Introduction
As one of the most common gastrointestinal
malignancies, gastric cancer is one of the leading causes of cancer
deaths worldwide (1). There are over
950,000 new cases and over 720,000 deaths per year in the world
(2). Early symptoms of gastric
cancer are not visible, and the early diagnosis rate of gastric
cancer is generally low. Most patients are diagnosed as advanced
gastric cancer at the time of initial clinical visit. Moreover,
cancer cells have serious invasion of local target lesions and
easily metastasize, so the efficacy is generally poor (3). Data show that the five-year total
survival rate of gastric cancer is low (4). According to Japanese guidelines for the
treatment of gastric cancer (5), for
unresectable advanced gastric cancer and recurrent cancer,
chemotherapy is able to achieve great tumor degeneration, but it is
still difficult to completely cure. Therefore, it is of vital
importance to explore new treatment methods to improve the
prognosis of patients with gastric cancer.
Interventional radiology, a method of using catheter
and guidewire puncture or directly through human body tube under
the guidance of imaging technology to deepen focus on angiography
or direct local administration of drugs, has a history of more than
50 years, and due to the minimally invasive execution, as well as
the advantages of high safety and good efficacy in angiography, it
has been used in angiography, stent transplantation and regional
cancer treatment (6). Literature has
shown that interventional therapy is a minimally invasive treatment
with good efficacy for gastric cancer patients who cannot be
treated by surgery and have distant metastasis, which can inhibit
tumor development and bring hope to the majority of patients
(7). However, interventional therapy
cannot fully meet the treatment needs of patients, and there are
still some limitations, such as high technical requirements of many
specific therapeutic targets for operators, low tumor clearance
rate, and easy postoperative recurrence (8,9).
Existing studies have shown that Helicobacter
pylori can attack different cell proteins for a long time to
affect the host's inflammatory response, thus leading to the
occurrence of chronic gastritis, peptic ulcer and even gastric
cancer (10). At present, the most
common cause of gastric cancer is chronic inflammation caused by
Helicobacter pylori (11).
Cytokines are an important part of tumor-related inflammation.
Literature shows that as common inflammatory cytokines,
interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10
(IL-10) all play a certain role in promoting the development of
gastric cancer (12–14). In addition, a study has shown that
these three inflammatory cytokine gene polymorphisms are associated
with the occurrence and development of gastric cancer and
gastroduodenal diseases related to Helicobacter pylori
(15). Various studies have pointed
out that the IL-6 is the key protein between inflammation and
gastric cancer and other cancers and proinflammatory cytokines
associated with gastric cancer state (16,17). The
expression of IL-8 and IL-10 in gastric carcinoma is associated
with Helicobacter pylori infection and lymph node metastasis
(18). Therefore, IL-6, IL-8 and
IL-10 were selected for this study. Currently, there are many
studies on the stages and prognosis of gastric cancer with IL-6,
IL-8 and IL-10, but few on the interventional treatment after
chemotherapy.
The present study tested the levels of IL-6, IL-8
and IL-10 in the serum of patients before and after treatment, to
explore the clinical efficacy of interventional therapy after
chemotherapy in patients with gastric cancer and the effect of
interventional therapy on inflammatory factors in peripheral blood
serum of patients, in order to provide clinical reference for the
treatment of patients with gastric cancer.
Patients and methods
Subjects
A retrospective analysis of 429 patients with
gastric cancer treated in Xiangyang No. 1 People's Hospital, Hubei
University of Medicine (Xiangyang, China) from July 2008 to
December 2014 was performed. Among them, 220 patients received
interventional therapy after chemotherapy as the experimental
group, and 209 patients received chemotherapy alone as the control
group. There were 177 males and 43 females in the experimental
group, aged 36–77 years, with an median age of (55.32±15.25) years.
The follow-up duration was 3–36 months, with an average follow-up
duration of (24.94±2.81) months. There were 167 males and 42
females in the control group, aged 32–76 years, with an median age
of (56.75±14.89) years and an average follow-up duration of
(24.82±3.64) months.
Inclusion criteria were: i) Aged 30–80 years; ii)
according to the criteria of Eastern Collaborative Oncology Group
(ECOG) in the United States (19),
the score ≤2; iii) gastric adenocarcinoma was diagnosed by
pathological examination, and clinical, imaging and
histopathological data were complete. Exclusion criteria were: i)
pregnant or lactating women; ii) patients with obvious
contraindications in interventional treatment, such as massive
ascites, hemogram and abnormal coagulation function; iii) existence
of other systemic malignant tumors; iv) patients with diseases that
could affect the evaluation of efficacy.
The present study was approved by the Ethics
Committee of Xiangyang No. 1 People's Hospital, Hubei University of
Medicine. The patients were explained in detail the contents of the
experiment. The patients or their guardians agreed and signed a
complete informed consent.
Experimental methods
Experimental reagents and
instruments
Oxaliplatin for injection (Chengdu Changqing
pharmaceutical Co., Ltd., H20020648); Tetrahydrofolic acid (Xiamen
Yanke biotechnology Co., Ltd., YKIR-14390); Drachen capecitabine
tablet (Qilu Pharmaceutical Co., Ltd., H20133361); Carcinoembryonic
antigen (CEA) radioimmunoassay (RIA) kit (Shanghai X-Y
Biotechnology Co., Ltd., xy-302); CA-199 Radioimmunoassay kit
(Shanghai Xinfan Biotechnology Co., Ltd., XFFMA10-33); IL-6
enzyme-linked immunosorbent assay (ELISA) kit (Shanghai Jingkang
Bioengineering Co., Ltd., JKSJ-2176); IL-8 ELISA detection kit
(Shanghai YBIO Biotechnology Co., Ltd., IC-IL8-p); IL-10 ELISA
detection kit (Shanghai Shock Biological Co., Ltd., HZ-IL10-Gu); R
RIA counter (Anhui USTC Zonkia Scientific Instruments Co., Ltd.,
GC-2010); FLUOstar Omega automatic multifunctional microplate
reader (Bio-Gene Technology Co., Ltd., FLUOstar Omega); Desktop
high-speed centrifuge TG16-WS (Beijing Tiderader Technology Co.,
Ltd., TG16-WS); Panasonic medical cryogenic refrigerator (Sanyo
Corporation, MDF-U5412); Spectrophotometer (Shanghai Genesci
Medical Technology Co., Ltd., OD-1000+).
Chemotherapy methods before
treatment
The routine chemotherapy regimen used in the
experimental and control groups was FOLFOX regimen (20). The specific drugs used were:
Oxaliplatin for injection, drug concentration: 135
mg/m2, intravenous drip for 2 h, d1; Tetrahydrofolic
acid, drug concentration: 200 mg/m2, intravenous drip
for 2 h, d1-5; Capecitabine tablet, drug concentration: 1250
mg/m2, intravenous drip for 2 h, d1-5. The patients
underwent the first chemotherapy after the outpatient examination
and assessment of their condition. If the patient had no obvious
discomfort after chemotherapy, routine chemotherapy was carried out
according to this scheme. It was repeated every 4 weeks, 6
times.
Interventional methods
After 6 courses, 3–4 courses of continuous treatment
were performed in the patients of the control group. During the
process, the treatment was stopped when the patients experienced
obvious discomfort. The blood of patients in the control group was
collected 3 weeks after treatment.
