Introduction
Cervical cancer (CC) is one of the most prevalent
and malignant types of cancer in women, and is the second most
common malignancy in women with >50 million new cases each year
(1). Although cervical screening
programs in certain developed countries have contributed to the
detection and prompt treatment of cervical abnormalities, the
morbidity and mortality of CC has continued to increase on a yearly
basis, particularly in developing countries (2).
Tumor biomarkers have been used for the clinical
diagnosis of cancer during the early stages, and have been
demonstrated to greatly improve the effectiveness of treatments and
thus the quality of life. Of these biomarkers, squamous cell
carcinoma antigen (SCCA) is a predictive and prognostic factor for
CC (3). However, SCCA exhibits low
sensitivity, resulting in a relatively high risk of missed
diagnosis of CC during screening (4,5). A
previous study indicated that the levels of SCCA were also
increased in other types of cancer, including breast cancer
(5). Sugar chain antigen 125
(CA-125) was used for the diagnosis of ovarian carcinoma and its
sensitivity was primarily based on the staging of ovarian
malignancies (6). However, it has
been reported that the expression levels of CA-125 were also
elevated in other malignancies, including ~10.4% of benign ovarian
tumors (7). Therefore, it is
necessary to screen for more specific indicators that could predict
the prognosis and assist in the diagnosis of CC.
Researchers have attempted to identify more accurate
and simple serum indices, such as serum vascular endothelial growth
factor levels (8) and pre-treatment
serum hemoglobin levels (9), to
assist in the diagnosis and prognosis of CC. However, there remains
a lack of specific predictors for the pathogenesis of CC and thus
more useful markers are required for improved early diagnosis.
Therefore, based on the results of the aforementioned previous
studies (8,9), the present study further investigated
the predictive factors associated with CC progression.
The aim of the present study was to identify
biomarkers to predict and detect progression of CC through
re-analysis of clinical serum indices using a multistage approach.
A total of four serological tumor markers, including sugar chain
antigen 199 (CA-199), CA-125, α-fetoprotein (AFP) and
carcino-embryonic antigen (CEA) were selected in the present study
to assess their diagnostic specificity for CC. Furthermore, the
present study also analyzed three potential metabolic factors,
including alkaline phosphatase (ALP), cholesterol and triglyceride
(TG) for the prediction of CC progression. The present study
systemically analyzed the association between seven types of serum
indices and CC, in order to assess whether they could be used as
early prediction factors of CC.
Patients and methods
Study population
The present study included 259 patients with CC and
390 healthy women recruited from June 2012 to June 2017 at The
Affiliated Hospital of Jiangsu University (Zhenjiang, China). The
characteristics of patients with CC and healthy control women are
summarized in Table I. The
pathological classification of CC was based on the morphology of
cervical tissues and cells, and confirmed by two independent
pathologists at The Affiliated Hospital of Jiangsu University.
Patients with CC were divided into three groups: i) Stage I; ii)
Stage II; and iii) Stage III/IV, according to the International
Federation of Gynecology and Obstetrics (FIGO) staging system
(10). Additionally, patients with
CC and healthy controls were divided into three age groups: <40
years old, 40–50 years old and >50 years old. The present study
was approved by The Ethics Committee for Biomedical Research at
Affiliated Hospital of Jiangsu University (approval no. 20120019),
and conducted according to the guidelines for clinical
retrospective studies (11). All
participants were fully informed of the purpose and significance of
the study. Written informed consent was obtained from all the
participants for participation.
 | Table I.Characteristics of normal controls
and patients with CC. |
Table I.
Characteristics of normal controls
and patients with CC.
|
|
| CC |
|---|
|
|
|
|
|---|
| Characteristic | Normal | SCC | AC | ASC |
|---|
| Participants, n
(%) | 390 | 235 (90.7%) | 23 (8.9%) | 1 (0.4%) |
| Age, mean ±
standard deviation | 35.8±9.7 | 50.7±11.3 | 50.8±11.0 | 38 |
| Age, n |
| <40 | 258 | 32 | 5 | 1 |
| 40–50 | 94 | 102 | 7 | N/A |
| >50 | 38 | 101 | 11 | N/A |
| FIGO stage, n
(%) | N/A | 235 (90.7%) | 23 (8.9%) | 1 (0.4%) |
| I | N/A | 119 (45.9%) | 16 (6.2%) | N/A |
| II | N/A | 90 (34.7%) | 6 (2.3%) | N/A |
| III/IV | N/A | 26 (10.1%) | 1 (0.4%) | 1 (0.4%) |
Data collection
The accrual data collection was retrospective.
Inclusion criteria for patients with CC were: i) Patients diagnosed
with CC; ii) FIGO Stage I–IV; iii) data collection prior to radical
surgery for CC from patients not treated with radiotherapy or
chemotherapy; and iv) no abnormal fever or infection present.
Exclusion criteria for patients with CC were: i) Data collection
following radical surgery for CC or postoperative recurrent cases
of CC; ii) patients who have undergone radiotherapy or
chemotherapy; iii) patients with other malignant tumors, including
ovary masses and uterine fibroids; iv) patients with other
diseases, including hepatitis, chronical disorder, inflammatory
infection, endometriosis, uterine fibroid and cervical
intraepithelial neoplasia, which would have affected serum indices
used in the present study and v) patients exceeding the normal
upper limit of aspartate aminotransferase (AST) and alanine
transferase (ALT). The average age of patients with CC tested for
serum indices was 50.6±11.3 years. Data for healthy women were
collected from the physical examination center, with an average age
of 35.8±9.7 years. No organic lesions or abnormal alterations were
identified during the physical examination of the healthy
women.
Hematoxylin and eosin (H&E)
staining
Briefly, cervical tissues were fixed in 4%
paraformaldehyde at room temperature for 40 min, embedded in
paraffin and cut into 5-µm-thick sections. After deparaffinization
and rehydration, sections were stained with hematoxylin solution
for 5 min followed by 5 dips in 1% acid ethanol (1% HCl in 70%
ethanol) and then rinsed in distilled water at room temperature.
Then the sections were stained with eosin solution for 3 min and
followed by dehydration with graded alcohol and clearing in xylene
at room temperature. The samples were mounted with neutral balsam
and visualized using a bright-field fluorescent microscope (×100
magnification; Axioskop 2 Plus; Carl Zeiss AG, Oberkochen,
Germany).
