Introduction
Clinically, neuroblastoma, also known as
neurocytoma, is one of the most common extracranial malignant solid
tumors in children, accounting for 6–10% of all tumors in children
(1,2). The incidence in children <15 years
of age is 9,700,000 every year in the United States, with >50%
in infants before the age of 2 years (3). Derived from neural stem cells,
neuroblastoma belongs to neuroendocrine tumors. Its primary site is
often concealed and extensive, which brings certain difficulties to
clinical diagnosis. In addition, tumor cells have extremely high
malignancy, prone to early multiple metastases (4,5).
As a group of non-coding RNAs consisting of
approximately 19–25 nucleotides in length, miRNAs can horizontally
regulate the expression of multiple genes after transcription, thus
functioning as oncogenes or tumor suppressor genes (6–8). A study
has confirmed that the pathological process of tumors is related to
the expression of serum miRNAs that play an important role in the
occurrence, development, diagnosis and prognosis of tumors
(9). In recent years, research has
shown that miR-181c may play a key role in the occurrence and
development of tumors and autoimmune diseases (10). For example, low expression in a
variety of tumors shows a negative regulatory effect on the
proliferation and metastasis of tumor cells, such as nasopharyngeal
carcinoma (11). However, studies on
miR-181c in neuroblastoma are rare. This study analyzed the
expression of miR-181c in neuroblastoma patients and its effect on
the proliferation of neuroblastoma M17 cells, in order to provide
clinical value in the occurrence and development, prediction and
prognosis, and diagnosis and treatment of neuroblastoma.
Patients and methods
Clinical information
Fifty-seven neuroblastoma patients admitted to
Weifang People's Hospital (Weifang, China) from January 2013 to
December 2017 were selected, and their cancer tissues and normal
adjacent tissues were obtained. In total, 32 males and 25 females
were included in the study, 0–27 years of age, with an average age
of 5.64±2.37 years. Inclusion criteria: all cancer tissue specimens
were confirmed by pathology, and the adjacent tissues were
confirmed to be without cancer or inflammatory cell infiltration;
patients with complete records were included; and patients who had
not received relevant medical treatment in other hospitals.
Exclusion criteria: patients during pregnancy or lactation;
patients with other severe diseases or tumors; patients with
communication disorders or cognitive impairment. The study was
approved by the Ethics Committee of Weifang People's Hospital.
Patients or their families signed the informed consent form and
cooperated with the medical staff to complete the relevant medical
treatment. The basic information of patients is shown in Table I.
 | Table I.Basic information of 57 neuroblastoma
patients [n (%)]. |
Table I.
Basic information of 57 neuroblastoma
patients [n (%)].
| Factors | Cases (n=57) |
|---|
| Age (years) |
|
|
<15 | 45 (78.95) |
| ≥15 | 12 (21.05) |
| Sex |
|
| Male | 32 (56.14) |
|
Female | 25 (43.86) |
| Arthralgia |
|
| Yes | 39 (68.42) |
| No | 18 (31.58) |
| Periorbital dark
circles |
|
| Yes | 21 (36.84) |
| No | 36 (63.16) |
| Pathological
typing |
|
| NB less
matrix type | 18 (31.58) |
| GNB rich
matrix type | 23 (40.35) |
| GN mature
type | 11 (19.30) |
| 3NB
nodule type | 5 (8.77) |
| Incidence site |
|
| Adrenal
gland | 23 (40.35) |
|
Cervix | 2 (3.51) |
| Pleural
cavity | 11 (19.30) |
| Abdominal
cavity | 18 (31.58) |
| Pelvic
cavity | 2 (3.51) |
|
Others | 1 (1.75) |
| INRGSS |
|
| Stage
L1 | 22 (38.60) |
| Stage
L2 | 23 (40.35) |
| Stage
M | 10 (17.54) |
| Stage
MS | 2 (3.51) |
Main materials
Human neuroblastoma M17 cells [human neuroblastoma
cell line BE(2)-M17; cat. no. CL-0262] were purchased from Shanghai
Chunmai Biotechnology Co.; RPMI-1640 medium was purchased from
Hyclone (GE Healthcare Life Sciences); reverse transcription kit
from Beyotime Institute of Biotechnology; RT-qPCR kit from Takara
Biotechnology Co., Ltd.; TRIzol kit from Shanghai Pufei
Biotechnology Co., Ltd.; fetal bovine serum (FBS) and dimethyl
sulfoxide (DMSO) from Sigma-Aldrich (Merck KGaA); UV-3100PC
spectrophotometer from Shanghai MeiPuda Co., Ltd.; Lipofectamine
2000 transfection reagent from Invitrogen (Thermo Fisher
Scientific, Inc.), and MTT detection kit from Thermo Fisher
Scientific, Inc.