After 6 courses, patients in the experimental group
received interventional surgery. Patients fasted for >8 h one
night before surgery and were given iodine allergy skin test early
in the morning the next day. Atropine (0.5 mg) was injected
intramuscularly 30 min before surgery, and 2% lidocaine was used
for local infiltration anesthesia. Femoral artery was successfully
punctured by Seldinger technique (21). The hook was set and the metal
guidewire were placed on the celiac trunk. The puncture site was
pressed adequately, and externally, the power injector was used to
perform high pressure angiography at the same time. If the target
vessel diameter was too small, and the intubation was difficult,
then the microguide wire and microcatheter were used for
intubation. According to the different location of the patient's
tumor, different parts were selected to perform interventional
intubation angiography, and the specific dose was determined by the
thickness, blood flow and blood supply of the interventional
vessels.
Patients in the experimental group were injected
intravenously with antiemetic drugs 15–30 min before experiment,
and then interventional surgery of arterial infusion chemotherapy
was performed. The embolic agent (gelfoam particles) was injected
into the target artery for chemoembolization, and hepatic arterial
targeted chemoembolization was performed in patients with hepatic
metastasis. After embolization, a second angiography was performed
to observe the development of the lesion in real time. The surgery
was completed when no imaging of the tumor feeding artery was
found. In this study, each patient received at least one
intervention, with an average of three interventions. After
treatment, routine primary nursing care was given after
interventional surgery, fasting and water prohibition for 24 h,
with bedside electrocardiogram (ECG) monitoring in real time.
Patients with liver metastases were treated with liver protection,
and postoperative wound bleeding at the femoral artery was closely
monitored. The lower limbs were fixed for 8 h and the patients
rested in bed for 24 h. Care workers massaged the lower limbs once
every 2 h to promote blood circulation in the lower extremities and
prevent bedsore and thrombosis. At the same time, the control group
continued to use conventional chemotherapy, as detailed in
‘Experimental reagents and instruments’. Blood samples were
taken 3 weeks after treatment.
Collection and treatment of blood
samples
From each patient blood was collected twice, two
tubes each time, for radioimmunoassay for tumor markers and ELISA
method for inflammatory factors, respectively. The samples were
collected 2 days before the intervention and 3 weeks after the last
intervention in the experimental group, and 2 days before treatment
and 3 weeks after the end of the last chemotherapy in the control
group. Blood samples were collected and processed as follows: 2 ml
elbow vein blood was extracted by vacuum blood sampling needle at a
single time from patients after at least 8 h on an empty stomach
and stored in a refrigerator at 4°C for 45 min. The condensed blood
was then centrifuged at 3,000 × g at 20°C for 15 min. The slurry in
test tube was absorbed carefully to obtain the serum which then was
stored in a cryogenic refrigerator at −80°C.
Laboratory examination of tumor
markers in serum
The serum samples were collected from the
experimental and control groups, dissolved at room temperature and
diluted with 450 µl 0.9% NaCl saline. Then NaHCO3 was
added to the test tube to adjust pH to ~7.0. Finally, 100 µl of
liquid in test tube was added into CEA and carbohydrate antigen
19-9 (CA19-9) kit, mixed, and placed at 4°C overnight. An immune
counter was used to measure and count.
Determination of concentrations of
IL-6, IL-8 and IL-10 in serum
The concentrations of IL-6, IL-8 and IL-10 in the
collected serum samples were determined by ELISA. Blank control
well, sample well and standard well were set first. Sample (50 µl)
was added to the standard well, and 40 µl 0.05 M pH 9.0 buffer
solution was dripped into the sample well. Sample (10 µl) was added
to the sample well and mixed gently. The polystyrene plate was
sealed by sealing membrane and placed for 30 min at 37°C. The
sealing membrane was opened, and the liquid in the reaction well
was discarded, then the reaction pore was dried. The well was
washed by the washing buffer diluted by distilled water 3 times, 3
min each time. Except the blank well, 50 µl enzyme-labeled reagents
in the kit was added to each reaction well. Then the chromogenic
agents A and B were added to the three wells, 50 µl each in turn,
and the liquid in each well was mixed gently. Reaction stop buffer
(50 µl) was added to each well to terminate the reaction, and
yellow appeared in the reaction well. Within 15 min, the optical
density (OD value) of each reaction well was measured by
spectrophotometer at the wavelength of 450 nm with a blank well as
a zero setting reference. The standard curve was used to calculate
the concentrations of IL-6, IL-8 and IL-10 in serum.
Observation indexes
i) According to the modified Response Evaluation
Criteria In Solid Tumors (RECIST) (22), the efficacy of patients can be
divided into: Complete remission (CR): All the target lesions have
disappeared and the short diameter of all pathological lymph nodes
are decreased to <10 mm; Partial remission (PR): The diameter
and baseline level of the target lesion are reduced to 30%;
Stability of disease (SD): The condition of target lesion is
between PR and progression of disease (PD); PD: In the whole
experiment, diameter sums of all target lesions relatively
increased at least 20%, and the absolute value of the diameter sums
increased at least 5 mm. The disease control rate (DCR) =
(CR+PR+SD)/total number of cases × 100%. ii) According to Common
toxicity criteria (23), the degree
of nausea, vomiting and liver and kidney damage caused by the use
of drugs in patients with gastric cancer was graded, and the
incidence rate of toxic and side effects was compared between the
experimental and control groups. iii) Telephone follow-up was
conducted once a month to ask patients about their survival.
Statistical analysis
The experimental data were analyzed by SPSS 17.0
software (SPSS Inc.). The figures were made with GraphPadPrism 7
(Beijing Huanzhongruichi Technology Co., Ltd.). The enumeration
data were represented by percentage (%). Chi-square (χ2)
test was used for comparison between the groups, and partitions of
χ2 method was used for pairwise comparison. The
measurement data were expressed as (mean ± SD) and were first
tested for normality. t-test was used when the data conformed to
the normal distribution, and the rank-sum test was used when the
data were not in the normal distribution. Pearson's correlation
analysis was used to test the correlation between CEA, CA-199 and
IL-6, IL-8 and IL-10. Survival analysis was performed using
Kaplan-Meier. Log-rank test was used to test, and univariate and
multivariate analyses were performed on the prognostic factors of
patients using Cox regression model of single factor analysis. At
P<0.05, the difference was considered statistically
significant.
Results
General clinical data comparison
As shown in Table I,
there was no difference in age, body mass index (BMI) and follow-up
duration and other factors between the experimental and control
groups (P>0.05). According to the American Joint Committee on
Cancer (AJCC) pathological staging gastric cancer (24), in the experimental group, 109
patients were at stage I+II, and 111 patients were at stage
IIIa+IIIb; while in the control group, 116 patients were at stage
I+II, and 93 patients were at stage IIIa+IIIb. According to the
Borrmann type (25), in the
experimental group, 54 patients were at stage I+II, and 166
patients were at stage III+IV; while in the control group, 51
patients were at stage I+II, and 158 patients were at stage III+IV.
There was no statistical difference between the experimental and
control groups in terms of tumor stage, Borrmann type,
differentiation degree, history of Helicobacter pylori
infection, history of peptic ulcer, and preexperimental treatment
(P>0.05).
 | Table I.Comparison of general clinical data
between the experimental and control groups (mean ± SD) [n
(%)]. |
Table I.
