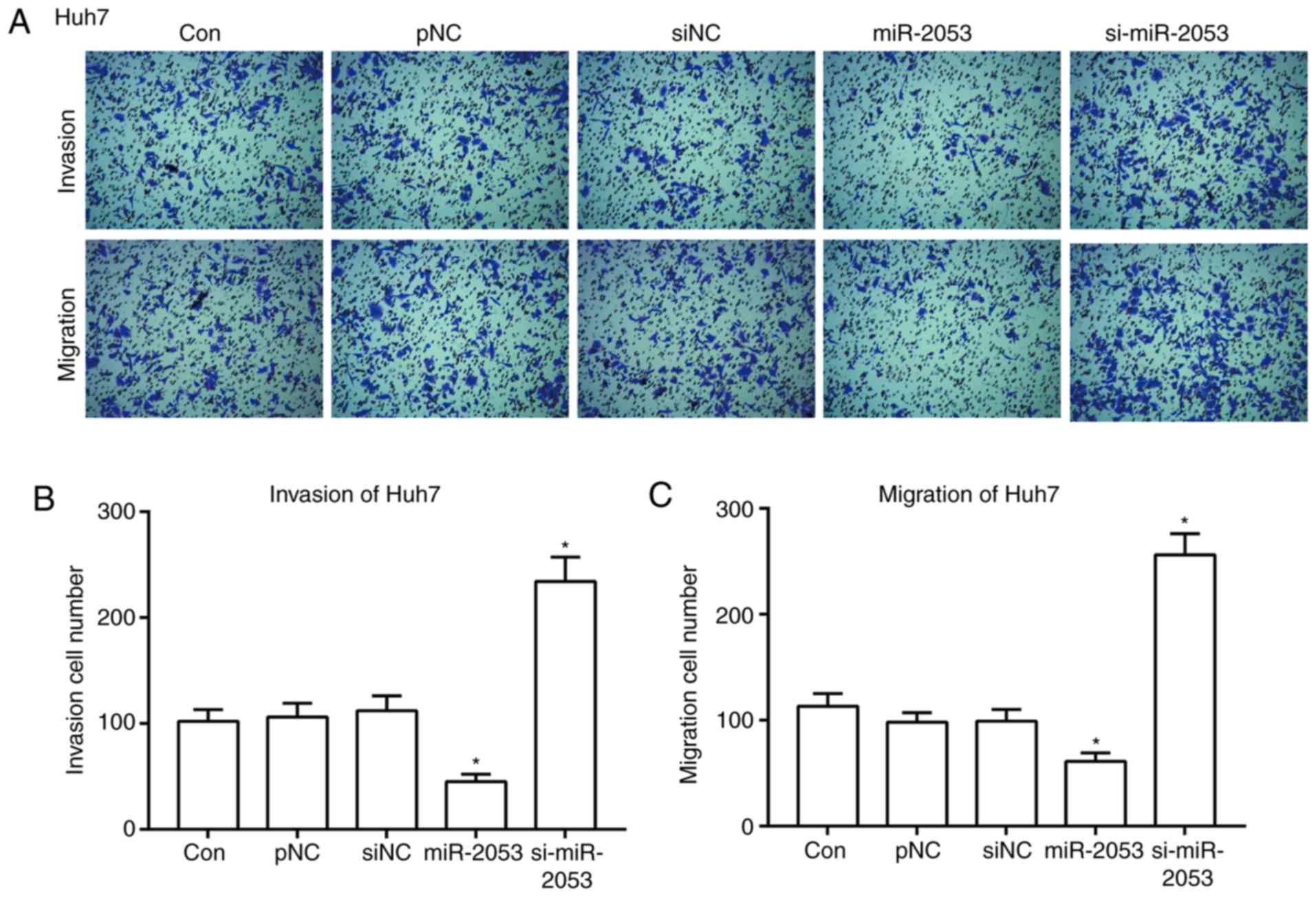 | Figure 3.miR-2053 inhibits Huh7 cell migration
and invasion in vitro. After 24 h of transfection, Transwell
and Matrigel assays were performed to assess cell migration and
invasion (A) Magnification, ×100. Quantification of the number of
(B) invasive and (C) migratory cells derived from 5 randomly chosen
fields. *P<0.05, miR-2053 group vs. pNC group, si-miR-2053 group
vs. siNC group; n=3. Con, untransfected group; pNC, cells
transfected with pCMV-MIR empty vector; miR-2053, cells transfected
with pCMV-MIR- miR-2053 vector, miR-2053 overexpression group;
siNC, cells transfected with pCMV-MIR-siRNA (non-coding siRNA)
vector; si-miR-2053, cells transfected with pCMV-MIR-si-miR-2053,
miR-2053 knockdown group. |
miR-2053 promotes apoptosis in Huh7
cells
Apoptosis plays an important role in the process of
tumor growth (24). The effect of
miR-2053 overexpression or knockdown on apoptosis was investigated
in Huh7 cells. As shown in Fig. 4,
16.69% of cells overexpressing miR-2053 were positive for Annexin V
and considered apoptotic. Of the cells transfected with
si-miR-2053, 8.00% were apoptotic, while the siNC group contained
11.00% apoptotic cells (Fig. 4A and
