Introduction
Acute myeloid leukemia (AML) is a heterogeneous
malignant clonal disease originating from hematopoietic stem or
myeloid progenitor cells (1). AML is
the most common type of leukemia in adults, with an incidence rate
of 3.7 per 100,000 worldwide, as it progresses rapidly, with the
natural course of the disease being only 11–20 weeks (2). Despite recent advances in the
diagnosis, treatment and prognosis of AML, the overall survival
rate remains <50% (3). Therefore,
further studies are required, in order to examine the underlying
mechanisms of AML pathogenesis and prognosis, as well as the search
for novel treatments. Following extensive research on the
pathogenesis of AML over the past few years, abnormal epigenetic
modifications have been reported as an important mechanism for the
occurrence and development of leukemia (4,5). Long
non-coding RNA (lncRNA), a recently discovered epigenetic
modification mechanism, serves a critical role in cell function and
gene regulation (6,7).
lncRNAs are longer than 200 nucleotides, and are a
members of the non-protein-coding RNA family. Numerous studies have
demonstrated that lncRNA serves an important role in the
pathogenesis and progression of AML (8,9).
lncRNA-CCDC26 is located at chromosomal locus 8q24 and is
associated with low-grade glioma, chronic myeloid leukemia-derived
K562 cells and pediatric AML (10–13).
Furthermore, previous studies have reported that CCDC26, also
referred to as retinoid modification (RAM), is upregulated in AML
cell lines (HL60 and THP-1), while CCDC26 regulates the
differentiation and apoptosis of the acute monocytic leukemia cell
line PLB985, which is induced by retinoic acid (12,14).
These results have indicated that CCDC26 is
associated with AML; however, the expression level and the clinical
significance of lncRNA-CCDC26 in adult patients with AML remains
unclear. The aim of the present study was therefore to examine the
expression of CCDC26 in clinical bone marrow samples of AML and AML
cell lines, and evaluate its role in the progression and poor
prognosis of AML.
Materials and methods
Patient samples
Bone marrow samples from 93 patients who were
diagnosed with AML, based on the World Health Organization
Morphology, Immunology, Cytogenetics Molecular biology
classification criteria (15), were
collected at the Guangzhou First People's Hospital (Guangzhou,
China) between November 2014 and March 2018. All participants
provided their written informed consent to participate in this
study. The age of the patients ranged from 11 to 81 years, with an
average of 40 years, including 51 females and 42 males. Among the
93 patients, 45 were at the stage of initial diagnosis of AML, 7
were relapses, 24 post-chemotherapy and 17 post-hematopoietic stem
cell transplantation (HSCT). The inclusion criteria were as
follows: i) Blasts ≥20% of bone marrow nucleated cells (ANC); ii)
blasts <20% of ANC, however with t(15;17), t(8;21) or inv
(16)/t(16;16); and iii) clearly
diagnosed AML. The exclusion criteria were as follows: i) Age
<18 years; and ii) incomplete follow-up information. Mononuclear
cells (MNCs) were isolated from bone marrow samples with Ficoll (GE
Healthcare Life Sciences, Little Chalfont, UK) and stored at −80°C
with TRIzol® reagent (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) for as long as required. MNCs isolated from the
bone marrow included monocytes, lymphocytes, hematopoietic stem
cells, and hematopoietic progenitor cells. Bone marrow samples from
ten anonymized healthy volunteers were collected between March 2017
and August 2017 and were included as the control samples. The age
ranged from 23 to 45 years, with an average of 31 years, and
included 5 females and 5 males. The present study was approved by
the Ethics Committee of Guangzhou First People's Hospital
(Guangzhou, China).
The clinical characteristics of the patients are
listed in Table I. Clinical data
were followed up until May 1, 2018. The median follow-up time for
surviving patients was 388 days (range, 122–1,275 days).
 | Table I.Association between CCDC26 expression
and clinicopathological factors of acute myeloid leukemia
(n=45). |
Table I.
Association between CCDC26 expression
and clinicopathological factors of acute myeloid leukemia
(n=45).
|
| CCDC26
expression |
| CCDC26
expression |
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | High n=36 (%) | Low n=9 (%) |
P-valuea | Mean ± standard
deviation |
P-valueb |
|---|
| Sex |
|
| 0.117 |
| 0.412 |
|
Male | 21 (58.3) | 2 (22.2) |
| 7.942±8.551 |
|
|
Female | 15 (41.7) | 7 (77.8) |
| 6.039±6.701 |
|
| Age (years) |
|
| 0.654 |
| 0.031c |
|
≥60 | 9 (25.0) | 1 (11.1) |
| 13.667±10.455 |
|
|
<60 | 27 (75.0) | 8 (88.9) |
| 5.111±5.528 |
|
| Anemia |
|
| 0.514 |
| 0.026c |
|
Yes | 30 (83.3) | 6 (66.7) |
| 7.815±8.277 |
|
| No | 6 (16.7) | 3 (33.3) |
| 3.799±3.126 |
|
| Fever |
|
| 0.573 |
| 0.697 |
|
Yes | 26 (72.2) | 5 (55.6) |
| 6.707±7.796 |
|
| No | 10 (27.8) | 4 (44.4) |
| 7.687±7.644 |
|
| Hemorrhage |
|
| 0.204 |
| 0.854 |
|
Yes | 17 (47.2) | 7 (77.8) |
| 6.811±7.889 |
|
| No | 19 (52.8) | 2 (22.2) |
| 7.242±7.612 |
|
| Extramedullary
infiltration |
|
| 0.106 |
| 0.538 |
|
Yes | 25 (69.4) | 3 (33.3) |
| 6.455±7.076 |
|
| No | 11 (30.6) | 6 (66.7) |
| 7.929±8.722 |
|
| WBC
(×109/l) |
|
| 1.000 |
| 0.893 |
|
≥40 | 10 (27.8) | 2 (22.2) |
| 7.271±8.126 |
|
|
<40 | 26 (72.2) | 7 (77.8) |
| 6.918±7.634 |
|
| LDH (U/l) |
|
| 1.000 |
| 0.654 |
|
>240 | 30 (83.3) | 7 (77.8) |
| 6.77±7.368 |
|
|
≤240 | 6 (16.7) | 2 (22.2) |
| 8.133±9.454 |
|
| Risk
stratification |
|
| 0.002d |
| 0.003d |
|
Poor/Intermediate | 23 (63.9) | 0 (0.0) |
| 9.886±8.176 |
|
|
Favorable | 13 (36.1) | 9 (100) |
| 3.419±5.248 |
|
| Remission |
|
| 0.226 |
| 0.030c |
| PR or
NR | 9 (25.0) | 0 (0.0) |
| 11.926±8.395 |
|
| CR | 27 (75.0) | 9 (100) |
| 5.783±7.085 |
|
Risk stratification was based on the cytogenetic and
molecular anomaly background of the National Comprehensive Cancer
Network Guidelines for the treatment of acute myeloid leukemia
(16). Since only 3 patients at
initial-diagnosis stage were of intermediate risk, risk
stratification was performed by classifying patients into
poor/intermediate and favorable risk groups.
