Introduction
Colorectal cancer (CRC) is a common gastrointestinal
cancer that affects more than 900,000 patients each year, and its
incidence is ranked third of all cancers (1,2).
Colorectal cancer is mainly divided into adenocarcinoma, mucinous
adenocarcinoma and undifferentiated carcinoma (3). It is necessary to further investigate
the pathogenesis and biological features of colorectal cancer.
MicroRNAs (miRNAs) are a class of non-coding RNAs
that mediate gene expression at the post-transcriptional level
(4,5). Growing evidence demonstrates that
miRNAs could act as oncogenes or tumor suppressors in diverse
tumors including colorectal cancer (2,6,7). Furthermore, increasing miRNAs has been
reported to play an important role in colorectal cancer, such as
miR-144, miR-495, miR-590, miR-6803 (8–11).
miR-148a has been reported to be downregulated in many kinds of
tumors including breast cancer, renal cell carcinoma and
endometrial cancer (12–14). According to Li et al (12), miR-148a promotes apoptosis and
inhibits growth of breast cancer. Feng et al (15) reported similar findings that miR-148a
suppressed the proliferation and migration in pancreatic cancer
cells. In addition, miR-148a could directly bind to 3′UTR of target
mRNAs to effect cell progression, and the target genes including
BCL-2, AKT2, IQGAP1 and ErbB3 (12–15). Our
study explored the pivotal roles of miR-148a and ErbB3 on the
proliferation and migration of colorectal cancer.
ErbB3, known as HER3, is a transmembrane tyrosine
kinase receptor that is the only member of the ErbB receptor family
that lacks tyrosine kinase activity, and containing other three
members: ErbB1 (EGFR, HER1), ErbB2 (Neu, HER2), and ErbB4 (HER4)
(16–19). Inactivation of ErbB3 promotes cell
apoptosis and inhibits the growth and invasiveness of lung
adenocarcinoma cell (20).
Similarly, Appert-Collin et al (21) found that ErbB3 was overexpressed and
promoted migration and invasion in gliomas and in non-small cell
lung carcinoma (21). Silence of
ErbB3 reduced the proliferation and tumor growth in osteosarcoma
cells. Considering all the findings, we verified the hypothesis
that miR-148a regulated the proliferation and migration through
suppressing the expression of ErbB3 in CRC cells. miR-148a has been
found to be downregulated in CRC tissues and cell lines, and it has
an inverse correlation between the expression of miR-148a and ErbB3
in CRC tissues. We subsequently found that miR-148a targeted the
3′UTR of ErbB3 mRNA and regulated the expression of ErbB3 in CRC
cells. Moreover, we discovered that downregulation of miR-148a
caused repression of ErbB3, thereby suppressing the proliferation
and migration of colorectal cancer cells.
Patients and methods
Patients and clinical samples
Fifty-one pairs of human colorectal cancer tissue
samples and corresponding paracancerous tissues were obtained from
colorectal cancer patients at the First Affiliated Hospital of
Xi'an Jiaotong University (Xi'an, China) from 2015 to 2017. The
tissues were immediately snap-frozen in liquid nitrogen and stored
in a −80°C freezer. None of these patients had local or systemic
treatment before operation.
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Xi'an Jiaotong
University (Human ethic no. 2015-06). Patients who participated in
this research had complete clinical data. The signed informed
consents were obtained from the patients or the guardians.
Cell lines and culture condition
Human colorectal cancer cell lines LoVo (cat. no.
CCL-229) and SW480 (cat. no. CCL-228) and the normal colon cell
line CCD-18Co (cat. no. CRL-1459) were obtained from the American
Type Culture Collection (ATCC; Manassas, VA, USA). All the cancer
and normal cells were cultured in RPMI-1640 medium containing 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C and 5% CO2.
RNA isolation and RT-qPCR
Total RNAs (from tissues and cell lines) were
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Waltham, MA, USA) containing total miRNAs, following
the manufacturers instructions of MagMAX™ and mirVana™ total RNA
isolation kits (both from Thermo Fisher Scientific, Inc.). The
temperature conditions for reverse transcription were as follows:
37°C for 15 min and 85°C for 5 sec. Thermoscript RT-qPCR system
(Takara Biotechnology Co., Ltd., Dalian, China) were utilized to
perform reverse-transcription. SYBR Prime Script miRNA RT-qPCR kit
or SYBR premix kit (Takara Biotechnology Co.) were applied to
analyze qPCR on an Applied Biosystems 7300 sequence detection
system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Relative expression levels of mRNA and miRNA were calculated using
2−ΔΔCq method (22). The
relative quantification of ErbB3 mRNA was normalized by GAPDH. The
premier sequences were: For miR-148a, F:
5′-ACACTCCAGCTGGGTCAGTGCACTACA-GAA-3′, and R:
5′-TGGTGTCGTGGAGTCG-3′; for U6, F: 5′-TCCGATCGTGAAGCGTTC-3′, and R:
5′-GTGCAGGGTCCGAGGT-3′; for ErbB3, F: 5′-TTCCGAGATGGGCAACTCTC-3′,
and R: 5′-CTTGCAGACTTCGTGACAGG-3′; for GADPH, F:
5′-GAAGGTGAAGGTCGGAGTC-3′ and R: 5′-ATCCAGTGCAGGGTCCGAGG-3′. The
reactions were incubated in a 96-well plate at 95°C for 5 min,
followed by 40 cycles of 95°C for 30 sec, 60°C for 30 sec, and 72°C
for 30 sec.
