Introduction
Tongue squamous cell carcinoma (TSCC) is one of the
most common oral cancer types, usually characterized by the early
occurrence of lymph node metastasis and poor prognosis (1–4). Though
the precise mechanism of TSCC tumorigenesis remains unclear, it has
become clear that the initiation and progression of tumors depends
not only on the epithelial cells, but also on the interactions
between the tumor stroma and tumor cells (5,6). Cancer
associated fibroblasts (CAFs), also named ‘activated fibroblasts’,
are the most abundant stromal cell types of the tumor stroma
(7,8). CAFs modulate tumor angiogenesis,
remodeling of the extracellular matrix and
epithelial-to-mesenchymal transition, and also contribute to tumor
recurrence and drug resistance (9,10), and
thus play a major role in the initiation and progression of
carcinomas (6,11). Therefore, CAFs would be effective
targets for cancer treatment; the present study on CAFs may be
beneficial for investigating the mechanisms of tumorigenesis and
tumor progression.
CAFs have been identified to differ phenotypically
and functionally from normal fibroblasts (NFs) (12,13).
Compared with fibroblasts from normal oral mucosal tissue, CAFs are
characterized by higher expression of α-SMA and a distinct
morphology (13). This is why NFs
are often used as the control when CAFs are studied (14). However, whether the behavior,
phenotype and function of peri-tumor fibroblasts (PTFs) are
different from CAFs in TSCC is not clear. In the present study,
CAFs and PTFs were cultured from specimens of TSCC and peri-tumor
tissues (confirmed by histology), from which fibroblasts were
purified, and subsequently their differential biological
characteristics by means of morphology observation, cell surface
marker [α-smooth muscle actin (α-SMA), vimentin and cytokeratin 19
(CK19)] expression, cell viability and pro-carcinogenic cytokine
expression was investigated. This information, on the one hand,
will provide some benefits in further understanding stromal cell
changes in tumor development, and, in turn, understanding the
mesenchymal-epithelium interactions in carcinogenesis. On the other
hand, it may provide further experimental evidence for PTFs as a
control in the examination of CAFs in TSCC.
Materials and methods
Tissue collection
TSCC specimens and ‘histologically normal’
peri-tumor tissues (at least 1 cm from the outer tumor margin) were
collected from primary tumors from 6 patients (2 males and 4
females, age 40–65 years) who underwent surgical resection at the
Qilu Hospital of Shandong University between March 2016 and January
2017. The inclusion criteria included: Age 18–75 years; a Karnofsky
score (15) ≥60%, and a life
expectancy ≥3 months; white blood cell ≥4.0×109/l,
absolute neutrophil count ≥2.0×109/l, platelet count
≥100×109/l, and hemoglobin ≥100 g/l; alanine
transaminase and aspartate transaminase <2.5× the upper limit of
normal, total bilirubin and serum creatinine <1.5× the upper
limit of normal. Patients were excluded if they had distant
metastasis or other types of cancer, had undergone surgery
involving primary tumor or lymph nodes (except diagnostic biopsy),
had received prior radiotherapy or chemotherapy, presented with
other malignancies within 5 years, or had creatinine clearance
<30 ml/min. All the resections (TSCC and peri-tumor tissues)
were confirmed by clinical and histopathological examination,
according to the World Health Organization and the 2010 criteria of
the International Union Against Cancer (16), All research procedures were approved
by the Medical Ethics Committee of Qilu Hospital, Shandong
University (2016015) and informed consent was obtained from all the
participants.
Separation, cultivation and
purification of CAFs and PTFs
The obtained tissues (tumor or peri-tumor) were
immersed in Dulbecco's modified Eagle's medium (DMEM; HyClone; GE
Healthcare Life Sciences) supplemented with 2%
penicillin-streptomycin (HyClone; GE Healthcare Life Sciences) for
at least 30 min, and then washed with PBS (HyClone; GE Healthcare
Life Sciences) three times. The tissues were minced with scalpels
into 1.0×1.0×1.0 mm3 fragments and tiled on the bottom
of a culture bottle. The cells were cultured in DMEM supplemented
20% FBS (HyClone; GE Healthcare Life Sciences) and 1%
penicillin-streptomycin. After 3 h, the culture bottle was turned
over. Cultures were incubated at 37°C in a humidified atmosphere of
5% CO2 and cell growth was observed under an inverted
microscope (magnification, ×100). Upon reaching 90% confluence, the
areas which epithelioid cells had grown in clusters were marked on
the culture bottle when the cells were observed under the
phase-contrast microscope (magnification, ×100), and according to
the markers, epithelioid cells were wiped with a cotton stick
dipped in 10 µl of trypsin. The remaining cells were digested with
0.25% trypsinase for 1 min; digestion was terminated with 3 ml of
medium containing serum, and cells were sub-cultured into fresh
bottles. Cells at passage (P)3-P5 were used in subsequent
experiments.
Cell morphology observation
Morphology of the purified P3-P5 CAFs and PTFs was
observed using a phase-contrast microscope.
Immunohistochemistry (IHC)
Coverslips with attached fibroblasts (at a density
of 3×104 cells/cm2) were fixed with 4%
paraformaldehyde for 10–20 min at room temperature. IHC procedures
using the SP kit (cat. no. SP9002; OriGene Technologies, Inc.) were
performed according to the manufacturer's instructions. The fixed
cells were washed in PBS three times, permeabilized with 0.05%
Triton for 5 min, and blocked with 4% bovine serum albumin (cat.
no. A8010; Beijing Solarbio Science & Technology Co., Ltd.) for
30 min at 37°C. Subsequently, the coverslips were incubated at 4°C
in a moist chamber overnight with the following primary mouse
anti-human monoclonal antibodies: Anti-human CK19 (dilution, 1:100;
cat. no. BM3267), anti-human vimentin (dilution, 1:100; cat. no.