RT-qPCR detection of miR-181c
expression
One-step extraction method was used to extract total
RNA from neuroblastoma cancer tissues and adjacent tissues using
the TRIzol kit. The specific steps were carried out in strict
accordance with the manufacturer's instructions. UV-3100PC
spectrophotometer was used to measure the concentration and purity
of the extracted RNA, and 1% denaturing agarose gel electrophoresis
to detect the integrity of extracted RNA. The extracted RNA was
reverse transcribed to obtain cDNA, that was used as a template for
the experiment. The primer sequence was designed and synthesized by
Shanghai Shenggong Bioengineering Co., Ltd., with β-actin as the
internal reference gene. RNA (1 µg) was added to the reverse
transcription system, and the reverse transcription procedure was
37°C for 15 min, 85°C for 5 sec, and 4°C for 10 min. RT-qPCR:
amplification was performed on a Light Cycler Real-time PCR
instrument (Bio-Rad Laboratories, Inc.) with the reverse
transcription product cDNA as a template. Reaction system: 10 µl of
SYBR Premix Ex Taq (2X) (Takara Biotechnology Co., Ltd.), each of
0.4 µl of 5′ and 3′ primers, 2.0 µl of DNA template, sterilized,
double distilled water added to 20 µl. Reaction conditions:
pre-denaturation at 95°C for 30 sec, denaturation at 95°C for 5
sec, and at 60°C for 20 sec. Melting conditions: 95°C for 30 sec,
65°C for 15 sec, and 95°C for 20 sec. Each group of samples was
repeated 3 times. The 2−∆Cq method was used to analyze
the expression level of miR-181c in the samples (12). Primer sequences are shown in Table II.
 | Table II.Primer sequences of miR-181c and
internal reference. |
Table II.
Primer sequences of miR-181c and
internal reference.
| Genes | Upstream primers | Downstream
primers |
|---|
| miR-181c |
5′-CGAAGCTTATGTTCAGGACCA |
5′-CCCTCGAGGGGCGCAGAT |
|
| AACGATCTGCGC-3′ |
CGTTTGGTCCTGAACAT-3′ |
| β-actin |
5′-GAGACCTTCAACACCCCAGC-3′ |
5′-ATGTCACGCACGATTTCCC-3′ |
MTT assay detection of
proliferation
Human neuroblastoma M17 cells were cultured and
proliferated in RPMI-1640 medium. M17 cells were cultured at 37°C,
with pH 6.8–7.4 and 5% CO2. miR-181c was subjected to
PCR amplification and purification. The PCR product was then
recovered. The product and the vector plasmid containing the green
fluorescent protein reporter gene were separately digested and
purified by Age and EcoRI, and the products were ligated
overnight at 16°C to obtain the vector. The vector was used to
transform DH5a competent bacteria, and the positive clones were
screened and sent to Shanghai Jima Pharmaceutical Technology Co.,
Ltd. for sequencing. miR-181c was transfected into cells in strict
accordance with the liposome Lipofectamine 2000 instructions.
miR-181c expression vectors were divided into the miR-181c group
and the blank group with blank vector (without tumor suppressor),
cultured together with human neuroblastoma M17 cells in RPMI-1640
medium, in an incubator at 37°C and 5% CO2 for 24 h.
Untreated M17 cells were used as the control group.
After transfection, human neuroblastoma M17 cells
were prepared into a single-arranged cell suspension. Cells were
routinely seeded and cultured in a 96-well culture plate. A part of
the cultured cells was taken out, and 20 µl of MTT (5 mg/ml)
solution were added, and continuously cultured at 37°C for 4 h. The
supernatant containing impurities was aspirated, and the DMSO
preparation was added, shaken for 15 min on a horizontal shaker. An
ELISA instrument was used to measure the OD values at a wavelength
of 480 nm at 24, 48 and 72 h, and the growth curve was plotted.