Protein extraction and western blot
analysis
Total proteins were extracted from tissue specimens
and cell lines on ice using RIPA lysis buffer containing 1%
proteinase inhibitor (Beyotime Institute of Biotechnology, Haimen,
China). Same amount of proteins (50 µg) were separated using
8% SDS polyacrylamide gels, which were quantitated by BCA protein
assay kit (Solarbio, Beijing, China); and then the protein blots
were transferred onto polyvinylidene difluoride membrane (PVDF;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). After blocking in
5% at room temperature for 1.5 h non-fat milk, the membrane was
incubated with rabbit anti-ErbB3 monoclonal antibody (cat. no.
32121; dil, 1:1,000; Abcam, Cambridge, UK) and anti-GAPDH mouse
monoclonal antibody (cat. no. 8795; 1:4,000; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany), which was used as internal reference. We
visualized the proteins using ECL detection kit (GE Healthcare,
Chicago, IL, USA) on Bio-Rad Gel Doc XR instrument (Bio-Rad
Laboratories, Inc.).
Cell proliferation assay
The cellular proliferative capacity was performed
using 3-(4,5-dimethylthiazol-2-yl)-2,5-dipheny-ltetrazolium bromide
(MTT; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and dimethyl
sulfoxide (DMSO; Beijing Solarbio Science & Technology Co.,
Ltd.) solutions. The applicable cells were seeded into 96-well
plates and cultured in 37°C incubator for 24, 48, 72 and 96 h. The
cells were added with 10 µl MTT solution for 4 h and then 150 µl
DMSO in order to solubilize the formazan crystals. The optical
density was measured by a microplate reader (BioTek China, Beijing,
China) at wavelength of 490 nm.
Transwell assay
Transwell assay utilized 8 µm pore inserts covered
with or without Matrigel (BD Biosciences, Franklin Lakes, NJ, USA)
to detect the invasion and migratory abilities in a 24-well plate,
in upper and lower chambers. Subsequently, cell suspension prepared
in serum-free medium was added into the upper chamber, whereas 500
µl RPMI-1640 medium was added into the lower chamber. After
migrating for 24 h, the non-migrating cells were removed using
cotton swab. The migratory or invasive cells were fixed in 4%
paraformaldehyde and then stained with 0.1% crystal violet. The
cell number in five random fields were counted under a microscope
(Olympus, Tokyo, Japan) and the average number calculated.
Transfection
Cells were transfected with miR-148a mimic, and
miR-148a inhibitor to up or down regulate the miR-148a expression,
and pcDNA3.1-ErbB3 vectors were employed to overexpress ErbB3
(Shanghai GenePharma Co., Ltd., Shanghai, China).
LoVo cells (4×106 cells/well) were seeded
into a 6-well plate at 80% density, using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). After transfected for
48 h, the cells were harvested for western blot analysis or
RT-qPCR.
Plasmid construction and luciferase
reporter assay
TargetScan (http://www.targetscan.org/vert_71/) was utilized to
predict the target genes of miR-148a and to confirm that ErbB3 was
a target gene of miR-148a. ErbB3 mRNA containing the putative
miR-148a binding sites on 3′UTR segment was cloned into firefly
luciferase vector pmirGlo (pmirGlo-ErbB3-WT, WT). QuickChange
Site-Directed Mutagenesis kit (Agilent Technologies, Inc., Santa
Clara, CA, USA) was applied to mutate the binding sites from
UGCACUG to ACGUGAC (pmirGlo-ErbB3-MUT, MUT).
LoVo cells were seeded into 6-well plates and
cultured overnight, and then co-transfected with miR-148a or
control and pmirGlo-ErbB3-WT or pmirGlo-ErbB3-MUT vectors with
Renilla luciferase report vector as the internal control. After 48
h, dual-luciferase assay kit (Promega Corporation, Madison, WI,
USA) was employed to perform the luciferase activity.
Statistical analysis
Statistical analysis was executed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Statistical analysis was
performed using Student's t-test between two groups and
Kruskall-Wallis test with Bonferonni's post hoc test for multiple
groups. Differences were considered statistically significant at
P<0.05.