BM0135), and anti-human α-SMA (dilution, 1:100; cat. no. BM0002;
all Wuhan Boster Biological Technology, Ltd.). PBS was used as the
negative control, in place of the primary antibody. After
incubation with HRP-conjugated rabbit-anti-mouse secondary antibody
(dilution, 1:100 cat. no. BA1048; Wuhan Boster Biological
Technology, Ltd.) for 30 min at room temperature, the coverslips
were counterstained with Mayer hematoxylin (cat. no. G1080; Beijing
Solarbio Science & Technology Co., Ltd.) and observed under a
light microscope (magnification, ×400; Olympus BX53; Olympus
Corporation, Tokyo, Japan,). Cells showing light-brown or
yellow-brown grains in the cytoplasm were classified as positively
stained.
Western blot analysis
CAFs/PTFs were seeded into 6-well plates
(1×105 cells/well) overnight. Following this, whole-cell
proteins were extracted using RIPA buffer (cat. no. R0020; Beijing
Solarbio Science & Technology Co., Ltd.) and quantitated using
a bicinchoninic acid protein assay kit (cat. no. PC0020; Beijing
Solarbio Science & Technology Co., Ltd.), according to the
manufacturer's protocols; the proteins (10 µg/lane) from different
samples were separated via 10% SDS-PAGE (cat. no. AR0138; Wuhan
Boster Biological Technology, Ltd.) and transferred to
polyvinylidene fluoride (PVDF) membranes (cat. no. ISEQ00010; EMD
Millipore). The PVDF membranes were blocked with blocking buffer
containing 5% skimmed milk for 1 h at room temperature before
incubation with the primary α-SMA antibody (dilution, 1:200; cat.
no. BM0002; Wuhan Boster Biological Technology, Ltd.) overnight at
4°C. Protein bands were detected using the appropriate secondary
antibodies (dilution, 1:2,000; cat. no. BA1092; Wuhan Boster
Biological Engineering Co., Wuhan, China) for 1 h at 37°C. and ECL
reagents (cat. no. 17-373SP; EMD Millipore; Merck KGaA) according
to the manufacturer's protocol. Quantitative analyses were
performed using ImageJ software (version 1.46; National Institutes
of Health, Bethesda). GAPDH (dilution, 1:2,000; cat. no.
60004-1-Ig; ProteinTech Group, Inc.) was used as an internal
control.
Cell viability analysis
Purified CAFs and PTFs (4×103 cells/well)
were loaded onto 96-well plates in 100 µl complete medium (DMEM
containing 1% penicillin-streptomycin and 10% FBS) and maintained
in a CO2 incubator at 37°C for 24 h. Each group
contained five parallel wells, as well as a negative control
(without cells). Cells were monitored for 7 consecutive days using
a Cell Counting Kit-8 (CCK-8) kit (Dojindo Molecular Technologies,
Inc.) following the manufacturer's instructions. The CCK-8 agent
(10 µl) was added to each well and incubated for 1 h at 37°C in the
dark. Absorbance values [optical density (OD)] values at 450 nm
were measured using a microplate reader.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
CAFs and PTFs were seeded into 6-well plates
(1.5×105 cells/well) and cultured in DMEM supplemented
with 10% FBS for 24 h. Total RNA from these fibroblasts was
isolated using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific), and 1 µg of the extracted RNA was used to synthesize
cDNA according to the manufacturer's recommendations for
PrimeScript RT master mix (Takara Biotechnology Co., Ltd.). The
mRNA levels of secreted matrix metalloproteinase 2 (MMP2), stromal
cell derived factor1 (SDF-1) and transforming growth factor β1
(TGFβ1) were determined using a SYBR PremixTaq II kit (Takara
Biotechnology Co., Ltd.) and a Roche 480 Light Cycler. GAPDH was
used as a reference gene. The oligonucleotide sequences of the qPCR
primers were as follows: GAPDH: Forward 5′-GCACCGTCAAGGCTGAGAAC-3′
and reverse 5′-TGGTGAAGACGCCAGTGGA-3′; MMP2: Forward
5′-CCTGCAAGTTTCCATTCCGC-3′ and reverse 5′-AACAGTGGACATGGCGGTC-3′;
SDF-1: Forward 5′-ATTCTCAACACTCCAAACTGTGC-3′ and reverse
5′-ACTTTAGCTTCGGGTCAATGC-3; and TGFβ1: Forward
5′-CGACTCGCCAGAGTGGTTAT-3′ and reverse 5′-CGGTAGTGAACCCGTTGATGT-3′.
Relative mRNA changes of the target genes were calculated using the
2−ΔΔCq method (17).
ELISA
CAFs and PTFs were cultured in 6-well plates
(1.5×105 cells/well) with serum-free DMEM for 24 h,
after which the conditioned medium (CM) was harvested for ELISA
analysis using the method described by Wang et al (18). The concentrations of MMP2, SDF-1 and
TGFβ1 in CAFs- or PTFs-derived CM were determined using ELISA kits
(cat. no. 70-EK1M022, 70-EK11192 and 70-EK1812, respectively;
Multisciences Biotech Co., Ltd.) according to the manufacturer's
instructions.
Statistical analysis
Statistical analysis was performed using SPSS 21.0
software (IBM Corp.). Values were compared using the Student's
t-test. Data are presented as the means ± standard deviation
P<0.05 was considered to indicate a statistically
significant difference.