Detection of serum indices
For detection of serum indices, 24 h prior to
commencement of the program, and 48 h after the last exercise
training session and after 12 h of fasting, 5-ml blood samples were
collected from the brachial veins of the participants between 8 and
10 a.m. Samples were poured into testing tubes without an
anticoagulant, to allow the serum to separate. To minimize the time
the samples were kept in laboratory conditions, after a 5-min
incubation at room temperature, the sample was immediately
centrifuged with 1,500 × g at 4°C for 5 min and the serum solution
was divided after blood clotting. Subsequently, the resulting serum
was used to determine the levels of CA-199, CA-125, AFP, CEA, ALP,
cholesterol, TG, ALT, AST and estradiol. Serum indices were
measured at the same time-point using an immunochemiluminescence
detection system (i2000-SR; Abbott Pharmaceutical Co., Ltd., Lake
Bluff, IL, USA) and an automated biochemical analyzer (AU5800;
Beckman Coulter, Inc., Brea, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cervical tissues using
RNAiso Plus (Takara Biotechnology Co., Ltd., Dalian, China)
according to the manufacturer's protocol. Total RNA was reverse
transcribed into cDNA at 37°C for 15 min followed by 85°C for 5 sec
using a Superscript RT kit (Takara Biotechnology Co., Ltd.) and
amplified using the SYBR Premix Ex Tag™ kit (cat. no.
RR420B; Takara Biotechnology Co., Ltd.), under the following
conditions: Pre-denaturation at 95°C for 30 sec; 40 cycles of
denaturation at 95°C for 3 sec, annealing at 60°C for 30 sec and
extension at 72°C for 60 sec. The following primers were used for
the amplification of tissue non-specific isozyme ALP (TNALP):
Forward, 5′-ACTCTCCGAGATGGTGGTGGTG-3′ and reverse,
5′-CGTGGTCAATTCTGCCTCCTTCC-3′. GAPDH was used as an internal
control with the following primers: Forward,
5′-ACCCAGAAGACTGTGGATGG-3′ and reverse, 5′-TTCAGCTCAGGGATGACCTT-3′.
The relative changes in gene expression were calculated using the
2−ΔΔCq method (12).
Statistical analysis
Data are presented as the mean ± standard deviation.
Odds ratios (ORs) and 95% confidence intervals (CIs) were analyzed
using logistic regression. Differences between two groups were
calculated using a Student's t-test. Differences for the comparison
of multiple groups were calculated using one-way ANOVA with a
post-hoc Bonferroni test. Data were analyzed using SPSS software
(version 22; IBM Corp., Armonk, NY, USA). Experiments were repeated
at least three times. P<0.05 was considered to indicate a
statistically significant difference.
Results
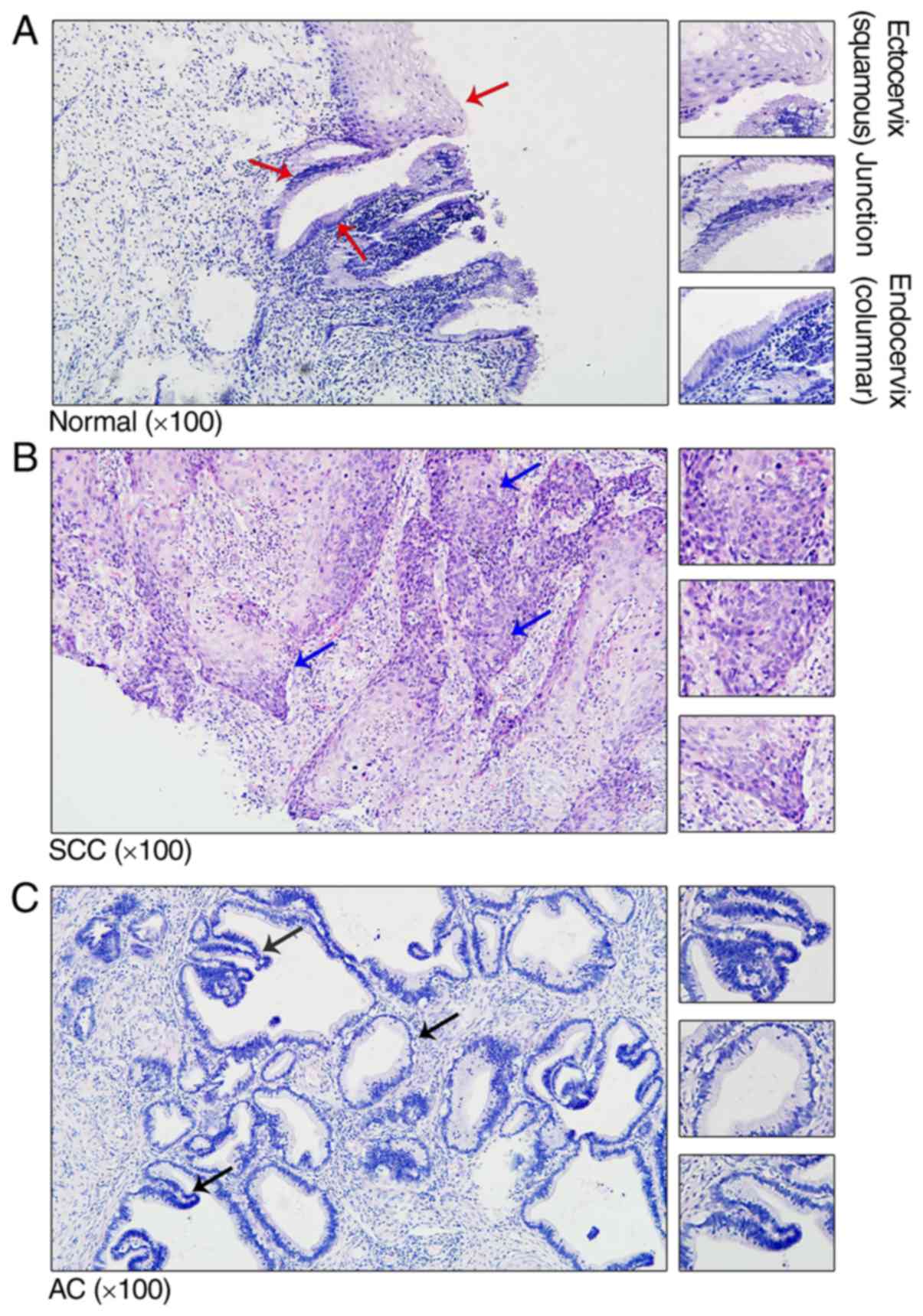
Structure of cervical tissue in normal
controls and patients with CC
To investigate the structure of cervical tissue in
the normal controls and patients with CC, cervical tissue was
stained using H&E. Histological analysis of the
ectoendocervical squamocolumnar (SC) junction from normal adults
revealed a discrete population of cuboidal epithelial cells at the
interface of the ectocervix and endocervix (Fig. 1A). Previously, gene expression
profiling separated the SC junction cells from the squamous and
columnar cells, indicating significant differences between the two
types of cells (13). In patients
with cervical squamous cell carcinoma (SCC) and cervical
adenocarcinoma (AC), the SC junction disappeared and was replaced
with tumor cells (Fig. 1B and
C).