Results
The relationship of miR-148a and ErbB3
in colorectal cancer
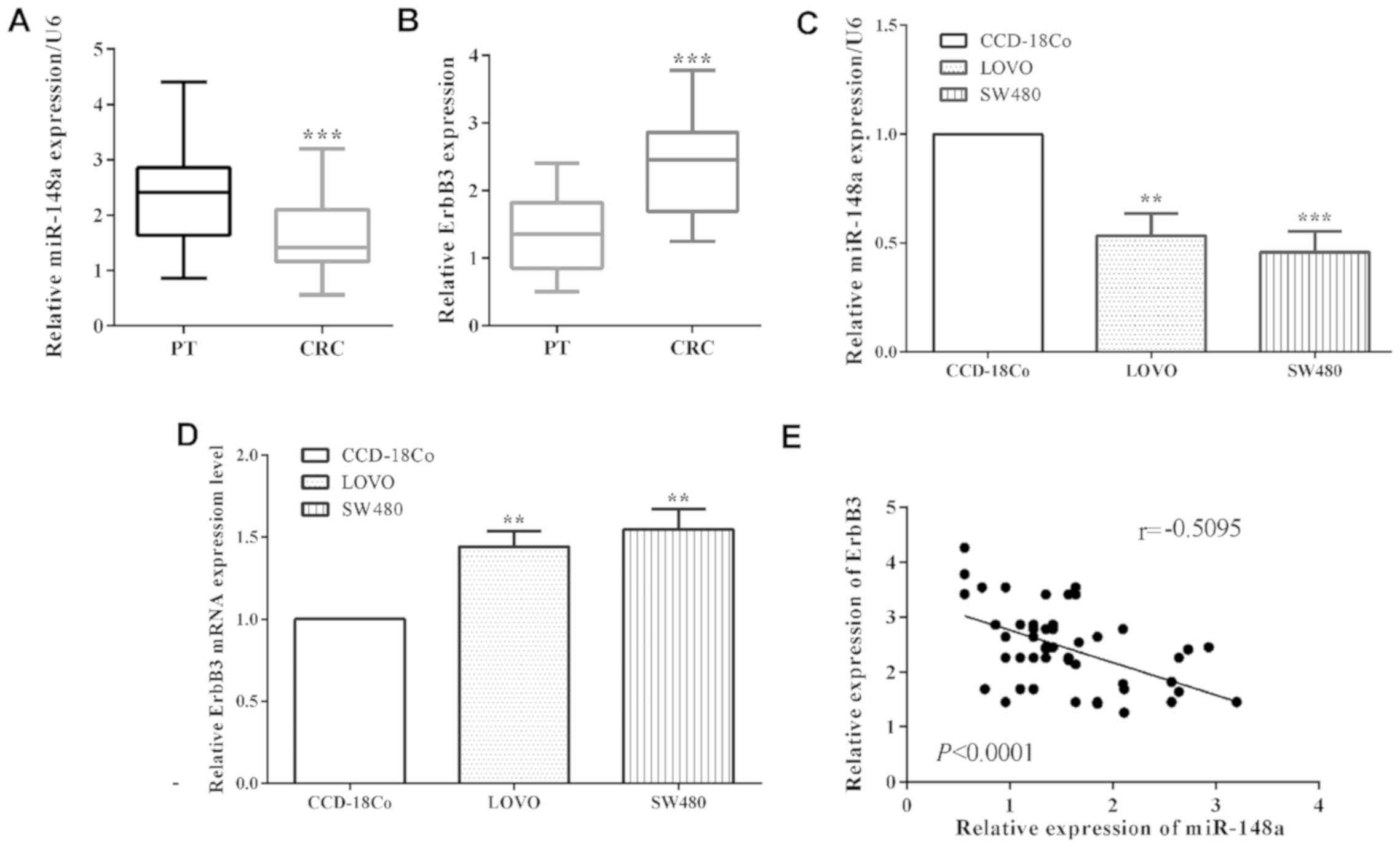
The mRNA level of miR-148a in colorectal cancer
tissues was determined to be lower than that in corresponding
paracancerous tissue samples (P<0.0001) (Fig. 1A). Due to the limitation of the
experiment condition, we did not evaluate the expression of ErbB3
in paraffin block. However, RT-qPCR was performed to calculate the
expression of ErbB3 in colorectal cancer tissue. The expression of
ErbB3 was higher in colorectal cancer tissues than that in the
corresponding paracancerous tissues (P<0.0001) (Fig. 1B). Moreover, in colorectal cancer
LoVo cell lines (P=0.0014) and SW480 (P=0.0006), the mRNA level of
miR-148a was also reduced versus the normal colon cells CCD-18Co
performed by RT-qPCR (Fig. 1C). On
the contrary, the mRNA level of ErbB3 of CRC cell lines LoVo
(P=0.0012) and SW480 (P=0.0016) were upregulated compared to
CCD-18Co cells (Fig. 1D). In
addition, the mRNA level of miR-148a had a negative relationship
with ErbB3 (P<0.0001; r=0.5095) in colorectal cancer tissues
(Fig. 1E). Our results showed that
miR-148a is downregulated while ErbB3 was upregulated in NPC
cells.
