Introduction
Gastric cancer is the second most common cancer
following lung cancer in China, as well as the second major cause
of cancer death around the world (1). The onset of gastric cancer is
considered as a gradual process caused by the abnormal activation
of oncogene and inactivation of tumor suppressor gene (TSG). Once
the oncogene is activated, carcinogenesis will be promoted by
giving growth advantages. On the contrary, TSG is able to inhibit
tumor growth and often inactivated during this process (2). The abnormal inactivation of TSG may be
the result of combined effect of genetic and epigenetic changes.
For example, hypermethylation of the promoter can lead to TSG
transcriptional silencing, which is of great significance in
tumorigenesis (3).
Fibulin is an extracellular matrix protein with
multiple functions located on chromosome 3p21.1. It plays an
important role in stabilizing the structure of basement membrane,
elastic fibers and loose connective tissues and regulating the
signal transduction between cells and between cells and matrix
(4). Moreover, fibulin can also
regulate cell morphology, growth, adhesion and movement. In human
cancers, such as prostate cancer, some fibulin proteins are out of
regulation (5), in which fibulin-1
and fibulin-2 are found to be downregulated in such cancers as
prostate and breast cancer and play roles as TSG (6). However, the role of fibulin-2 in
gastric cancer is still unclear.
β-catenin, a protein with dual function, has cell
adhesion and signal transmission activity, was first discovered in
the study on cell adhesion molecule E-cadherin (7). β-catenin is an indispensable part of
the Wnt/β-catenin signaling pathway and plays a key role in the
occurrence and development of gastric cancer (8). Previous studies have revealed that
there is a direct or indirect regulatory relation between fibulin
protein family and β-catenin, both of which exert an important
influence on the development of gastric cancer (9).
The present study aims to further understand the
roles of fibulin-2 and β-catenin in the pathogenesis of gastric
cancer, and clarify whether there are significant differences in
their expressions in gastric cancer and whether there is a
correlation between them, so as to provide new ideas for the
clinical diagnosis and treatment of gastric cancer.
Materials and methods
General data
A total of 49 cases of gastric cancer specimens and
corresponding para-carcinoma tissue specimens (>5 cm away from
the edge of cancer tissues and pathologically confirmed as normal
gastric mucosa) obtained via surgical resection in the Pathology
Department of Heping Hospital Affiliated to Changzhi Medical
College (Changzhi, China) from March 2013 to August 2017 were
collected, fixed with 4% neutral formalin, routinely embedded into
paraffin and serially sliced into 4 µm sections, followed by
immunohistochemical labeling. After collection, the tissues were
divided into 2 parts. One part of tissues was immediately placed
into an Eppendorf (EP) tube containing ribonucleic acid (RNA)
protective solution and then stored at −80°C for subsequent
experiments. The other part was added with tissue lysis solution to
extract the total protein, and the protein expression level was
detected via western blot analysis. The 49 gastric cancer patients
were aged 34–87 years with an average age of 60 years, and there
were 26 males and 23 females. In terms of tissue differentiation
degree, there were 26 cases of high and moderate differentiation
and 23 cases of low and no differentiation. In terms of
tumor-node-metastasis (TNM) stage, there were 20 cases in stage
I–II and 29 cases in stage III–IV. None of the patients underwent
chemotherapy, radiotherapy and immunotherapy before the operation,
and all of them were diagnosed via routine histopathology after
operation.
This study was approved by the Ethics Committee of
Heping Hospital Affiliated to Changzhi Medical College. Informed
consent for the specimen collection was obtained from patients.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Gastric cancer and para-carcinoma tissues were
ground with liquid nitrogen, and 1 ml TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was added to
extract total RNA. The upper-layer liquid was taken, transferred
into a 2.5 ml EP tube, placed on ice for 15 min and added with 200
µl chloroform, followed by centrifugation at 12,000 × g and 4°C for
15 min. Then, the upper-layer liquid was taken carefully,
transferred into a new EP tube, added with 200 µl isopropanol,
vibrated gently several times and placed on ice for 10 min,
followed by centrifugation at 12,000 × g and 4°C for 15 min. After
the supernatant was discarded, 2 ml 75% ethanol was added to wash
RNA, followed by centrifugation at 12,000 × g and 4°C for 10 min.
