Introduction
Primary liver cancer is the fifth most common type
of cancer and the third leading cause of cancer-related mortality
worldwide, with hepatocellular carcinoma (HCC) accounting for
>90% of cases (1). Although
surgical resection is the standard treatment modality for HCC, its
use is limited, as the majority of patients, even in those with
small-sized tumors, also have associated severe liver dysfunction
(2,3). Liver transplantation provides an
alternative curative treatment for small unresectable HCC in
patients with small-sized tumors. However, the shortage of liver
grafts limits the applicability of this approach (4).
Radiofrequency ablation (RFA) is a previously
developed technique for ablation of liver tumors and is well
tolerated in patients with unresectable HCC (5). In 2012, a randomized controlled trial
also revealed that percutaneous RFA was as effective as hepatic
resection in terms of overall survival and recurrence-free survival
times in the treatment of patients with small HCC tumors (<4 cm)
(6). Despite its technical
simplicity and safety, the recurrence rate following RFA remains
high (48–95%) (7–9) as the complete ablation of the tumor is
difficult to achieve (9). To
investigate the biological characteristics of residual cancer
following RFA, a model of insufficient RFA is important.
In the present study, an orthotopic nude mouse model
of HCC was developed to assess the metastatic potential of residual
cancer following insufficient RFA and to improve the current animal
model.
Materials and methods
Cell line
The green fluorescent protein (GFP)-expressing
HCCLM3 cell line (HCCLM3-GFP), derived from HCCLM3 cells
(established by the Liver Cancer Institute of Fudan University,
Shanghai, China) (10), was used for
in vivo experiments. The cells were maintained in Dulbecco's
modified Eagle's medium with 10% fetal bovine serum and 100 mg/ml
penicillin G (all from Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 37°C in a humidified atmosphere containing 5%
CO2.
Animal model
A total of 24 male athymic BALB/c nu/nu mice,
weighing 18–20 g at 4–6 weeks of age, were obtained from the
Shanghai Laboratory Animal Centre Co., Ltd. (Shanghai, China). All
mice were housed in specific pathogen-free conditions (temperature,
26–28°C; atmosphere, 0.65 cm H2O; 10/14 h light/dark
cycle; with ad libitum access to food and water). All animal
protocols were approved by the Ethical Committee on Animal
Experiments of the Animal Care Committee of Fudan University
(Shanghai, China). All efforts were made to minimize animal
suffering.
Equipment
The main equipment comprised a 1500X RF Generator
(RITA Medical Systems, Inc.; AngioDynamics, Inc., Latham, NY, USA),
retractable multiple hook RFA needles (Fig. 1; RITA Medical Systems, Inc.;
AngioDynamics, Inc.), and RITA grounding pads (RITA Medical
Systems, Inc.; AngioDynamics, Inc.).
Establishment of an orthotopic
transplantation tumor model of HCC in nude mice
The HCCLM3-GFP cells (1×107) were
subcutaneously inoculated into the right flanks of two 4-week-old
BALB/c nu/nu male mice. Subcutaneous ectopic tumors were harvested
upon reaching ~1 cm in diameter, after 3–4 weeks. Prior to
subcutaneous ectopic tumor removal, general anesthesia was induced
with 50 mg/kg pelltobarbitalum natricum (Haoran Biological
Technology Co., Ltd., Shanghai, China) administered by
intraperitoneal injection. Following the removal of the necrotic
tissues, the tumors were cut into ~1-mm3 sections under
aseptic conditions. Following anesthesia with pelltobarbitalum
natricum, at the aforementioned dose, the abdominal cavity of the
mice was opened and the left liver lobe was made accessible.
Ophthalmic ligature forceps were used to create a small tunnel
under the surface of the left liver lobe and the trimmed tumor was
placed into the tunnel. Finally, the opening of the tunnel was
closed by a 6-0 non-absorbable suture. The nude mice bearing
xenografts were randomly divided into a sham-operated group (n=12)
and an insufficient RFA group (n=12) when the orthotopic
transplantation tumor had reached ~0.5 cm in diameter, ~3 weeks
after implantation.