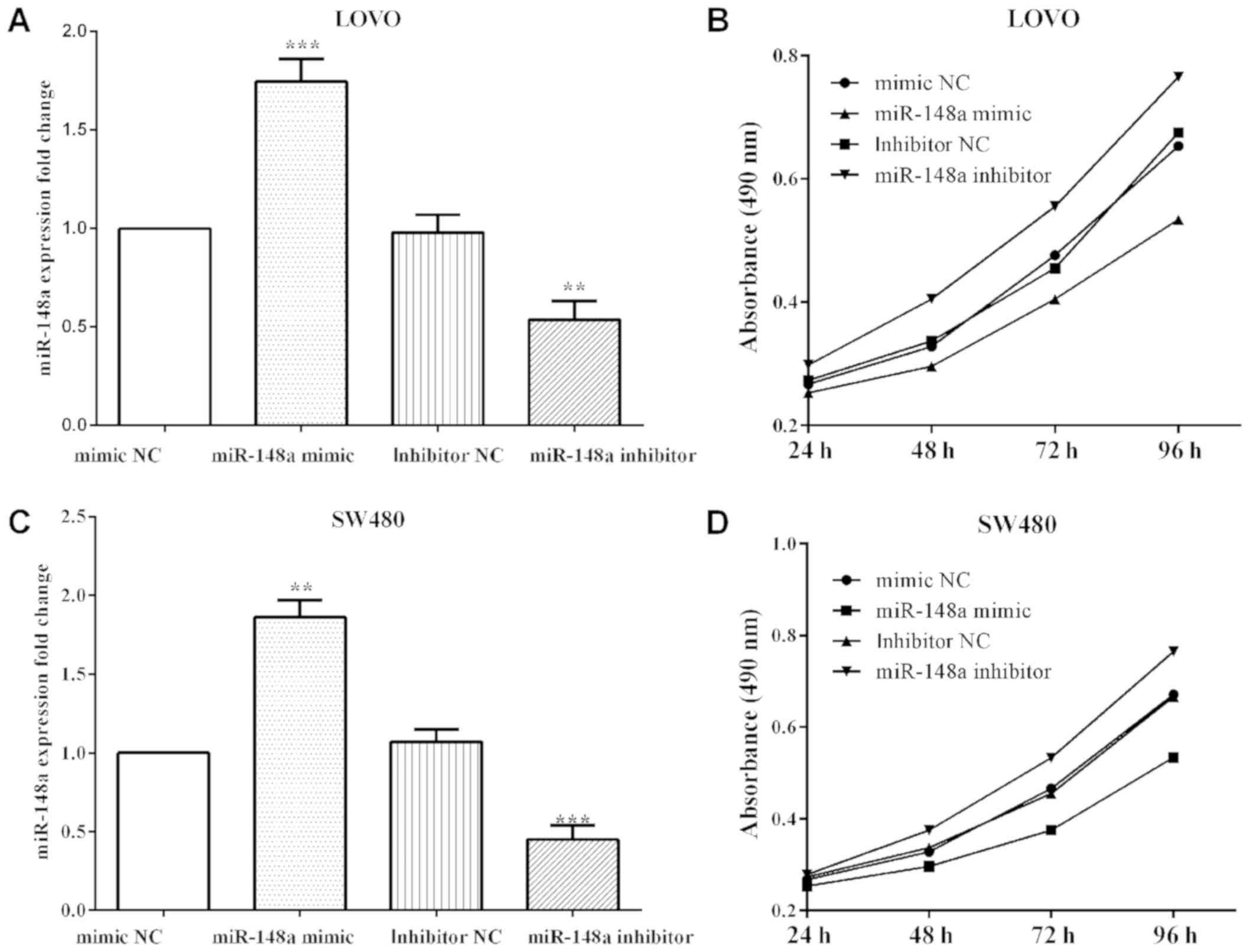
miR-148a inhibits proliferation of
colorectal cancer LoVo cells
To overexpress or knockdown miR-148a, miR-148a
mimics (P=0.0004 and P=0.0026) or inhibitor (P=0.0043 and P=0.0008)
were transfected, as well as their negative control in LoVo and
SW480 cells and then MTT and Transwell assays were performed
(Fig. 2A and C). The proliferative
absorbancy was reduced (P<0.0001) when transfected with miR-148a
mimic, while increased (P<0.0001) when transfected with miR-148a
inhibitor in LoVo cells (Fig. 2B).
Similar findings to the results in LoVo cells were found where
miR-148a mimic decreased the proliferation (P<0.0001), and
miR-148a inhibitor increased in SW480 (P<0.0001) (Fig. 2D).
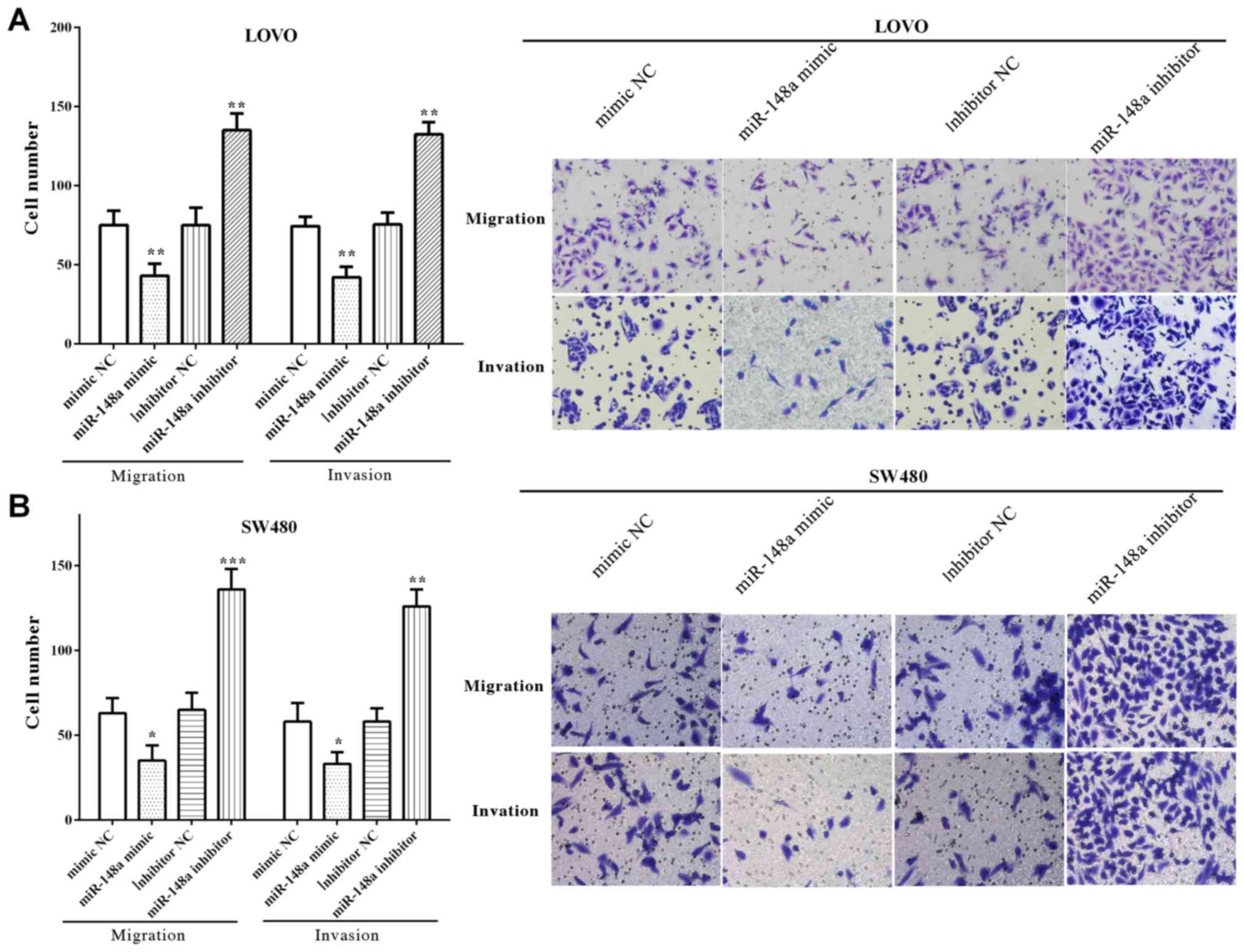
miR-148a inhibits migration and
invasion of colorectal cancer LoVo and SW480 cells
The migratory and invasive number of LoVo cells was
decreased (P=0.0095 and P=0.0052) after upregulated miR-148a,
whereas enhanced (P=0.0024 and P=0.0035) when downregulated with
miR-148a (Fig. 3A). Similarly, cell
migration and invasion were reduced by miR-148a mimic (P=0.0342 and
P=0.0284), whereas increased by miR-148a inhibitor (P=0.0002 and
P=0.0075) (Fig. 3B). All the results
demonstrated that miR-148a inhibited the migration and invasion of
LoVo and SW480 cells.
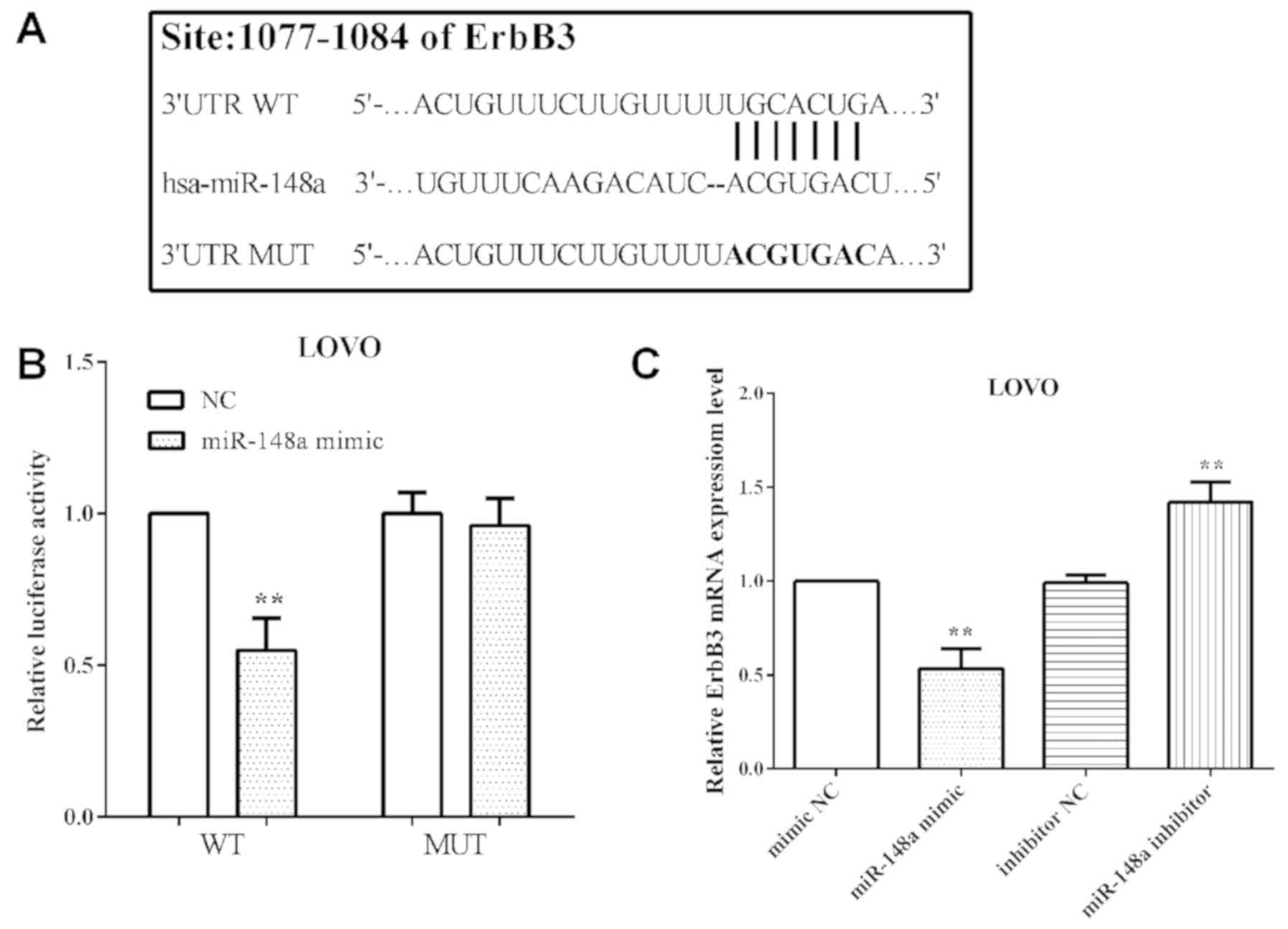
miR-148a targets ErbB3 and inhibits
its expression
TargetScan software predicted that ErbB3 was a
direct target gene of miR-148a and the binding sequence was
UGCACUGA, which was located at 1077 to 1084 on 3′UTR of mRNA. The
binding sequences were then mutated from