B). The mechanisms of miR-2053-induced apoptosis was further
determined by analyzing the expression of 4 apoptosis-related
proteins including BCL2, BAX, caspase-9 and cleaved caspase-3
(caspase-3-p17). BCL2 is an anti-apoptotic protein and BAX is a
pro-apoptotic protein, while caspase-9 and activated caspase-3 also
play key roles in the process of apoptosis (25,26). The
results showed that overexpression of miR-2053 downregulated the
level of BCL2 and upregulated BAX, caspase-9 and activated
caspase-3 expression compared with that in the pNC group.
Transfection with si-miR-2053 exhibited the opposite effect
(Fig. 4C). Thus, these results
strongly indicate that miR-2053 induces apoptosis in Huh7 cells
through activating caspase-3 and caspase-9.
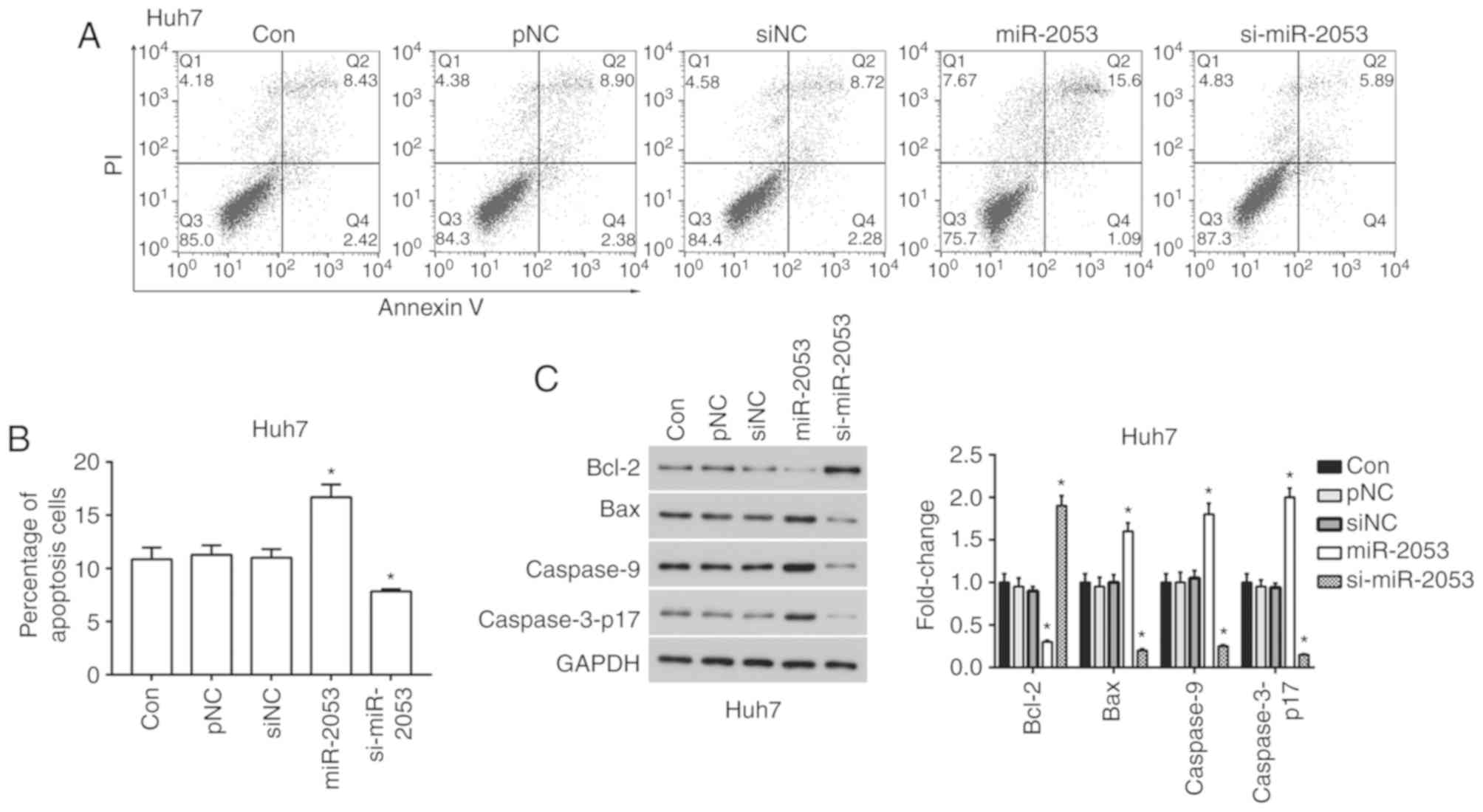 | Figure 4.miR-2053 promotes apoptosis in Huh7
cells. (A) After 24 h of transfection, cell apoptosis was measured
by flow cytometry after incubation with FITC-Annexin V and PI
solution. (B) Percentage of cells positive for Annexin V and PI.
(C) Protein expression levels of BCL2, BAX, caspase-9 and cleaved
caspase-3. *P<0.05, miR-2053 group vs. pNC group, si-miR-2053
group vs. siNC group; n=3. Con, untransfected group; pNC, cells