Comparison of general clinical data
between the experimental and control groups (mean ± SD) [n
(%)].
| Clinical
factors | Experimental group
(n=220) | Control group
(n=209) |
t/χ2 | P-value |
|---|
| Follow-up duration
(month) |
24.94±2.81 |
24.82±3.64 | 0.324 | 0.750 |
| BMI
(kg/m2) |
24.68±1.36 |
24.81±1.14 | 1.076 | 0.285 |
| Heart rate
(time/minute) | 103.51±12.36 | 104.24±11.54 | 0.632 | 0.528 |
| Age (years) |
|
| 0.072 | 0.788 |
|
≤50 | 87
(39.55) | 80
(38.28) |
|
|
|
>50 | 133 (60.45) | 129 (61.72) |
|
|
| Sex [n (%)] |
|
| 0.201 | 0.886 |
|
Male | 177 (80.45) | 167 (79.90) |
|
|
|
Female | 43
(19.55) | 42
(20.10) |
|
|
| Tumor stage |
|
| 1.525 | 0.217 |
|
I+II | 109 (49.55) | 116 (55.50) |
|
|
|
IIIa+IIIb | 111 (50.45) | 93
(44.50) |
|
|
| Borrmann type |
|
| 0.001 | 0.972 |
|
I+II | 54
(24.55) | 51
(24.40) |
|
|
|
III+IV | 166 (75.45) | 158 (75.60) |
|
|
| Degree of
differentiation |
|
| 0.207 | 0.901 |
|
Differentiated | 35
(15.91) | 33
(15.79) |
|
|
| Poorly
differentiated | 106 (48.18) | 105 (50.24) |
|
|
|
Undifferentiated | 79
(35.91) | 71
(33.97) |
|
|
| History of
Helicobacter pylori infection |
|
| 0.077 | 0.781 |
| No | 104 (47.27) | 96
(45.93) |
|
|
|
Yes | 116 (52.73) | 113 (54.07) |
|
|
| History of peptic
ulcer |
|
| 0.012 | 0.913 |
| No | 102 (46.36) | 98
(46.89) |
|
|
|
Yes | 118 (53.64) | 111 (53.11) |
|
|
| Chemotherapy cycle
before experiment |
|
| 0.001 | 0.989 |
| ≤4 | 178 (80.91) | 169 (80.86) |
|
|
|
>4 | 42
(19.09) | 40
(19.14) |
|
|
| Prophylactic
anti-inflammatory therapy |
|
| 1.780 | 0.182 |
| No | 12
(5.45) | 6
(2.87) |
|
|
|
Yes | 208 (94.55) | 203 (97.13) |
|
|
| Smoking
history |
|
| 0.155 | 0.694 |
| No | 101 (45.91) | 92
(44.02) |
|
|
|
Yes | 119 (54.09) | 117 (55.98) |
|
|
| Serum creatinine [n
(%)] |
|
| 0.008 | 0.983 |
| <133
µmol/l | 64
(20.09) | 61
(29.19) |
|
|
| ≥133
µmol/l | 156 (70.91) | 148 (70.81) |
|
|
| Blood urea nitrogen
[n (%)] |
|
| 0.004 | 0.947 |
|
<7.14 mmol/l | 49
(22.27) | 46
(22.01) |
|
|
| ≥7.14
mmol/l | 171 (77.73) | 163 (77.99) |
|
|
Comparison of tumor markers between
the experimental and control groups after treatment
The concentrations of CEA in the serum of the
experimental and control groups 2 days before treatment were
(5.98±2.57) and (6.14±2.53) µg/l; The concentrations of CA19-9 in
serum of the experimental and control groups 2 days before
treatment were (42.35±1.76) and (42.15±1.04) U/ml. The
concentrations of CEA in the serum of the experimental and control
groups 3 weeks after treatment were (5.42±2.03) and (6.02±2.61)
µg/l. The concentrations of CEA and CA19-9 in the serum of the
experimental and control groups 3 weeks after treatment were
(39.62±1.37) and (41.97±1.28) U/ml. There was no significant
difference in the concentrations of CEA and CA19-9 between the
experimental and control groups before treatment (P>0.05). The
concentrations of CEA and CA19-9 in the experimental group after
treatment were lower than that before treatment (P<0.05), and
there was no significant difference in the control group before and
after treatment (P>0.05). The concentrations of CEA and CA19-9
in the experimental group after treatment was lower than that in
the control group at the same time (P<0.05). Specific data are
shown in Table II.
 | Table II.Comparison of tumor markers between
the experimental and control groups (mean ± SD). |
Table II.
Comparison of tumor markers between
the experimental and control groups (mean ± SD).
| Factors | Experimental group
(n=220) | Control group
(n=209) | t | P-value |
|---|
| CEA (µg/l) |
| 2 days
before treatment |
5.98±2.57 |
6.14±2.53 |
0.649 |
0.516 |
| 3 weeks
after treatment |
5.42±2.03a,c |
6.02±2.61 |
2.665 |
0.008 |
| CA19-9 (U/ml) |
| 2 days
before treatment | 42.35±1.76 | 42.15±1.04 |
1.424 |
0.155 |
| 3 weeks
after treatment |
39.62±1.37b,d | 41.97±1.28 | 18.332 | <0.001 |
Comparison of efficacy between the
experimental and control groups
In the experimental group, there were 12 cases of
CR, 68 cases of PR, 113 cases of SD, and 27 cases of PD, and the
DCR was 87.73% (193/220) after treatment. In the control group,
there were 6 cases of CR, 28 cases of PR, 110 cases of SD, and 65
cases of PD, and the DCR was 68.90% (144/209) after treatment.
There was no significant difference in CR and SD patients between
the experimental and control groups (P>0.05). There were
significantly more PR patients in the experimental group than the
control group, and significantly less PD patients in the
experimental group than in the control group. The DCR of the
experimental group was significantly higher than that of the
control group (P<0.001) (Table
III).
 | Table III.Comparison of efficacy between the
experimental and control groups [n (%)]. |
Table III.
Comparison of efficacy between the
experimental and control groups [n (%)].
| Items | Experimental group
(n=220) | (n=209) | Control group
χ2 | P-value |
|---|
| CR | 12
(5.45) | 6
(2.87) |
|
|
| PR | 68
(30.91) | 28
(13.40) |
|
|
| SD | 113 (51.36) | 110 (52.63) |
|
|
| PD | 27
(12.27) | 65
(31.10) |
|
|
| DCR |
87.73 |
68.90 | 22.552 | <0.001 |
Grade of the side effects between the
experimental and control groups
According to the Common toxicity criteria, grade
III–IV of severe side effects in the experimental group and the
control group were compared. The incidence of side effects in the
experimental group was significantly lower than that in the control
group, P<0.05, which suggested that interventional therapy could
alleviate the side effects in patients with gastric cancer.
Detailed information is provided in Table IV.
 | Table IV.Grade of side effects between the
experimental and control groups [n (%)]. |
Table IV.