5′-…ACUGUUUCUUGUUUUUGCACUGA…-3′ to 5′-…ACUGUUUCUUGUUUUACGUGACA…-3′,
as shown in Fig. 4A.
The luciferase reporter assay was performed to
confirm that ErbB3 was a direct target of miR-148a. We
co-transfected pmirGlo-miR-148a mimic or negative control and
pmirGlo-ErbB3-WT or pmirGlo-ErbB3-MUT into LoVo cells and measured
the luciferase reporter activity. The results showed that miR-148a
suppressed (P=0.0018) pmirGlo-ErbB3-WT reporter activity, but not
pmirGlo-ErbB3-MUT (P=0.05762) (Fig.
4B), which illustrated ErbB3 wild-type binding to miR-148a,
whereas not that of the mutant.
The miR-148a mimic or inhibitor was transfeted to
alter the expression of miR-148a and to determined the mRNA level
of ErbB3. As expected, transfection of miR-148a mimic decreased
(P=0.0016) the mRNA level of ErbB3 in LoVo cells. Simultaneously,
the mRNA level of ErbB3 was increased (P=0.0027) with miR-148a in
LoVo cells (Fig. 4C).
ErbB3 reverses partial function of
miR-148a
To explore whether ErbB3 re-expression could reverse
the inhibitory effects of miR-148a on ErbB3 and cell proliferation
and migration miR-148a mimic was transfected into LoVo cells, along
with ErbB3 overexpression plasmid (pcNA31-ErbB3) or negative
control. The cells transfected with miR-148a mimic and pcNA31-ErbB3
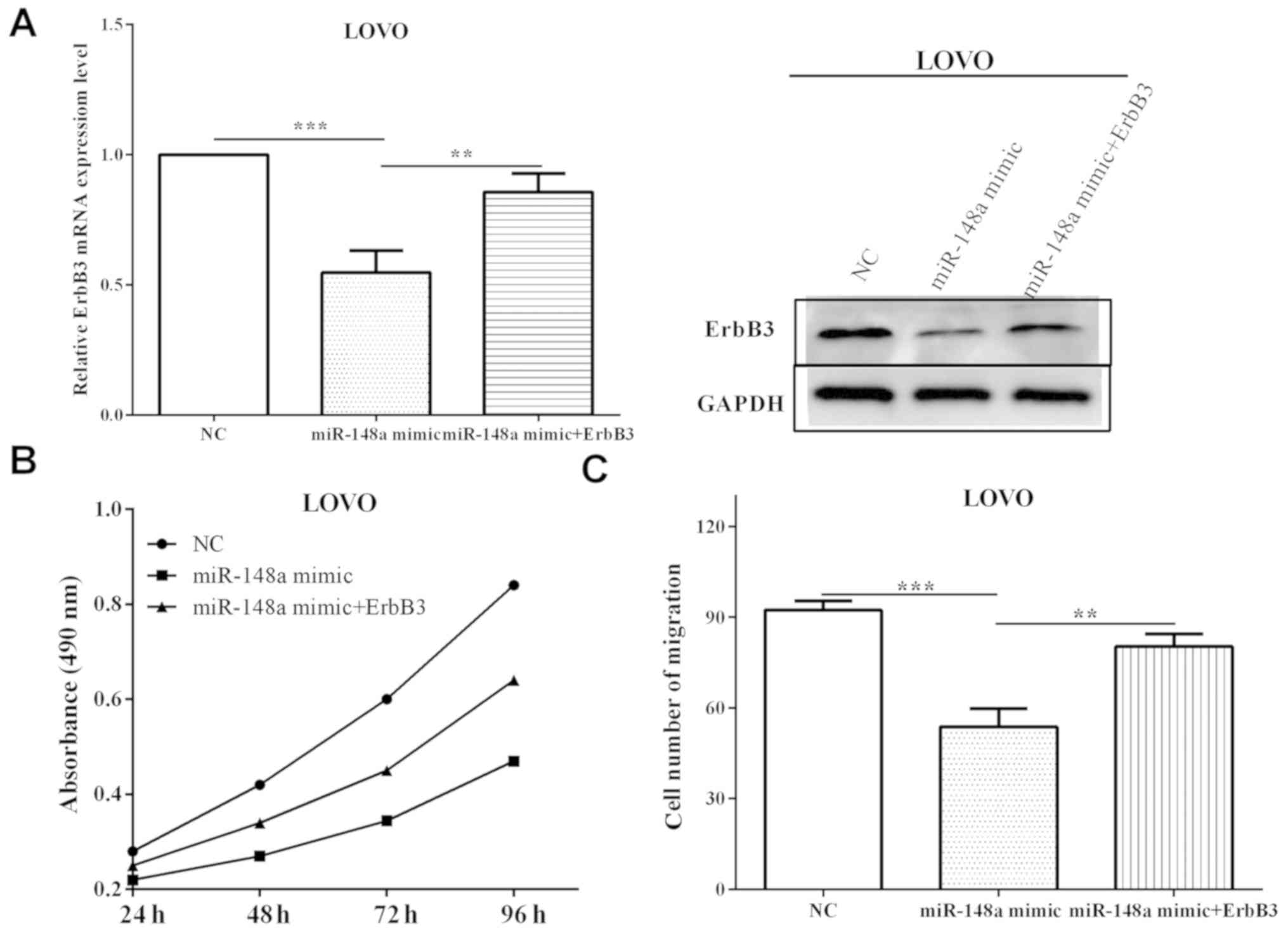
exhibited higher (P=0.0084) ErbB3 level compared with cells
transfected with miR-148a in mRNA and protein levels (Fig. 5A), suggesting that ErbB3 rescued the
suppression by miR-148a. Subsequently, the changes of proliferation
and migration affected by miR-148a mimic and ErbB3 were measured.