transfected with pCMV-MIR empty vector; miR-2053, cells transfected
with pCMV-MIR-miR-2053 vector, miR-2053 overexpression group; siNC,
cells transfected with pCMV-MIR-siRNA (non-coding siRNA) vector;
si-miR-2053, cells transfected with pCMV-MIR-si-miR-2053, miR-2053
knockdown group; FITC, fluorescein isothiocyanate; PI, propidium
iodide; BCL2, B-cell lymphoma 2, BAX, BCL-2-associated X
protein. |
miR-2053 overexpression inhibits
activation of the PI3K/AKT signaling pathway in Huh7 cells
The PI3K signaling pathway serves a central role in
the progression of HCC, participating in proliferation and
metastasis (27). According to our
previous results, it was demonstrated that miR-2053 overexpression
decreased cell proliferation, migration and invasion. This effect
may be mediated through PI3K signaling. Therefore, the impact of
miR-2053 overexpression on the PI3K/AKT signaling pathway was
assessed. It was found that the ratio of phosphorylated (p) AKT to
total AKT, the ratio of p-mTOR to total mTOR, and the levels of p70
and Cyclin D1 were downregulated in the miR-2053 group (Fig. 5). These results highlight an
association between miR-2053 and the PI3K/AKT signaling pathway in
Huh7 cells.