The liquid was discarded and the RNA precipitate was dried at room
temperature. Finally, an appropriate amount of RNase-free water was
added to dissolve the RNA precipitate, and the RNA concentration
was measured using a spectrophotometer. According to the
experimental procedure provided by Takara (article no. 2690A), 1 µg
RNA was taken for reverse transcription, and complementary
deoxyribonucleic acid (cDNA) obtained was stored at −20°C. The mRNA
level of each index was detected according to instructions of the
All-in-One™ qPCR Mix kit. The relative expression level of mRNA of
each index was calculated as follows: 2−ΔCq [ΔCq = Cq
(target gene) - Cq (glyceraldehyde-3-phosphate dehydrogenase,
GAPDH)] (10). The corresponding
primer sequences are shown in Table
I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene name | Primer sequences |
|---|
| Fibulin-2 |
5′-GAGATCCCTGAGAGTGGCACTGAGG-3′ |
|
|
3′-GAGAAGGCACTCATCCTGGTCATCG-3′ |
| β-catenin |
5′-CAAGGTGGGTGATGCCCTGAAGGAG-3′ |
|
|
3-CGTCTGCACGGTCTTGAACTGGTCGTA-3′ |
| GAPDH |
5′-AGGTCGGTGTGAACGGATTTG-3′ |
|
|
3-GTAGACCATGTAGTTGAGGTCA-3′ |
Immunohistochemistry (IHC)
After 49 cases of gastric cancer and para-carcinoma
tissue specimens were fixed with 4% formaldehyde, routinely
dehydrated and embedded into paraffin, the paraffin-embedded
tissues were sliced into 4 µm sections, followed by IHC using
streptavidin-peroxidase (SP) staining: After dewaxing with xylene
and dehydration with gradient ethanol, antigen retrieval was
performed using sodium citrate buffer via microwave, and the
peroxidase was blocked with 3% H2O2 blocker.
The sections were sealed with 10% donkey serum, phosphate-buffered
saline (PBS) was added as the negative control, and the primary
rabbit anti-human fibulin-2 and β-catenin polyclonal antibodies
(cat. nos. ab251662 and ab16051, respectively; Abcam; diluted at
1:200) were added dropwise, followed by incubation in a wet box at
4°C overnight. The next day, sections were washed with PBS 3 times,
and incubated with the secondary goat anti-rabbit polyclonal
antibody (cat. no. ab6721; Abcam; diluted at 1:200), followed by
color development via diaminobenzidine (DAB) and photography under
a microscope. The brown and dark brown nuclei under the microscope
indicated positive cells, and the number of positive cells was
counted. The number of positive cells/total number of cells in the
visual field >5% indicated positive expression.
Cell culture and plasmid
transfection
Human gastric cancer AGS and SGC-790 cell lines were
purchased from the Chinese Academy of Sciences. The two kinds of
cell lines were cultured in Dulbeccos modified Eagles medium (DMEM;
Hyclone; GE Healthcare) and added with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin sodium
and 100 µg/ml streptomycin (Hyclone; GE Healthcare). The cell
culture flask was placed in an incubator containing 5%
CO2 at 37°C.
Gastric cancer AGS and SGC-790 cells in the
logarithmic growth phase were taken and digested with 0.25%
trypsin. The cell density was adjusted, and cells were inoculated
into a 24-well plate (1×105/well) and cultured for
another 12 h, followed by transfection according to the
instructions of Lipofectamine 2000. In the experiment, the cells
were divided into the pcDNA-fibulin-2 group and control group.
pcDNA-fibulin-2 (sequence: 5′-GTTAUUTACUCGTTGCGGUA-3′,
5′-CUTTAGACUTTUGUGAGAUA-3′) was produced by Shanghai GenePharma
Co., Ltd.
Western blot analysis
After treatment, the cells were scraped off,
transferred into a 2.5 ml EP tube and added with 150 µl mixture of
radioimmunoprecipitation assay (RIPA) and protease inhibitor
cocktail (Sigma-Aldrich; Merck KGaA), followed by ultrasonic
dispersion using an ultrasonic apparatus (40 A, 3 sec/time,
repeated 3 times). After centrifugation at 12,000 × g at 4°C for 15
min, the supernatant was taken as the tissue protein. The protein
concentration was measured using the bicinchoninic acid (BCA) kit
(Beyotime Institute of Biotechnoogy). After denaturation, the total
protein was separated using 10% acrylamide gel, transferred onto a
0.22 µm nitrocellulose membrane (EMD Millipore) for 1.5 h, sealed
with 5% skim milk for 1 h and incubated with rabbit anti-human
fibulin-2, β-catenin, cyclin D1, C-myc and GAPDH polyclonal
antibodies (cat. nos. ab251662, ab16051, ab226977, ab39688 and
ab9485, respectively; Abcam; diluted at 1:1,000) overnight. Then
the band was incubated with the goat anti-rabbit IgG secondary
polyclonal antibody (cat. no. ab6721; Abcam; diluted at 1:300) for
1 h, and the target protein band was developed using the ECL system
(Bio-Rad Laboratories). The relative content of the target protein
was calculated as: gray value (target protein)/gray value
(corresponding internal reference band).