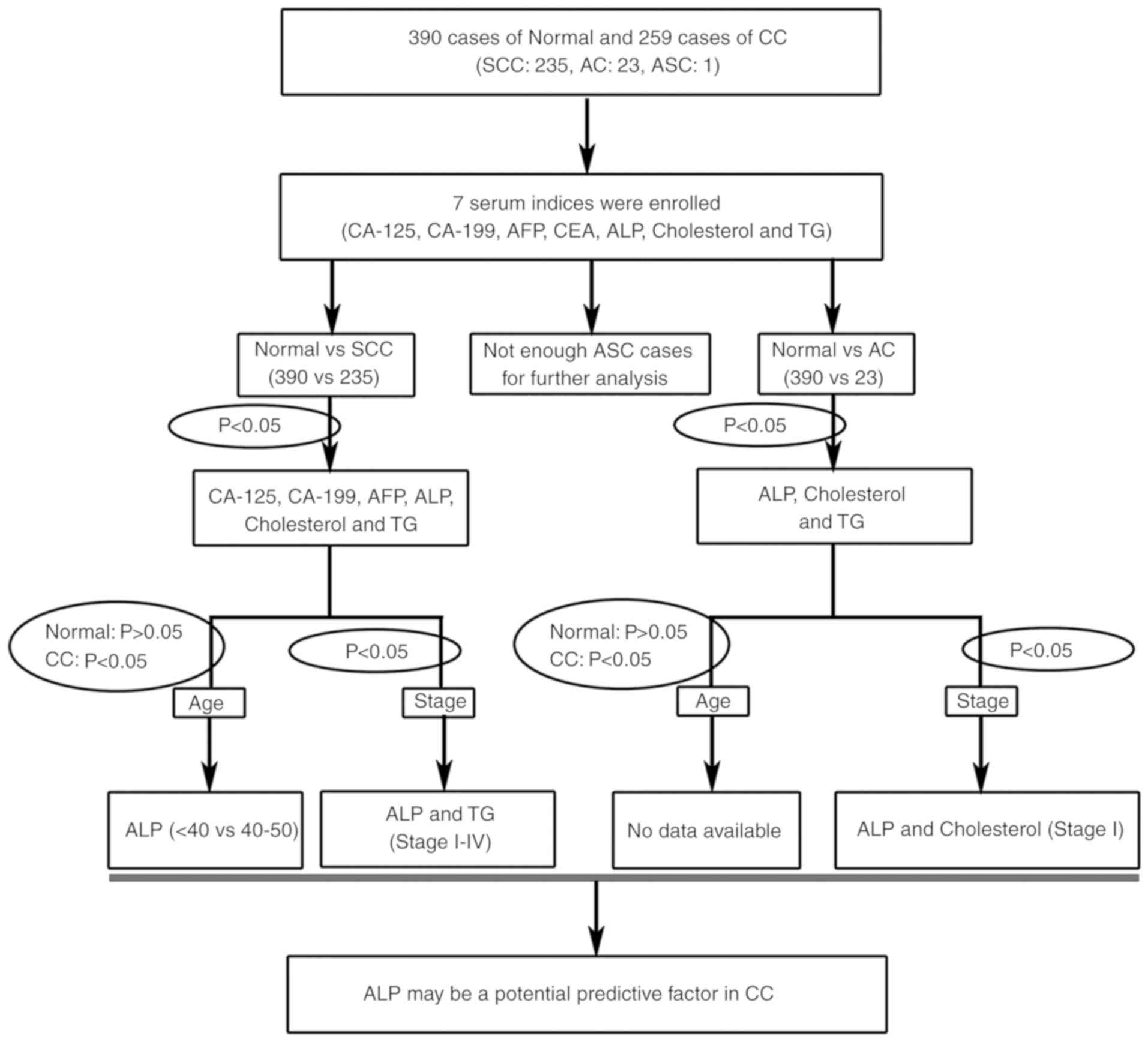
Re-analysis of potential serum indices
for CC
To identify novel clinical predictive factors for
patients with CC, the present study systemically used a multistage
re-analysis strategy of seven serum indices (Fig. 2). A total of four serological tumor
markers, including CA-199, CA-125, AFP and CEA were used in the
present study to assess their specificity for CC. Furthermore,
three potential metabolic factors, including ALP, cholesterol and
TG were also analyzed for the prediction of CC progression.
The expression levels of CA-125 (P=0.035), CA-199
(P=0.011), AFP (P=0.043), ALP (P<0.001), cholesterol (P=0.017)
and TG (P<0.001) were significantly different between healthy
women and patients with SCC. Furthermore, for healthy women and
patients with AC, ALP (P=0.001), cholesterol (P=0.002) and TG
(P=0.015) expression levels changed significantly (Table II).
 | Table II.Comparison of the seven serum indices
in normal controls and patients with cervical cancer. |
Table II.
Comparison of the seven serum indices
in normal controls and patients with cervical cancer.
|
| Normal | SCC | Normal vs. SCC | AC | Normal vs. AC |
|---|
|
|
|
|
|
|
|
|---|
| Serum index | n | Mean ± SD | n | Mean ± SD | OR (95% CI) | P-value | n | Mean ± SD | OR (95% CI) | P-value |
|---|
| CA-125 | 246 | 16.530±8.573 | 137 | 29.923±73.543 | 1.028
(1.011–1.044) | 0.035 | 16 | 24.644±29.386 | 1.037
(1.007–1.068) | 0.288 |
| CA-199 | 220 | 10.614±7.762 | 114 | 16.603±24.190 | 1.026
(1.008–1.044) | 0.011a | 12 | 10.497±6.714 | 0.998
(0.925–1.077) | 0.959 |
| AFP | 387 | 3.487±2.475 | 129 | 3.008±1.764 | 0.897
(0.806–0.998) | 0.043a | 15 | 3.053±2.036 | 0.909
(0.687–1.203) | 0.503 |
| CEA | 388 | 2.426±2.807 | 134 | 10.178±67.004 | 1.096
(1.039–1.157) | 0.183 | 16 | 3.619±3.654 | 1.073
(0.976–1.181) | 0.216 |
| ALP | 237 | 53.236±16.262 | 218 | 72.168±24.482 | 1.052
(1.039–1.065) |
9.58×10−20c | 21 | 65.990±23.815 | 1.032
(1.011–1.054) | 0.001b |
| Cholesterol | 388 | 4.645±0.823 | 160 | 4.876±1.093 | 1.316
(1.076–1.610) | 0.017a | 18 | 5.258±0.928 | 2.295
(1.332–3.956) | 0.002b |
| TG | 388 | 1.105±0.526 | 158 | 1.679±1.074 | 3.226
(2.325–4.477) |
1.14×10−9c | 18 | 1.708±0.940 | 2.774
(1.594–4.827) | 0.015a |
Age variation and CC
Age is a significant risk factor for tumorigenesis
(14). The results of the present
study indicated that body homeostasis was altered with age. In
normal control women, CA-125, CA-199, ALP, cholesterol and TG
indices were significantly different (P<0.05) between the <40
years old age group and the >50 years old age group (Table III).
 | Table III.Comparison of seven serum indices by
age variation in normal controls. |
Table III.