Results
Separation, cultivation and
purification of CAFs and PTFs
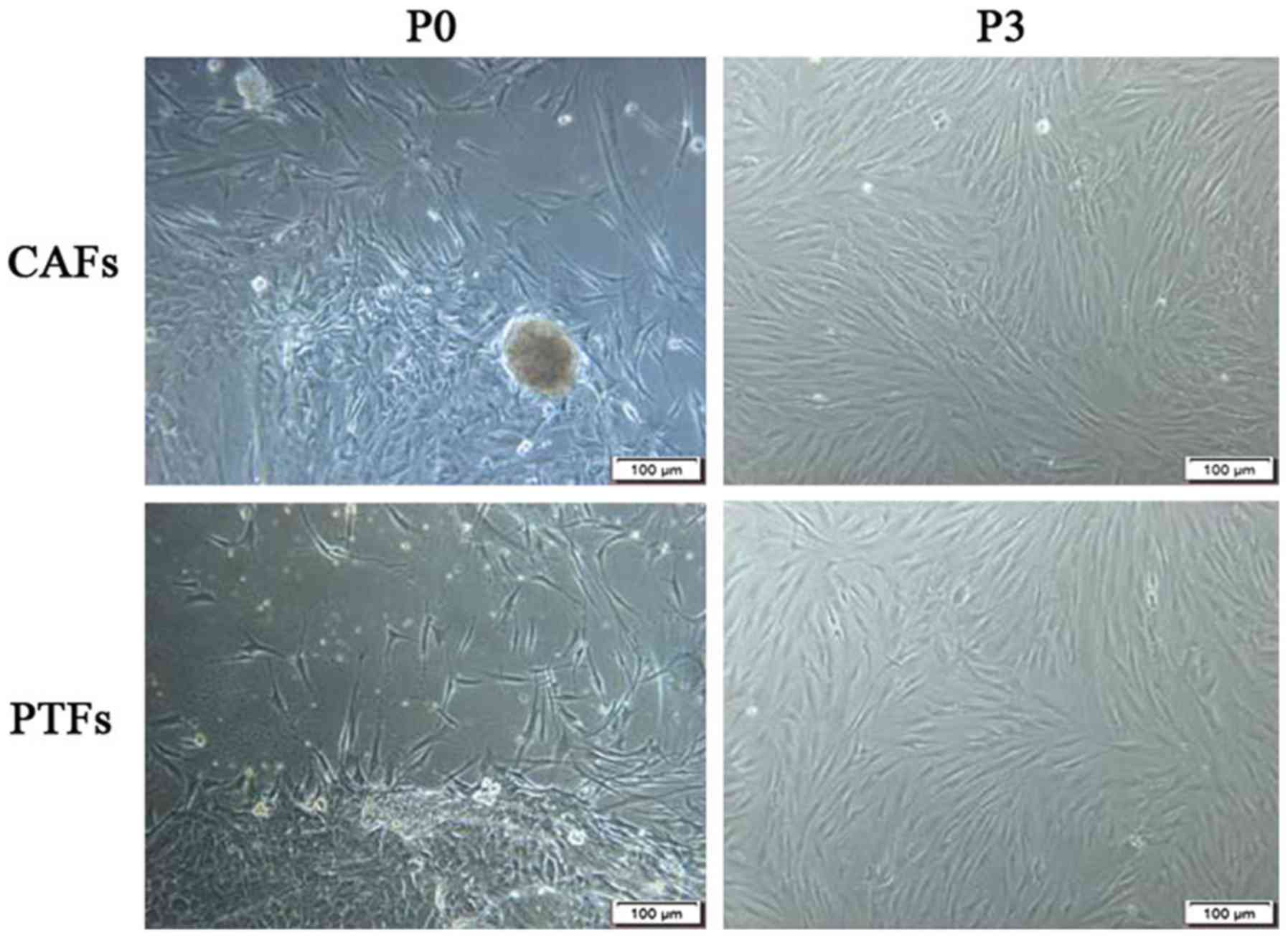
The epidermoid cells and/or fibroblasts grew from
the edge of the tissue. The epithelioid cells grew in clusters and
exhibited dominant growth in the first few days, while the
fibroblasts (CAFs or PTFs) grew radially along the tissue block or
surrounding the clusters of epidermoid cells. The time taken for
CAFs to migrate out of the tissue block is usually 4–6 days, faster
than for PTFs (5–7 days after inoculation). It took 10–12 days for
CAFs and 12–14 days for PTFs to reach 90% confluence in a Petri
dish. In P0-P2 cells, the fibroblasts mixed with the epithelioid
cells. A mechanical curettage method (13) combined with trypsinization was used
to purify these fibroblasts for P0-P2, and no epithelioid cells or
tumor cells were mixed in with the cells after the P3 generation
(Fig. 1).