Grade of side effects between the
experimental and control groups [n (%)].
|
| III–IV |
|---|
|
|
|
|---|
| Factors | Experimental group
(n=220) | (n=209) | Control group
χ2 | P-value |
|---|
| Nausea | 23.18 (51/220) | 40.19 (84/209) | 14.382 |
0.001 |
| Vomiting | 20.45 (45/220) | 37.80 (79/209) | 15.688 | <0.001 |
| Hepatic damage | 10.45 (23/220) | 25.84 (54/209) | 17.219 | <0.001 |
| Renal damage | 22.27 (49/220) | 31.10 (65/209) |
4.281 |
0.039 |
Comparison of serum levels of IL-6,
IL-8 and IL-10 before and after treatment between the experimental
and control groups
There was no significant difference in serum IL-6,
IL-8 and IL-10 concentrations between the two groups 2 days before
treatment (P>0.05). The serum levels of IL-6, IL-8 and IL-10 in
the experimental group were significantly lower than those in the
control group 3 weeks after treatment (P<0.05). The serum levels
of IL-6, IL-8 and IL-10 in the experimental group 3 weeks after
treatment were significantly decreased compared with those 2 days
before treatment (P<0.05). Specific data are shown in Table V.
 | Table V.Comparison of relative indicators
before and after treatment between the experimental and control
groups (mean ± SD). |
Table V.
Comparison of relative indicators
before and after treatment between the experimental and control
groups (mean ± SD).
| Factors | Before
treatment | After
treatment | t | P-value |
|---|
| IL-6 (pg/ml) |
|
Experimental group
(n=220) | 78.34±25.12 |
58.23±15.75d |
9.875 | <0.001 |
| Control
group (n=209) | 78.56±29.45 |
79.36±28.34a |
0.775 |
0.286 |
| IL-8 (pg/ml) |
|
Experimental group
(n=220) | 89.36±5.36 |
61.12±18.42e | 21.793 | <0.001 |
| Control
group (n=209) | 89.04±5.41 |
89.86±6.67b |
1.402 |
0.162 |
| IL-10 (pg/ml) |
|
Experimental group
(n=220) | 69.57±5.32 |
67.26±5.23f |
30.441 | <0.001 |
| Control
group (n=209) | 68.65±5.36 |
68.37±5.46c |
0.593 |
0.553 |
Correlation analysis of tumor markers
with IL-6, IL-8 and IL-10 concentrations
Pearson's correlation test was used to analyze the
correlation between CEA, CA19-9 and IL-6, IL-8 and IL-10
concentrations before and after the experiment. The results showed
that the serum CEA concentration of the experimental group was
positively correlated with the serum IL-6, IL-8 and IL-10
concentrations of patients before treatment (r=0.420, 0.397, 0.503,
P<0.001). Before treatment, the serum CA19-9 concentration of
the experimental group was positively correlated with the serum
IL-6, IL-8 and IL-10 concentrations (r=0.410, 0.257, 0.468,
P<0.001). After treatment, the serum CEA concentration of
patients in the experimental group was positively correlated with
IL-6, IL-8 and IL-10 concentrations (r=0.417, 0.355, 0.334,
P<0.001). After treatment, the serum CA19-9 concentration of
patients in the experimental group was positively correlated with
IL-6, IL-8 and IL-10 concentrations (r=0.324, 0.249, 0.408,
P<0.001). After treatment, the correlation between CEA, CA19-9
and IL-6, IL-8 and IL-10 decreased slightly but not significantly
(Fig. 1).
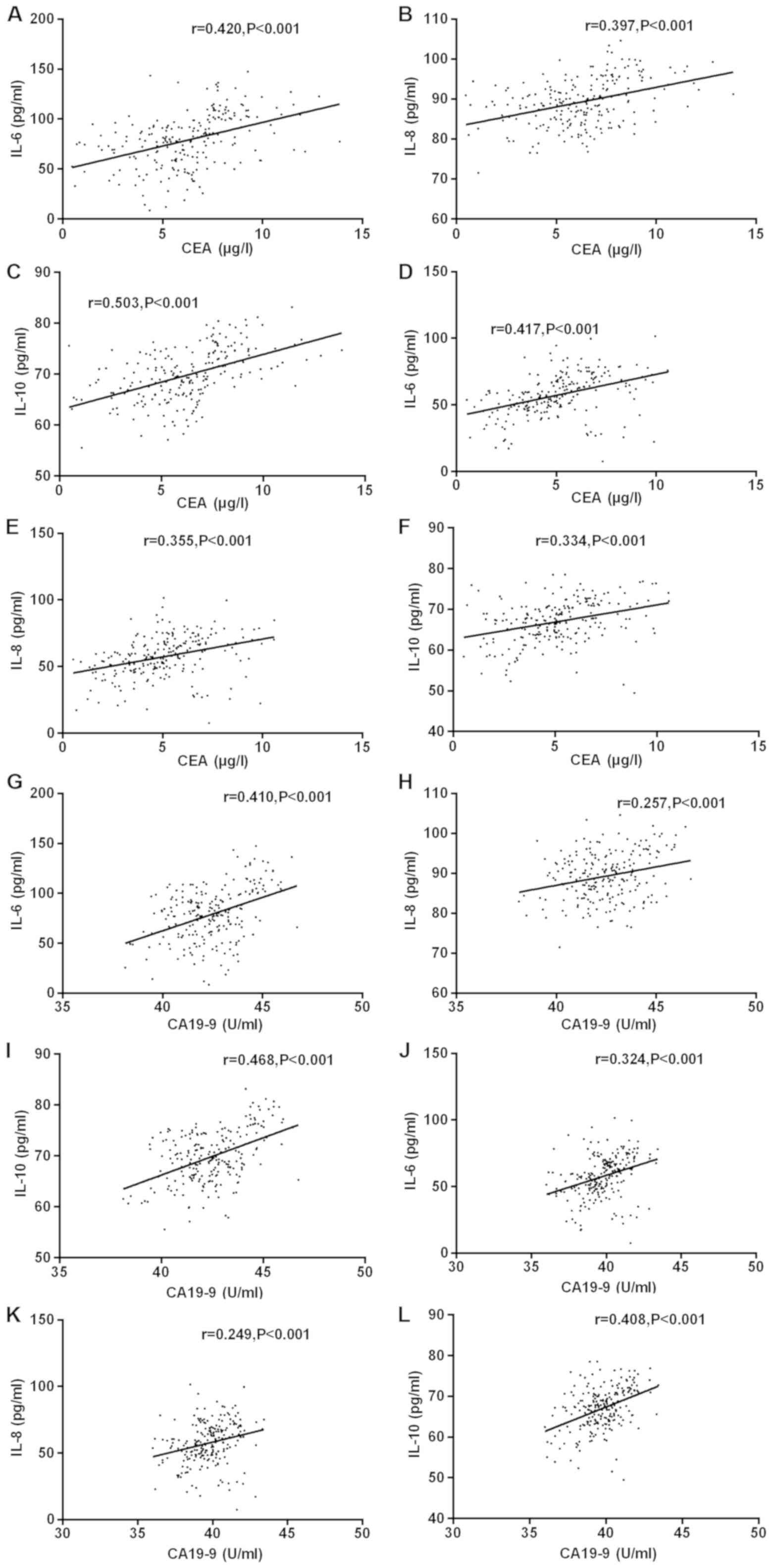 | Figure 1.Correlation analysis of tumor markers
with IL-6, IL-8 and IL-10 concentration before and after the
experiment was performed by Pearson's correlation test. Serum CEA
concentration of the experimental group before treatment was
positively correlated with the serum IL-6, IL-8 and IL-10
concentrations of the patients (r=0.420, 0.397, 0.503; P<0.001).