Comparison of seven serum indices by
age variation in normal controls.
|
| Age |
|
|
|---|
|
|
|
|
|
|---|
|
| <40 years | >50 years | <40 years vs.
>50 years |
|---|
|
|
|
|
|
|---|
| Serum index | n | Mean ± SD | n | Mean ± SD | OR (95% CI) | P-value |
|---|
| CA-125 | 163 | 17.189±8.718 | 27 | 11.888±5.823 | 0.868
(0.794–0.950) | 0.003b |
| CA-199 | 150 | 10.528±7.794 | 21 | 14.414±9.310 | 1.051
(1.001–1.104) | 0.038a |
| AFP | 257 | 3.240±2.151 | 38 | 3.602±2.350 | 1.069
(0.932–1.226) | 0.339 |
| CEA | 257 | 2.355±3.005 | 38 | 2.469±1.974 | 1.012
(0.910–1.126) | 0.821 |
| ALP | 150 | 51.600±15.236 | 27 | 67.630±21.335 | 1.047
(1.023–1.070) |
7.57×10−4c |
| Cholesterol | 257 | 4.480±0.807 | 37 | 5.203±0.663 | 3.143
(1.945–5.078) |
3.71×10−7c |
| TG | 257 | 1.015±0.466 | 37 | 1.353±0.410 | 3.244
(1.740–6.045) |
3.75×10−5c |
To study the early diagnostic factors that are
independent of age, the present study aimed to identify serum
indices using two criteria: There was no statistical difference in
normal control patients (P>0.05); and there was a statistical
difference in patients with CC (P<0.05; Table IV; Fig.
2). Comparing the <40 years old age group with the 40–50
years old age group ALP did not change significantly in normal
women, but increased significantly in patients with SCC. Taken
together, the present data demonstrated that ALP may be a potential
predictive factor for the occurrence and progression of CC,
particularly in women with SCC among the 40–50 years old age
group.
 | Table IV.Comparison of the seven serum indices
by age variation in normal controls, patients with SCC and patients
with AC. |
Table IV.
Comparison of the seven serum indices
by age variation in normal controls, patients with SCC and patients
with AC.
| A, Normal |
|---|
|
|---|
|
| Age |
|
|
|---|
|
|
|
|
|
|---|
|
| <40 years | 40–50 years | <40 years vs.
40–50 years |
|---|
|
|
|
|
|
|---|
| Serum index | n | Mean ± SD | n | Mean ± SD | OR (95% CI) | P-value |
|---|
| CA-125 | 163 | 17.189±8.718 | 56 | 16.850±8.691 | 0.995
(0.960–1.032) | 0.802 |
| CA-199 | 150 | 10.528±7.794 | 49 | 9.249±6.476 | 0.975
(0.930–1.023) | 0.301 |
| AFP | 257 | 3.240±2.151 | 92 | 4.129±3.180 | 1.136
(1.039–1.243) | 0.014a |
| CEA | 257 | 2.355±3.005 | 93 | 2.605±2.529 | 1.028
(0.952–1.109) | 0.474 |
| ALP | 150 | 51.600±15.236 | 60 | 50.850±12.791 | 0.996
(0.976–1.018) | 0.737 |
| Cholesterol | 257 | 4.480±0.807 | 94 | 4.876±0.776 | 1.852
(1.360–2.523) |
4.84×10−5c |
| TG | 257 | 1.015±0.466 | 94 | 1.252±0.650 | 2.214
(1.400–3.502) | 0.002b |
|
| B, SCC |
|
|
| Age |
|
|
|
|
|
|
|
|
| <40
years | 40–50
years | <40 years vs.
40–50 years |
|
|
|
|
|
| Serum
index | n | Mean ±
SD | n | Mean ±
SD | OR (95%
CI) | P-value |
|
| CA-125 | 14 | 19.107±8.060 | 54 | 21.213±21.154 | 1.007
(0.971–1.044) | 0.717 |
| CA-199 | 11 | 14.271±11.415 | 46 | 19.029±28.154 | 1.009
(0.976–1.043) | 0.587 |
| AFP | 12 | 4.603±3.513 | 53 | 2.722±1.187 | 0.660
(0.473–0.920) | 0.093 |
| CEA | 12 | 4.047±8.508 | 54 | 3.991±6.923 | 0.999
(0.915–1.090) | 0.981 |
| ALP | 28 | 56.843±13.926 | 97 | 64.723±18.471 | 1.028
(1.001–1.056) | 0.039a |
| Cholesterol | 18 | 4.508±0.691 | 67 | 4.563±1.015 | 1.065
(0.610–1.858) | 0.828 |
| TG | 18 | 1.273±0.678 | 67 | 1.395±0.713 | 1.313
(0.579–2.974) | 0.518 |
|
| C, AC |
|
|
| Age |
|
|
|
|
|
|
|
|
| <40
years | 40–50
years | <40 years vs.
40–50 years |
|
|
|
|
|
| Serum
index | n | Mean ±
SD | n | Mean ±
SD | OR (95%
CI) | P-value |
|
| CA-125 | 4 | 17.625±10.356 | 4 | 37.000±35.849 | 1.040
(0.962–1.124) | 0.365 |
| CA-199 | 4 | 14.740±8.105 | 3 | 12.550±6.856 | 0.950
(0.753–1.198) | 0.722 |
| AFP | 4 | 2.115±1.051 | 3 | 4.023±1.932 | 4.236
(0.351–51.105) | 0.149 |
| CEA | 4 | 5.020±6.482 | 4 | 2.880±2.929 | 0.890
(0.624–1.269) | 0.569 |
| ALP | 5 | 58.180±7.811 | 6 | 66.167±41.732 | 1.011
(0.965–1.058) | 0.686 |
| Cholesterol | 4 | 4.878±0.970 | 5 | 4.850±0.768 | 0.953
(0.168–5.395) | 0.963 |
| TG | 4 | 1.360±0.920 | 5 | 1.604±0.544 | 1.808
(0.218–15.012) | 0.633 |
FIGO staging and CC
To investigate the association between FIGO staging
and CC, the present study further analyzed serological CA-125,
CA-199, AFP, ALP, cholesterol and TG levels in patients with SCC,
and measured ALP, cholesterol and TG in patients with AC.