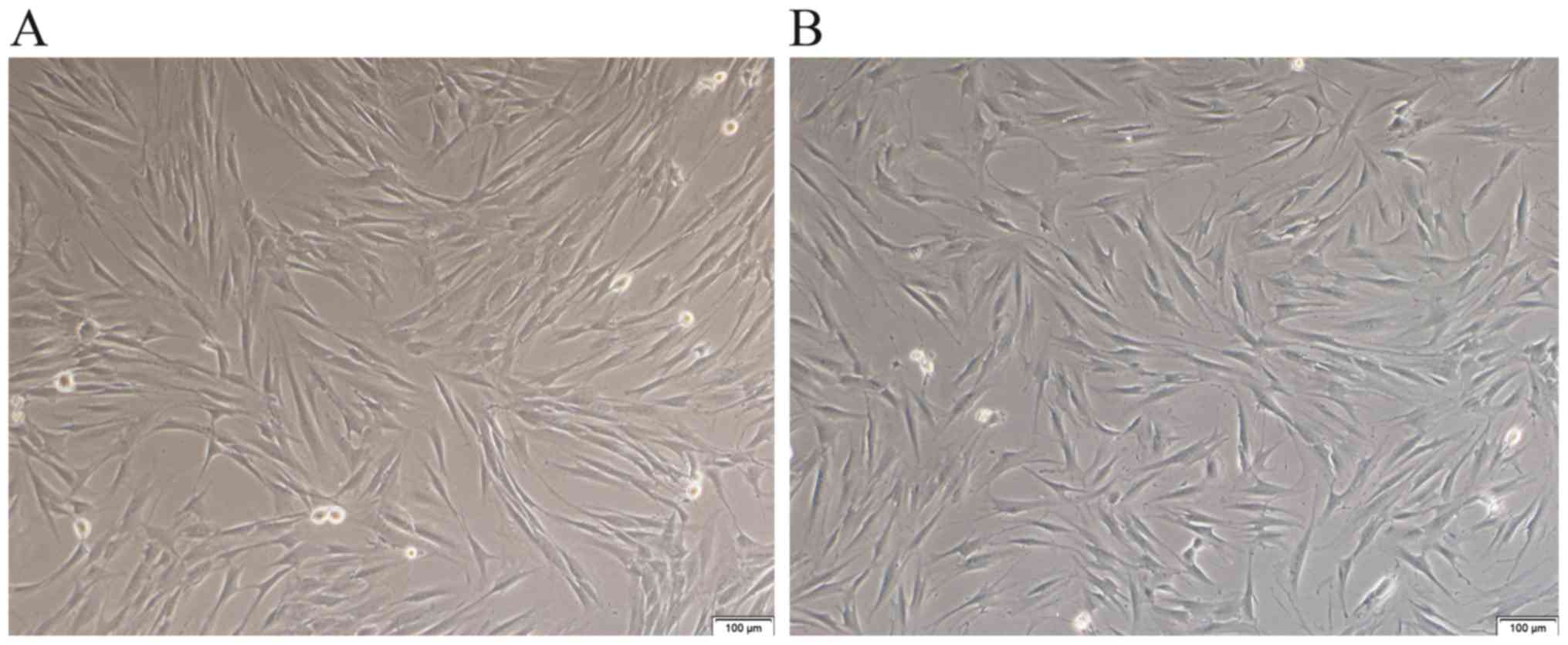
Different morphology of CAFs and
PTFs
There were differences in the morphology and growth
mode between CAFs and PTFs. Morphological observation of CAFs and
PTFs under an inverted microscope showed that the fibroblasts had a
long spindle shape. CAFs usually have small cytoplasmic
protrusions, which are accumulated and overlap in a disorderly
arrangement, and presented visible loss of contact inhibition,
which is characteristic of the proliferation of malignant cells
(19). By contrast, PTFs seem to
have more cytoplasmic processes and were nearly invariable with
respect to size; the cells showed obvious contact and density
inhibition (Fig. 2).
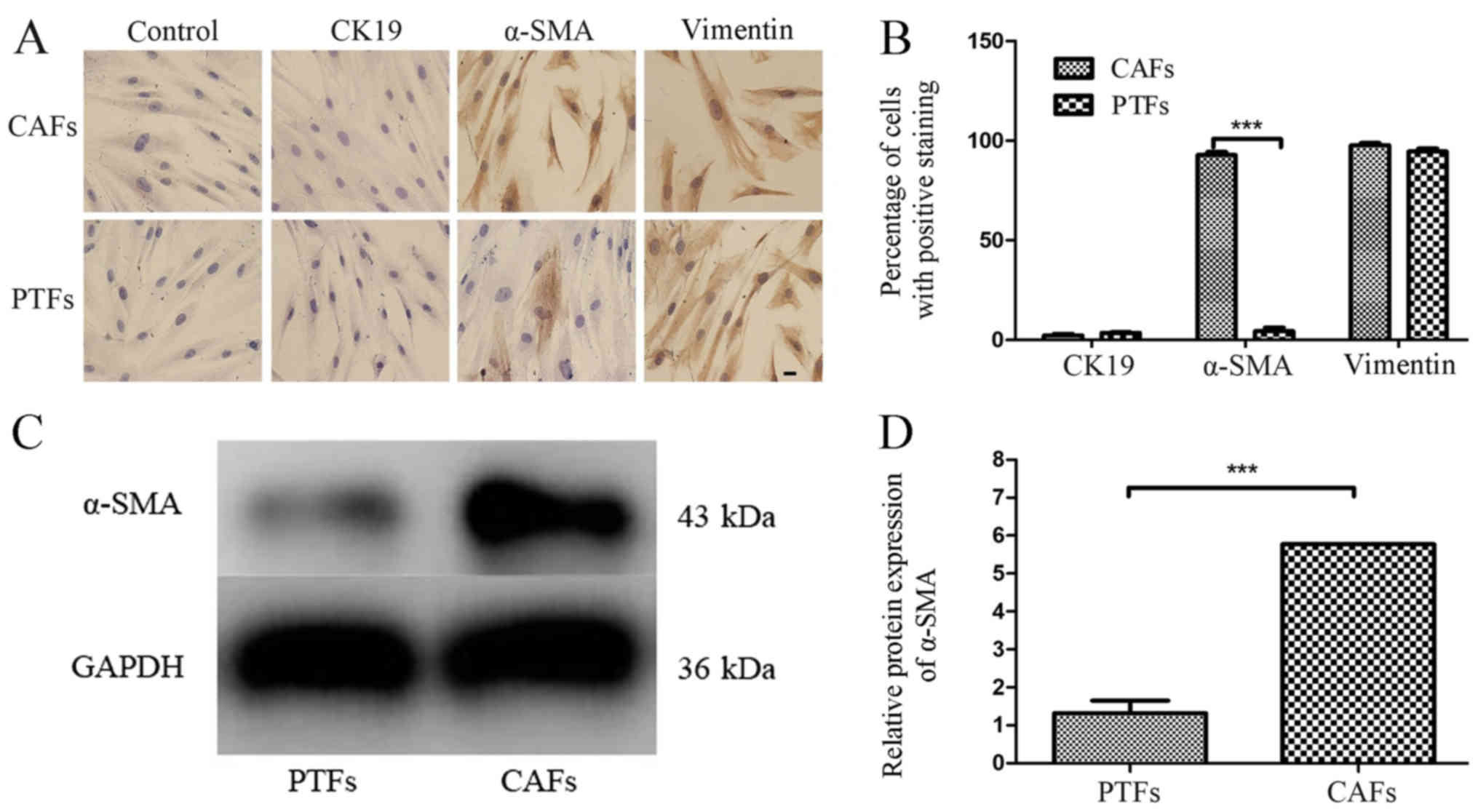
Different immunophenotypical
characterization of PTFs and CAFs
To observe the immunophenotype of PTFs and CAFs, the
expression of vimentin, α-SMA (markers for mesenchymal cells) and
CK19 (marker for epithelial cells) were detected in these
fibroblasts using IHC staining. The IHC results showed that both
CAFs and PTFs had positive expression of vimentin and negative
expression of CK19. CAFs positively expressed α-SMA, while PTFs
showed negative or only partly positive expression for α-SMA
(Fig. 3A). The percentages of CAFs
and PTFs with positive expression of CK19, α-SMA or vimentin were