Public data from the gene expression
omnibus (GEO) database
Evaluation of the CCDC26 expression level was
performed using the publicly available GEO database (https://www.ncbi.nlm.nih.gov/geo/). GSE85030 was
downloaded from the report of Lei et al (17), which contained 6 AML and 2 normal
control samples.
Extraction of bone marrow MNCs
Bone marrow samples were superimposed on the surface
of 5 ml Ficoll (1.077 g/ml; GE Healthcare Life Sciences), and
horizontal centrifugation at 670 × g, at 25°C for 20 min was
subsequently performed in the centrifuge (Centrifuge 5810;
Eppendorf, Hamburg, Germany). Following centrifugation, the liquid
was divided into three layers, with a narrow white cloudy layer
mainly composed of MNCs at the interface between the upper and
middle layers. The cloudy layer was aspirated into another
centrifuge tube, and the MNCs were washed twice with RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc.). The proportion of MNCs was
estimated on May-Grünwald-Giemsa-stained cytocentrifugate
preparations using light microscopy at ×400 magnification. A
two-step process was performed for staining, firstly 50%
May-Grünwald at 25°C for 3–5 min, secondly 10% buffered Giemsa
solution for 10–30 min then running water for 1–3 min. The MNCs
selected for analysis contained a minimum of 90% blasts following
separation. Pellets of 2–10,000,000 MNCs were stored in
TRIzol® reagent and immediately frozen at −80°C.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from MNCs using TRIzol
reagent, according to the manufacturer's protocol. An RT kit
(Promega Corporation, Madison, WI, USA) was used to reverse
transcribe total RNA to cDNA, following the manufacturer's
protocol. The reverse transcription method was a 2-step process,
with a total 20 µl. Firstly, RNA 500 ng, 0.5 µl oligo (dT) (0.5
µg/reaction), 0.5 µl random primer (0.5 µg/reaction) and RNase free
double distilled water (ddH2O), up to 5 µl, was added
together and incubated at 70°C for 5 min, and subsequently rapidly
cooled on ice for 5 min. Secondly, 4.0 µl GoScriptTM 5X
reaction buffer, 1.7 µl MgCl2 (final concentration 2.0
mM), 1.0 µl 0.5 mM dNTPs, 0.3 µl ribonuclease inhibitor (20 U), 1.0
µl reverse transcriptase, and ddH2O to a total of 15 µl
was subsequently added. After mixing, samples were incubated at
42°C for 60 min and inactivated at 70°C for 15 min. RT-qPCR was
carried out with SYBR Green Master Mix (Promega Corporation). The
qPCR was performed at a total 10 µl and included the following: 5
µl GoTaq 2X Master Mix, 0.5 µl forward primer (10 µM), 0.5 µl
reverse primer (10 µM), 2 µl cDNA (diluted 10-fold) and 2 µl
ddH2O. The following thermocycling conditions were used
for the qPCR: Intital denaturation at 95°C for 10 min; 40 cycles of
95°C 15 sec and 60°C for 1 min. Primer sequences were as follows:
18SrRNA, 5′-CGGCGGCTTTGGTGACTCTAGA-3′ forward and
5′-CCTGCTGCCTTCCTTGGATGTG-3′ reverse; CCDC26,
5′-CCTTGTACAGTGTTGCCTCAGC-3′ forward and
5′-GCAGTCTTCGGCATTCTCCCA-3′ reverse. The results were normalized to
the expression of 18SrRNA and are presented as a fold change
(2−ΔΔCq) (18). Each
experiment was repeated in triplicate.
Screening for differentially expressed
mRNAs
The differentially expressed mRNAs between AML and
normal control samples in GSE85030 were screened using GEO2R
(https://www.ncbi.nlm.nih.gov/geo/geo2r/). The criteria
for screening were adjusted P<0.05 and
|log2FC|>1.
Weighted gene co-expression network
analysis (WGCNA)
WGCNA, which can identify clusters (modules) of
highly correlated genes, is a systematic biology method used to
describe correlation patterns among genes in microarray samples
(19). Compared to Pearson's
correlation coefficient, WGCNA uses the soft threshold to provide
more extensive and accurate correlations between genes. The R
package WGCNA (19) was used to
construct a weighted correlation network between lncRNA-CCDC26 and
differentially expressed mRNAs. The module colors under the
dendrogram represent the module assignment determined by the
Dynamic Tree Cut. The k-core score was used to determine the core
genes of the co-expression networks. The lncRNA-co-expressed mRNA
cluster was obtained with a soft threshold of 0.85 for further
functional analysis of lncRNA-CCDC26.
Pathway analysis and protein-protein
interaction (PPI) network establishment
The WGCNA-calculated lncRNA-CCDC26-co-expressed
mRNAs were subjected to pathway analysis and PPI network
establishment. The Database for Annotation, Visualization and
Integrated Discovery (DAVID; http://david.ncifcrf.gov/) tool was used for Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway analysis. The PPI
network of CCDC26-co-expressed mRNAs was established using Search
Tool for the Retrieval of Interacting Genes/Proteins (STRING;
http://string-db.org/). The highly important
nodes are represented by the central nodes in the network; nodes in
the network represent genes, and edges represent interactions
between nodes.