CA19-9 concentration was positively correlated with IL-6, IL-8 and
IL-10 concentrations in patients' serum (r=0.410, 0.257, 0.468;
P<0.001). After treatment, the serum CEA concentration of the
experimental group was positively correlated with the serum IL-6,
IL-8 and IL-10 concentrations (r=0.417, 0.355, 0.334; P<0.001),
and CA19-9 concentration was positively correlated with the serum
IL-6, IL-8 and IL-10 concentrations (r=0.324, 0.249, 0.408;
P<0.001). (A) Correlation between IL-6 and CEA before treatment.
(B) Correlation between IL-8 and CEA before treatment. (C)
Correlation between IL-10 and CEA before treatment. (D) Correlation
between IL-6 and CEA after treatment. (E) Correlation between IL-8
and CEA after treatment. (F) Correlation between IL-10 and CEA
after treatment. (G) Correlation between IL-6 and CA19-9 before
treatment. (H) Correlation between IL-8 and CA19-9 before
treatment. (I) Correlation between IL-10 and CA19-9 before
treatment. (J) Correlation between IL-6 and CA19-9 after treatment.
(K) Correlation between IL-8 and CA19-9 after treatment. (L)
Correlation between IL-10 and CA19-9 after treatment. |
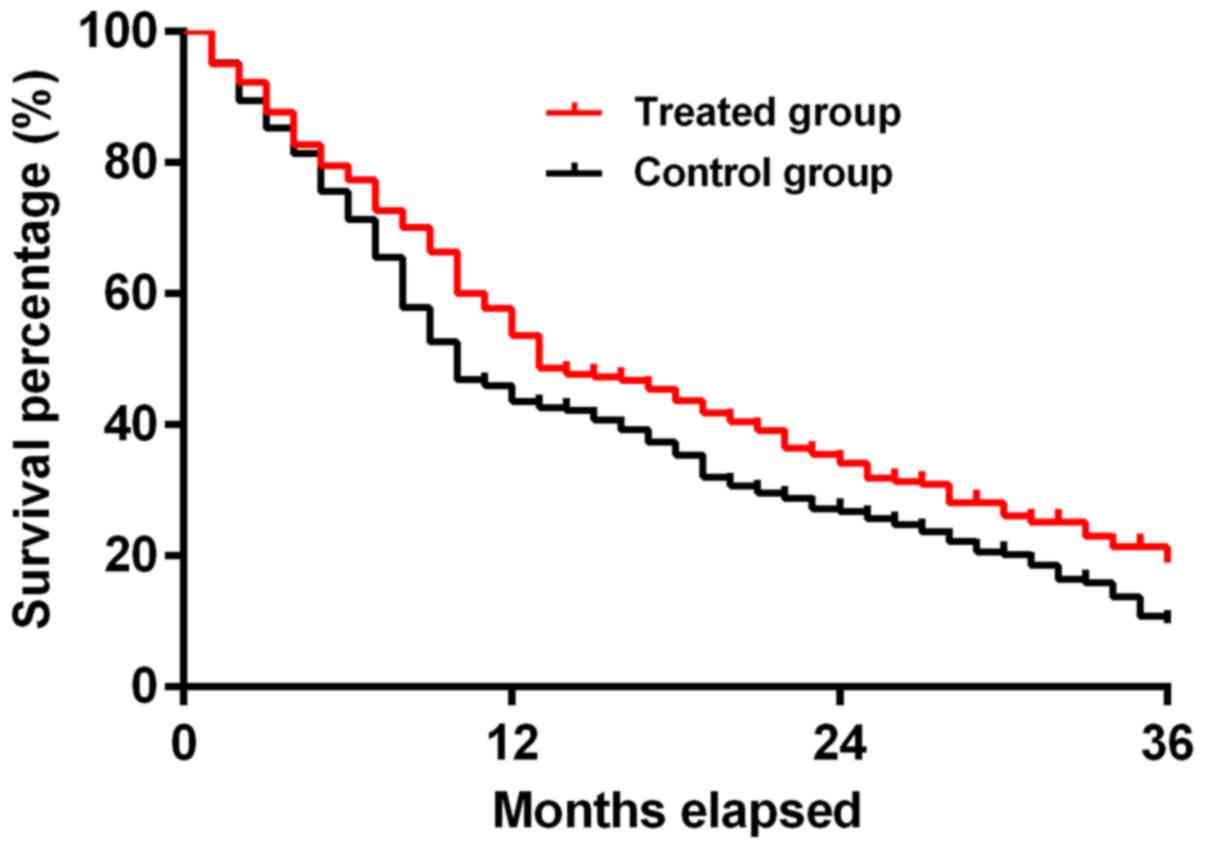
Comparison of survival analysis
between the experimental and control groups
The median overall survival (mOS) in the
experimental group was 13 months (95% CI, 1.057–1.598), and the mOS
in the control group was 10 months (95% CI, 0.6256–0.9458). The
3-year overall survival rate in the two groups was 16.36 and 9.09%,
respectively. The median survival time and overall survival rate in
the experimental group were significantly higher than those in the
control group (χ2=6.440, P<0.05), and the difference
was statistically significant. The survival curve is shown in
Fig. 2. The annual survival rate was
obtained by comparing the number of survivors per year with the
total number of individuals. The 1-, 2- and 3-year survival rates
in the experimental group were higher than those in the control
group (P<0.05) (Table VI and
Fig. 2).
 | Table VI.Comparison of survival analysis
between the experimental and control groups [n (%)]. |
Table VI.
Comparison of survival analysis
between the experimental and control groups [n (%)].
|
Case/percentage | 1-year | 2-year | 3-year | χ2 | P-value |
|---|
| Experimental group
(n=220) | 127 (57.73) | 78
(35.45)b | 36
(16.36)b,c | 81.354 | <0.001 |
| Control group
(n=209) | 96
(45.93)a | 55
(26.32)a,b | 19
(9.09)a–c | 71.880 | <0.001 |
Factors affecting postoperative survival of patients
after the experimental intervention treatment was analyzed by Cox
regression model. The results of single factor analysis showed that
age, Borrmann type, differentiation degree and the history of
Helicobacter pylori infection were associated with the
prognosis and survival of patients with gastric cancer treated by
interventional therapy (P<0.05); and sex, tumor stage history of
peptic ulcer, experiment before chemotherapy cycle, preventive
anti-inflammatory therapy, smoking history, serum creatinine and
blood urea nitrogen were not related to the prognosis and survival
of patients with gastric cancer treated by interventional therapy
(P>0.05), as shown in Table
VII. Multivariate analysis results showed that the age
(P=0.041), Borrmann type (P=0.026), the degree of differentiation
(P=0.005) and Helicobacter pylori infection (P=0.029) were
associated with the prognosis and survival of patients with gastric
cancer treated by interventional therapy. Patients aged >50
years, with infiltrating ulcer type or diffuse infiltration type,
undifferentiated type, and free of Helicobacter pylori
infection had poor prognosis (Table
VIII).
 | Table VII.Univariate analysis of factors
influencing postoperative survival of gastric cancer patients. |
Table VII.