calculated (Fig. 3B). The results
show that the percentage of α-SMA-positive cells in CAFs was
significantly higher compared with that in PTFs (92.8±2.05 vs.
4.4±2.5%; P<0.001). However, there was no significant
difference in the percentage of positive staining for CK19 (2.4±1.0
vs. 3.5±0.6%; P>0.05) and vimentin (97.7±1.5 vs.
94.5±2.2%; P>0.05) in CAFs compared with that in PTFs
(Fig. 3B). The expression of α-SMA
in CAFs and PTFs was further confirmed using western blot analysis
for quantitative analysis, and the results showed that the
expression of α-SMA in CAFs was 4-fold higher compared with that in
PTFs (P<0.001; Fig. 3C and
D). These data demonstrate that α-SMA may serve as an excellent
marker for distinguishing CAFs from PTFs.
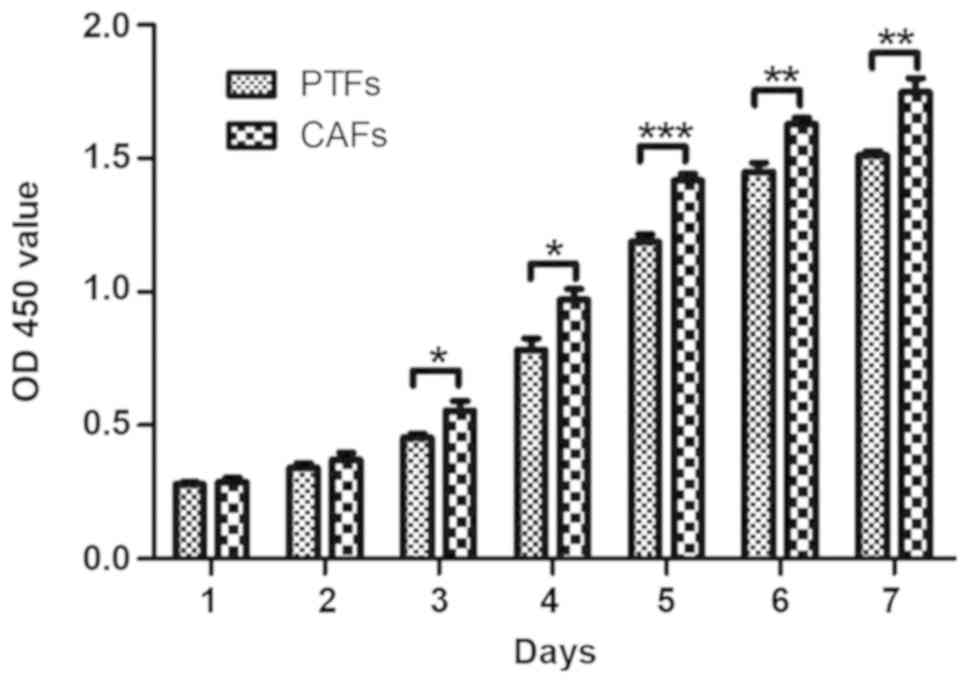
Different viability of CAFs and
PTFs
A CCK-8 kit was used to detect the viability of CAFs
and PTFs. To ensure the same culture conditions, equal quantities
of the cells (4×103) were inoculated in 96 well plates,
and the time of CCK-8 incubation and detection was the same every
day. From day 3–7 after inoculation, the OD450 value of CAFs
increased compared with that of PTFs. These results showed that the
viability of CAFs was significantly higher compared with that of
PTFs (P<0.05, P<0.01 and P<0.001; Fig. 4).