Statistical analysis
All statistical analysis was conducted using SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA), GraphPad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA) and R software 3.5.1
(https://www.r-project.org/). Student's
t-test and χ2 tests were performed to compare the
significance of differences between two groups, as appropriate.
One-way ANOVA test followed by the Bonferroni test was used for
multiple comparisons. Overall survival rates were calculated via
the Kaplan-Meier method and the log-rank test was used for
comparison. Receiver operating characteristic (ROC) curves were
constructed using the survival ROC package (20) in R software. The data are presented
as mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
Expression of CCDC26 is upregulated in
AML
The relative expression level of CCDC26 was detected
in 93 AML bone marrow samples by RT-qPCR and normalized to 18rRNA.
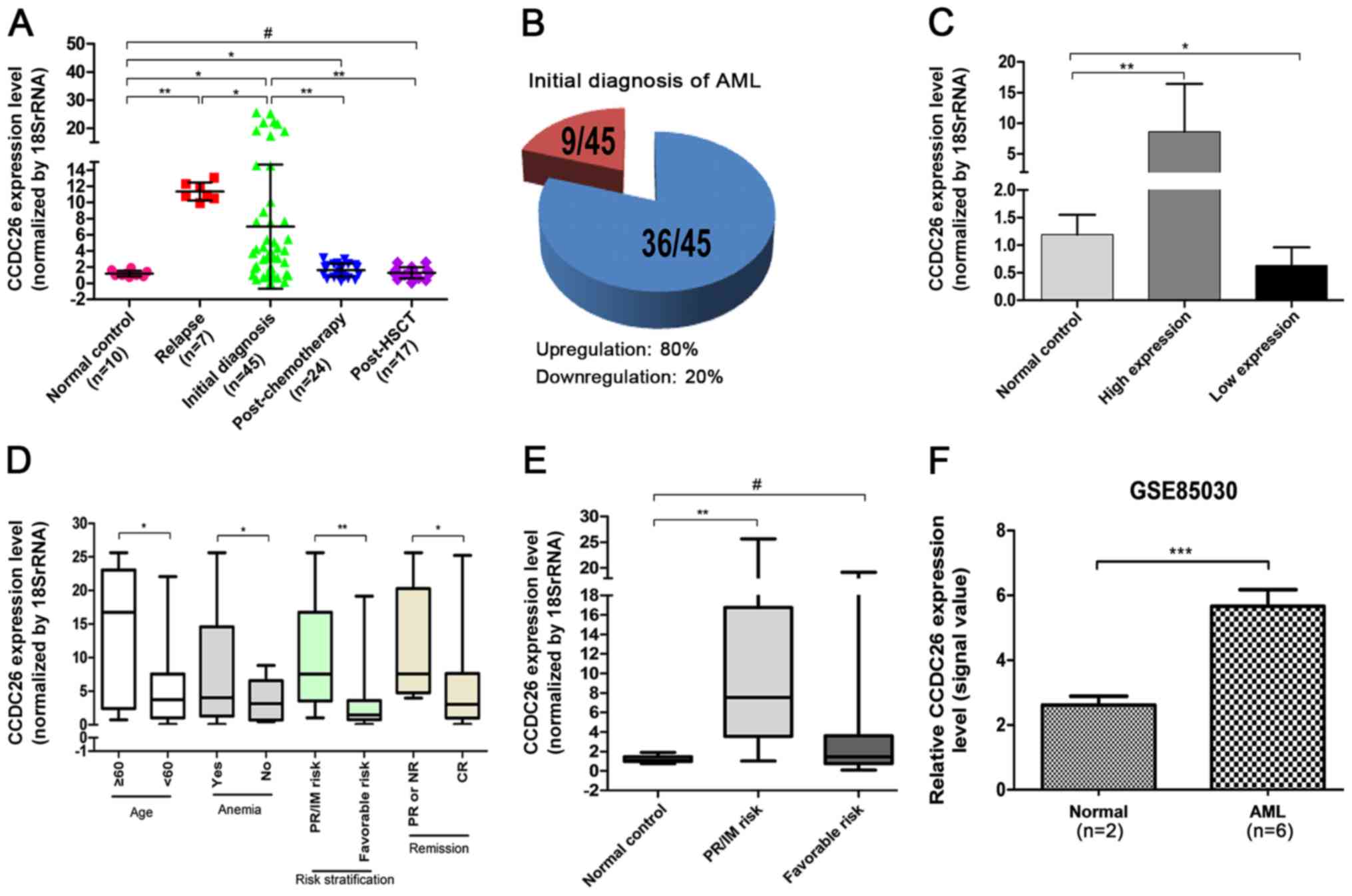
As indicated in Fig. 1A and B,
CCDC26 expression was significantly upregulated in 80% of patients
with an initial AML diagnosis (P=0.018) and in patients who had
relapsed (P=0.001). At the same time, the expression of CCDC26 in
patients with AML relapses was significantly higher compared with
patients with an initial AML diagnosis (P=0.041). The level of
CCDC26 expression was distinctly decreased in patients with AML
following chemotherapy (P=0.001) and HSCT (P=0.002) compared with
the patients with an initial AML diagnosis. In addition, compared
with the normal control, the expression of CCDC26 in patients with
AML following chemotherapy remained high (P=0.045), however there
was no significant difference in patients with AML following
transplantation (P=0.725). These results indicated that the
relative expression of CCDC26 in patients with AML was upregulated,
but returned to normal levels following effective treatment. As
indicated in Fig. 1C, the absolute
value of CCDC26 for upregulation in AML was 7.415 compared with the
normal control (P=0.006), while the absolute value of CCDC26 for
downregulation in AML was 0.562 compared with the normal control
(P=0.039).
This result was confirmed in the publicly available
GEO expression dataset GSE85030. The relative expression level of
CCDC26 in AML was higher compared with that in the normal control
(Fig. 1F).