Univariate analysis of factors
influencing postoperative survival of gastric cancer patients.
| Factors | Cases | Overall
survival | P-value |
|---|
| Age (years) |
|
|
0.008 |
|
≤50 | 87 | 15 (12.6–17.2) |
|
|
>50 | 133 | 10 (8.4–11.2) |
|
| Sex |
|
|
0.866 |
|
Male | 177 | 13 (11.3–15.4) |
|
|
Female | 43 | 13 (12.4–14.9) |
|
| Tumor stage |
|
|
0.452 |
|
I+II | 109 | 13 (11.0–15.0) |
|
|
IIIa+IIIb | 111 | 13 (9.7–16.4) |
|
| Borrmann type |
|
| <0.001 |
|
I+II | 54 | 17 (16.2–17.8) |
|
|
III+IV | 166 | 9
(7.7–10.3) |
|
| Degree of
differentiation |
|
| <0.001 |
|
Differentiated | 35 | 15 (12.6–10.2) |
|
| Low
differentiated | 106 | 8
(5.8–10.2) |
|
|
Undifferentiated | 79 | 5
(3.9–6.1) |
|
| History of
Helicobacter pylori infection |
|
| <0.001 |
| No | 104 | 16 (14.6–18.6) |
|
|
Yes | 116 | 8
(5.3–10.7) |
|
| History of peptic
ulcer |
|
|
0.692 |
| No | 102 | 12 (10.3–14.4) |
|
|
Yes | 118 | 13 (8.4–15.8) |
|
| Chemotherapy cycle
before experiment |
|
|
0.111 |
| ≤4 | 178 | 12 (10.5–13.5) |
|
|
>4 | 42 | 13 (9.5–16.4) |
|
| Prophylactic
anti-inflammatory therapy |
|
|
0.673 |
|
Yes | 208 | 13 (10.3–14.7) |
|
| No | 12 | 12 (10.2–13.6) |
|
| Smoking
history |
|
|
0.301 |
| No | 101 | 13 (10.6–15.5) |
|
|
Yes | 119 | 13 (10.2–14.8) |
|
| Serum
creatinine |
|
|
0.561 |
| <133
µmol/l | 64 | 13 (9.3–15.1) |
|
| ≥133
µmol/l | 156 | 13 (9.7–14.2) |
|
| Blood urea
nitrogen |
|
|
0.717 |
|
<7.14 mmol/l | 49 | 13 (10.8–14.2) |
|
| ≥7.14
mmol/l | 171 | 10 (7.4–13.6) |
|
 | Table VIII.Multivariate analysis of factors
influencing postoperative survival of gastric cancer patients. |
Table VIII.
Multivariate analysis of factors
influencing postoperative survival of gastric cancer patients.
| Factors | Regression
coefficient | Standard error | Wald value | HR | 95% CI | P-value |
|---|
| Age (≤50 years,
>50 years) |
0.064 | 0.032 | 4.159 | 1.067 | 0.003–1.135 | 0.041 |
| Borrmann type
(I+II, III+IV) |
0.294 | 0.132 | 4.951 | 1.342 | 1.036–1.740 | 0.026 |
| Degree of
differentiation (differentiated, low differentiated,
undifferentiated) |
0.466 | 0.165 | 7.926 | 1.593 | 1.152–2.204 | 0.005 |
| History of
Helicobacter pylori infection (no, yes) | −1.511 | 0.690 | 4.789 | 0.221 | 0.057–0.854 | 0.029 |
Discussion
In modern society, the aging of population is
becoming more obvious. As the incidence of gastric cancer is
increasing with age, the number of elderly gastric cancer patients
is also increasing. The elderly patients have poor immunity, poor
surgical tolerance and difficulty in recovery (26). For patients who cannot tolerate
surgery, the most important clinical treatment method is still
chemotherapy, but most of the chemotherapy drugs, such as
paclitaxel and epirubicin, have relatively obvious toxic side
effects, and cannot be used for long time periods (27). Therefore, it is important to search
for new and safe treatment methods for the prognosis and survival
of gastric cancer patients.
Infusion chemoembolization, a common interventional
therapy, takes chemotherapeutic drugs through blood vessels to the
tumor, while using gelfoam particles to block tumor blood vessels
directly, and at the same time using lipiodol to immerse cancer
cells directly in high concentrations of drugs to accurately strike
cancer cells, which makes up for the insufficient intensity of
local target lesions in systemic intravenous chemotherapy (28). However, there are few studies on
interventional therapy for patients with gastric cancer, so we
focused on the analysis of the efficacy of interventional therapy
for patients with gastric cancer after chemotherapy, in order to
provide clinical reference for patients with gastric cancer.
It was found that the serum level of tumor marker
CEA and CA19-9 in patients treated with interventional therapy was
significantly lower than that in the control group (P<0.05),
indicating that interventional therapy may cause the change of CEA
and CA19-9 in patients. Research shows that CEA is the best tumor
marker for gastrointestinal malignancies at present, with low cost
and high sensitivity. Its diagnostic accuracy of tumor metastasis
and recurrence is higher than other serologic biomarkers (29). CA19-9 is also a common tumor marker
in the detection of gastric cancer. It has been reported that the
detection of CEA and CA19-9 in serum before surgery can more
effectively detect the recurrence of gastric cancer (30). In addition, Lee et al
(31) found that the level of CEA
and CA19-9 in serum could assess the prognosis of patients after
chemotherapy, and the decreased concentration of these two tumor
markers was related to the improvement of patients' survival rate.
Although the study was aimed at cholangiocarcinoma, another
malignancy of the digestive tract, it can also support our study
that interventional therapy can reduce the serum marker
concentration of patient and possibly improve the prognosis of
patient.
The results showed that patients treated with
interventional therapy had better efficacy, less side effects and
longer survival time than patients with systemic chemotherapy only.
The effective rate of treatment in the experimental group was
87.73%, the cumulative 1-, 2- and 3-year survival rates were 57.73,
35.45 and 16.36%, respectively, and the median survival time was 13
months. It is believed that the interventional therapy can focus on
the lesions and has a better effect on killing cancer tissue, thus
achieving a better efficacy. The relative literature showed that
compared with peripheral intravenous administration, interventional
therapy can better locate the treatment site to the tumor target
lesion, rather than the tumor adjacent tissue, and can guarantee
the drug concentration in the target lesion area, therefore
inhibiting tumor growth (32).
However, for patients with gastric cancer, the most alarming
complication is gastrointestinal hemorrhage (33). Research has shown that in the case of
malignant tumor hemorrhage the expected survival and quality of
life of patients should be considered. Since 1970s, arterial
embolization has been used to treat refractory hemorrhage in cancer
patients (34). A study has shown
that patients with gastric cancer hemorrhage treated by endoscopy
are prone to recurrence and re-hemorrhage; at this point, arterial
embolization should be considered, which may lead to better
efficacy (35).
Univariate and multivariate analyses were performed
on Cox regression model of prognostic factors in patients with
gastric cancer. It was found that age, Borrmann type,
differentiation degree and history of Helicobacter pylori
infection were associated with interventional therapy on the
prognosis of patients with gastric cancer survival (P<0.05), and
age (P=0.041), Borrmann type (P=0.026), the degree of
differentiation (P=0.005) and Helicobacter pylori infection
(P=0.029) are independent prognostic factors for patients with
interventional therapy. Patients aged over 50 years, with Borrmann
III+IV type and undifferentiated type, and free of Helicobacter
pylori infection had poor prognosis. This study indicated that
patients with gastric cancer who were not infected with
Helicobacter pylori before operation had poor prognosis and
lower survival rate than patients with Helicobacter pylori
infection. Negative Helicobacter pylori is an independent
prognostic factor for poor prognosis (36,37),
which are consistent with our results. We believe that the
prognosis of patients with Helicobacter pylori infection can
improve activation of antitumor immunity. However, a study has
pointed out that Helicobacter pylori is one of the main
causes of gastric cancer, and positive Helicobacter pylori
can be an independent factor for the prognosis and survival of
gastric cancer patients (38). The
connection may still need further study. In another study the
follow-up analysis of 3,966 patients with gastric cancer, showed
that Borrmann I–IV survival rate was 68.1, 67.5, 55.2 and 31.8%.