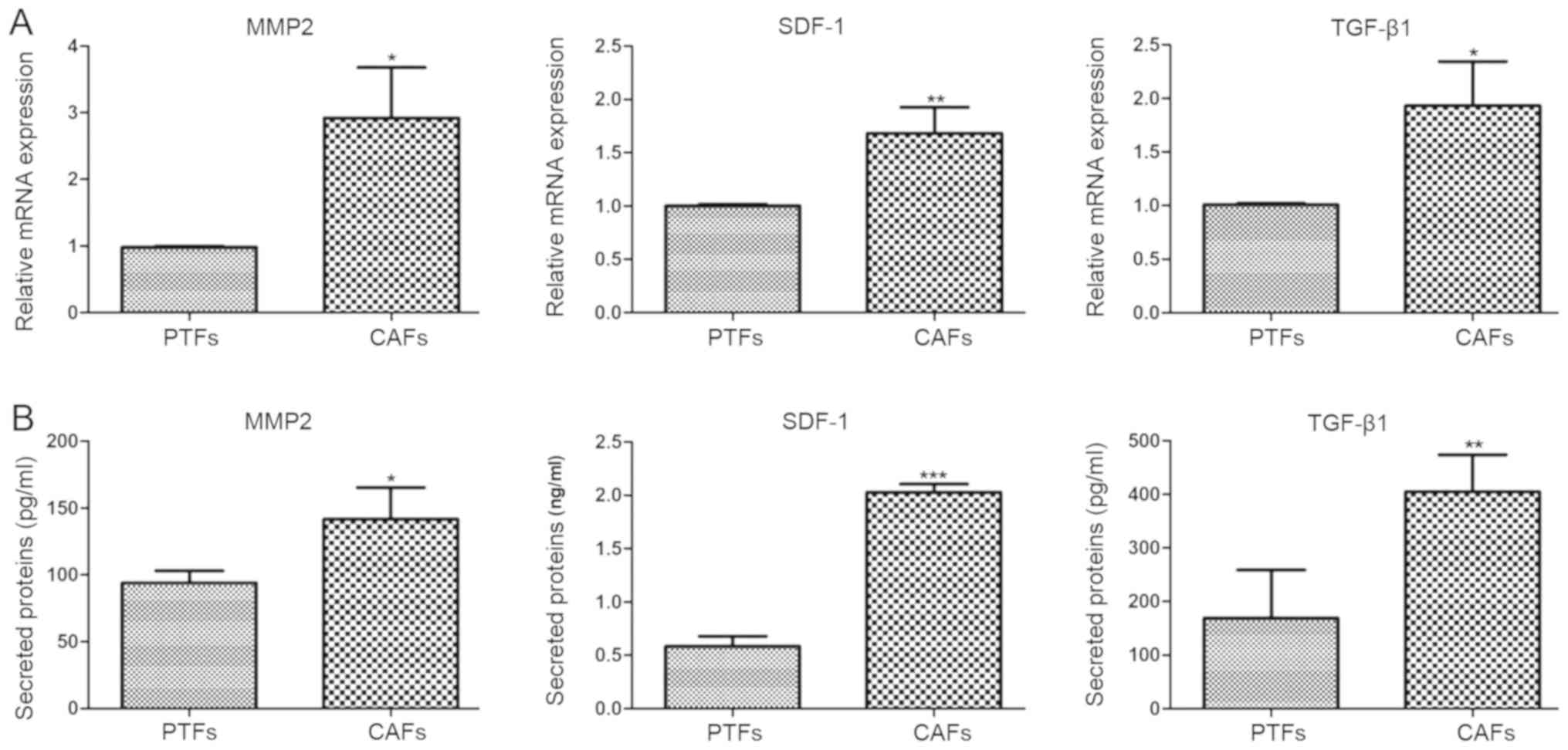
Differential expression of
pro-carcinogenic cytokines in CAFs and PTFs
CAFs and PTFs were cultured in DMEM containing 10%
FBS for 24 h. To detect the mRNA expression of pro-carcinogenic
cytokines (MMP2, SDF-1 and TGFβ1) RT-qPCR was performed. The
expression of MMP2, SDF-1 and TGFβ1 in CAFs were 2.97-, 1.68- and
1.92-fold higher compared with that in PTFs (P<0.05, P<0.01,
P<0.05, respectively; Fig. 5A).
Since these pro-carcinogenic cytokines are secretory proteins, the
levels of solubilized MMP2, SDF-1 and TGFβ1 protein in the CM of
CAFs and PTFs were assessed using corresponding ELISA kits. The
concentrations of MMP2, SDF-1 and TGFβ1 in CAFs were 1.51-, 3.47-
and 2.40-fold higher compared with those in PTFs. Thus, it was
confirmed that CAFs expressed significantly higher mRNA and protein
levels of MMP2, SDF-1 and TGF β1 compared with PTFs (P<0.05,
P<0.001 and P<0.01, respectively; Fig. 5B).
Discussion
There are several differences between CAFs and NFs;
in particular, cell morphology, proliferation, secretory function
and the expression of certain surface markers (such as α-SMA;
fibroblast activation protein, FAP; fibroblast specific protein 1,
FSP1) (20,21). However, whether these features
distinguish CAFs from PTFs in TSCC is still unclear. During
carcinogenesis, the changes occurring within cells are not only
restricted to epithelial cells, but also occur in stromal cells.