Statistical analysis
GraphPad Prism 5.01 statistical software (GraphPad
Software, Inc.) was used for the analysis of the experimental
results. The independent-samples t-test was used for the comparison
of difference in samples between two groups. Chi-square test was
used for the comparison of difference in indexes between two
groups. The correlation between fibulin-2 and β-catenin protein
expression in gastric cancer was detected via Spearman's analysis.
P<0.05 indicates the difference was statistically
significant.
Results
Expression of Fibulin-2 and β-catenin
detected via RT-PCR
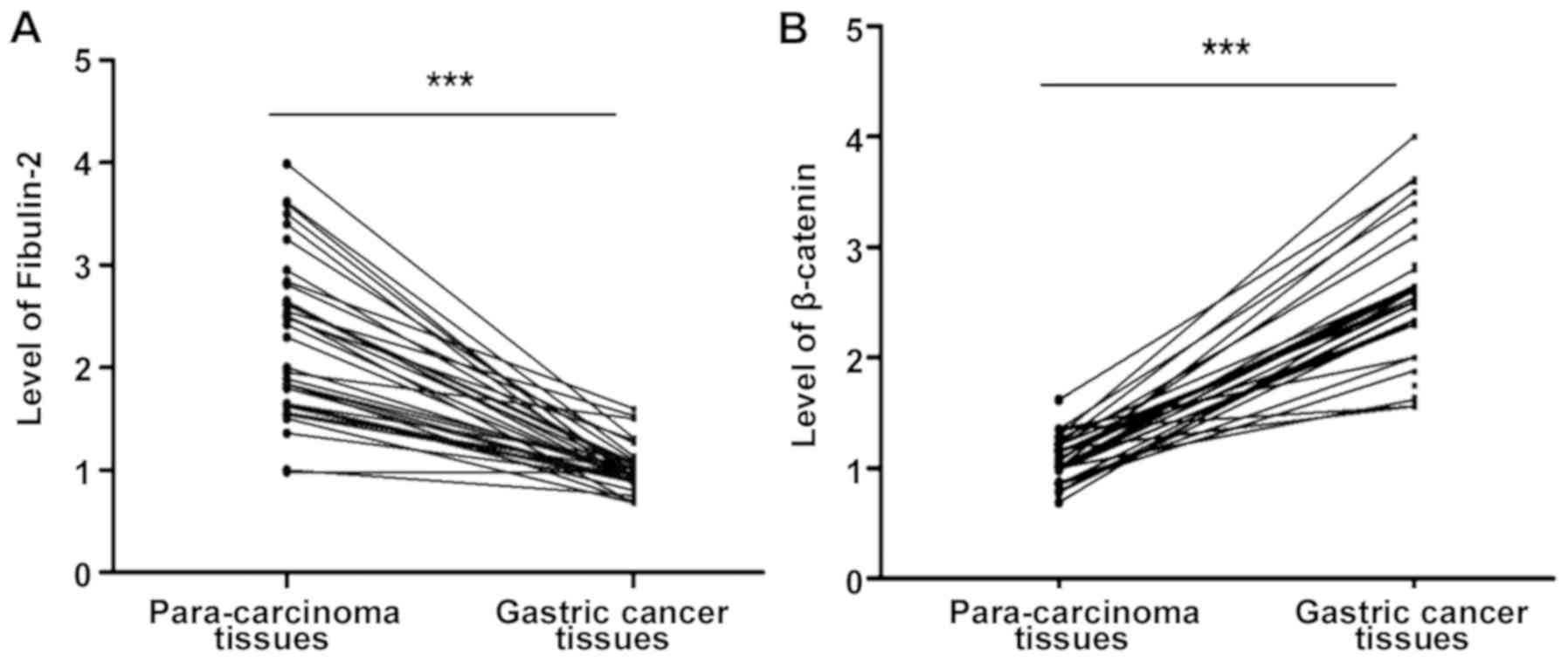
Fibulin-2 and β-catenin mRNA levels in gastric
cancer and para-carcinoma tissues were quantitatively detected via
RT-PCR. It was found that the fibulin-2 mRNA level in gastric
cancer tissues was significantly lower than that in para-carcinoma
tissues, while the β-catenin mRNA level in gastric cancer tissues
was significantly higher than that in para-carcinoma tissues
(P<0.05), and the mean differences were up to 2.25- and
3.02-fold higher, respectively (P<0.05; Fig. 1).