For patients with SCC (Table V), only ALP and TG levels increased
significantly from Stage I to Stage III/IV CC compared with the
normal controls. Furthermore, only ALP and cholesterol levels
significantly increased in patients with FIGO Stage I AC compared
with the normal controls (Table
VI).
 | Table V.Comparison of the serum indices by
The International Federation of Gynecology and Obstetrics staging
in normal controls and patients with SCC. |
Table V.
Comparison of the serum indices by
The International Federation of Gynecology and Obstetrics staging
in normal controls and patients with SCC.
|
| Normal | SCC (Stage I) | Normal vs. Stage I
SCC | SCC (Stage II) | Normal vs. Stage II
SCC | SCC (Stage
III/IV) | Normal vs. Stage
III/IV SCC |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Serum index | n | Mean ± SD | n | Mean ± SD | OR (95% CI) | P-value | n | Mean ± SD | OR (95% CI) | P-value | n | Mean ± SD | OR (95% CI) | P-value |
|---|
| CA-125 | 246 | 16.530±8.573 | 55 | 15.629±8.479 | 0.987
(0.951–1.024) | 0.481 | 59 | 28.295±27.882 | 1.045
(1.024–1.068) | 0.002b | 23 | 68.283±170.741 | 1.039
(1.011–1.068) | 0.16 |
| CA-199 | 220 | 10.614±7.762 | 45 | 18.647±27.147 | 1.035
(1.011–1.06) | 0.055 | 49 | 14.215±20.104 | 1.023
(0.998–1.049) | 0.223 | 20 | 17.853±26.946 | 1.037
(1.004–1.071) | 0.246 |
| AFP | 387 | 3.487±2.475 | 52 | 3.348±2.222 | 0.975
(0.860–1.107) | 0.7 | 57 | 2.984±1.429 | 0.886
(0.755–1.040) | 0.135 | 20 | 2.195±0.820 | 0.457
(0.263–0.796) | 0.02a |
| ALP | 237 | 53.236±16.262 | 109 | 69.613±24.087 | 1.045
(1.030–1.060) |
1.33×10−9c | 84 | 74.073±24.918 | 1.054
(1.038–1.070) |
1.07×10−10c | 25 | 76.908±24.400 | 1.055
(1.034–1.077) |
6.49×10−5c |
| Cholesterol | 388 | 4.645±0.823 | 69 | 4.947±0.988 | 1.498
(1.115–2.011) | 0.007b | 71 | 4.932±1.196 | 1.414
(1.073–1.863) | 0.056 | 20 | 4.433±1.002 | 0.731
(0.422–1.269) | 0.267 |
| TG | 388 | 1.105±0.526 | 69 | 1.745±1.325 | 2.857
(1.917–4.257) |
1.77×10−4c | 69 | 1.654±0.888 | 3.054
(2.084–4.476) |
3.72×10−6c | 20 | 1.535±0.623 | 2.433
(1.370–4.324) |
4.61×10−4c |
 | Table VI.Comparison of serum indexes by The
International Federation of Gynecology and Obstetrics staging in
normal controls and patients with AC. |
Table VI.
Comparison of serum indexes by The
International Federation of Gynecology and Obstetrics staging in
normal controls and patients with AC.
|
| Normal | Stage I AC | Normal vs. Stage I
AC | Stage II AC | Normal vs. Stage II
AC | Stage III/IV | Normal vs. AC
(Stage III/IV) |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Serum index | n | Mean ± SD | n | Mean ± SD | OR (95% CI) | P-value | n | Mean ± SD | OR (95% CI) | P-value | n | Mean ± SD | OR (95% CI) | P-value |
|---|
| ALP | 237 | 53.236±16.262 | 15 | 62.227±15.191 | 1.028
(1.001–1.055) | 0.038a | 6 | 75.400±38.388 | 1.042
(1.011–1.073) | 0.217 | 0 | 0 | N/A | N/A |
| Cholesterol | 388 | 4.645±0.823 | 12 | 5.223±0.960 | 2.178
(1.142–4.153) | 0.017a | 5 | 5.172±0.969 | 2.056
(0.767–5.512) | 0.156 | 1 | 6.100 | 5.071
(0.826–31.145) | 0.078 |
| TG | 388 | 1.105±0.526 | 12 | 1.794±1.130 | 2.798
(1.539–5.084) | 0.059 | 5 | 1.512±0.412 | 2.131
(0.858–5.290) | 0.086 | 1 | 1.650 | 2.305
(0.437–12.167) | 0.302 |
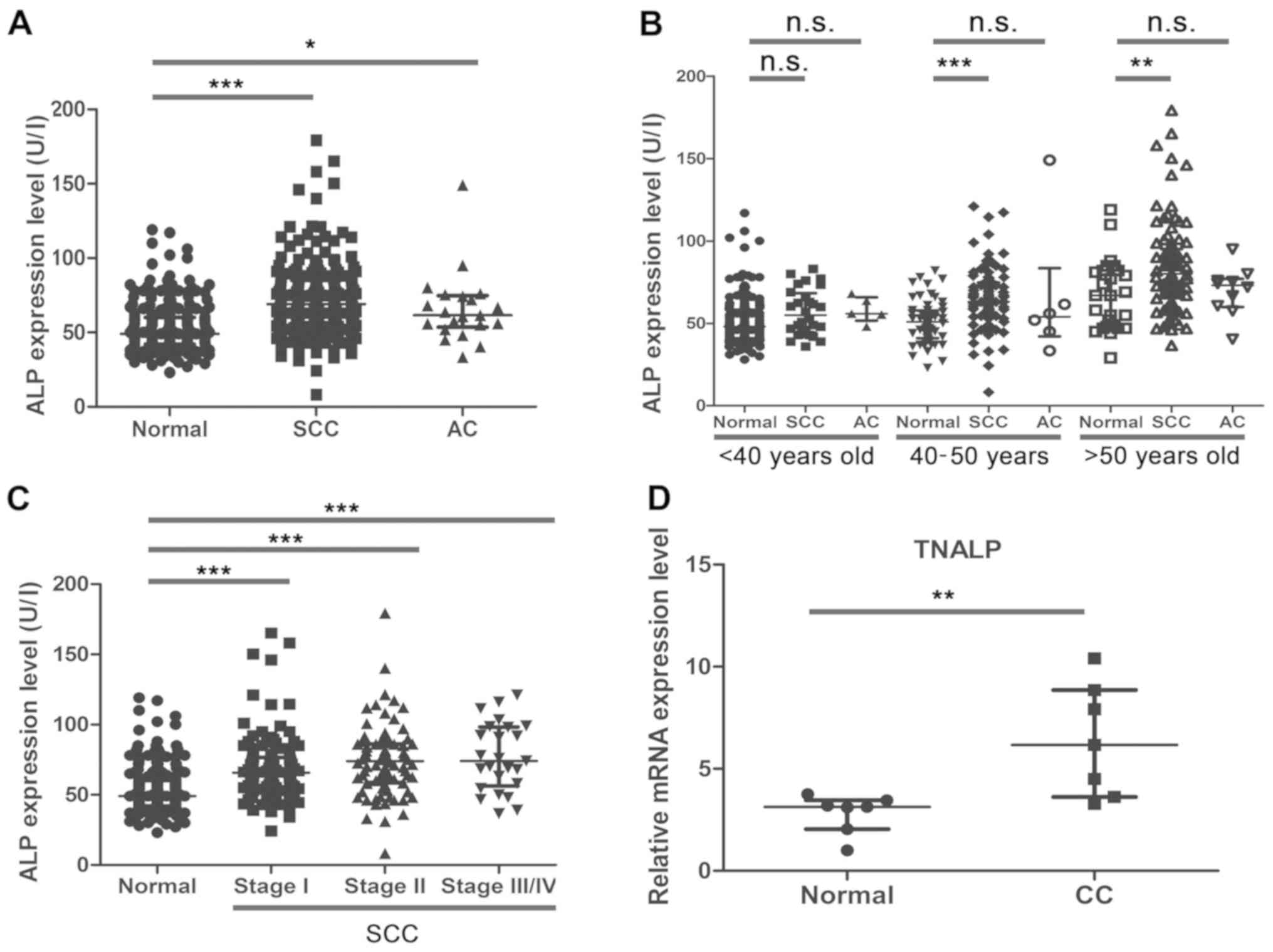
Expression pattern of ALP in CC
Using the re-analysis strategy, the present study
demonstrated that ALP was a potential factor, which may predict the
tumorigenesis of CC. Despite the ALP expression levels not
exceeding the upper limits of the normal recommended level, the
present results indicated that the ALP expression level increased
in patients with SCC (P<0.001) and AC (P<0.05) compared with
the normal controls (Fig. 3A). As
shown in Fig. 3B, the ALP expression
level was significantly increased between the normal controls and
patients with SCC in the 40–50 years old group (P<0.001) and the
>50 years old group (P<0.01). Although the expression levels
of ALP also increased with age between the normal controls and
patients with AC, the difference was not statistically
significant.