CCDC26 upregulation is associated with
age, anemia, risk stratification and remission
The association between CCDC26 expression and
clinical parameters of patients with an initial AML diagnosis was
investigated. Higher CCDC26 expression was observed in patients
with old age (≥60 years), anemia, poor/intermediate risk and
partial remission or no remission, while the expression was lower
in patients at a young age (<60 years), without anemia,
favorable risk and complete remission (CR), (P=0.031, 0.026, 0.003
and 0.03, respectively) (Fig. 1D and
Table I). No association was
observed between the expression level of CCDC26 and other clinical
factors, including sex, fever, hemorrhage, white blood cells (WBC),
lactate dehydrogenase (LDH) or extramedullary infiltration.
Compared with the normal control, the expression of CCDC26 in
patients with AML with poor/intermediate risk was significantly
increased (P=0.002), however there was no significant difference in
the expression of CCDC26 in patients with AML with favorable-risk
stratification (P>0.05; Fig.
1E).
Association between
clinicopathological factors and CCDC26 expression, analyzed using
the χ2 test
The expression level of CCDC26 was divided into two
groups: High (above normal control) and low (under normal control).
The χ2 test was used to evaluate differences in clinical
parameters between the two groups. As presented in Table I, the expression of CCDC26 is
associated with risk stratification (P=0.002). In the present
study, no significant difference was observed in the association
between CCDC26 expression and other clinical parameters, including
sex, age, anemia, fever, hemorrhage, WBC, LDH, extramedullary
infiltration and remission.
CCDC26 upregulation is associated with
poor prognosis in patients with AML
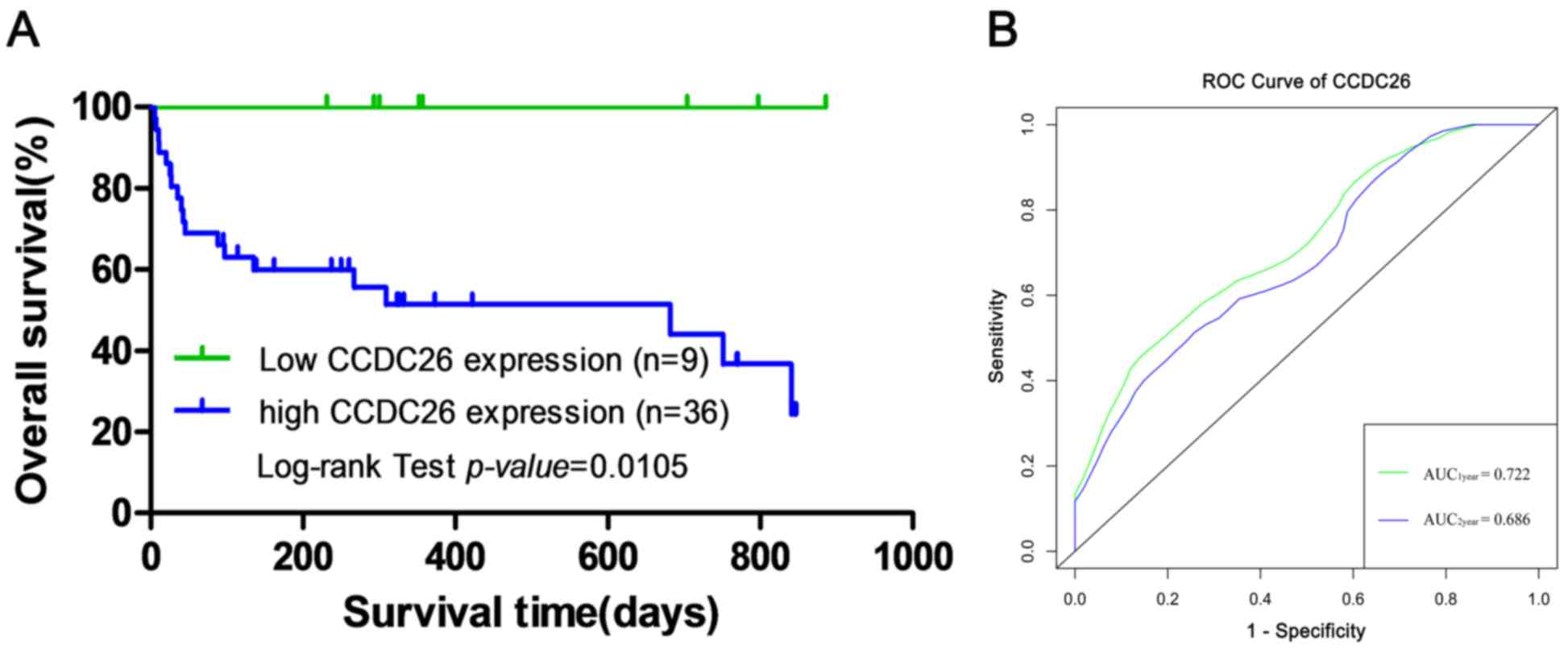
To examine the association between CCDC26 expression
levels and the prognosis of patients with AML, Kaplan-Meier
survival analysis and the log-rank test were performed to evaluate
the association between CCDC26 expression and overall survival.
Kaplan-Meier survival analysis indicated a significant difference
in overall survival between the CCDC26 high- and low-expression
groups in 45 patients with an initial AML diagnosis (log-rank test;
χ2=6.550; P=0.0105; Fig.
2A). The ROC curve was used to evaluate the predictive ability
of CCDC26. As presented in Fig. 2B,
the AUC1year and AUC2year of CCDC26 according
to the ROC curve were 0.722 and 0.686, respectively, which
indicated that the expression level of CCDC26 can be used to
predict overall survival in AML.