Expression of Fibulin-2 and β-catenin
protein in gastric cancer and para-carcinoma tissues detected via
IHC
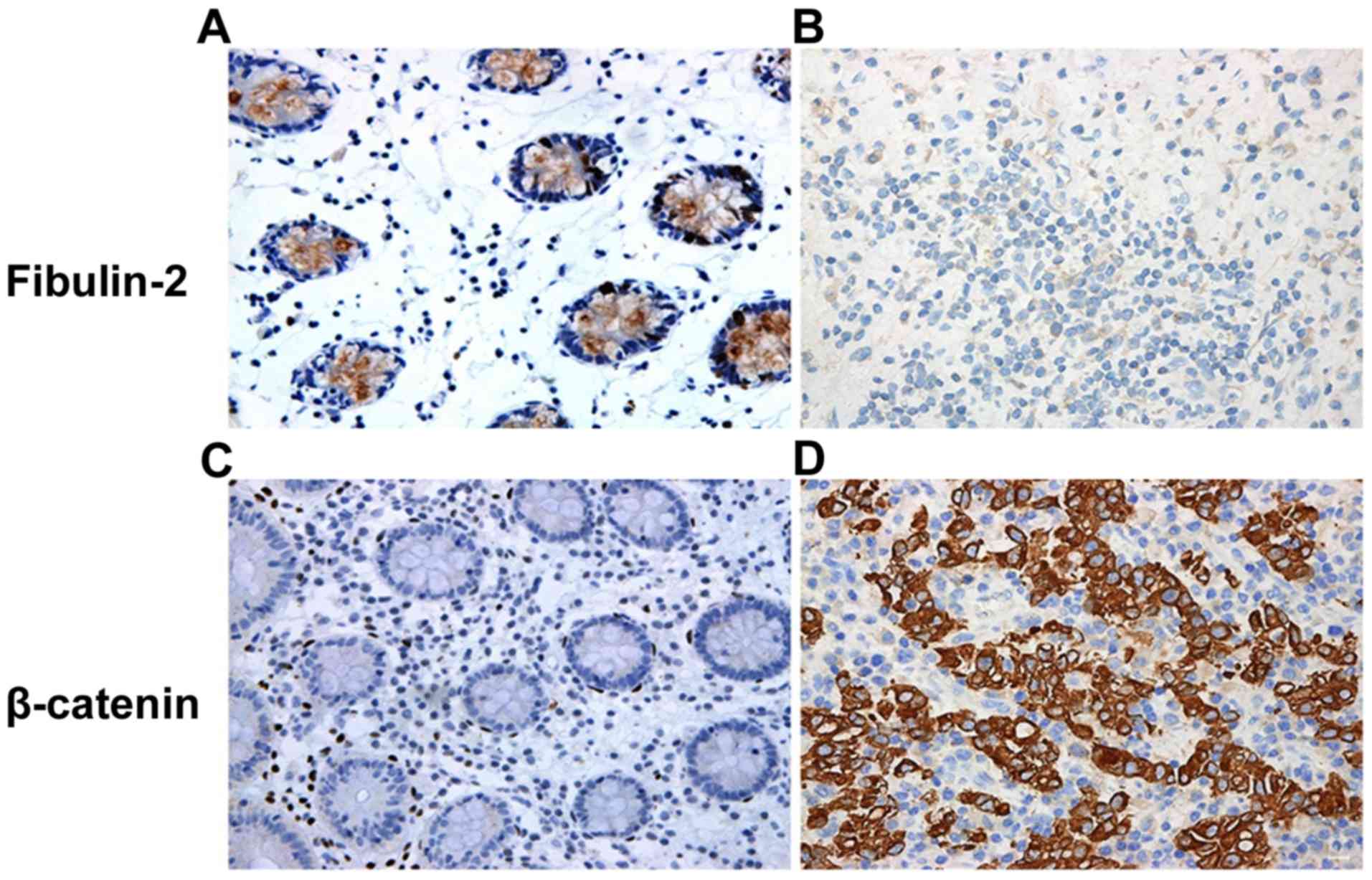
As shown in Fig. 2,
both fibulin-2 and β-catenin proteins were located in the
cytoplasm, and the cytoplasm of positive cells were evident as
brown yellow or dark brown color to varying degrees. The positive
rate of fibulin-2 protein was 73.47% (36/49) in gastric cancer
tissues and 16.33% (8/49) in para-carcinoma tissues, indicating
that fibulin-2 protein is significantly deleted in gastric cancer
tissues (P<0.001). The positive rate of β-catenin protein was
77.55% (38/49) in gastric cancer tissues and 28.57% (14/49) in
para-carcinoma tissues, suggesting that β-catenin protein is
significantly upregulated in gastric cancer tissues (P<0.001)
(Table II).
 | Table II.Expression of fibulin-2 and β-catenin
protein in gastric cancer and para-carcinoma tissues. |
Table II.