Borrmann type III+IV patients had higher percentage of serosal
invasion, serous diffusion and undifferentiated ratio than Borrmann
type I+II tumor patients, and Borrmann type III+IV patients were
prone to lymph node involvement (39), showing that Borrmann type is an
independent prognostic factor in patients with gastric cancer.
Finally, differentiation degree and age have also been found as
independent prognostic factors of gastric cancer patients in other
studies (40). The prognosis of
patients with low differentiation and older age is generally poor.
The above support our views that age, Borrmann classification,
degree of differentiation, and history of Helicobacter
pylori infection were related to the prognosis of patients with
interventional gastric cancer, and more attention should be paid to
patients with these risk factors in clinical practice.
After intervention, compared with the control group,
the levels of IL-6, IL-8 and IL-10 in the experimental group were
significantly decreased, indicating that interventional therapy has
some anti-inflammatory effects in patients. As an inflammatory
medium with many different functions, IL-6 has been shown to be
involved in tumor metastasis and local invasion of gastric cancer,
which can induce vascular endothelial growth factor and promote the
activity of tumors (41). IL-8 is a
chemokine produced by various malignant tumor cells, which can play
a role in tumor formation and immunity. A study revealed that IL-8
level in patient serum was correlated with tumor load, and the
monitoring of the IL-8 level was of great significance for
prognosis (42). IL-10 is considered
to be a tolerant cytokine, which can inhibit the production of
pro-inflammatory cytokines in medullary cells such as macrophages
and the stimulation ability of T cells. In addition, a study has
shown that it has a non-superfluous tolerance effect in intestinal
immunity, which may induce the occurrence of rectal inflammation
(43). We found a significant
reduction in the three factors after interventional therapy, which
can also explain that intervention may be able to regulate the
body's immune function, thereby causing a better curative effect.
However, due to the limitation of the experimental conditions, we
did not study how interventional therapy leads to the lower
expression of inflammatory markers.
The study results also showed that the expression of
tumor markers CEA and CA19-9 and inflammatory factors IL-6, IL-8,
IL-10 in both before and after the experiment present a positive
correlation (P<0.05). After treatment, the correlation between
CEA and CA19-9 and IL-6, IL-8 and IL-10 declined slightly, but
without a statistically significant difference (P>0.05). There
was an association between IL-6, IL-8, IL-10 and the digestive
system tumors. This may be related to the inflammatory response
during tumorigenesis, or may be the result of some unknown factors.
Currently, there are few reports on correlation between these three
inflammatory factors and CEA, CA19-9. Our study indicated that
there was a positive correlation. It may be a coincidence, but the
result is very interesting, and may provide more potential options
for serum biological markers. The mechanisms of the two between
tumor markers and interleukins can be one of the key research
directions in the future.
There are still some limitations in this study.
First, the data which were retrospectively obtained were sometimes
inevitably interfered by subjective factors. Second, because the
general clinical stages of patients were relatively late, the
period of drug intervention was not entirely optimal, and the
survival feedback received was not fully comprehensive. Third, the
time span of this study was long, and there were some inevitable
situation such as loss of visits to patients.
Therefore, interventional therapy has a good
tolerance and a high effective rate for gastric cancer patients. It
can also alleviate the adverse reactions and is worthy of clinical
promotion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PW drafted the manuscript and was responsible for
chemotherapy. PW and JW contributed to interventional therapy. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Xiangyang No. 1 People's Hospital, Hubei University of
Medicine (Xiangyang, China). Signed informed consents were obtained
from the patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dong X, Wang G, Zhang G, Ni Z, Suo J, Cui
J, Cui A, Yang Q, Xu Y and Li F: The endothelial lipase protein is
promising urinary biomarker for diagnosis of gastric cancer. Diagn
Pathol. 8:452013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen L, Li J, Xu J, Pan H, Dai G, Qin S,
Wang L, Wang J, Yang Z, Shu Y, et al: Bevacizumab plus capecitabine
and cisplatin in Chinese patients with inoperable locally advanced
or metastatic gastric or gastroesophageal junction cancer:
Randomized, double-blind, phase III study (AVATAR study). Gastric
Cancer. 18:168–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003–2005: A population-based study. Int J Cancer.
136:1921–1930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Japanese Gastric Cancer Association:
Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric
Cancer. 20:1–19. 2017. View Article : Google Scholar
|
|
6
|
Baum RA and Baum S: Interventional
radiology: A half century of innovation. Radiology. 273
(Suppl):S75–S91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vogl TJ, Gruber-Rouh T, Eichler K,
Nour-Eldin NE, Trojan J, Zangos S and Naguib NN: Repetitive
transarterial chemoembolization (TACE) of liver metastases from
gastric cancer: Local control and survival results. Eur J Radiol.
82:258–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chehab MA, Brinjikji W, Copelan A and
Venkatesan AM: Navigational tools for interventional radiology and
interventional oncology applications. Semin Intervent Radiol.
32:416–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu DSK, Raman SS, Limanond P, Aziz D,
Economou J, Busuttil R and Sayre J: Influence of large peritumoral
vessels on outcome of radiofrequency ablation of liver tumors. J
Vasc Interv Radiol. 14:1267–1274. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang F, Meng W, Wang B and Qiao L:
Helicobacter pylori-induced gastric inflammation and gastric
cancer. Cancer Lett. 345:196–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rugge M, Fassan M and Graham DY:
Epidemiology of gastric cancer. Gastric Cancer: Principles and
Practice. Strong VE: 1st. Springer; Cham, Switzerland: pp. 23–34.
2015, View Article : Google Scholar
|
|
12
|
Fu XL, Duan W, Su CY, Mao FY, Lv YP, Teng
YS, Yu PW, Zhuang Y and Zhao YL: Interleukin 6 induces M2
macrophage differentiation by STAT3 activation that correlates with
gastric cancer progression. Cancer Immunol Immunother.
66:1597–1608. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li W, Zhou Y, Yang J, Zhang X, Zhang H,
Zhang T, Zhao S, Zheng P, Huo J and Wu H: Gastric cancer-derived
mesenchymal stem cells prompt gastric cancer progression through
secretion of interleukin-8. J Exp Clin Cancer Res. 34:52. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pachnia D, Drop B, Dworzańska A,
Kliszczewska E and Polz-Dacewicz M: Transforming growth factor-β,
interleukin-10, and serological markers in EBV-associated gastric
carcinoma. Anticancer Res. 37:4853–4858. 2017.PubMed/NCBI
|
|
15
|
Kang JM, Kim N, Lee DH, Park JH, Lee MK,
Kim JS, Jung HC and Song IS: The effects of genetic polymorphisms
of IL-6, IL-8, and IL-10 on Helicobacter pylori-induced
gastroduodenal diseases in Korea. J Clin Gastroenterol. 43:420–428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taniguchi K and Karin M: IL-6 and related
cytokines as the critical lynchpins between inflammation and
cancer. Semin Immunol. 26:54–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang SP, Wu MS, Shun CT, Wang HP, Lin MT,
Kuo ML and Lin JT: Interleukin-6 increases vascular endothelial
growth factor and angiogenesis in gastric carcinoma. J Biomed Sci.