Co-expression module
establishment
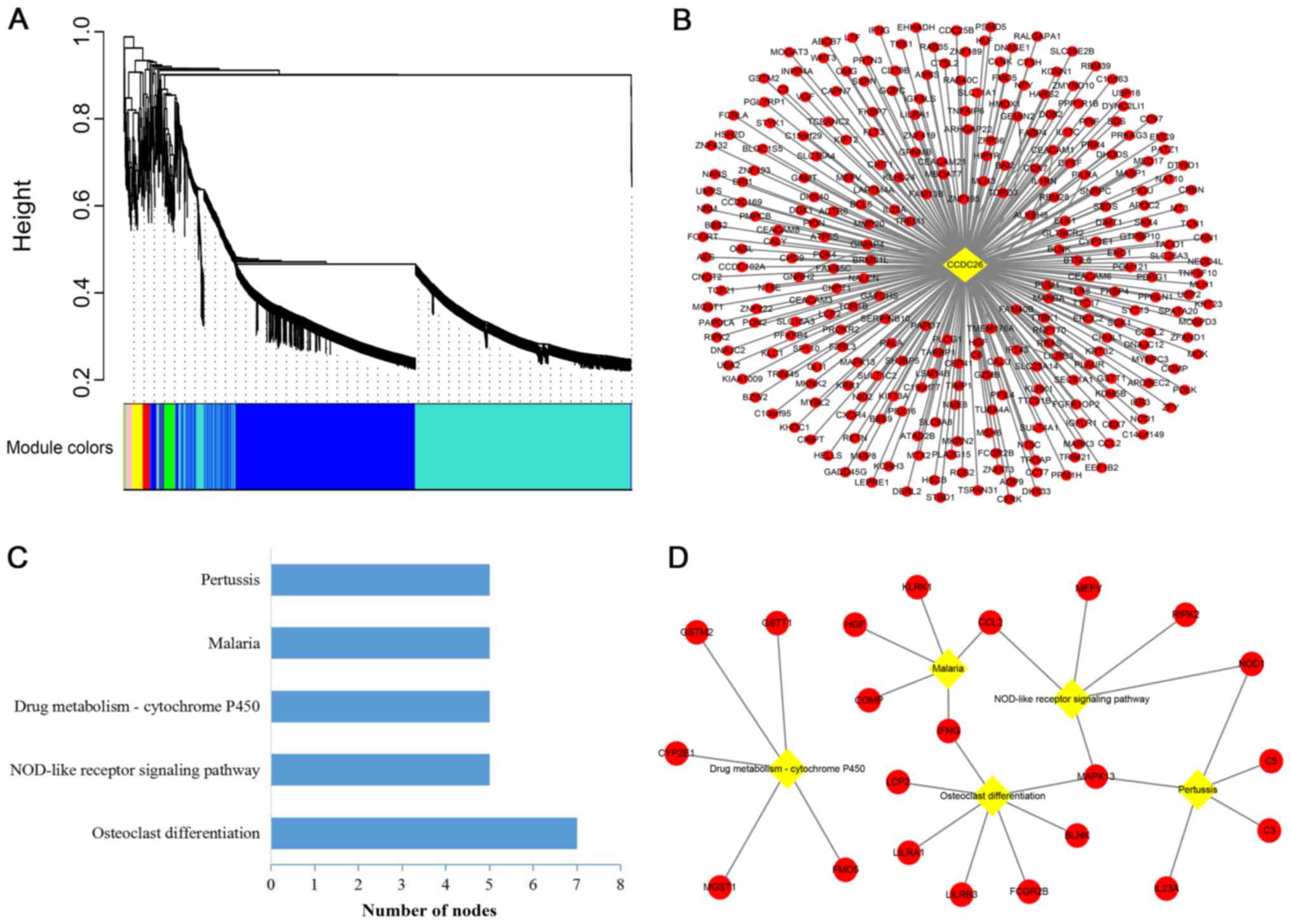
WGCNA is a systematic biology method used to find
clusters (modules) of highly correlated genes and examine the
potential biological patterns. CCDC26 and a total of 5,020
differentially expressed mRNAs in GSE85030 were involved in the
construction of a weighted gene co-expression network. As indicated
in Fig. 3A, the cluster dendrogram
contained nine co-expression modules (gray, red, green, yellow,
black, pink, brown, blue and turquoise). The co-expression network
of CCDC26 was in the turquoise module, which contained 289 mRNAs
(Fig. 3B).
Pathway analysis and PPI network
establishment of co-expressed genes
To predict the potential biological processes of
CCDC26 in AML, KEGG was used to enrich and analyze 289
CCDC26-co-expressed genes in the DAVID database. According to the
KEGG terms, CCDC26 was enriched in five important pathways,
including ‘osteoclast differentiation’, ‘NOD-like receptor
signaling pathway’, ‘drug metabolism-cytochrome P450’, ‘malaria’
and ‘pertussis’ (Fig. 3C).
‘Osteoclast differentiation’ and ‘NOD-like receptor signaling
pathway’ were considered to serve the most important roles in the
network, since the other pathways strongly depended on their
linkages (Fig. 3D).