Soft agar colony formation assay to
determine proliferation
The bottom agar was prepared. Fully melted 5% agar
and fresh complete medium (pre-warmed at 37°C) were mixed evenly at
a ratio of 1:9 at 40°C, and then added to a Petri dish (60-mm
diameter) containing 0.5 ml of agar medium per dish. The agar was
completely solidified at room temperature. miR-181c was used to
transfect cells in strict accordance to the manufacturer's
instructions of liposome Lipofectamine 2000. The miR-181c
expression vector was divided into miR-181c group and blank group
of blank vector (no virus group). Human neuroblastoma M17 cells
were added to the medium and cultured to prepare a cell suspension.
The cell suspension was repeatedly blown to fully disperse the
cells. Then the upper layer of agar was prepared. A total of 1.5 ml
of 100 cell suspension was transferred per dish to a small beaker
at 37°C and 40°C, 5% agar was added in equal volume and mixed, and
then added to Petri dish with the bottom agar. The mixture was
incubated at 37°C, 5% CO2 for 72 h. There were 10
culture dishes in each group, and colony formation was observed at
24, 48 and 72 h.
Statistical analysis
SPSS 19.6 statistical software [Boyizhixun (Beijing)
Information Technology Co., Ltd.] was used for the analyzing and
processing the data. The basic enumeration data of patients were
expressed as percentage [n (%)], and the expression level of
miR-181c as mean ± standard deviation (mean ± SD). t-test was used
for comparison between two groups. Variance analysis was used for
comparison among multiple groups, and LSD test was the post hoc
test used. Repeated measures ANOVA was used for comparison between
different time points within a group, and LSD test was used as post
hoc test. Multivariate logistic regression was used to analyze risk
factors. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of miR-181c in
neuroblastoma cancer tissues and adjacent tissues after
transfection
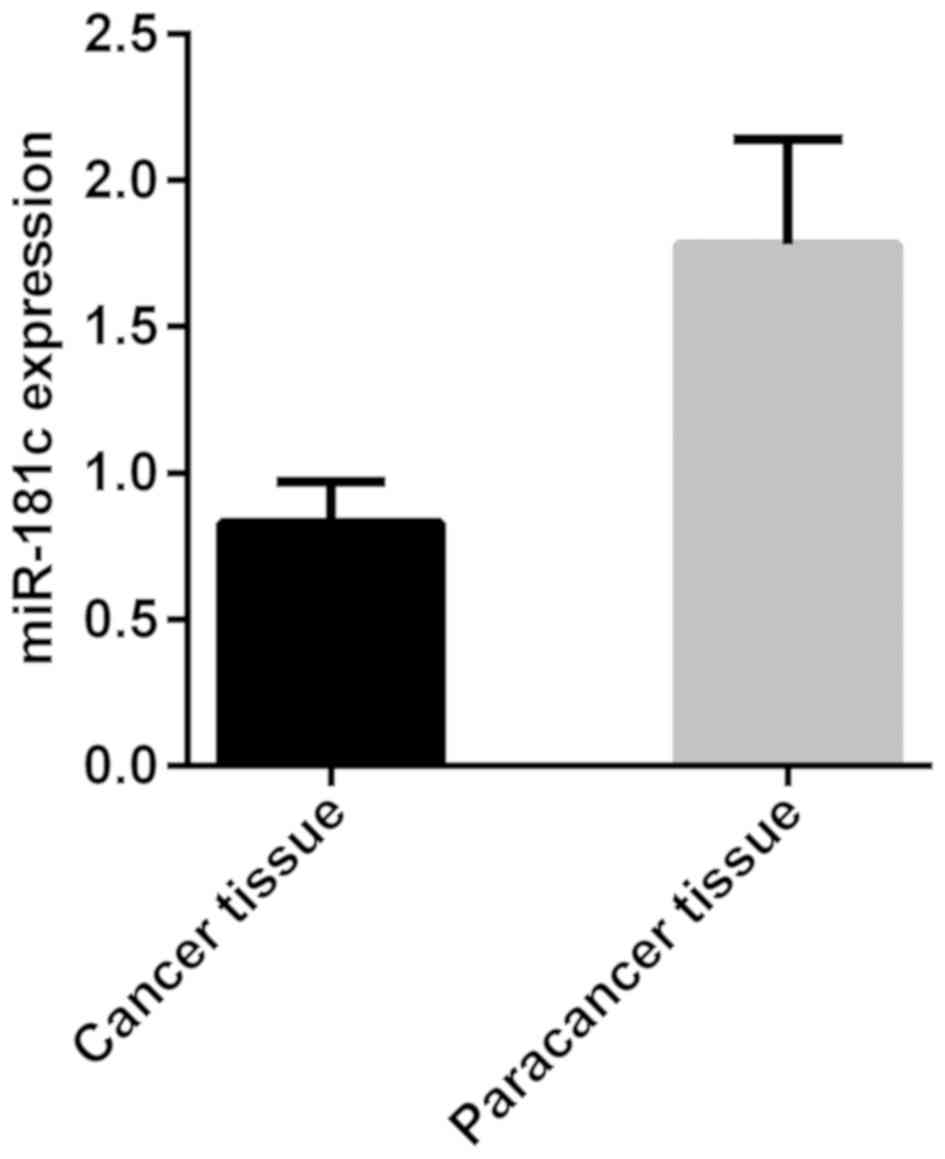
The results of RT-qPCR detection of miR-181c showed
that the expression level of miR-181c was 0.83±0.14 in
neuroblastoma cancer tissues and 1.78±0.36 in adjacent tissues. The
expression level of miR-181c was significantly lower in cancer