Insufficient RFA
Insufficient RFA was performed 3 weeks after tumor
implantation. Following anesthesia, the abdomen was prepared with
iodine and alcohol scrub, and draped with sterile towels.
Initially, the mouse was placed on a conductive metal plate and its
limbs were fixed. RITA grounding pads were adhered to the back of
the metal plate. Good electrical conductivity was maintained
between the metal plate and the back of the nude mouse. The
abdominal cavity of the mouse was opened to expose the left liver
lobe and the transplanted tumor. During implantation, the graft was
embedded under the capsule of the left lobe of the liver, such that
after 3 weeks, the protruding transplanted tumors were visible with
the naked eye on the surface of the liver. The center straight
needle of the RF probe with an active diameter of 4 mm was inserted
into the tumor, and normal saline was dripped into the puncture
site to maintain good conductivity. An active diameter of 4 mm for
the probe was determined to be optimal based on preliminary
experiments (data not shown). Taking into account the weight and
volume of the tumor, RFA was performed using a low-energy protocol,
in which the output power was 5 W and the duration was 30 sec.
Following RFA, the abdominal cavity of the mice was closed using
5-0 non-absorbable sutures. The control group underwent a sham
surgery by inserting a needle electrode into the tumor without
performing RFA.
Tumor volume and survival time of nude
mice
To evaluate tumor growth and metastasis, 6 nude mice
from the 2 aforementioned groups were sacrificed 4 weeks after
either insufficient RFA treatment or a sham operation. Tumors were