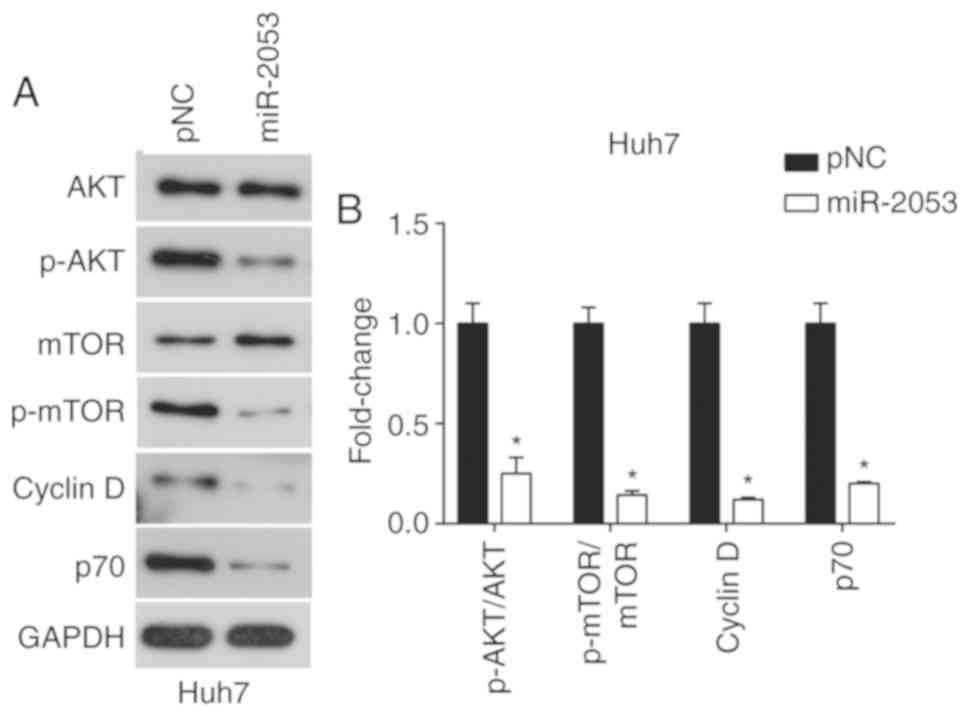 | Figure 5.miR-2053 inhibits the PI3K signaling
pathway. (A) Protein expression levels of total and p-AKT, total
and p-mTOR, cyclin D1 and p70. (B) Ratios of p-AKT/AKT and
p-mTOR/mTOR, and the expression of cyclin D1 and p70. n=3,
*P<0.05 vs. pNC control group. pNC, cells transfected with
pCMV-MIR empty vector; miR-2053, cells transfected with pCMV-MIR-
miR-2053 vector, miR-2053 overexpression group; p, phosphorylated;
AKT, serine/threonine protein kinase; mTOR, Mammalian target of
rapamycin. |
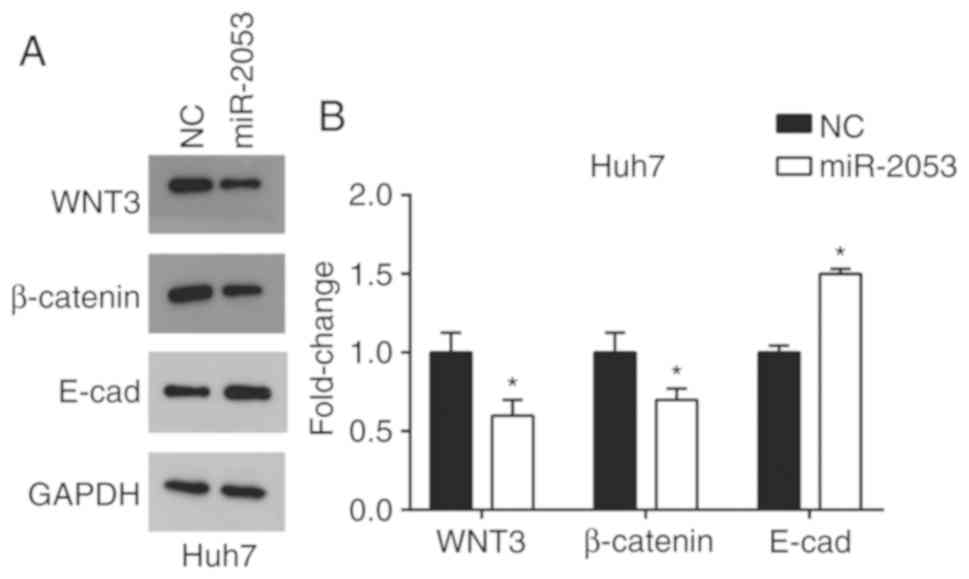
miR-2053 inhibits activation of the
Wnt/β-catenin signaling pathway in Huh7 cells
Previous studies have found that miRNAs promote HCC
invasion and metastasis via the Wnt/β-catenin signaling pathway
(28,29). The results of the present study
demonstrated that overexpression of miR-2053 resulted in a
reduction in Wnt3 protein levels. It was also revealed that
β-catenin was decreased and E-cadherin was increased in
miR-2053-overexpressing Huh7 cells compared with that in the pNC
group (Fig. 6). These results
indicate that miR-2053 may impact HCC progression through the
Wnt/β-catenin signaling pathway.