Following changes in epithelial cancer cells, various cells, such
as resident fibroblasts, adipocytes, epithelial cells, endothelial
cells or bone marrow derived mesenchymal stem cells, were
phenotypically and functionally modified and differentiated into
CAFs in the TME (22,23). Cancer cells play an important role in
inducing the transformation of local fibroblasts into CAFs, and in
endowing CAFs with pro-carcinogenic effects by secreting cytokines
such as TGFβ and IL-1β (24), thus
leading to differences between CAFs and PTFs in terms of cell
morphology, viability, secretory function and surface marker
expression.
To study the changes to stromal cells during tumor
progression, the purified fibroblast populations were verified
according to cell morphology under a microscope, and using
immunostaining, cell viability analysis and pro-carcinogenic
cytokine expression. Observation under a phase-contrast microscope
showed that, compared with CAFs, PTFs performed more cytoplasmic
processes, were invariable in size and showed contact and density
inhibition.
Previous studies have reported that CAFs express
vimentin (mesenchymal marker) and α-SMA (a specific marker of
active fibroblasts), but do not express cytokeratin (maker of
epithelial cells) (13,14,25),
though they are usually derived from various origins. Therefore,
the expression of vimentin, α-SMA and cytokeratin was detected to
identify the immunophenotype of the primarily cultured fibroblasts.
α-SMA is not only a widely used marker for identifying CAFs, but is
also an independent prognostic marker, which significantly
correlated with metastasis, recurrence and mortality in oral
squamous cell carcinoma (OSCC) (26–28). It
has been reported that the expression of α-SMA in PTFs is variable
in different organs (29,30). In the present study, the expression
of α-SMA in purified CAFs was significantly higher compared with
that in PTFs in TSCC detected by IHC. Western blot analysis further
verified that CAFs expressed high levels of α-SMA relative to that
in PTFs. These results confirmed that the expression of α-SMA is
increased step by step in PTFs and CAFs, which indicated that the
fibroblasts may perform partial immunophenotypic changes earlier
than the epithelial cells.
A study on the morphological, functional and genetic
alteration of NFs, PTFs and CAFs in normal tissue, peri-tumor
tissue and tumoral tissue would be beneficial to further reveal the
processes involved in tumor progression. In general, peri-tumor
tissue described in the literature refers to the tissues with no
abnormal histological appearance at a certain distance around the
tumor boundary. In a previous study, fibroblasts derived from
normal oral mucosa or para-cancerous tissue at least 2 cm away from
the outer tumor margin were more commonly used as control cells
(31). In the present study,
fibroblasts derived from peri-tumor tissues 1 cm away from the
outer tumor margin were not only significantly different from the
biological characteristics of CAFs, but also retained the
morphology and function of the tongue tissue to a greater
extent.
Paracrine signaling is an important way for tumor
stromal cells to participate in tumorigenesis and development. In
the present study, CAFs showed significant pro-carcinogenic
potential compared with that in PTFs. It has recently become widely
accepted that CAFs could be an efficient target for cancer therapy
(32). Drugs that can regulate the
phenotype and function of CAFs cells may be used in the treatment
of tumors. In future studies, it is necessary to further explore
the differential performance in chemosensitivity between CAFs and
PTFs, which will be beneficial to the therapy of TSCC.
In conclusion, PTFs are distinguishable from CAFs in
terms of their biological characteristics, and PTFs would be
suitable control cells in a study investigating the role of CAFs in
tumorigenesis and tumor progression.
Acknowledgements
Not applicable.
Funding
This investigation was supported by Natural Science
Foundation of China (grant no. 81702684), Beijing, China, and
Shandong Medical and Health Science and Technology Development Plan
Project, Jinan, Shandong (grant no. 2018WS112).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PB and XZ performed the experiment and wrote the
manuscript. QS and CY were responsible for the design of the
experiment. MY, LL, GF and PY analyzed the experimental data and
assisted in the revision of the manuscript. XD, MW, and SL assisted
with the statistical analysis. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All research procedures were approved by the Medical
Ethics Committee of Qilu Hospital, Shandong University (approval
no. 2016015) and informed consent was obtained from all the
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qiu K, Huang Z, Huang Z, He Z and You S:
miR-22 regulates cell invasion, migration and proliferation in
vitro through inhibiting CD147 expression in tongue squamous cell
carcinoma. Arch Oral Biol. 66:92–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsushima N, Sakashita T, Homma A,
Hatakeyama H, Kano S, Mizumachi T, Kakizaki T, Suzuki T and Fukuda
S: The role of prophylactic neck dissection and tumor thickness
evaluation for patients with cN0 tongue squamous cell carcinoma.
Eur Arch Otorhinolaryngol. 273:3987–3992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang T, Liang L, Liu X, Wu JN, Chen J, Su
K, Zheng Q, Huang H and Liao GQ: TGFβ1-Smad3-Jagged1-Notch1-Slug
signaling pathway takes part in tumorigenesis and progress of
tongue squamous cell carcinoma. J Oral Pathol Med. 45:486–493.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rodrigues-Lisoni FC, Peitl P Jr, Vidotto
A, Polachini GM, Maniglia JV, Carmona-Raphe J, Cunha BR, Henrique
T, Souza CF, Teixeira RA, et al: Genomics and proteomics approaches
to the study of cancer-stroma interactions. BMC Med Genomics.
3:142010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jia CC, Wang TT, Liu W, Fu BS, Hua X, Wang
GY, Li TJ, Li X, Wu XY, Tai Y, et al: Cancer-associated fibroblasts
from hepatocellular carcinoma promote malignant cell proliferation
by HGF secretion. PLoS One. 8:e632432013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du H and Che G: Genetic alterations and
epigenetic alterations of cancer-associated fibroblasts. Oncol
Lett. 13:3–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xouri G and Christian S: Origin and
function of tumor stroma fibroblasts. Semin Cell Dev Biol.