11:517–527. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang XY, Chan WY, Whitney BM, Fan DM,
Chow JH, Liu Y, Ng EK and Chung SC: Changes of interleukin
expression correlate with Helicobacter pylori infection and
lymph node metastases in gastric carcinoma. Diagn Mol Pathol.
11:135–139. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malalasekera A, Tan CSY, Phan V, Yip PY,
Vardy J, Clarke S and Kao S: Eastern Cooperative Oncology Group
score: Agreement between non-small-cell lung cancer patients and
their oncologists and clinical implications. Cancer Treat Commun.
5:17–21. 2016. View Article : Google Scholar
|
|
20
|
Vincent MD, Breadner D, Cripps MC, Jonker
DJ, Klimo P, Biagi JJ, Lam W, O'Connell A, Whiston F, Stitt L, et
al: Phase I/II trial of dose-reduced capecitabine in elderly
patients with advanced colorectal cancer. Curr Oncol. 24:e261–e268.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Shi H, Yang G, Han G, Zhao M, Duan
X, Mi L, Han X, Li N, Shi J, et al: Combined intra-arterial and
intravenous chemotherapy for unresectable, advanced gastric cancer
has an improved curative effect compared with intravenous
chemotherapy only. Oncol Lett. 15:5662–5670. 2018.PubMed/NCBI
|
|
22
|
Kim HS, Kim JW, Kim JH, Choi DR, Han AR,
Kim MJ, Kim BC and Zang DY: Single-lesion measurement per organ for
assessing tumor response in advanced gastric cancer. Oncology.
88:69–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Trotti A, Byhardt R, Stetz J, Gwede C,
Corn B, Fu K, Gunderson L, McCormick B, Morrisintegral M, Rich T,
et al: Common toxicity criteria: version 2.0. An improved reference
for grading the acute effects of cancer treatment: Impact on
radiotherapy. Int J Radiat Oncol Biol Phys. 47:13–47. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth Edition AJCC Cancer Staging Manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Wang X, Kou H, He H, Lu M, Zhou L
and Yang Y: Comparing single oral contrast-enhanced ultrasonography
and double contrast-enhanced ultrasonography in the preoperative
Borrmann classification of advanced gastric cancer. Oncotarget.
9:8716–8724. 2017.PubMed/NCBI
|
|
26
|
Liu G, Jian F, Wang X and Chen L:
Fast-track surgery protocol in elderly patients undergoing
laparoscopic radical gastrectomy for gastric cancer: A randomized
controlled trial. Onco Targets Ther. 9:3345–3351. 2016.PubMed/NCBI
|
|
27
|
Shi J, Gao P, Song Y, Chen X, Li Y, Zhang
C, Wang H and Wang Z: Efficacy and safety of taxane-based systemic
chemotherapy of advanced gastric cancer: A systematic review and
meta-analysis. Sci Rep. 7:53192017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Baere T, Arai Y, Lencioni R, Geschwind
JF, Rilling W, Salem R, Matsui O and Soulen MC: Treatment of liver
tumors with lipiodol TACE: Technical recommendations from experts
opinion. Cardiovasc Intervent Radiol. 39:334–343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Acharya A, Markar SR, Matar M, Ni M and
Hanna GB: Use of tumor markers in gastrointestinal cancers: Surgeon
perceptions and cost-benefit trade-off analysis. Ann Surg Oncol.
24:1165–1173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee EC, Yang JY, Lee KG, Oh SY, Suh YS,
Kong SH, Yang HK and Lee HJ: The value of postoperative serum
carcinoembryonic antigen and carbohydrate antigen 19-9 levels for
the early detection of gastric cancer recurrence after curative
resection. J Gastric Cancer. 14:221–228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee DW, Im SA, Kim YJ, Yang Y, Rhee J, Na
II, Lee KH, Kim TY, Han SW, Choi IS, et al: CA19-9 or CEA decline
after the first cycle of treatment predicts survival in advanced
biliary tract cancer patients treated with S-1 and cisplatin
chemotherapy. Cancer Res Treat. 49:807–815. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanehira Y, Togami K, Tada H and Chono S:
Tumor distribution and anti-tumor effect of doxorubicin following
intrapulmonary administration to mice with metastatic lung tumor. J
Drug Deliv Sci Technol. 33:143–148. 2016. View Article : Google Scholar
|
|
33
|
Pucheanu X and Beuran M: Bleeding gastric
cancer in young and elderly patients. J Med Life. 8:356–360.
2015.PubMed/NCBI
|
|
34
|
Niekamp A, Sheth RA, Kuban J, Avritscher R
and Ganguli S: Palliative embolization for refractory bleeding.
Semin Intervent Radiol. 34:387–397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim YI and Choi IJ: Endoscopic management
of tumor bleeding from inoperable gastric cancer. Clin Endosc.
48:121–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jung DH, Lee YC, Kim JH, Chung H, Park JC,
Shin SK, Lee SK, Kim HI, Hyung WJ and Noh SH: Postoperative
Helicobacter pylori infection as a prognostic factor for
gastric cancer patients after curative resection. Gut Liver.
11:635–641. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Postlewait LM, Squires MH III, Kooby DA,
Poultsides GA, Weber SM, Bloomston M, Fields RC, Pawlik TM,
Votanopoulos KI, Schmidt CR, et al: Preoperative Helicobacter
pylori infection is associated with increased survival after
resection of gastric adenocarcinoma. Ann Surg Oncol. 23:1225–1233.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang WJ, Du Y, Zhao X, Ma LY and Cao GW:
Inflammation- related factors predicting prognosis of gastric
cancer. World J Gastroenterol. 20:4586–4596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li C, Oh SJ, Kim S, Hyung WJ, Yan M, Zhu
ZG and Noh SH: Macroscopic Borrmann type as a simple prognostic
indicator in patients with advanced gastric cancer. Oncology.
77:197–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zu H, Wang H, Li C and Xue Y:
Clinicopathologic characteristics and prognostic value of various
histological types in advanced gastric cancer. Int J Clin Exp
Pathol. 7:5692–5700. 2014.PubMed/NCBI
|
|
41
|
Gopinathan G, Milagre C, Pearce OM,
Reynolds LE, Hodivala-Dilke K, Leinster DA, Zhong H, Hollingsworth
RE, Thompson R, Whiteford JR, et al: Interleukin-6 stimulates
defective angiogenesis. Cancer Res. 75:3098–3107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sanmamed MF, Carranza-Rua O, Alfaro C,
Oñate C, Martín-Algarra S, Perez G, Landazuri SF, Gonzalez A, Gross
S, Rodriguez I, et al: Serum interleukin-8 reflects tumor burden
and treatment response across malignancies of multiple tissue
origins. Clin Cancer Res. 20:5697–5707. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Geginat J, Larghi P, Paroni M, Nizzoli G,
Penatti A, Pagani M, Gagliani N, Meroni P, Abrignani S and Flavell
RA: The light and the dark sides of Interleukin-10 in
immune-mediated diseases and cancer. Cytokine Growth Factor Rev.
30:87–93. 2016. View Article : Google Scholar : PubMed/NCBI
|