As demonstrated by the FIGO staging analysis, the
expression levels of ALP were significantly increased in patients
with SCC of different FIGO stages, when compared with the normal
controls (all P<0.001; Fig. 3C).
At present, the number of AC cases for comparison across the FIGO
stages was limited, and a larger sample size is required to further
confirm the significance of ALP and other serum indices. The
present study also detected the relative mRNA expression level of
the major type of ALP isozyme, TNALP, between the normal control
and CC tissues. The RT-qPCR results indicated that TNALP mRNA
expression levels were significantly increased in CC tissues
compared with normal cervical tissues (Fig. 3D; P<0.01). In summary, the results
demonstrated that ALP might be a reliable early predictive factor
for detecting the development of CC, particularly in 40–50 year-old
women.
Adjusted assessment of the serum
indices for CC
Liver function is an important factor associated
with progression of numerous diseases and tumorigenesis (15). A recent study demonstrated that
serological ALP and AST expression levels were significantly
increased in patients with gastric cancer (16). Similarly, the present study confirmed
that ALP expression levels were also increased in patients with CC.
To exclude the effects of liver dysfunction on ALP expression, the
present study also assessed seven serum indices between normal
control women and patients with CC using adjusted AST and ALT
factors (excluding cases exceeding the normal upper limit of AST
and ALT). The present data indicated that the seven serum indices
between the normal control women and patients with CC exhibited
similar results in the adjusted assessment (Table VII).
 | Table VII.Adjusted assessment of the serum
indices by aspartate aminotransferase and alanine transferase in
normal controls and patients with cervical cancer. |
Table VII.
Adjusted assessment of the serum
indices by aspartate aminotransferase and alanine transferase in
normal controls and patients with cervical cancer.
|
| Normal | SCC | Normal vs. SCC | AC | Normal vs. AC |
|---|
|
|
|
|
|
|
|
|---|
| Serum index | n | Mean ± SD | n | Mean ± SD | OR (95% CI) | P-value | n | Mean ± SD | OR (95% CI) | P-value |
|---|
| CA-125 | 238 | 16.692±8.634 | 118 | 29.815±77.010 | 1.028
(1.011–1.046) | 0.067 | 13 | 19.592±26.488 | 1.020
(0.981–1.060) | 0.701 |
| CA-199 | 214 | 10.613±7.723 | 99 | 15.477±21.506 | 1.026
(1.006–1.046) | 0.031a | 10 | 10.642±7.048 | 1.000
(0.921–1.087) | 0.991 |
| AFP | 374 | 3.510±2.502 | 113 | 2.904±1.645 | 0.862
(0.761–0.976) | 0.003b | 13 | 3.075±2.198 | 0.912
(0.679–1.224) | 0.537 |
| CEA | 375 | 2.431±2.812 | 117 | 11.202±71.674 | 1.099
(1.040–1.161) | 0.188 | 13 | 3.703±3.827 | 1.074
(0.972–1.186) | 0.114 |
| ALP | 229 | 52.856±16.006 | 200 | 71.745±23.407 | 1.055
(1.041–1.068) |
1.503×10−19c | 17 | 62.459±13.607 | 1.030
(1.005–1.057) | 0.017a |
| Cholesterol | 375 | 4.639±0.819 | 148 | 4.839±1.024 | 1.289
(1.040–1.598) | 0.035a | 14 | 5.306±0.958 | 2.436
(1.333–4.452) | 0.003b |
| TG | 375 | 1.100±0.523 | 146 | 1.655±1.081 | 3.160
(2.250–4.440) |
1.535×10−8c | 14 | 1.540±0.709 | 2.384
(1.256–4.525) | 0.038a |
Discussion
In the present study, to determine novel clinical
predictive factors for the development of CC, a multistage
re-analysis strategy of seven serum indices was systematically
conducted. The expression levels of CA-125, CA-199, ALP,
cholesterol and TG significantly increased in patients with SCC. In
addition, the expression levels of AFP were significantly decreased
in patients with SCC. In patients with AC, ALP, cholesterol and TG
expression levels significantly increased compared with the normal
controls. According to age variation (normal, P>0.05; CC,
P<0.05) and FIGO staging (P<0.05), the combined analysis
results demonstrated that ALP appears to serve a role in CC. ALP
expression significantly increased in patients with SCC and AC
compared with the normal controls, indicating its potential role in
the diagnosis of CC.