21:40–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karagiannis GS, Poutahidis T, Erdman SE,
Kirsch R, Riddell RH and Diamandis EP: Cancer-associated
fibroblasts drive the progression of metastasis through both
paracrine and mechanical pressure on cancer tissue. Mol Cancer Res.
10:1403–1418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beacham DA and Cukierman E: Stromagenesis:
The changing face of fibroblastic microenvironments during tumor
progression. Semin Cancer Biol. 15:329–341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xing F, Saidou J and Watabe K: Cancer
associated fibroblasts (CAFs) in tumor microenvironment. Front
Biosci (Landmark Ed). 15:166–179. 2011. View Article : Google Scholar
|
|
13
|
Liu Y, Hu T, Shen J, Li SF, Lin JW, Zheng
XH, Gao QH and Zhou HM: Separation, cultivation and biological
characteristics of oral carcinoma-associated fibroblasts. Oral Dis.
12:375–380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou B, Chen WL, Wang YY, Lin ZY, Zhang
DM, Fan S and Li JS: A role for cancer-associated fibroblasts in
inducing the epithelial-to-mesenchymal transition in human tongue
squamous cell carcinoma. J Oral Pathol Med. 43:585–592. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brezinski D, Stone PH, Muller JE, Tofler
GH, Davis V, Parker C, Hartley LH and Braunwald E: Prognostic
significance of the Karnofsky Performance Status score in patients
with acute myocardial infarction: Comparison with the left
ventricular ejection fraction and the exercise treadmill test
performance. The MILIS Study Group. Am Heart J. 121:1374–1381.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sobin LH and Wittekind C: TNM
classification of malignant tumours. 7th. Wiley-Blackwell; Hoboken,
NJ: pp. pp3092010
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Zhang F, Cui JY, Chen L, Chen YT
and Liu BW: CAFs enhance paclitaxel resistance by inducing EMT
through the IL6/JAK2/STAT3 pathway. Oncol Rep. 39:2081–2090.
2018.PubMed/NCBI
|
|
19
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Cao L, Wang H, Liu B, Zhang Q,
Meng Z, Wu X, Zhou Q and Xu K: Cancer-associated fibroblasts
enhance metastatic potential of lung cancer cells through
IL-6/STAT3 signaling pathway. Oncotarget. 8:76116–76128.
2017.PubMed/NCBI
|
|
21
|
Räsänen K, Virtanen I, Salmenperä P,
Grenman R and Vaheri A: Differences in the nemosis response of
normal and cancer-associated fibroblasts from patients with oral
squamous cell carcinoma. PLoS One. 4:e68792009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuzet SE and Gaggioli C: Fibroblast
activation in cancer: When seed fertilizes soil. Cell Tissue Res.
365:607–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shiga K, Hara M, Nagasaki T, Sato T,
Takahashi H and Takeyama H: Cancer-associated fibroblasts: Their
characteristics and their roles in tumor growth. Cancers (Basel).
7:2443–2458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leef G and Thomas SM: Molecular
communication between tumor-associated fibroblasts and head and
neck squamous cell carcinoma. Oral Oncol. 49:381–386. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Chen S, Wang W, Ning BF, Chen F,
Shen W, Ding J, Chen W, Xie WF and Zhang X: Cancer-associated
fibroblasts promote hepatocellular carcinoma metastasis through
chemokine-activated hedgehog and TGF-β pathways. Cancer Lett.
379:49–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dourado RC, Porto LPA, Leitão ÁCGH,
Cerqueira PSG, Dos Santos JN, Ramalho LMP and Xavier FCA:
Immunohisto-chemical characterization of Cancer-associated
fibroblasts in oral squamous cell carcinoma. Appl Immunohistochem
Mol Morphol. 26:640–647. 2018.PubMed/NCBI
|
|
27
|
Marsh D, Suchak K, Moutasim KA, Vallath S,
Hopper C, Jerjes W, Upile T, Kalavrezos N, Violette SM, Weinreb PH,
et al: Stromal features are predictive of disease mortality in oral
cancer patients. J Pathol. 223:470–481. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luksic I, Suton P, Manojlovic S, Virag M,
Petrovecki M and Macan D: Significance of myofibroblast appearance
in squamous cell carcinoma of the oral cavity on the occurrence of
occult regional metastases, distant metastases, and survival. Int J
Oral Maxillofac Surg. 44:1075–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mazzocca A, Dituri F, Lupo L, Quaranta M,
Antonaci S and Giannelli G: Tumor-secreted lysophostatidic acid
accelerates hepatocellular carcinoma progression by promoting
differentiation of peritumoral fibroblasts in myofibroblasts.
Hepatology. 54:920–930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hawsawi NM, Ghebeh H, Hendrayani SF,
Tulbah A, Al-Eid M, Al-Tweigeri T, Ajarim D, Alaiya A, Dermime S
and Aboussekhra A: Breast carcinoma-associated fibroblasts and
their counterparts display neoplastic-specific changes. Cancer Res.
68:2717–2725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou B, Zhuang XM, Wang YY, Lin ZY, Zhang
DM, Fan S, Li JS and Chen WL: Tumor necrosis factor α induces
myofibroblast differentiation in human tongue cancer and promotes
invasiveness and angiogenesis via secretion of stromal cell-derived
factor-1. Oral Oncol. 51:1095–1102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alkasalias T, Moyano-Galceran L,
Arsenian-Henriksson M and Lehti K: Fibroblasts in the tumor
microenvironment: Shield or Spear? Int J Mol Sci. 19(pii):
E15322018. View Article : Google Scholar : PubMed/NCBI
|