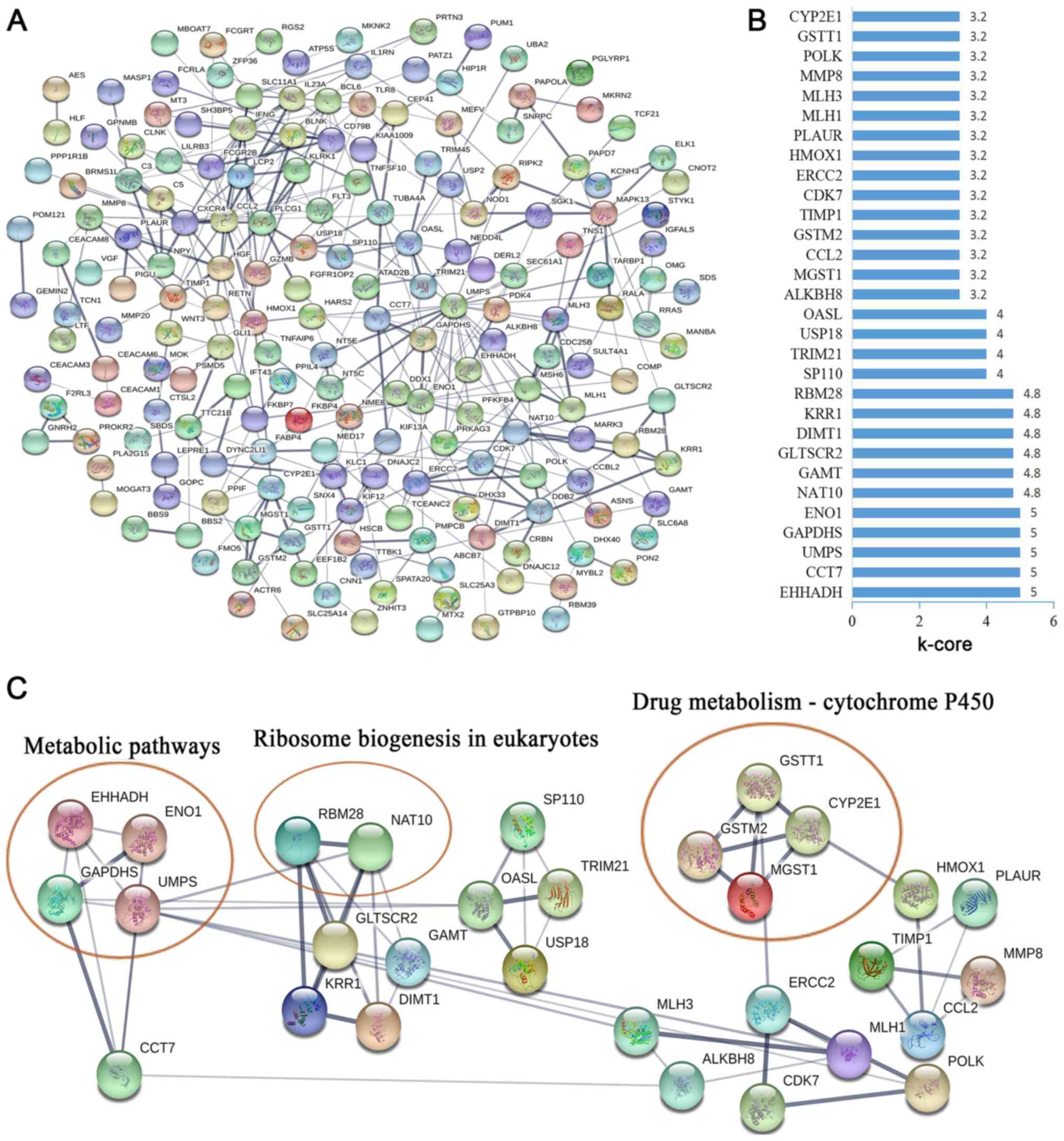
To further investigate the function of
CCDC26-co-expressed genes at the protein level, the STRING database
was used to screen for functional genes, thus providing a visual
annotated network revealing the structural and functional
properties of the proteins. The PPI network was composed of 187
nodes and 345 edges (Fig. 4A). The
k-core was used to determine the core genes of the PPI network, and
the 30 highest k-core genes were contained within four core
subnetworks (Fig. 4B). The four core
subnetworks were enriched in three pathways, including ‘metabolic
pathways’, ‘ribosome biogenesis in eukaryotes’ and ‘drug
metabolism-cytochrome P450’ (Fig.
4C).
Discussion
AML is a highly malignant tumor of the hematopoietic
system with substantial heterogeneity in cytogenetics and molecular
genetics (21). Over the past few
years, with the rapid development of molecular biology, researchers
have discovered that chromosomal abnormalities, and gene fusions
and mutations serve an important role in the clinical evaluation,
prognosis and treatment of patients with AML. However, these
molecular abnormalities are not yet comprehensively used as
clinical prognostic molecular markers and therapeutic targets
(22,23). Therefore, further studies are crucial
for identifying novel AML molecular markers and therapeutic targets
for guiding treatment or prognostic analysis.
Recently, the aberrant expression of lncRNAs has
emerged as an important factor in a number of biological processes
(e.g. cell proliferation and apoptosis) and human diseases,
particularly in cancer (17,24–26).
Studies have reported that lncRNA-CCDC26 not only serves an
important role in the development of low-grade glioma, but is also
significantly increased in the chronic myeloid leukemia cell line
K562, and pediatric AML and AML cell lines HL60 and THP-1 (10–13). In
addition, CCDC26, also known as RAM, regulates the differentiation
and apoptosis of the AML cell line PLB985, which is induced by
retinoic acid (12,14).
In the present study, it was reported that the level
of lncRNA-CCDC26 in AML was significantly higher compared with that
in healthy individuals. The high expression of CCDC26 in patients
with AML was distinctly associated with a number of clinical
parameters, including age, anemia, risk stratification and
remission. Furthermore, the high CCDC26 expression in AML was
associated with poor prognosis. These results indicated that high
CCDC26 expression was associated with AML oncogenesis and may serve
as a prognostic indicator for AML.
Despite the significant progress reported in the
treatment of AML, only 50–60% of elderly patients achieve CR
following chemotherapy, and the 5-year overall survival rate
following intensive induction chemotherapy is 15–30% (27,28). The
5-year overall survival rate of patients with AML with high-risk
factors has been reported to be only 13% (29). However, allogeneic (allo)-HSCT is an
effective treatment following chemotherapy remission (30). A study reported that patients aged
40–60 years, who achieved allo-HSCT remission, had a higher 5-year
overall survival rate compared with those who only achieved
chemotherapy remission (57 and 40%, respectively) (30). Chemotherapy cannot completely
eliminate quiescent leukemia cells and minimal residual disease was
still present (31,32). In the present study, although the
expression of CCDC26 could not fully return to normal levels, it
significantly decreased compared with that in patients with an
initial diagnosis of AML. However, during the transplantation
process, pretreatment with high-dose chemotherapy can eliminate a
number of quiescent leukemia cells in the bone marrow. Studies have
also reported that donor lymphocytes can play a graft-vs.-leukemia
role (33–35). In the present study, the expression
of CCDC26 following transplantation was lower compared with that in
patients with an initial AML diagnosis, and returned to normal
levels. These data indicated that CCDC26 could have a clinical
therapeutic effect.
A large number of clinical trials and retrospective
studies have reported that the 5-year survival rates for patients
with AML with poor-risk stratification are 2–14% (36–39). In
the present study, the expression level of CCDC26 in patients with
AML with poor/intermediate-risk stratification were higher compared
with that in healthy individuals, while the expression in patients
with AML with favorable-risk stratification was at a normal level.