ALP is a member of the superfamily of
metalloenzymes, which catalyze the hydrolysis of phosphoric acid.
In humans, there are four types of ALP isozyme, including TNALP,
which is primarily expressed in bone tissue, the liver and kidneys,
intestinal-type ALP, placental-type ALP and germ cell ALP (17). TNALPs in the bone tissue and liver
account for >90% of serum ALP and are the most abundantly
expressed isoforms in the serum (18). Previous studies have indicated that
ALP is a prognostic factor in predicting overall survival in
advanced prostate cancer (19,20).
However, the role and mechanism of ALP in tumor pathogenesis remain
unclear. A previous study demonstrated that ALP functioned as an
ectoenzyme in human osteosarcoma cells (21). Another previous study showed that the
tumor-derived alkaline phosphatase, biomineralization associated
(ALPL) gene, encoding the TNALP protein, was associated with
disease-free survival in metastatic prostate cancer and that ALPL
promoted the pathogenesis of prostate cancer (22). Osteoblasts abundantly express ALP,
which can hydrolyze inorganic pyrophosphate and thus, affect bone
mineralization, indicating its roles in osteoblastic activity
(23).
Recently, evidence indicated that TNALP could
regulate cranial base growth and synchondrosis maturation via
mitogen-activated protein kinase (MAPK) signalling (24). Previous studies have also indicated
the role of MAPK signaling in tumorigenesis (25–28), and
demonstrated that oncogenes promote tumor growth and metastasis
through the MAPK signaling pathway (29). Ectopic activation of the MAPK
signaling pathway enhances the progression of cancer (30). Furthermore, epidermal growth factor
receptor regulated matrix metalloproteinase 1 expression via the
MAPK/extracellular signal-regulated kinase signaling pathway in
SiHa cells, which has been shown to serve a role in tumor invasion,
metastasis and angiogenesis (31).
CC is a disease caused by multiple pathogenic
factors, including age, human papillomavirus (HPV) infection, FIGO
stage and menopausal status (32).
The present study provided a multiple-stage screening strategy for
exploring predictive factors by systematic re-analysis of serum
indices, and two key pathogenic factors were selected for the
second round of screening. ALP was identified as a potential
predictor for CC progression. Combined analysis of ALP with other
factors, including HPV infection and cytological examination may
optimize the diagnosis of CC. Furthermore, serum ALP levels are
determined as part of a routine examination in hospitals and the
present study suggested that even routine tests can reveal the
occurrence of CC, providing important information for early
diagnosis of CC. However, more accurate data acquisition is
required to confirm the results of the present study and further
elucidate the mechanism underlying the development of CC.
Additionally, estradiol was determined to be an important factor
for affecting ALP levels between normal women and CC in
pre-menopausal women (data not shown), and thus, the correlation
between estradiol and ALP levels in normal controls and patients
with CC requires further analysis in future studies.
The present study identified elevated expression
levels of ALP in patients with SCC and AC compared with the normal
controls. High levels of serum ALP may influence tumorigenesis and
may be an important predictive factor in CC. Further studies may
focus on the role and mechanism of increased expression of TNALP in
CC. The present study provided novel insight into determining an
association between ALP and CC.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant nos. 31701298 and
81402100), The Natural Science Foundation of Jiangsu Province
(grant no. BK20170562), The Key Research Foundation of Zhenjiang
Social Development (grant nos. SH2017013, SH2017020, SH2016028,
SH2016031 and SH2014026), The Key Research Foundation of Zhenjiang
Health Science and Technology (grant no. SHW2016001), The Science
Foundation of Doctorate Research of Affiliated Hospital of Jiangsu
University (grant no. jdfyRC2016005), Suzhou Key Medical Center
(grant no. SZZX201505), Suzhou Introduced Project of Clinical
Medical Expert Team (grant no. SZYJTD201708), Jiangsu Provincial
Medical Innovation Team (grant no. CXTDB2017013), The Foundation of
Health and Family Planning Commission of Jiangsu Province (grant
no. Q201408), The Foundation for Young Medical Talents of Jiangsu
province (grant no. QNRC2016840) and The Six Talent Peaks Project
in Jiangsu Province (grant no. WSW-007).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY, JF and XH contributed to the conception and
design of the study. QZ, BZ, XC, CS, YZ, XL, WC, MW and YY
contributed to the acquisition, analysis and interpretation of
data. XD, HL and CQ performed the morphological experiments. BX,
JL, BC and PY were involved in sample collection and performing
molecular experiments.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee for Biomedical Research at Affiliated Hospital of Jiangsu
University (approval no. 20120019), and conducted according to the
guidelines for clinical retrospective studies. All participants
were fully informed of the purpose and significance of the study.
Written informed consent was obtained from all the participants for
participation.
Patient consent for publication
All patients provided consent for the use of their
samples in scientific research.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CC
|
cervical cancer
|
|
FIGO
|
The International Federation of
Gynecology and Obstetrics
|
|
SCC
|
squamous cell carcinoma
|
|
AC
|
cervical adenocarcinoma
|
|
ASC
|
adenosquamous cervical carcinoma
|
|
TG
|
triglyceride
|
|
CA-125
|
sugar chain antigen 125
|
|
CA-199
|
sugar chain antigen 199
|
|
AFP
|
α fetoprotein
|
|
CEA
|
carcino-embryonic antigen
|
|
SC
|
ectoendocervical squamocolumnar
|
|
AST
|
aspartate aminotransferase
|
|
SCCA
|
squamous cell carcinoma antigen
|
|
ALP
|
alkaline phosphatase
|
|
TNALP
|
tissue non-specific isozyme ALP
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen P, Jiao L and Wang DB: Squamous cell
carcinoma antigen expression in tumor cells is associated with the
chemosensitivity and survival of patients with cervical cancer
receiving docetaxel-carboplatin-based neoadjuvant chemotherapy.
Oncol Lett. 13:1235–1241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Molina R, Filella X, Augé JM, Fuentes R,
Bover I, Rifa J, Moreno V, Canals E, Viñolas N, Marquez A, et al:
Tumor markers (CEA, CA 125, CYFRA 21-1, SCC and NSE) in patients
with non-small cell lung cancer as an aid in histological diagnosis
and prognosis. Comparison with the main clinical and pathological
prognostic factors. Tumour Biol. 24:209–218. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Catanzaro JM, Guerriero JL, Liu J, Ullman
E, Sheshadri N, Chen JJ and Zong WX: Elevated expression of
squamous cell carcinoma antigen (SCCA) is associated with human
breast carcinoma. PLoS One. 6:e190962011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sánchez Vega JF, Murillo Bacilio MDR,
Vintimilla Condoy AS, Palta González AM, Crespo Astudillo JA and
Mora-Bravo FG: Predictive equation of metastasis in patients with
malignant ovarian epithelial tumors with the Ca-125 marker. BMC
Cancer. 18:5872018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malati T: Tumour markers: An overview.