tissues than that in adjacent tissues, with a statistically
significant difference (t=18.570, P<0.001) (Fig. 1).
Association of miR-181c expression
level with clinical and pathological features
The expression level of miR-181c was not associated
with sex (P=0.632). miR-181c expression was lower in patients
<15 years of age than that in patients ≥15 years of age, with a
statistically significant difference (P=0.003). miR-181c expression
was lower in patients with poor differentiation than that in
patients with moderate and high differentiation, with a
statistically significant difference (P=0.007). Also, the
expression of miR-181c was lower in patients with lymph node
metastasis than that in patients without lymph node metastasis,
with a statistically significant difference (P=0.002), was lower in
patients with distant metastasis than that in patients without
distant metastasis, with a statistically significant difference
(P=0.013), and was lower in patients with stage M+MS than that in
patients with stage L1+L2, with a statistically significant
difference (P<0.001) (Table
III).
 | Table III.Association of miR-181c expression
with clinical and pathological features. |
Table III.
Association of miR-181c expression
with clinical and pathological features.
| Variables | n | miR-181c | t value | P-value |
|---|
| Age (years) |
|
| 3.106 |
0.003 |
|
<15 | 45 | 0.74±0.12 |
|
|
| ≥15 | 12 | 0.89±0.23 |
|
|
| Sex |
|
| 0.481 |
0.632 |
| Male | 32 | 0.82±0.16 |
|
|
|
Female | 25 | 0.84±0.15 |
|
|
| Differentiation
degree |
|
| 2.783 |
0.007 |
| Poor | 34 | 0.71±0.25 |
|
|
| Moderate
and high | 23 | 0.87±0.14 |
|
|
| Lymph node
metastasis |
|
| 3.309 |
0.002 |
|
Yes | 26 | 0.72±0.26 |
|
|
| No | 31 | 0.89±0.11 |
|
|
| Distant
metastasis |
|
| 2.554 |
0.013 |
|
Yes | 39 | 0.74±0.15 |
|
|
| No | 18 | 0.87±0.23 |
|
|
| INRGSS |
|
| 3.630 | <0.001 |
| Stage
L1+L2 | 45 | 0.96±0.18 |
|
|
| Stage
M+MS | 12 | 0.75±0.17 |
|
|
Multivariate logistic regression
analysis of neuroblastoma
Age, differentiation degree, lymph node metastasis,
distant metastasis, and International Neuroblastoma Risk Group
Staging System (INRGSS) were independent risk factors for
neuroblastoma (P<0.05) (Table
IV).
 | Table IV.Multivariate logistic regression
analysis of neuroblastoma. |
Table IV.