Expression of fibulin-2 and β-catenin
protein in gastric cancer and para-carcinoma tissues.
|
|
| Fibulin-2 | β-catenin |
|---|
|
|
|
|
|
|---|
| Group | n | Negative n, (%) | Positive n, (%) | Negative n, (%) | Positive n, (%) |
|---|
| Gastric cancer
tissues | 49 | 36 (73.47) | 13 (26.53) | 11 (22.45) | 38 (77.55) |
| Para-carcinoma
tissues | 49 | 8 (16.33) | 41 (83.67) | 35 (71.43) | 14 (28.57) |
| χ2 |
| 9.81 |
| 6.84 |
|
| P-value |
| 0.001 |
| 0.001 |
|
Correlation analysis between fibulin-2
and β-catenin
The correlation between fibulin-2 and β-catenin
protein expression in gastric cancer was analyzed via Spearman's
correlation analysis, and it was found that they had a negative
correlation (r=−0.361, P=0.003; Table
III).
 | Table III.Correlation between fibulin-2 and
β-catenin in gastric cancer tissues. |
Table III.
Correlation between fibulin-2 and
β-catenin in gastric cancer tissues.
|
| Fibulin-2 |
|
|
|---|
|
|
|
|
|
|---|
| Protein
expression | Negative (36) | Positive (13) | r | P-value |
|---|
| β-catenin |
|
|
|
|
| Negative (11) | 6 | 10 | −0.361 | 0.003 |
| Positive (38) | 27 | 6 |
|
|
Correlation of fibulin-2 and β-catenin
expression with clinicopathological features of patients
As shown in Table
IV, the expression levels of fibulin-2 and β-catenin were not
associated with the sex, age, differentiation degree, depth of
infiltration and TNM stage of gastric cancer patients (P>0.05),
but closely related to the tumor size and lymph node metastasis
(P<0.05).
 | Table IV.Association of fibulin-2 and β-catenin
expression with clinicopathological characteristics of
patients. |
Table IV.
Association of fibulin-2 and β-catenin
expression with clinicopathological characteristics of
patients.
|
|
| Fibulin-2 |
|
| β-catenin |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Index | n | Negative (13) | Positive (36) | P-value | n | Positive (38) | Negative (11) | P-value |
|---|
| Sex |
|
|
| 0.245 |
|
|
| 0.315 |
| Male | 26 | 6 | 20 |
| 27 | 21 | 6 |
|
|
Female | 23 | 7 | 16 |
| 22 | 17 | 5 |
|
| Age (years) |
|
|
| 0.135 |
|
|
| 0.262 |
| ≤60 | 24 | 8 | 16 |
| 23 | 18 | 5 |
|
|
>60 | 25 | 5 | 20 |
| 26 | 20 | 6 |
|
| Tumor size
(cm) |
|
|
| 0.038 |
|
|
| 0.019 |
| ≤3 | 16 | 4 | 12 |
| 19 | 10 | 9 |
|
|
>3 | 33 | 9 | 24 |
| 30 | 28 | 2 |
|
| Differentiation
degree |
|
|
| 0.224 |
|
|
| 0.092 |
| High
and moderate differentiation | 26 | 6 | 20 |
| 20 | 13 | 7 |
|
| Low and