excised and their largest (a) and shortest (b) diameters were
measured to calculate tumor volume as follows: Tumor volume=a ×
b2/2 (11). To evaluate
the survival time, the remaining mice in each group were maintained
until death.
Lung metastasis and intrahepatic
metastasis (IHM) evaluation
To evaluate the metastatic potential of residual
tumor following insufficient RFA, the area of green fluorescence,
which indicated the lung metastatic nodes, was measured. The images
of GFP-positive metastatic foci were captured by stereoscopic
fluorescence microscopy (magnification, ×10; Leica Microsystems
GmbH, Wetzlar, Germany). The lung tissues were sectioned (4 µm)
serially, and hematoxylin and eosin staining (room temperature;
hematoxylin, 10 min; eosin, 30 sec) confirmed the aforementioned
results. IHM was observed by fluorescent imaging and quantified as
number per liver.
Statistical analysis
Data were analyzed using SPSS 22.0 (IBM Corp.,
Armonk, NY, USA). The results are expressed as the mean ± standard
deviation. Statistical analysis was performed using Student's
t-test. The Kaplan-Meier method with log-rank test was used for
survival analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
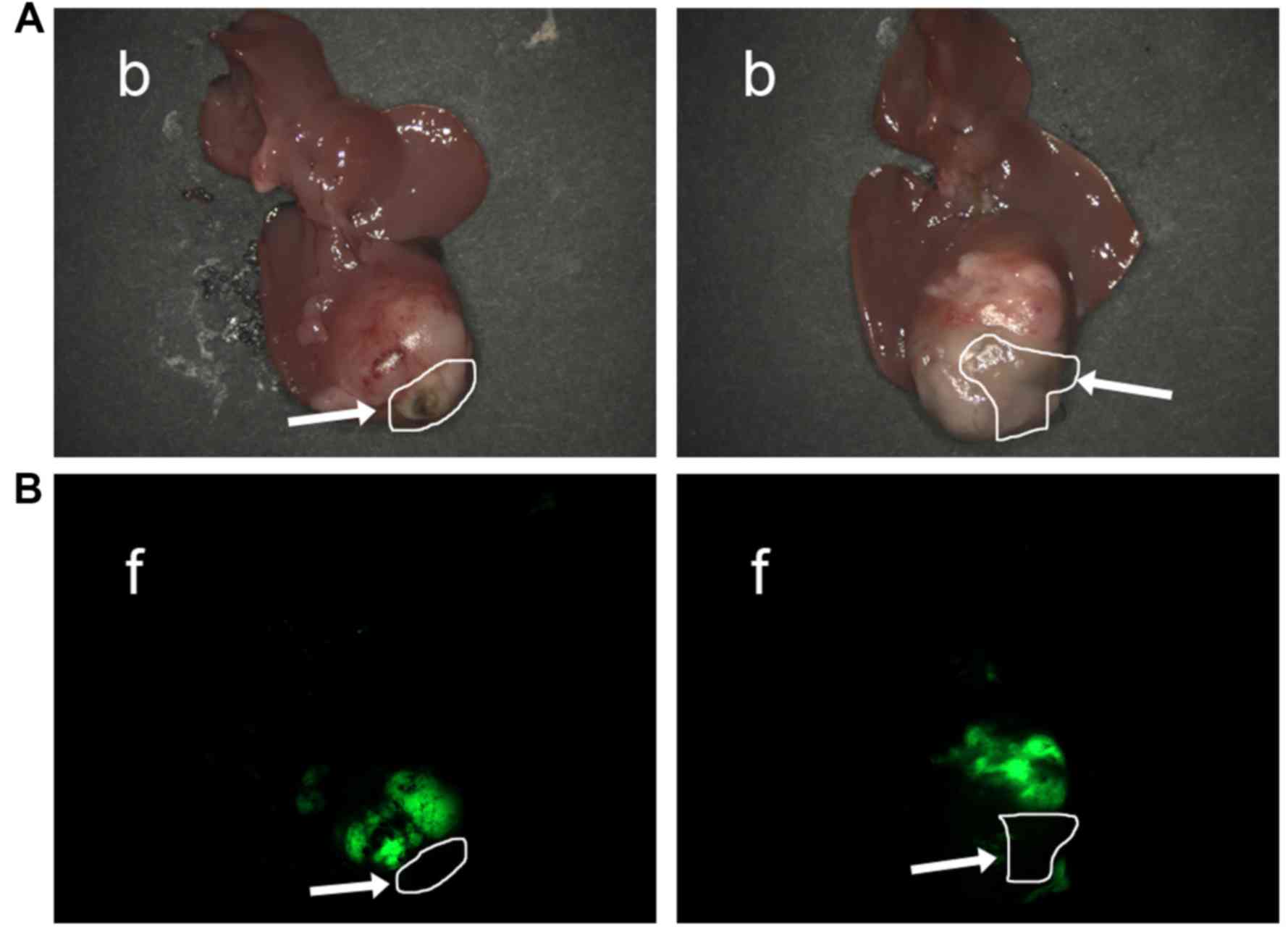
Establishment of insufficient RFA
orthotopic nude mouse model of HCC
No nude mice died following insufficient RFA. If
ablation of necrotic tissue and residual tumor was clearly observed
under fluorescence microscopy, it revealed that insufficient RFA
was successful (Fig. 2), and the
success rate in the insufficient RFA group was 100%. In the control
group, all the nude mice were alive until the evaluation of
metastasis.