Multivariate logistic regression
analysis of neuroblastoma.
| Factors | β | Standard error | W | P-value | OR (95% CI) |
|---|
| Age (years) | 1.703 | 0.558 |
2.968 | 0.003 | 1.316
(0.714–3.007) |
| Differentiation
degree | 2.571 | 0.194 | 11.832 | <0.001 | 2.724
(1.307–6.994) |
| Lymph node
metastasis | 1.885 | 0.157 | 11.653 | <0.001 | 2.592
(1.243–6.628) |
| Distant
metastasis | 1.046 | 0.804 |
1.137 | 0.036 | 0.508
(0.271–1.361) |
| INRGSS | 1.587 | 0.092 | 17.468 | <0.001 | 4.346
(2.031–10.617) |
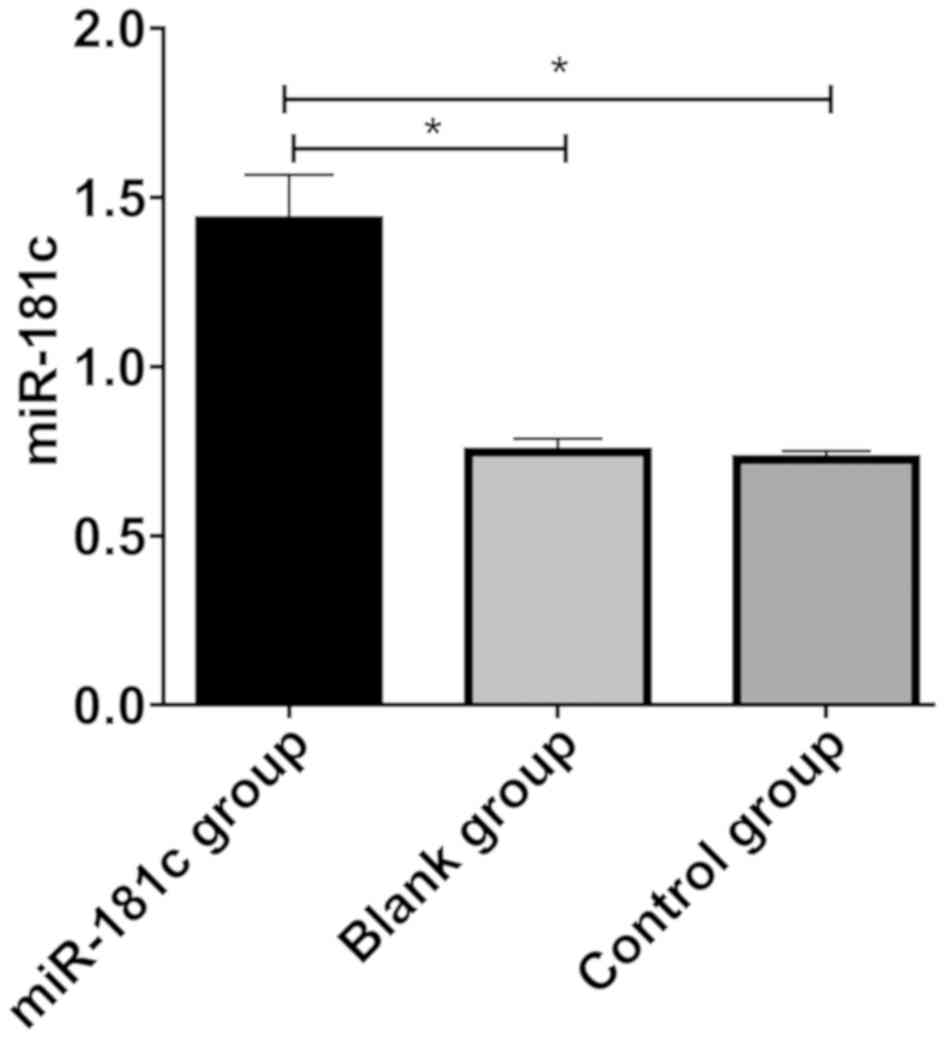
miR-181c transfection results
After transfection, the expression level of miR-181c
in the M17 cells of miR-181c group was significantly higher than
that of the blank and control groups (P<0.05). There was no
significant difference between the blank and the control group
(P>0.05), indicating that the transfection was successful
(Fig. 2).