Discussion
Hepatocellular carcinoma is the third leading cause
of cancer-associated mortality and the incidence of viral
infections is increasing worldwide (30). Research has shown that miRNAs are
abnormally expressed in HCC, and are involved in the regulation of
tumor development and malignant changes (31). Certain miRNAs serve as promoters in
hepatic tumorigenesis. Conversely, several miRNAs function as tumor
suppressors. For example, miR-221 blockage prompts HCC survival
(32,33), whereas miR-148b suppresses cell
proliferation and invasion in HCC by targeting the Wnt1/β-catenin
pathway (34). Studies have found
that miR-132 inhibits cell proliferation, invasion and migration of
HCC (35). These findings indicate
that miRNAs are intimately involved in the progression of HCC
malignancy.
In the present study, it was shown that the
overexpression of miR-2053 significantly inhibited proliferation,
colony formation, cell migration and invasion, and induced cell
apoptosis in a human HCC cell line. Knockdown of miR-2053 induced
the opposite changes, increasing proliferation and migration, and
reducing apoptosis. These results suggest that miR-2053 may
function as a tumor suppressor in HCC. The molecular mechanisms
underlying the role of miR-2053 in HCC cells remains unclear.
Previous studies have shown that the PI3K/AKT
pathway is one of the most altered oncogenic pathways in tumor
development, including HCC (36,37). The
downstream effector, mTOR serves an important role in hepatitis and
HCC development. Previous studies have demonstrated that AKT/mTOR
serves an important role in the cell cycle of tumor cells (38,39).
Therefore, the phosphorylation of AKT and mTOR, as well as
expression of cell cycle regulators Cyclin D1 and p70, was examined
in the present study. The results demonstrated that miR-2053
overexpression significantly reduced the levels of phosphorylated
AKT and mTOR, and the levels of Cyclin D1 and p70 in Huh7 cells,
suggesting that miR-2053 may inhibit HCC proliferation through
regulating the AKT/mTOR signaling pathway.
Wnt3 belongs to the Wnt1 class of ligands and
stimulates the canonical Wnt/β-catenin pathway (18,40).
Wnt/β-catenin is involved in HCC progression. TRIM37 overexpression
promotes cell migration and metastasis in HCC by activating
Wnt/β-catenin signaling (41). It
has also been found that the downregulation of miR-200a induced
epithelial-mesenchymal transition phenotypes and influenced
proliferation, migration and invasion of SGC790 and U251 cells
through targeting the β-catenin pathway (42). Here, miR-2503 overexpression
inhibited the expression of Wnt3 and β-catenin, and increased
E-cadherin levels, downstream molecules in the Wnt signaling
pathway. Taken together, these data demonstrated that miR-2053 may
function as a tumor suppressor in HCC development by regulating
cell migration and invasion.
In conclusion, it was demonstrated that miR-2053
overexpression inhibited Huh7 cell proliferation, colony formation,
cell migration and invasion, and induced cell apoptosis.
Additionally, the PI3K/AKT/ and Wnt/β-catenin signaling pathways
were regulated by miR-2053 in this HCC cell line. Therefore,
miR-2053 may be a potential target for novel invasive HCC
treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TS, KM, CZ, JY, JL participated in the experiments,
data analysis, manuscript design and writing. All authors have read
and approved this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davis GL, Dempster J, Meler JD, Orr DW,
Walberg MW, Brown B, Berger BD, O'Connor JK and Goldstein RM:
Hepatocellular carcinoma: Management of an increasingly common
problem. Proc (Bayl Univ Med Cent). 21:266–280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Di Pardo BJ, Bronson NW, Diggs BS, Thomas
CR Jr, Hunter JG and Dolan JP: The global burden of esophageal
cancer: A disability-adjusted life-year approach. World J Surg.