Furthermore, the prognosis of patients with AML with relapsed or
refractory AML is poor, and the 3-year overall survival is
estimated at <10% (40,41). It was indicated that the expression
of CCDC26 in relapsed patients was significantly higher compared
with that in patients with an initial AML diagnosis. These findings
suggested that CCDC26 can serve as an indicator of risk
stratification in AML.
In order to further investigate the functions of
CCDC26, co-expression and PPI networks were constructed in the
present study, and KEGG pathway analysis was performed. The
analysis revealed that CCDC26 was involved in the regulation of a
number of pathways, including ‘osteoclast differentiation’,
‘NOD-like receptor signaling pathway’, ‘metabolic pathways’,
‘ribosome biogenesis in eukaryotes’ and ‘drug metabolism-cytochrome
P450’, which served an important role in the pathogenesis and
progression of AML.
In conclusion, CCDC26 was indicated to be
upregulated in patients with AML, and this upregulation was
associated with the progression of AML and poor prognosis. These
results suggested that lncRNA-CCDC26 may serve as a novel biomarker
for monitoring the progression and predicting the clinical outcome
of patients with AML. However, further studies are required in
order to determine additional biological characteristics of CCDC26
and their potential involvement in the underlying mechanisms of AML
in larger cohort studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81500126), the
Medical Science and Technology of Research Project of Guangdong
(grant no. 2013B021800065) and the Natural Science Foundation of
Guangdong Province, China (grant no. 2015A030313727) awarded to
CW.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW and SW designed the study. CC and PW performed
the experiments, CC, PW, WM, YZ, WZ, TD, MZ and XC analyzed the
data. CC, PW, CW and SW wrote the manuscript. All authors reviewed
the manuscript and approved the final version.
Ethics approval and consent to
participate
All research conducted was in line with generally
accepted ethical principles and was approved by the Research Ethics
Committee of Guangzhou First People's Hospital (Guangzhou, China).
The collection and analysis of AML samples was performed in
accordance with the principles outlined in the Declaration of
Helsinki. All participants provided their written informed consent
to participate in this study. The consent procedure was approved by
the aforementioned ethics committee. Personal information for the
samples involved in the study was anonymized.
Patient consent for publication
Written informed consent was provided by each
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Estey E and Döhner H: Acute myeloid
leukaemia. Lancet. 368:1894–1907. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deschler B and Lübbert M: Acute myeloid
leukemia: Epidemiology and etiology. Cancer. 107:2099–2107. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferrara F: Unanswered questions in acute
myeloid leukaemia. Lancet Oncol. 5:443–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ntziachristos P, Mullenders J, Trimarchi T
and Aifantis I: Mechanisms of epigenetic regulation of leukemia
onset and progression. Adv Immunol. 117:1–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Woods BA and Levine RL: The role of
mutations in epigenetic regulators in myeloid malignancies. Immunol
Rev. 263:22–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han BW and Chen YQ: Potential pathological
and functional links between long noncoding RNAs and hematopoiesis.
Sci Signal. 6:re52013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mer AS, Lindberg J, Nilsson C, Klevebring
D, Wang M, Grönberg H, Lehmann S and Rantalainen M: Expression
levels of long non-coding RNAs are prognostic for AML outcome. J
Hematol Oncol. 11:522018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen ZH, Wang WT, Huang W, Fang K, Sun YM,
Liu SR, Luo XQ and Chen YQ: The lncRNA HOTAIRM1 regulates the
degradation of PML-RARA oncoprotein and myeloid cell
differentiation by enhancing the autophagy pathway. Cell Death
Differ. 24:212–224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li S, Jin T, Zhang J, Lou H, Yang B, Li Y,
Chen C and Zhang Y: Polymorphisms of TREH, IL4R and CCDC26 genes
associated with risk of glioma. Cancer Epidemiol. 36:283–287. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Hui Y, Li X and Jia Q: Silencing
of lncRNA CCDC26 restrains the growth and migration of glioma cells
in vitro and in vivo via targeting miR-203. Oncol Res.
26:1143–1154. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hirano T, Yoshikawa R, Harada H, Harada Y,
Ishida A and Yamazaki T: Long noncoding RNA, CCDC26, controls
myeloid leukemia cell growth through regulation of KIT expression.
Mol Cancer. 14:902015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Radtke I, Mullighan CG, Ishii M, Su X,
Cheng J, Ma J, Ganti R, Cai Z, Goorha S, Pounds SB, et al: Genomic
analysis reveals few genetic alterations in pediatric acute myeloid
leukemia. Proc Natl Acad Sci USA. 106:12944–12949. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin W, Rossin A, Clifford JL and
Gronemeyer H: Co-resistance to retinoic acid and TRAIL by insertion
mutagenesis into RAM. Oncogene. 25:3735–3744. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Swerdlow SH, Campo E, Harris NL, et al:
WHO classifcation of tumours of haematopoietic and lymphoid
tissues. Lyon: IARC; 2008
|
|
16
|
O'Donnell MR, Tallman MS, Abboud CN,
Altman JK, Appelbaum FR, Arber DA, Bhatt V, Bixby D, Blum W, Coutre
SE, et al: Acute myeloid Leukemia, Version 3.2017, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
15:926–957. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lei L, Xia S, Liu D, Li X, Feng J, Zhu Y,
Hu J, Xia L, Guo L, Chen F, et al: Genome-wide characterization of
lncRNAs in acute myeloid leukemia. Brief Bioinform. 19:627–635.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Langfelder P and Horvath S: WGCNA: An R
package for weighted correlation network analysis. BMC
Bioinformatics. 9:5592008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heagerty PJ, Lumley T and Pepe MS:
Time-dependent ROC curves for censored survival data and a
diagnostic marker. Biometrics. 56:337–344. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salerno L, Romeo G, Modica MN, Amata E,
Sorrenti V, Barbagallo I and Pittalà V: Heme oxygenase-1: A new
druggable target in the management of chronic and acute myeloid
leukemia. Eur J Med Chem. 142:163–178. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Valk PJ, Verhaak RG, Beijen MA, Erpelinck
CA, Barjesteh van Waalwijk van Doorn-Khosrovani S, Boer JM,
Beverloo HB, Moorhouse MJ, van der Spek PJ, Löwenberg B and Delwel
R: Prognostically useful gene-expression profiles in acute myeloid
leukemia. N Engl J Med. 350:1617–1628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hussaini MO, Mirza AS, Komrokji R, Lancet
J, Padron E and Song J: Genetic landscape of acute myeloid leukemia
interrogated by Next-generation Sequencing: A large cancer center
experience. Cancer Genomics Proteomics. 15:121–126. 2018.PubMed/NCBI
|
|
24
|
Chen L, Wang W, Cao L, Li Z and Wang X:
Long Non-coding RNA CCAT1 Acts as a competing endogenous RNA to
regulate cell growth and differentiation in acute myeloid leukemia.