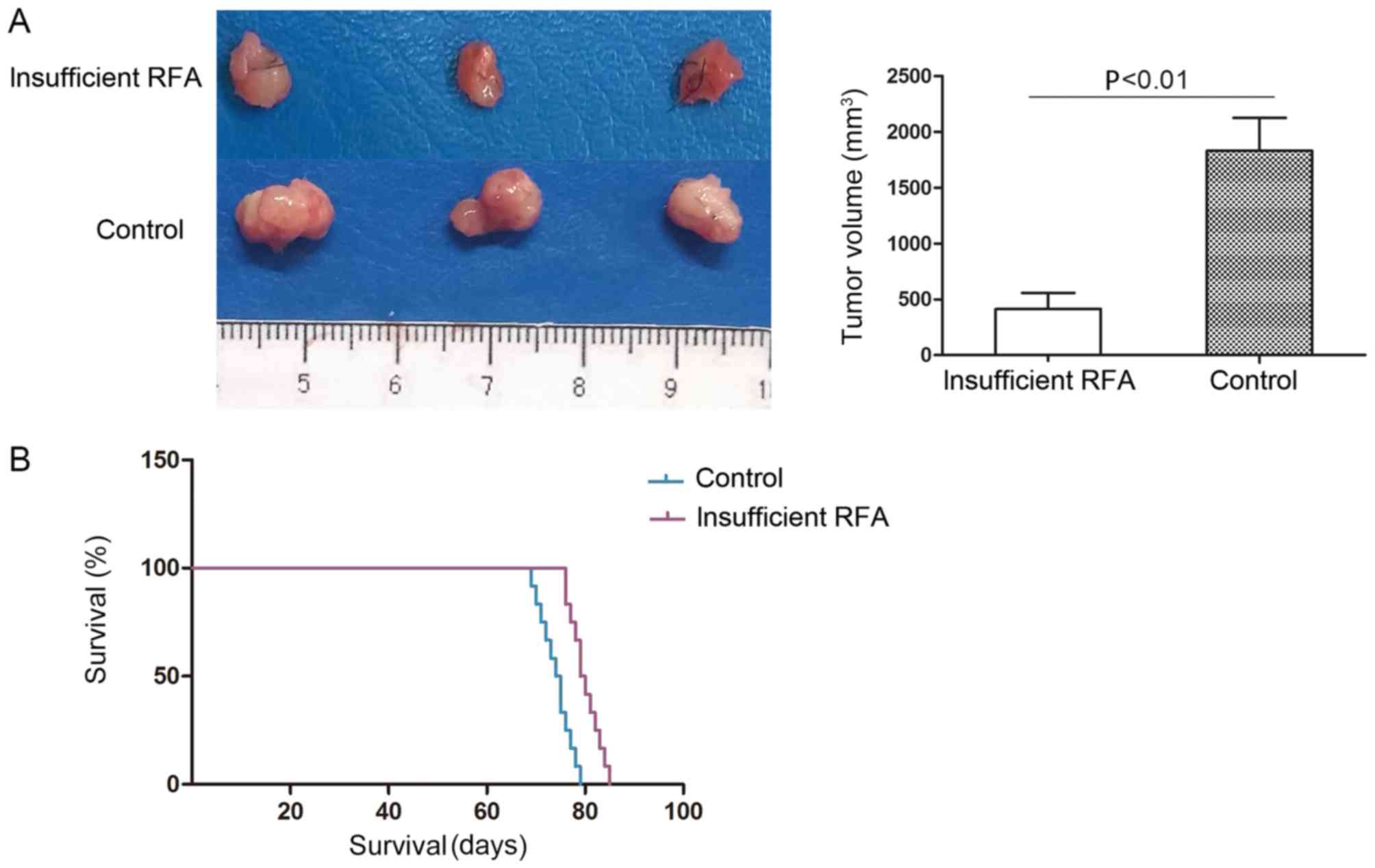
Insufficient RFA reduces tumor volume
and prolongs survival time in nude mice
The tumor volume of the HCCLM3-GFP-derived
xenografts was 449.58±350.75 mm3 in the insufficient RFA
group, which was significantly smaller compared with that of the
matched sham-operated controls (1788.66±608.80 mm3;
P<0.05; Fig. 3A). The mean
survival time in the insufficient RFA group was significantly
longer compared with that in the sham-operated control group
(80.8±3.5 days vs. 75.0±3.3 days; P<0.05; Fig. 3B).