40:395–401. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang J, Yan L and Wang W: Current status
of multimodal & combination therapy for hepatocellular
carcinoma. Indian J Med Res. 136:391–403. 2012.PubMed/NCBI
|
|
4
|
Thorgeirsson SS and Grisham JW: Molecular
pathogenesis of human hepatocellular carcinoma. Nat Genet.
31:339–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Felekkis K, Touvana E, Stefanou C and
Deltas C: MicroRNAs: A newly described class of encoded molecules
that play a role in health and disease. Hippokratia. 14:236–240.
2010.PubMed/NCBI
|
|
6
|
Wahid F, Shehzad A, Khan T and Kim YY:
MicroRNAs: Synthesis, mechanism, function, and recent clinical
trials. Biochim Biophys Acta. 1803:1231–1243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mott JL: MicroRNAs involved in tumor
suppressor and oncogene pathways: Implications for hepatobiliary
neoplasia. Hepatology. 50:630–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fendler A, Stephan C, Yousef GM and Jung
K: MicroRNAs as regulators of signal transduction in urological
tumors. Clin Chem. 57:954–968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roy S, Benz F, Luedde T and Roderburg C:
The role of miRNAs in the regulation of inflammatory processes
during hepatofibrogenesis. Hepatobiliary Surg Nutr. 4:24–33.
2015.PubMed/NCBI
|
|
10
|
Szabo G and Bala S: MicroRNAs in liver
disease. Nat Rev Gastroenterol Hepatol. 10:542–552. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu SH and Ghoshal K: MicroRNAs in liver
health and disease. Curr Pathobiol Rep. 1:53–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pandey R, Jackson L, Ma G and Ahmed RP:
Identification of MicroRNAs inducing adult cardiomyocyte
proliferation. Circulation. 130:A163142014.
|
|
13
|
Tsai WL and Chung RT: Viral
hepatocarcinogenesis. Oncogene. 29:2309–2324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steelman LS, Chappell WH, Abrams SL, Kempf
RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F,
Mazzarino MC, et al: Roles of the Raf/MEK/ERK and
PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity
to therapy-implications for cancer and aging. Aging (Albany NY).
3:192–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galuppo R, Ramaiah D, Ponte OM and Gedaly
R: Molecular therapies in hepatocellular carcinoma: What can we
target? Dig Dis Sci. 59:1688–1697. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vilchez V, Turcios L, Marti F and Gedaly
R: Targeting Wnt/beta-catenin pathway in hepatocellular carcinoma
treatment. World J Gastroenterol. 22:823–832. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qu C, He, Lu X, Dong L, Zhu Y, Zhao Q,
Jiang X, Chang P, Jiang X, Wang L, et al: Salt-inducible Kinase
(SIK1) regulates HCC progression and WNT/beta-catenin activation. J
Hepatol. 64:1076–1089. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chu Y, Fan W, Guo W, Zhang Y, Wang L, Guo
L, Duan X, Wei J and Xu G: MiR-1247-5p functions as a tumor
suppressor in human hepatocellular carcinoma by targeting Wnt3.
Oncol Rep. 38:343–351. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu Z, Wang Z, Li F, Yang J and Tang L:
MiR-138 modulates prostate cancer cell invasion and migration via
Wnt/β-catenin pathway. Mol Med Rep. 17:3140–3145. 2018.PubMed/NCBI
|
|
20
|
Tanabe H, Hayashi M, Sugimoto H, Kurimoto
K, Hirabayashi S, Kanda M, Takami H, Niwa Y, Iwata N, Kobayashi D,
et al: Abstract 3429: Oncogenic function of miR-23b-3p in
hepatocellular carcinoma. Cancer Res. 77:3429. 2017.
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fritz V and Fajas L: Metabolism and
proliferation share common regulatory pathways in cancer cells.
Oncogene. 29:4369–4377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Curran S and Murray GI: Matrix
metalloproteinases in tumor invasion and metastasis. J Pathol.
189:300–308. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Symonds H, Krall L, Remington L,
Saenz-Robles M, Lowe S, Jacks T and Van Dyke T: p53-Dependent
apoptosis suppresses tumor growth and progression in vivo. Cell.