no differentiation | 23 | 7 | 16 |
| 29 | 25 | 4 |
|
| Depth of
infiltration |
|
|
| 0.261 |
|
|
| 0.085 |
|
T1-T2 | 22 | 5 | 17 |
| 21 | 17 | 4 |
|
|
T3-T4 | 27 | 8 | 19 |
| 28 | 21 | 7 |
|
| Lymph node
metastasis |
|
|
| 0.041 |
|
|
| 0.023 |
| No | 19 | 8 | 11 |
| 20 | 11 | 9 |
|
|
Yes | 30 | 5 | 25 |
| 29 | 27 | 2 |
|
| TNM stage |
|
|
| 0.108 |
|
|
| 0.081 |
|
I–II | 20 | 9 | 11 |
| 22 | 17 | 5 |
|
|
III–IV | 29 | 4 | 25 |
| 27 | 21 | 6 |
|
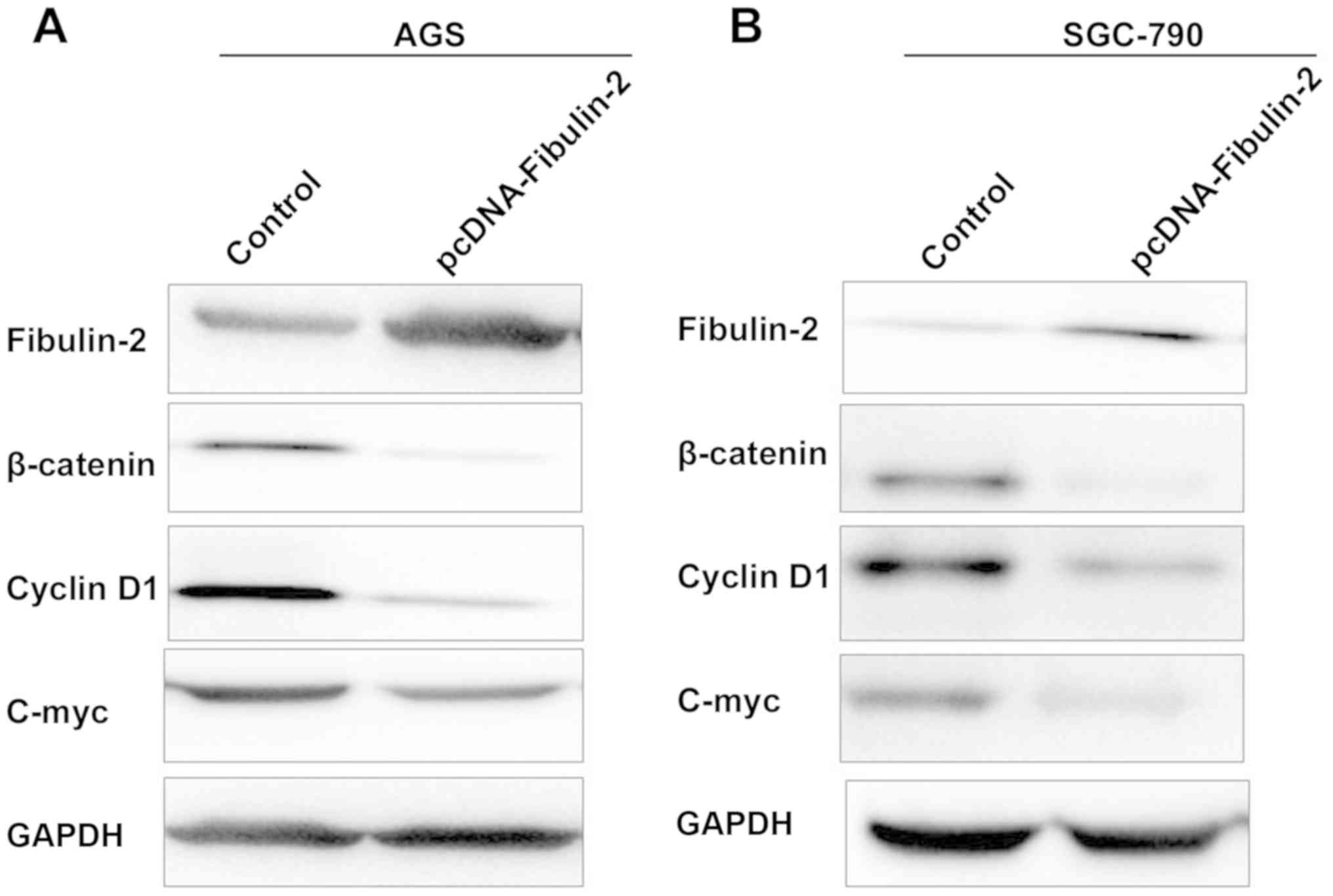
Overexpression of fibulin-2
significantly downregulated β-catenin
According to literature reports, fibulin-2
expression is low in AGS and SGC-790 cell lines. Therefore,
fibulin-2 overexpression plasmid was transfected into the two
cells. As shown in Fig. 3, after
overexpression of fibulin-2, protein expression of β-catenin,
cyclin D1 and c-Myc was obviously downregulated (P<0.05).
Discussion
Previous findings revealed that fibulin-2 is one of
the 64 genes overexpressed in solid tumors from different sources
(11). However, fibulin-2 silencing
was found in studies on colorectal cancer (23 cases), liver cancer
(24 cases), prostate cancer (25 cases) and nasopharyngeal cancer
(26 cases) (12–14). According to recent studies, type I
transmembrane glycoprotein whose expression is enhanced in
pancreatic cancer interacts with fibulin-2 via the nestin-like
domain, and fibulin-2 is one of the related genes promoting
pancreatic cancer MC-4 cell-mediated metastasis (15). The proteomic analysis of the mouse
model of tumor showed that fibulin-2 may be a tissue and plasma
biomarker for breast cancer. In addition, several studies have
demonstrated that the inactivation of fibulin-2 is correlated with
the tumor progression (16,17). The 10K array analysis of human
genomic map revealed that the chromosome 3p25.1 region (fibulin-2)
in patients with esophageal squamous cell carcinoma is often
deleted, indicating that fibulin-2 is a potential TSG (18). Studies on liver cancer have suggested
that the anti-angiogenic effect of fibulin-2 is accompanied by the
downregulation of two pro-angiogenic factors, vascular endothelial
growth factor and matrix metalloproteinase-2 (19). According to subsequent epigenetic
research, the inactivation of fibulin-2 in a variety of tumors is
caused by the abnormal increase of methylation level (20). However, neither expression nor
function of fibulin-2 in gastric cancer has been clear as yet.