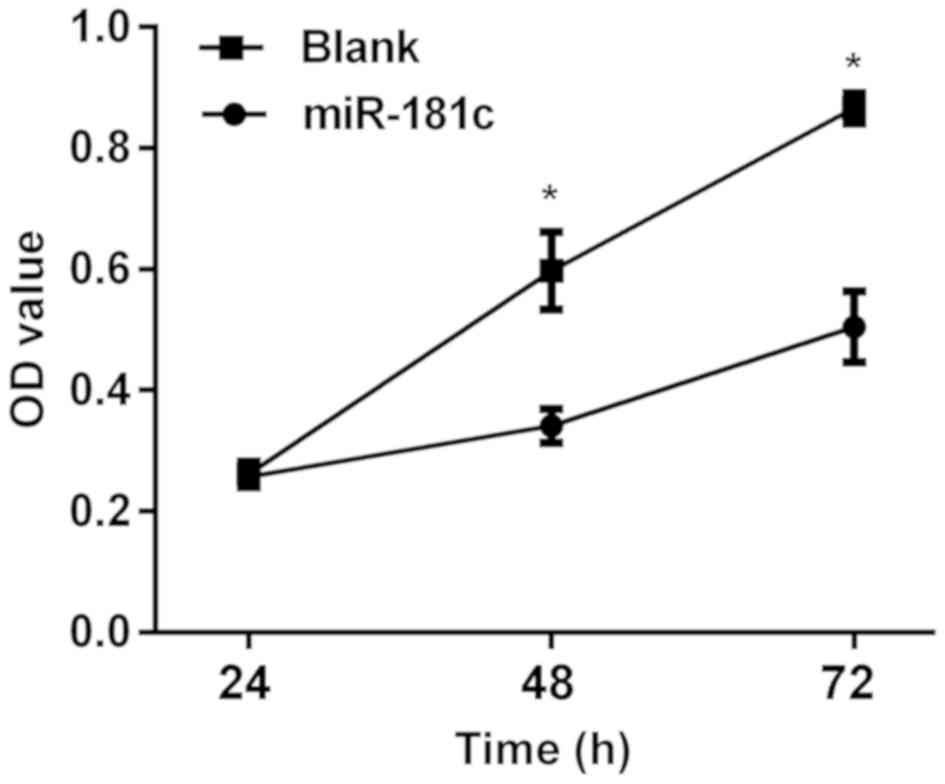
Effects of miR-181c on neuroblastoma
M17 cells
Following miR-181c transfection into M17 cells, the
results of MTT assay showed that there was no significant
difference between the two groups in the proliferation of M17 cells
at 24 h (P>0.05). After 48 h, differences between the two groups
arose. The proliferation of M17 cells was significantly lower in
the miR-181c group than that in the blank group (P<0.05)
(Fig. 3). Following miR-181c
transfection into M17 cells, the results of colony formation assay
showed that there was no significant difference between the two
groups (P>0.05). The colonies at 48 and 72 h in the miR-181c
groups were significantly lower than those in the blank group
(P<0.05) (Table V).
 | Table V.Analysis of colony formation (%). |
Table V.
Analysis of colony formation (%).
| Time (h) | miR-181c group
(n=10) | Blank group
(n=10) | t value | P-value |
|---|
| 24 | 10.65±5.62 | 11.57±6.81 | 0.329 | 0.746 |
| 48 | 21.33±7.68 | 35.65±9.66 | 3.669 | 0.002 |
| 72 | 47.25±8.54 |
88.36±10.98 | 9.346 | <0.001 |
Discussion
Neuroblastoma is a common malignant tumor in
children, with increasing incidence in recent years, causing great
harm to society (13). Without
obvious features and specificity in early neuroblastoma, >50% of
patients have reached the high-risk status of middle and late
stages when diagnosed, missing the best treatment time (14). In addition, early neuroblastoma can
metastasize in children, so >80% of patients are diagnosed with
distant metastasis, resulting in poor prognosis and poor long-term
survival rate (15). Therefore,
finding tumor markers with high sensitivity and accuracy for
neuroblastoma has become a hot research topic in clinical
practice.