Indian J Clin Biochem. 22:17–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lebrecht A, Ludwig E, Huber A, Klein M,
Schneeberger C, Tempfer C, Koelbl H and Hefler L: Serum vascular
endothelial growth factor and serum leptin in patients with
cervical cancer. Gynecol Oncol. 85:32–35. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fuso L, Mazzola S, Marocco F, Ferrero A,
Dompè D, Carus AP and Zola P: Pretreatment serum hemoglobin level
as a predictive factor of response to neoadjuvant chemotherapy in
patients with locally advanced squamous cervical carcinoma: A
preliminary report. Gynecol Oncol. 99 (Suppl 1):S187–S191. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhatla N, Aoki D, Sharma DN and
Sankaranarayanan R: Cancer of the cervix uteri. Int J Gynaecol
Obstet. 143 (Suppl 2):S22–S36. 2018. View Article : Google Scholar
|
|
11
|
US Preventive Services Task Force, ; Curry
SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni
CA, Epling JW Jr, Kemper AR, et al: Screening for cervical cancer:
US preventive services task force recommendation statement. JAMA.
320:674–686. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herfs M, Yamamoto Y, Laury A, Wang X,
Nucci MR, McLaughlin-Drubin ME, Münger K, Feldman S, McKeon FD,
Xian W and Crum CP: A discrete population of squamocolumnar
junction cells implicated in the pathogenesis of cervical cancer.
Proc Natl Acad Sci USA. 109:10516–10521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quinn BA, Deng X, Colton A, Bandyopadhyay
D, Carter JS and Fields EC: Increasing age predicts poor cervical
cancer prognosis with subsequent effect on treatment and overall
survival. Brachytherapy. 18:29–37. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang C, Wang H, Ning Z, Xu L, Zhuang L,
Wang P and Meng Z: Serum liver enzymes serve as prognostic factors
in patients with intrahepatic cholangiocarcinoma. Onco Targets
Ther. 10:1441–1449. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fuchs CS, Muro K, Tomasek J, Van Cutsem E,
Cho JY, Oh SC, Safran H, Bodoky G, Chau I, Shimada Y, et al:
Prognostic factor analysis of overall survival in gastric cancer
from two phase III studies of second-line ramucirumab (REGARD and
RAINBOW) using pooled patient data. J Gastric Cancer. 17:132–144.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haarhaus M, Brandenburg V, Kalantar-Zadeh
K, Stenvinkel P and Magnusson P: Alkaline phosphatase: A novel
treatment target for cardiovascular disease in CKD. Nat Rev
Nephrol. 13:429–442. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Magnusson P, Degerblad M, Sääf M, Larsson
L and Thorén M: Different responses of bone alkaline phosphatase
isoforms during recombinant insulin-like growth factor-i (IGF-I)
and during growth hormone therapy in adults with growth hormone
deficiency. J Bone Miner Res. 12:210–220. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Metwalli AR, Rosner IL, Cullen J, Chen Y,
Brand T, Brassell SA, Lesperance J, Porter C, Sterbis J and McLeod
DG: Elevated alkaline phosphatase velocity strongly predicts
overall survival and the risk of bone metastases in
castrate-resistant prostate cancer. Urol Oncol. 32:761–768. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sonpavde G, Pond GR, Berry WR, de Wit R,
Armstrong AJ, Eisenberger MA and Tannock IF: Serum alkaline
phosphatase changes predict survival independent of PSA changes in
men with castration-resistant prostate cancer and bone metastasis
receiving chemotherapy. Urol Oncol. 30:607–613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fedde KN, Lane CC and Whyte MP: Alkaline
phosphatase is an ectoenzyme that acts on micromolar concentrations
of natural substrates at physiologic pH in human osteosarcoma
(SAOS-2) cells. Arch Biochem Biophys. 264:400–409. 1998. View Article : Google Scholar
|
|
22
|
Rao SR, Snaith AE, Marino D, Cheng X, Lwin
ST, Orriss IR, Hamdy FC and Edwards CM: Tumour-derived alkaline
phosphatase regulates tumour growth, epithelial plasticity and
disease-free survival in metastatic prostate cancer. Br J Cancer.
116:227–236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Millán JL: The role of phosphatases in the
initiation of skeletal mineralization. Calcif Tissue Int.
93:299–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nam HK, Sharma M, Liu J and Hatch NE:
Tissue nonspecific alkaline phosphatase (TNAP) regulates cranial
base growth and synchondrosis maturation. Front Physiol. 8:1612017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shao J, Wang C, Li L, Liang H, Dai J, Ling
X and Tang H: Luteoloside inhibits proliferation and promotes
intrinsic and extrinsic pathway-mediated apoptosis involving MAPK
and mTOR signaling pathways in human cervical cancer cells. Int J
Mol Sci. 19:E16642018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hua FF, Liu SS, Zhu LH, Wang YH, Liang X,
Ma N and Shi HR: MiRNA-338-3p regulates cervical cancer cells
proliferation by targeting MACC1 through MAPK signaling pathway.
Eur Rev Med Pharmacol Sci. 21:5342–5352. 2017.PubMed/NCBI
|
|
27
|
Sun Q, Liang Y, Zhang T, Wang K and Yang
X: ER-α36 mediates estrogen-stimulated MAPK/ERK activation and
regulates migration, invasion, proliferation in cervical cancer
cells. Biochem Biophys Res Commun. 487:625–632. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li S, Ma YM, Zheng PS and Zhang P: GDF15
promotes the proliferation of cervical cancer cells by
phosphorylating AKT1 and Erk1/2 through the receptor ErbB2. J Exp
Clin Cancer Res. 37:802018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang XL, Liu KY, Lin FJ, Shi HM and Ou ZL:
CCL28 promotes breast cancer growth and metastasis through
MAPK-mediated cellular anti-apoptosis and pro-metastasis. Oncol
Rep. 38:1393–1401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang C, Li P, Xuan J, Zhu C, Liu J, Shan
L, Du Q, Ren Y and Ye J: Cholesterol enhances colorectal cancer
progression via ROS elevation and MAPK signaling pathway
activation. Cell Physiol Biochem. 42:729–742. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Z, Wang L, Du J, Li Y, Yang H, Li C,
Li H and Hu H: Lipid raft localization of epidermal growth factor
receptor alters matrix metalloproteinase-1 expression in SiHa cells
via the MAPK/ERK signaling pathway. Oncol Lett. 12:4991–4998. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cohen PA, Jhingran A, Oaknin A and Denny
L: Cervical cancer. Lancet. 393:169–182. 2019. View Article : Google Scholar : PubMed/NCBI
|