The activation of Wnt/β-catenin pathway is a key
carcinogenic event in the occurrence, development and metastasis of
tumors. β-catenin is a kind of oncoprotein generally located in the
cytoplasm, whose expression is inhibited by
ubiquitin/proteasome-mediated protein degradation (21). In adenomatous polyp, the glycogen
synthase kinase 3β (GSK3β)-mediated carcinogenic Wnt signal
promotes the accumulation and nuclear translocation of glycogen
synthase kinase 3β (GSK3β)-catenin, thus forming the complex.
Factors such as T-cell factor 4 (TCF-4) lead to the activation of
downstream targets, such as oncogenes C-myc and cyclin D1, and the
Wnt/β-catenin signal transduction is often activated in tumors,
thereby promoting the proliferation, survival and metastasis of
tumor cells (22,23).
In this study, 48 cases of gastric cancer were
collected. Both mRNA and protein levels of fibulin-2 in gastric
cancer tissues were significantly downregulated, while those of
β-catenin were significantly upregulated. The positive rate of
fibulin-2 protein was 73.47% in gastric cancer tissues and 16.33%
in para-carcinoma tissues, while positive rate of β-catenin protein
was 77.55% in gastric cancer tissues and 28.57% in para-carcinoma
tissues. Then the correlation between expression of fibulin-2 and
β-catenin protein in gastric cancer was analyzed via Spearman's
correlation analysis, and it was found that they were negatively
correlated (r=−0.361, P=0.003). Furthermore, gastric cancer AGS and
SGC-790 cell lines were selected and transfected with fibulin-2
overexpression plasmid, and β-catenin and its downstream regulatory
molecules (C-myc and cyclin D1) were detected via western blot
analysis. Results showed that β-catenin and its downstream
molecules were also inhibited, which is consistent with findings
obtained in clinical tissue specimens. According to previous
findings, fibulin-2 has the largest molecular weight in the fibulin
family, which can interact with various cytoplasmic proteins. Such
interactions may be critical in cell movement and proliferation
(24). It is hypothesized that the
downregulation of fibulin-2 promotes the proliferation of gastric
cancer through the abnormal activation of β-catenin, and the
β-catenin signal also plays an important role in gastric cancer.
The constantly high expression level of β-catenin in gastric cancer
cells leads to the activation of TCF-4 and the overexpression of
cyclin D1, thus promoting the proliferation of gastric cancer
cells. The specific molecular mechanisms remain unclear, but the
expression has correlation in tumors with larger volume and deeper
infiltration, further confirming the role of fibulin-2 in the
mechanism of proliferation of gastric cancer. The above results
indicate that the in vitro and in vivo functional
characteristics of fibulin-2 reveal the important antitumor effect
of fibulin-2 in cell proliferation, migration and invasion, which
provides the first clue for the functional and molecular
characteristics of fibulin-2 in tumor progression.
In conclusion, results of the present study indicate
that fibulin-2 plays an important role in inhibiting gastric cancer
invasion, which is mediated by the inhibition of β-catenin signal.
Further research on this pathway may provide potentially useful
information for the development of biological or pharmacological
drugs for metastatic lung cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
HM and CL performed the immunohistochemistry and
PCR. HM and YS were responsible for the cell culture and western
blotting. HM wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Heping Hospital Affiliated to Changzhi Medical College
(Changzhi, China) and informed consents were signed by the patients
or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li H, Jin X, Liu P and Hong W: Time to
local recurrence as a predictor of survival in unrecetable gastric
cancer patients after radical gastrectomy. Oncotarget.
8:89203–89213. 2017.PubMed/NCBI
|
|
2
|
Maciejowski J and de Lange T: Telomeres in
cancer: Tumour suppression and genome instability. Nat Rev Mol Cell
Biol. 18:175–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Inamura K: Major tumor suppressor and
oncogenic non-coding RNAs: Clinical relevance in lung cancer.
Cells. 6:122017. View Article : Google Scholar
|
|
4
|
Manders DB, Kishore HA, Gazdar AF, Keller
PW, Tsunezumi J, Yanagisawa H, Lea J and Word RA: Dysregulation of
fibulin-5 and matrix metalloproteases in epithelial ovarian cancer.