Mol Cells. 39:330–336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fernando TR, Contreras JR, Zampini M,
Rodriguez-Malave NI, Alberti MO, Anguiano J, Tran TM, Palanichamy
JK, Gajeton J, Ung NM, et al: The lncRNA CASC15 regulates SOX4
expression in RUNX1-rearranged acute leukemia. Mol Cancer.
16:1262017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gaidzik VI, Teleanu V, Papaemmanuil E,
Weber D, Paschka P, Hahn J, Wallrabenstein T, Kolbinger B, Köhne
CH, Horst HA, et al: RUNX1 mutations in acute myeloid leukemia are
associated with distinct clinico-pathologic and genetic features.
Leukemia. 30:2160–2168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Burnett AK, Milligan D, Goldstone A,
Prentice A, McMullin MF, Dennis M, Sellwood E, Pallis M, Russell N,
Hills RK, et al: The impact of dose escalation and resistance
modulation in older patients with acute myeloid leukaemia and high
risk myelodysplastic syndrome: The results of the LRF AML14 trial.
Br J Haematol. 145:318–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Löwenberg B, Ossenkoppele GJ, van Putten
W, Schouten HC, Graux C, Ferrant A, Sonneveld P, Maertens J,
Jongen-Lavrencic M, von Lilienfeld-Toal M, et al: High-dose
daunorubicin in older patients with acute myeloid leukemia. N Engl
J Med. 361:1235–1248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Versluis J, Hazenberg CLE, Passweg JR, van
Putten WL, Maertens J, Biemond BJ, Theobald M, Graux C, Kuball J,
Schouten HC, et al: Post-remission treatment with allogeneic stem
cell transplantation in patients aged 60 years and older with acute
myeloid leukaemia: A time-dependent analysis. Lancet Haematol.
2:e427–e436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cornelissen JJ, Versluis J, Passweg JR,
van Putten WL, Manz MG, Maertens J, Beverloo HB, Valk PJ, van
Marwijk Kooy M, Wijermans PW, et al: Comparative therapeutic value
of post remission approaches in patients with acute myeloid
leukemia aged 40–60 years. Leukemia. 29:1041–1050. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hira VVV, Van Noorden CJF, Carraway HE,
Maciejewski JP and Molenaar RJ: Novel therapeutic strategies to
target leukemic cells that hijack compartmentalized continuous
hematopoietic stem cell niches. Biochim Biophys Acta Rev Cancer.
1868:183–198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Behbehani GK, Samusik N, Bjornson ZB,
Fantl WJ, Medeiros BC and Nolan GP: Mass cytometric functional
profiling of acute myeloid leukemia defines Cell-cycle and
immunophenotypic properties that correlate with known responses to
therapy. Cancer Discov. 5:988–1003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kolb HJ, Schattenberg A, Goldman JM,
Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A,
Verdonck L, Niederwieser D, et al: Graft-versus-leukemia effect of
donor lymphocyte transfusions in marrow grafted patients. Blood.
86:2041–2050. 1995.PubMed/NCBI
|
|
34
|
Orti G, Barba P, Fox L, Salamero O, Bosch
F and Valcarcel D: Donor lymphocyte infusions in AML and MDS:
Enhancing the graft-versus-leukemia effect. Exp Hematol. 48:1–11.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dickinson AM, Norden J, Li S, Hromadnikova
I, Schmid C, Schmetzer H and Jochem-Kolb H: Graft-versus-Leukemia
effect following hematopoietic stem cell transplantation for
leukemia. Front Immunol. 8:4962017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Byrd JC, Mrózek K, Dodge RK, Carroll AJ,
Edwards CG, Arthur DC, Pettenati MJ, Patil SR, Rao KW and Watson
MS: Pretreatment cytogenetic abnormalities are predictive of
induction success, cumulative incidence of relapse, and overall
survival in adult patients with de novo acute myeloid leukemia:
Results from cancer and leukemia group B (CALGB 8461). Blood.
100:4325–4336. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Slovak ML, Kopecky KJ, Cassileth PA,
Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR,
Rowe JM, et al: Karyotypic analysis predicts outcome of
preremission and postremission therapy in adult acute myeloid
leukemia: A southwest oncology Group/Eastern cooperative oncology
group study. Blood. 96:4075–4083. 2000.PubMed/NCBI
|
|
38
|
Grimwade D, Walker H, Harrison G, Oliver
F, Chatters S, Harrison CJ, Wheatley K, Burnett AK and Goldstone
AH; Medical Research Council Adult Leukemia Working Party, : The
predictive value of hierarchical cytogenetic classification in
older adults with acute myeloid leukemia (AML): Analysis of 1065
patients entered into the United Kingdom medical research council
AML11 trial. Blood. 98:1312–1320. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grimwade D, Walker H, Oliver F, Wheatley
K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A and
Goldstone A: The importance of diagnostic cytogenetics on outcome
in AML: Analysis of 1,612 patients entered into the MRC AML 10
trial. The Medical Research Council Adult and Children's Leukaemia
Working Parties. Blood. 92:2322–2333. 1998.PubMed/NCBI
|
|
40
|
Döhner H, Weisdorf DJ and Bloomfield CD:
Acute myeloid leukemia. N Engl J Med. 373:1136–1152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rowe JM and Tallman MS: How I treat acute
myeloid leukemia. Blood. 116:3147–3156. 2010. View Article : Google Scholar : PubMed/NCBI
|