In this study, RT-qPCR was used to examine the
miR-181c expression in neuroblastoma cancer tissues and adjacent
tissues. The expression level of miR-181c was significantly lower
in neuroblastoma cancer tissues than that in adjacent tissues, with
a statistically significant difference. Moreover, it was found that
the expression level of miR-181c was not associated with sex, but
it was associated with age, differentiation degree, lymph node
metastasis, distant metastasis and INRGSS, with statistically
significant differences. Due to their stable biological
characteristics in tissues or serum of tumor patients, miRNAs are
more suitable as monitoring indicators for the diagnosis of
neuroblastoma and the invasion and deterioration of tumors
(16). Affecting the proliferation
and differentiation of tumor cells, the expression of most
oncogenes and tumor suppressor genes is associated with the
occurrence, development and prognosis of tumors (17,18).
According to the study of Guo et al (19) on neuroblastoma, the low expression of
miR-181c was significantly associated with tumor metastasis in
patients, and therefore could become a clinical diagnostic marker
for neuroblastoma. This is consistent with the result of the
present study, that the expression of miR-181c was significantly
lower in cancer tissues of neuroblastoma patients than that in
adjacent tissues. After transfection of miR-181c into M17 cells,
the results of MTT assay showed that there was no significant
difference between the two groups in the proliferation of M17 cells
at 24 h. After 48 h, differences between the two groups were
recorded. The proliferation of M17 cells was significantly lower in
the miR-181c group than that in the blank group. We showed that
miR-181c can inhibit the proliferation of M17 cells, and obtained
the influence and evaluation of miR-181c on neuroblastoma from the
proliferation direction of M17 cells, which is a major feature of
our research. Wang et al (20) showed that miRNAs, involved in all
biological processes in the body, have a strong regulatory effect
on the normal function of cells. They participate in the occurrence
and development of multiple tumors through regulating
proliferation, migration, apoptosis and angiogenesis of tumor cells
(21). Our experimental results are
also consistent with those of Li et al (22), according to which miR-181c inhibits
cell proliferation by targeting Smad7, and its high expression in
neuroblastoma cancer cells can inhibit their proliferation and
differentiation. Studies have shown that miR-181c can be encoded in
the nucleus, processed into a mature form in the cytoplasm,
translocated into the mitochondria, thereby regulating
mitochondrial gene expression (23).
Other studies have shown that miR-181c overexpression can inhibit
the transformation factor growth signal-β expression, thereby
inhibiting cancer cell proliferation (23). This study only used a gene in
vitro to study the effects of proliferation of neuroblastoma,
but did not study its mechanism, which is a future direction of the
research.
In the present study, there are also some
limitations as the sample size was small. Also, the clinical
information was collected from neuroblastoma patients in only one
hospital, so the conclusions obtained cannot fully and reliably
demonstrate the overall clinical features and prognosis. Therefore,
more in-depth research is needed.
Collectively, miR-181c expression was found to be
low in neuroblastoma tissues, and associated with age,
differentiation degree, lymph node metastasis, distant metastasis
and INRGSS. Also, miR-181c was shown to inhibit the proliferation
of neuroblastoma M17 cells. Therefore, miR-181c has certain
clinical significance in evaluating pathogenesis, early diagnosis
and treatment of neuroblastoma patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX drafted the manuscript. XX and QH performed PCR.
XX and JW were responsible for the MTT assay and the soft agar
colony formation assay. JW and GR collected and analyzed the
patient clinical information and assisted with statistical
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Weifang People's Hospital (Weifang, China). Patients who
participated in this research had complete clinical data. Informed
consents were obtained from the patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bosse KR and Maris JM: Advances in the
translational genomics of neuroblastoma: From improving risk
stratification and revealing novel biology to identifying
actionable genomic alterations. Cancer. 122:20–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He J, Wang F, Zhu J, Zhang R, Yang T, Zou
Y and Xia H: Association of potentially functional variants in the
XPG gene with neuroblastoma risk in a Chinese population. J Cell
Mol Med. 20:1481–1490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ham J, Costa C, Sano R, Lochmann TL,
Sennott EM, Patel NU, Dastur A, Gomez-Caraballo M, Krytska K, Hata
AN, et al: Exploitation of the apoptosis-primed state of
MYCN-amplified neuroblastoma to develop a potent and specific
targeted therapy combination. Cancer Cell. 29:159–172. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Speleman F, Park JR and Henderson TO:
Neuroblastoma: A tough nut to crack. Am Soc Clin Oncol Educ Book.