Oncotarget. 9:14251–14267. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lisi S, DAmore M, Scagliusi P, Mitolo V
and Sisto M: Anti-Ro/SSA autoantibody-mediated regulation of
extracellular matrix fibulins in human epithelial cells of the
salivary gland. Scand J Rheumatol. 38:198–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan H, Zhang J, Fu D and Zhu Y: Loss of
fibulin-2 expression is involved in the inhibition of breast cancer
invasion and forms a new barrier in addition to the basement
membrane. Oncol Lett. 14:2663–2668. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Melnik S, Dvornikov D, Müller-Decker K,
Depner S, Stannek P, Meister M, Warth A, Thomas M, Muley T, Risch
A, et al: Cancer cell specific inhibition of Wnt/β-catenin
signaling by forced intracellular acidification. Cell Discov.
4:372018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng YZ, Yao F, Li JJ, Mao ZF, Hu PT, Long
LY, Li G, Ji XD, Shi S, Guan DX, et al: RACK1 suppresses gastric
tumorigenesis by stabilizing the β-catenin destruction complex.
Gastroenterology. 142:812–823.e15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siersema PD: Esophageal cancer.
Gastroenterol Clin North Am. 37:pp. 943–964, x. 2008, https://doi.org/10.1016/j.gtc.2008.09.012
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yi CH, Smith DJ, West WW and Hollingsworth
MA: Loss of fibulin-2 expression is associated with breast cancer
progression. Am J Pathol. 170:1535–1545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khan SA, Dong H, Joyce J, Sasaki T, Chu ML
and Tsuda T: Fibulin-2 is essential for angiotensin II-induced
myocardial fibrosis mediated by transforming growth factor (TGF)-β.
Lab Invest. 96:773–783. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fontanil T, Rúa S, Llamazares M,
Moncada-Pazos A, Quirós PM, García-Suárez O, Vega JA, Sasaki T,
Mohamedi Y, Esteban MM, et al: Interaction between the ADAMTS-12
metalloprotease and fibulin-2 induces tumor-suppressive effects in
breast cancer cells. Oncotarget. 5:1253–1264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen R, Yi EC, Donohoe S, Pan S, Eng J,
Cooke K, Crispin DA, Lane Z, Goodlett DR, Bronner MP, et al:
Pancreatic cancer proteome: The proteins that underlie invasion,
metastasis, and immunologic escape. Gastroenterology.
129:1187–1197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fontanil T, Álvarez-Teijeiro S, Villaronga
MÁ, Mohamedi Y, Solares L, Moncada-Pazos A, Vega JA, García-Suárez
O, Pérez-Basterrechea M, García-Pedrero JM, et al: Cleavage of
Fibulin-2 by the aggrecanases ADAMTS-4 and ADAMTS-5 contributes to
the tumorigenic potential of breast cancer cells. Oncotarget.
8:13716–13729. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hergeth SP, Aicher WK, Essl M, Schreiber
TD, Sasaki T and Klein G: Characterization and functional analysis
of osteoblast-derived fibulins in the human hematopoietic stem cell
niche. Exp Hematol. 36:1022–1034. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Fu L, Zhang LY, Kwong DL, Yan L
and Guan XY: Tumor suppressor genes on frequently deleted
chromosome 3p in nasopharyngeal carcinoma. Chin J Cancer.
31:215–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Argraves WS, Greene LM, Cooley MA and
Gallagher WM: Fibulins: Physiological and disease perspectives.
EMBO Rep. 4:1127–1131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng YY, Jin H, Liu X, Siu JM, Wong YP,
Ng EK, Yu J, Leung WK, Sung JJ and Chan FK: Fibulin 1 is
downregulated through promoter hypermethylation in gastric cancer.
Br J Cancer. 99:2083–2087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Austinat M, Dunsch R, Wittekind C,
Tannapfel A, Gebhardt R and Gaunitz F: Correlation between
beta-catenin mutations and expression of Wnt-signaling target genes
in hepatocellular carcinoma. Mol Cancer. 7:212008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McCracken KW, Aihara E, Martin B, Crawford
CM, Broda T, Treguier J, Zhang X, Shannon JM, Montrose MH and Wells
JM: Wnt/β-catenin promotes gastric fundus specification in mice and
humans. Nature. 541:182–187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Bai B, Liu L, Ma P, Kong L, Yan J,
Zhang J, Ye Z, Zhou H, Mao B, et al: Novel β-carbolines against
colorectal cancer cell growth via inhibition of Wnt/β-catenin
signaling. Cell Death Discov. 1:150332015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brown K, Yang P, Salvador D, Kulikauskas
R, Ruohola-Baker H, Robitaille AM, Chien AJ, Moon RT and Sherwood
V: WNT/β-catenin signaling regulates mitochondrial activity to
alter the oncogenic potential of melanoma in a PTEN-dependent
manner. Oncogene. 36:3119–3136. 2017. View Article : Google Scholar : PubMed/NCBI
|