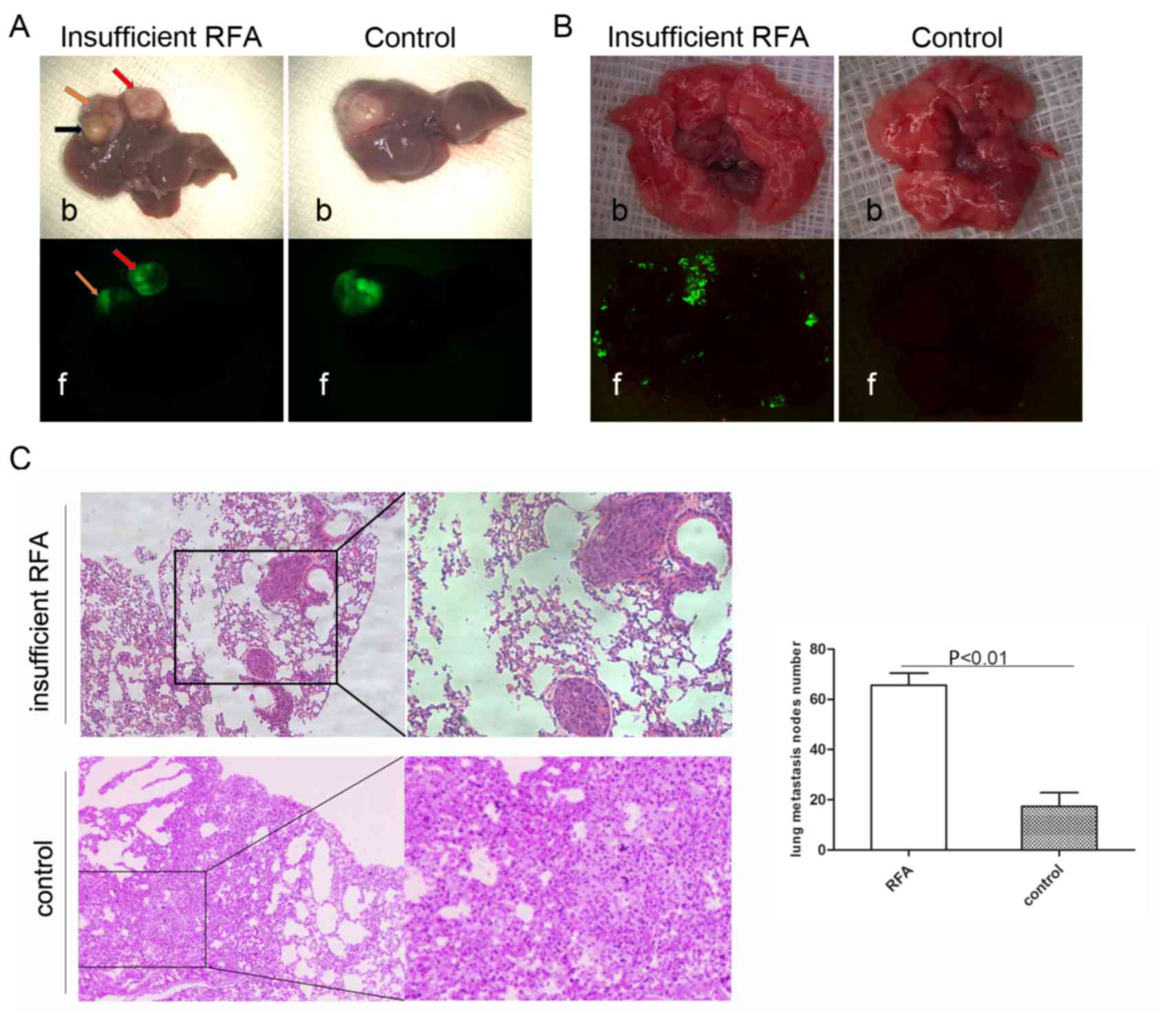
Insufficient RFA promotes invasiveness
and distant metastasis
Green fluorescence imaging revealed that
insufficient RFA promoted intrahepatic dissemination (Fig. 4A). The IHM rate in the insufficient
RFA group was 66.67% (4/6), but no IHM was observed in the control
group (P<0.05). The lung metastasis rate in the insufficient RFA
group was 100% (6/6), compared with 33.33% (2/6) in the control
group (P<0.05; Fig. 4B). Analysis
of serial lung paraffin sections indicated a significantly higher
incidence of lung metastasis in the insufficient RFA-treated mice
compared with that in the sham-operated mice (Fig. 4C).