78:703–711. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martinou JC and Youle R: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gui D, Guo Y, Wang F, Liu W, Chen J, Chen
Y, Huang J and Wang N: Astragaloside IV, a novel antioxidant,
prevents glucose-induced podocyte apoptosis in vitro and in vivo.
PLoS One. 7:e398242012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen JS, Wang Q, Fu XH, Huang XH, Chen XL,
Cao LQ, Chen LZ, Tan HX, Li W, Bi J and Zhang LJ: Involvement of
PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in
hepatocellular carcinoma: Association with MMP-9. Hepatol Res.
39:177–186. 2010. View Article : Google Scholar
|
|
28
|
Tuo H, Wang Y, Wang L, Yao B, Li Q, Wang
C, Liu Z, Han S, Yin G, Tu K and Liu Q: MiR-324-3p promotes tumor
growth through targeting DACT1 and activation of Wnt/β-catenin
pathway in hepatocellular carcinoma. Oncotarget. 8:65687–65698.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao N, Mu L, Yang W, Liu L, Liang L and
Zhang H: MicroRNA-298 represses hepatocellular carcinoma
progression by inhibiting CTNND1-mediated Wnt/β-catenin signaling.
Biomed Pharmacother. 106:483–490. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Balogh J, Victor D III, Asham EH,
Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM and Monsour
HP Jr: Hepatocellular carcinoma: A review. J Hepatocell Carcinoma.
3:41–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chu R, Mo G, Duan Z, Huang M, Chang J, Li
X and Liu P: MiRNAs affect the development of hepatocellular
carcinoma via dysregulation of their biogenesis and expression.
Cell Commun Signal. 12:452014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thurnherr T, Mah WC, Lei Z, Jin Y, Rozen
SG and Lee CG: Differentially expressed miRNAs in hepatocellular
carcinoma target genes in the genetic information processing and
metabolism pathways. Sci Rep. 6:200652016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park JK, Kogure T, Nuovo GJ, Jiang J, He
L, Kim JH, Phelps MA, Papenfuss TL, Croce CM, Patel T and
Schmittgen TD: MiR-221 silencing blocks hepatocellular carcinoma
and promotes survival. Cancer Res. 71:7608–7616. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang JG, Shi Y, Hong DF, Song M, Huang D,
Wang CY and Zhao G: MiR-148b suppresses cell proliferation and
invasion in hepatocellular carcinoma by targeting WNT1/beta-catenin
pathway. Sci Rep. 5:80872015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu K, Li X, Cao Y, Ge Y, Wang J and Shi
B: MiR-132 inhibits cell proliferation, invasion and migration of
hepatocellular carcinoma by targeting PIK3R3. Int J Oncol.
47:1585–1593. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Courtney KD, Corcoran RB and Engelman JA:
The PI3K pathway as drug target in human cancer. J Clin Oncol.
28:1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tu K, Liu Z, Yao B, Han S and Yang W:
MicroRNA-519a promotes tumor growth by targeting PTEN/PI3K/AKT
signaling in hepatocellular carcinoma. Int J Oncol. 48:965–974.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang F, Lee JT, Navolanic PM, Steelman
LS, Shelton JG, Blalock WL, Franklin RA and McCubrey JA:
Involvement of PI3K/Akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: A target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao N, Zhang Z, Jiang BH and Shi X: Role
of PI3K/AKT/mTOR signaling in the cell cycle progression of human
prostate cancer. Biochem Biophys Res Commun. 310:1124–1132. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim M, Lee HC, Tsedensodnom O, Hartley R,
Lim YS, Yu E, Merle P and Wands JR: Functional interaction between
Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin
signaling pathway in hepatocellular carcinoma cells. J Hepatol.
48:780–791. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang J, Yu C, Chen M, Tian S and Sun C:
Over-expression of TRIM37 promotes cell migration and metastasis in
hepatocellular carcinoma by activating Wnt/beta-catenin signaling.
Biochem Biophys Res Commun. 464:1120–1127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Juan S, Anling Z, Zhendong S, Ma F, Pu P,
Wang T, Zhang J, Kang C and Zhang Q: MicroRNA-200a suppresses the
Wnt/β-catenin signaling pathway by interacting with β-catenin. Int
J Oncol. 40:1162–1170. 2012.PubMed/NCBI
|