The proliferative absorbancy was increased (P=0.0032) when
re-transfected with ErbB3, compared with only transfected with
miR-148a mimic, which was decreased (P=0.00006) versus negative
control (Fig. 5B). Migration was
reduced (P=0.00009) when transfected with miR-148a mimic, and the
roles were partially reversed (P=0.0079) after re-transfected with
ErbB3 (Fig. 5C). Taken together,
these results suggest that miR-148a affects cell proliferative and
migratory abilities through directly mediating ErbB3.
Discussion
Colorectal cancer has a high incidence, and is
divided into adenocarcinoma, mucinous adenocarcinoma and
undifferentiated carcinoma (1,3).
Colorectal cancer has a high mortality rate and due to drug
resistance and relapse, the outcome is poor. Therefore, it is
urgent to identify new biomarkers for effective therapeutics of
CRC. MicroRNAs are a class of non-coding RNAs, which mediate gene
expression at post-transcriptional level (4,5).
Increasing evidence demonstrates that miRNAs play important roles
in cancer inhibition and promotion of colorectal cancer (2,6,7). miR-148a is downregulated and are
associated with several cellular processes including development,
differentiation, growth, migration and apoptosis (12–14). In
breast cancer, Li et al discovered that miR-148a promoted
apoptosis and inhibited growth (12). Similarly, Feng et al
demonstrated that miR-148a suppressed the proliferation and
migration in pancreatic cancer cells (15). Our results were consistent with these
findings, miR-148a was found to be downregulated in colorectal