Discussion
To investigate the invasiveness and metastatic
potential of a residual tumor following insufficient RFA, an
appropriate orthotopic animal model is required. The majority of
research utilizes the nude mice model and its use in investigating
a variety of HCC therapies is not novel. The orthotopic nude mouse
model of HCC was initially described in 1996 (12) and the orthotopic mouse model has
served as an ideal experimental tool to investigate HCC and its
treatment since 1998 (13). However,
there are few reports regarding the nude mouse model in RFA
research. To date, the majority of in vivo studies
investigating the biological behavior of RFA of tumors have used
nude mouse subcutaneous xenograft models or a rabbit orthotopic
model (14–17). Xu et al (15) inoculated HCC cells subcutaneously
into the right flank of mice. After 14 days, the subcutaneous
xenograft tumor was treated with a partial RFA strategy using 180
sec of RFA at 1 W once the tumor length had reached 12–15 mm. There
are no reports regarding the biological characteristics of residual
HCC following RFA using an orthotopic nude mouse model, to the best
of our knowledge.
An orthotopic nude mouse model is required and has
the potential to improve RFA research by imitating the in
vivo environment. This aspect is important as the invasiveness
and metastatic potential of HCC can be investigated under improved
conditions compared with subcutaneous xenografts in nude mice
(18,19). However, the use of this orthotopic
model for RFA is limited by the high mortality rate, as heat from
the RFA needle electrode kills the tumor cells, but also damages
the normal liver parenchyma that surrounds the tumor. In addition,
restricting the application of the orthotopic model is required due
to the highly complex surgical technique involved to maintain a
high tumor formation rate in the animal liver, thus experienced
laboratory technicians are essential. The orthotopic insufficient
RFA model designed in the present study resolved the two
aforementioned difficulties. The HCC nude mouse model (LCI-D20) was
initially established 20 years ago, and has a reliable and stable
tumor formation rate of 100% following orthotopic inoculation
(12). Minimizing mortality is a
vital step in model establishment. The third week (19–21 days)
following xenograft inoculation was identified as the optimum RFA
intervention time, as the xenograft tumor reached ~0.5 cm in
diameter. It is easier to achieve complete necrosis in smaller
tumors during RFA. By contrast, bigger tumors require more time to
reach the ideal state of insufficient RFA, leading to thermal
damage to the surrounding normal liver parenchyma and death. Open
abdominal rather than percutaneous RFA was selected in the present
study, as the latter approach burns the skin at the puncture point
and causes necrosis, which can cause death in a number of mice, as
aforementioned. However, the open approach carries an increased
risk of infection, but this is easily controlled by experienced
operators. In addition, with the open approach, the xenograft tumor
is observed directly without the requirement of ultrasonography, as
the tumor protrudes from the liver surface. Thus, the insufficient
RFA procedure is easier to perform. A series of preliminary
experiments as part of the present study, the parameters for RFA
were, including the preset temperature, power and duration time.
The RFA duration was the critical factor; for example, if the
duration time was >30 sec, the majority of nude mice suffered
complete tumor necrosis and death. The parameters chosen in the
present study avoided complete xenograft tumor necrosis and
guaranteed residual cancer.