35:e548–e557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shipley MM, Mangold CA and Szpara ML:
Differentiation of the SH-SY5Y human neuroblastoma cell line. J Vis
Exp. 108:531932016.
|
|
6
|
Wilczynska A and Bushell M: The complexity
of miRNA- mediated repression. Cell Death Differ. 22:22–33. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan R, Zhi Q, Zhao H, Han Y, Gao L, Wang
B, Kou Z, Guo Z, He S, Xue X, et al: Upregulated expression of
miR-106a by DNA hypomethylation plays an oncogenic role in
hepatocellular carcinoma. Tumour Biol. 36:3093–3100. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yen CS, Su ZR, Lee YP, Liu IT and Yen CJ:
miR-106b promotes cancer progression in hepatitis B
virus-associated hepatocellular carcinoma. World J Gastroenterol.
22:5183–5192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thind A and Wilson C: Exosomal miRNAs as
cancer biomarkers and therapeutic targets. J Extracell Vesicles.
5:312922016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gholamin S, Mirzaei H, Razavi SM,
Hassanian SM, Saadatpour L, Masoudifar A, Shahid Sales S and Avan
A: GD2-targeted immunotherapy and potential value of circulating
microRNAs in neuroblastoma. J Cell Physiol. 233:866–879. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maugeri M, Barbagallo D, Barbagallo C,
Banelli B, Di Mauro S, Purrello F, Magro G, Ragusa M, Di Pietro C,
Romani M, et al: Altered expression of miRNAs and methylation of
their promoters are correlated in neuroblastoma. Oncotarget.
7:83330–83341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nalavenkata SB, Sacks R, Adappa ND, Palmer
JN, Purkey MT, Feldman MD, Schlosser RJ, Snyderman CH, Wang EW,
Woodworth BA, et al: Olfactory neuroblastoma: Fate of the neck - a
long-term multicenter retrospective study. Otolaryngol Head Neck
Surg. 154:383–389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mao Y, Eissler N, Blanc KL, Johnsen JI,
Kogner P and Kiessling R: Targeting suppressive myeloid cells
potentiates checkpoint inhibitors to control spontaneous
neuroblastoma. Clin Cancer Res. 22:3849–3859. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ho WL, Hsu WM, Huang MC, Kadomatsu K and
Nakagawara A: Protein glycosylation in cancers and its potential
therapeutic applications in neuroblastoma. J Hematol Oncol.
9:1002016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohammadi M, Goodarzi M, Jaafari MR,
Mirzaei HR and Mirzaei H: Circulating microRNA: A new candidate for
diagnostic biomarker in neuroblastoma. Cancer Gene Ther.
23:371–372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fish JE, Santoro MM, Morton SU, Yu S, Yeh
RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY and Srivastava D:
miR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Png KJ, Halberg N, Yoshida M and Tavazoie
SF: A microRNA regulon that mediates endothelial recruitment and
metastasis by cancer cells. Nature. 481:190–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo J, Dong Q, Fang Z, Chen X, Lu H, Wang
K, Yin Y, Cai X, Zhao N, Chen J, et al: Identification of miRNAs
that are associated with tumor metastasis in neuroblastoma. Cancer
Biol Ther. 9:446–452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang S, Aurora AB, Johnson BA, Qi X,
McAnally J, Hill JA, Richardson JA, Bassel-Duby R and Olson EN: The
endothelial-specific microRNA miR-126 governs vascular integrity
and angiogenesis. Dev Cell. 15:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Madhavan D, Peng C, Wallwiener M, Zucknick
M, Nees J, Schott S, Rudolph A, Riethdorf S, Trumpp A, Pantel K, et
al: Circulating miRNAs with prognostic value in metastatic breast
cancer and for early detection of metastasis. Carcinogenesis.
37:461–470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Wang H, Li J and Yue W: MiR-181c
modulates the proliferation, migration, and invasion of
neuroblastoma cells by targeting Smad7. Acta Biochim Biophys Sin
(Shanghai). 46:48–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Das S, Bedja D, Campbell N, Dunkerly B,
Chenna V, Maitra A and Steenbergen C: miR-181c regulates the
mitochondrial genome, bioenergetics, and propensity for heart
failure in vivo. PLoS One. 9:e968202014. View Article : Google Scholar : PubMed/NCBI
|