cancer tissue samples and cells, while ErbB3 was upregulated. In
addition, miR-148a suppressed the proliferation and migration
through directly targeting ErbB3 in LoVo cells. However, there are
likely to be many factors governing proliferation of CRC cells,
thus, cell proliferation was partially suppressed by miR-148a.
ErbB3 is a transmembrane tyrosine kinase receptor,
which is a member of ErbB receptor family (16–19). In
lung adenocarcinoma, ErbB3 promotes cell apoptosis and inhibits
cell growth and invasiveness (20).
Similar findings were found by Appert-Collin et al (21), ErbB3 was usually overexpressed and
promoted cell migration and invasion in gliomas and non-small cell
lung carcinoma. However, in colorectal cancer miR-148a
downregulation contributed to carcinogenesis and cell invasion
(2). Our findings were consistent
with these findings. ErbB3 was overexpressed in colorectal cancer
tissues and cell lines LoVo and SE480. In CRC tissues, the mRNA
level of ErbB3 had a negative correlation with the expression of
miR-148a. Furthermore, Yu et al (23) elucidated that miR-148a inhibited
tumor angiogenesis through targeting ErbB3 and its downstream
signaling molecules. Similar findings were found by Feng et
al as miR-148 suppressed the proliferation and migration
through downregulating ErbB3 in pancreatic cancer cells (15). Our results were consistent with the
above findings, ErbB3 was a direct target of miR-148a and it
reversed partial function of miR-148a on cell proliferation and
migration. We propose that miR-148a impact the proliferation and
migration through directly targeting ErbB3 in colorectal cancer
cells.