The present study was designed to establish an
orthotropic nude mouse model and evaluate the invasiveness and
metastasis of residual HCC following insufficient RFA. The model
established in the present study is considered to be the first
orthotopic nude mouse model to investigate insufficient RFA. The
results demonstrated the significant influence of insufficient RFA
on tumor growth and the invasiveness of HCC. The results revealed
that insufficient RFA reduced xenograft tumor volume and prolonged
the survival time of the nude mice. The residual tumor demonstrated
more invasiveness and metastatic behavior in the insufficient
RFA-treated mice, and insufficient RFA was followed by increased
numbers of intrahepatic and lung metastatic nodules. The results
from the present study are consistent with previous studies that
revealed that insufficient RFA facilitated rapid progression of the
residual tumor (16,20,21). It
has been reported that angiogenesis induced by hypoxia-inducible
factor-1α/vascular endothelial growth factor (VEGF)-A following
hyperthermia may serve an important role in the rapid growth of the
residual tumor following incomplete RFA (22). Ke et al (16) indicated that incomplete RFA
facilitated the rapid progression of residual hepatic VX2 carcinoma
by inducing the overexpression of several molecular factors,
including proliferating cell nuclear antigen, matrix
metalloproteinase-9, VEGF, hepatocyte growth factor and
interleukin-6.
In conclusion, establishment of an insufficient RFA
orthotopic nude mouse model facilitates research into the
biological behavior of residual tumors and its underlying
mechanisms. The results of the present study, utilizing this novel
nude mouse model, shed novel insights on the pro-metastatic effects
of insufficient RFA of HCC. Further preclinical research is further
required to clarify the exact mechanism responsible for the
metastasis-enhancing potential of residual tumors.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81372314).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
NZ, HZ and YD performed the experiments. LW
participated in the study concept and design. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal protocols were approved by the Ethical
Committee on Animal Experiments of Animal Care Committee of Fudan
University (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dutta R and Mahato RI: Recent advances in
hepatocellular carcinoma therapy. Pharmacol Ther. 173:106–117.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawaguchi Y, Honda G, Endo I, Cherqui D
and Kokudo N: Current technical issues for surgery of primary liver
cancer. Liver Cancer. 6:51–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lau WY and Lai EC: The current role of
radiofrequency ablation in the management of hepatocellular
carcinoma: A systematic review. Ann Surg. 249:20–25. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shiina S, Tateishi R, Arano T, Uchino K,
Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y, et al:
Radiofrequency ablation for hepatocellular carcinoma: 10-year
outcome and prognostic factors. Am J Gastroenterol. 107:569–578.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng K, Yan J, Li X, Xia F, Ma K, Wang S,
Bie P and Dong J: A randomized controlled trial of radiofrequency
ablation and surgical resection in the treatment of small
hepatocellular carcinoma. J Hepatol. 57:794–802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park W, Chung YH, Kim JA, Jin YJ, Lee D,
Shim JH, Lee D, Kim KM, Lim YS, Lee HC, et al: Recurrences of
hepatocellular carcinoma following complete remission by
transarterial chemoembolization or radiofrequency therapy: Focused
on the recurrence patterns. Hepatol Res. 43:1304–1312. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lam VW, Ng KK, Chok KS, Cheung TT, Yuen J,
Tung H, Tso WK, Fan ST and Poon RT: Incomplete ablation after
radiofrequency ablation of hepatocellular carcinoma: Analysis of
risk factors and prognostic factors. Ann Surg Oncol. 15:782–790.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Yin X, Gan YH, Zhang BH, Zhang
JB, Chen Y, Xie XY, Ge NL, Wang YH, Ye SL and Ren ZG:
Radiofrequency ablation following first-line transarterial
chemoembolization for patients with unresectable hepatocellular
carcinoma beyond the Milan criteria. BMC Gastroenterol. 14:112014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang BW, Liang Y, Xia JL, Sun HC, Wang L,
Zhang JB, Tang ZY, Liu KD, Chen J, Xue Q, et al: Biological
characteristics of fluorescent protein-expressing human
hepatocellular carcinoma xenograft model in nude mice. Eur J
Gastroenterol Hepatol. 20:1077–1084. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Tang ZY, Qin LX, Wu XF, Sun HC,
Xue Q and Ye SL: High-dose and long-term therapy with
interferon-alfa inhibits tumor growth and recurrence in nude mice
bearing human hepatocellular carcinoma xenografts with high
metastatic potential. Hepatology. 32:43–48. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun FX, Tang ZY, Lui KD, Ye SL, Xue Q, Gao
DM and Ma ZC: Establishment of a metastatic model of human
hepatocellular carcinoma in nude mice via orthotopic implantation
of histologically intact tissues. Int J Cancer. 66:239–243. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bu W, Tang ZY, Sun FX, Ye SL, Liu KD, Xue
Q, Chen J and Gao DM: Effects of matrix metalloproteinase inhibitor
BB-94 on liver cancer growth and metastasis in a patient-like
orthotopic model LCI-D20. Hepatogastroenterology. 45:1056–1061.
1998.PubMed/NCBI
|
|
14
|
Kong J, Kong L, Kong J, Ke S, Gao J, Ding
X, Zheng L, Sun H and Sun W: After insufficient radiofrequency
ablation, tumor-associated endothelial cells exhibit enhanced
angiogenesis and promote invasiveness of residual hepatocellular
carcinoma. J Transl Med. 10:2302012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu M, Xie XH, Xie XY, Xu ZF, Liu GJ, Zheng
YL, Huang GL, Wang W, Zheng SG and Lü MD: Sorafenib suppresses the
rapid progress of hepatocellular carcinoma after insufficient
radiofrequency ablation therapy: An experiment in vivo. Acta
Radiol. 54:199–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ke S, Ding XM, Kong J, Gao J, Wang SH,
Cheng Y and Sun WB: Low temperature of radiofrequency ablation at
the target sites can facilitate rapid progression of residual
hepatic VX2 carcinoma. J Transl Med. 8:732010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakagawa H, Mizukoshi E, Iida N, Terashima
T, Kitahara M, Marukawa Y, Kitamura K, Nakamoto Y, Hiroishi K,
Imawari M and Kaneko S: In vivo immunological antitumor effect of
OK-432-stimulated dendritic cell transfer after radiofrequency
ablation. Cancer Immunol Immunother. 63:347–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Massazza G, Tomasoni A, Lucchini V,
Allavena P, Erba E, Colombo N, Mantovani A, D'Incalci M, Mangioni C
and Giavazzi R: Intraperitoneal and subcutaneous xenografts of
human ovarian carcinoma in nude mice and their potential in
experimental therapy. Int J Cancer. 44:494–500. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Niederberger M, DeLozier-Blanchet CD,
Hedinger CE and Walt H: Differences between subcutaneous and
intraperitoneal forms of three human testicular teratocarcinomas in
nude mice. Cancer. 61:1571–1578. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kasugai H, Osaki Y, Oka H, Kudo M and Seki
T; Osaka Liver Cancer Study Group, : Severe complications of
radiofrequency ablation therapy for hepatocellular carcinoma: An
analysis of 3,891 ablations in 2,614 patients. Oncology. 72 (Suppl
1):S72–S75. 2007. View Article : Google Scholar
|
|
21
|
Koda M, Murawaki Y, Hirooka Y, Kitamoto M,
Ono M, Sakaeda H, Joko K, Sato S, Tamaki K, Yamasaki T, et al:
Complications of radiofrequency ablation for hepatocellular
carcinoma in a multicenter study: An analysis of 16 346 treated
nodules in 13 283 patients. Hepatol Res. 42:1058–1064. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kong J, Kong J, Pan B, Ke S, Dong S, Li X,
Zhou A, Zheng L and Sun WB: Insufficient radiofrequency ablation
promotes angiogenesis of residual hepatocellular carcinoma via
HIF-1α/VEGFA. PLoS One. 7:e372662012. View Article : Google Scholar : PubMed/NCBI
|