In conclusion, we established that miR-148a was
downregulated while ErbB3 was upregulated in colorectal cancer
tissues and cell lines. miR-148a was found to suppress the
proliferative and migratory abilities by directly regulating the
expression of ErbB3 in colorectal cancer cells. The newly
identified miR-148a/ErbB3 axis provides novel insight into
pathogenesis of colorectal cancer, and represents a potential
target for colorectal cancer treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS as the corresponding author contributed to the
conception of the study. WZ and JZ, contributed significantly to
analysis and manuscript preparation. GW, performed the data
analyses and wrote the manuscript. KY and GW, helped perform the
analysis with constructive discussions. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Xi'an Jiaotong
University (Xi'an, China) (Human ethic no. 2015-06). Patients who
participated in this research had complete clinical data. The
signed informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peng YC, Lin CL, Hsu WY, Chang CS, Yeh HZ,
Liao SC and Kao CH: The risk of colorectal cancer is related to
frequent hospitalization of IBD in an Asian population: Results
from a nationwide study. QJM. 108:457–463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hibino Y, Sakamoto N, Naito Y, Goto K, Oo
HZ, Sentani K, Hinoi T, Ohdan H, Oue N and Yasui W: Significance of
miR-148a in colorectal neoplasia: Downregulation of miR-148a
contributes to the carcinogenesis and cell invasion of colorectal
cancer. Pathobiology. 82:233–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jass JR, Love SB and Northover JM: A new
prognostic classification of rectal cancer. Lancet. 1:1303–1306.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Leva G and Croce CM: Roles of small
RNAs in tumor formation. Trends Mol Med. 16:257–267. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Doleshal M, Magotra AA, Choudhury B,
Cannon BD, Labourier E and Szafranska AE: Evaluation and validation
of total RNA extraction methods for microRNA expression analyses in
formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 10:203–211.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao R, Li C and Chai B: miRNA-144
suppresses proliferation and migration of colorectal cancer cells
through GSPT1. Biomed Pharmacother. 74:138–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan L, Yao J and Qiu J: miRNA-495
suppresses proliferation and migration of colorectal cancer cells
by targeting FAM83D. Biomed Pharmacother. 96:974–981. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim CW, Oh ET, Kim JM, Park JS, Lee DH,
Lee JS, Kim KK and Park HJ: Hypoxia-induced microRNA-590-5p
promotes colorectal cancer progression by modulating matrix
metalloproteinase activity. Cancer Lett. 416:31–41. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan S, Jiang Y, Liang C, Jin C, Duan Q, Xu
D, Yang L, Zhang X, Ren B and Jin P: Exosomal miR-6803-5p as
potential diagnostic and prognostic marker in colorectal cancer. J
Cell Biochem. 119:4113–4119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Q, Ren P, Shi P, Chen Y, Xiang F, Zhang
L, Wang J, Lv Q and Xie M: MicroRNA-148a promotes apoptosis and
suppresses growth of breast cancer cells by targeting B-cell
lymphoma 2. Anticancer Drugs. 28:588–595. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao H, Liu Z, Wang R, Zhang X, Yi W, Nie
G, Yu Y, Wang G and Zhu M: miR-148a suppresses human renal cell
carcinoma malignancy by targeting AKT2. Oncol Rep. 37:147–154.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong P, Ihira K, Xiong Y, Watari H, Hanley
SJ, Yamada T, Hosaka M, Kudo M, Yue J and Sakuragi N: Reactivation
of epigenetically silenced miR-124 reverses the
epithelial-to-mesenchymal transition and inhibits invasion in
endometrial cancer cells via the direct repression of IQGAP1
expression. Oncotarget. 7:20260–20270. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng H, Wang Y, Su J, Liang H, Zhang CY,
Chen X and Yao W: MicroRNA-148a suppresses the proliferation and
migration of pancreatic cancer cells by down-regulating ErbB3.
Pancreas. 45:1263–1271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campbell MR, Amin D and Moasser MM: HER3
comes of age: new insights into its functions and role in
signaling, tumor biology, and cancer therapy. Clin Cancer Res.
16:1373–1383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guy PM, Platko JV, Cantley LC, Cerione RA
and Carraway KL III: Insect cell-expressed p180erbB3 possesses an
impaired tyrosine kinase activity. Proc Natl Acad Sci USA.
91:8132–8136. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sierke SL, Cheng K, Kim HH and Koland JG:
Biochemical characterization of the protein tyrosine kinase
homology domain of the ErbB3 (HER3) receptor protein. Biochem J.
322:757–763. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jura N, Shan Y, Cao X, Shaw DE and Kuriyan
J: Structural analysis of the catalytically inactive kinase domain
of the human EGF receptor 3. Proc Natl Acad Sci USA.
106:21608–21613. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sithanandam G, Fornwald LW, Fields J and
Anderson LM: Inactivation of ErbB3 by siRNA promotes apoptosis and
attenuates growth and invasiveness of human lung adenocarcinoma
A549. Oncogene. 24:1847–1859. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Appert-Collin A, Hubert P, Crémel G and
Bennasroune A: Role of ErbB receptors in cancer cell migration and
invasion. Front Pharmacol. 6:2832015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu J, Li Q, Xu Q, Liu L and Jiang B:
MiR-148a inhibits angiogenesis by targeting ERBB3. J Biomed Res.
25:170–177. 2011. View Article : Google Scholar : PubMed/NCBI
|