Introduction
Oral cancer is one of the most common cancers in the
head as well as neck region, and is a major public health issue
worldwide. It develops on the tongue, lips, gum, salivary glands,
buccal mucosa, palate, and even the floor of mouth. There were
300,000 estimated new cases in 2012 worldwide, with two thirds
developing in men (1). Although oral
cancer is ranked as the 15th most common cancer, it is often not
diagnosed until a very advanced stage and the lethality of 50% has
caused much concern (1). Moreover,
recent data suggested that the incidence of oral cancer has highly
increased in the last two decades (2,3). The
vast majority of oral cancer is derived from the multi-layered
squamous epithelium and is referred to as oral squamous cell
carcinoma (OSCC). Three major lifestyle factors, namely areca nut
chewing, alcohol consumption, and smoking are generally considered
as main causative factors for oral cancer. Genetic confounders,
however, are of great interest but remain unclear.
Polymorphisms are prevalent genetic loci, which play
a vital role during the process of human traits. Single-nucleotide
polymorphisms (SNPs) are low penetrate genetic variants, which are
found to be associated with cancer susceptibility (4). DNA repair genes prevent the genome from
being damaged, which are due to endogenic or environmental
carcinogens. The defect in DNA repair system is proved to induce
pre-cancerous lesions and cancers (5). In this regard, it is natural to make
the hypothesis that part of the genetic variants with DNA repairing
genes may influence the DNA repair ability and infer susceptibility
of oral cancer. X-ray repair cross-complementing group 3
(XRCC3), located mainly on chromosome 14q32.3, belongs to
the RAD51 family. It has been proven to participate in double
strand break and homologous recombination repair (6). It is one of the most important genes
related to DNA repair. The Thr241Met (18607C/T, rs861539)
polymorphism, a C/T substitution in exon seven, has resulted in a
Thr-to-Met amino-acid alteration. It has drawn wide attention as
its variant influences repair capacity for DNA damage, and has been
confirmed to be related to increased risk of various cancers
(7–9). Moreover, previous research has
investigated the association regarding Thr241Met polymorphism to
the risk of oral cancer. However, the data are inconclusive
(10–16). For example, the 241Met allele showed
a >3-fold increased risk for oral cancer in Thai population
(10), while no significant
association was identified in Brazilian patients (13). This discrepancy may be due to various
aspects. From a statistical perspective, studies with relatively
inadequate sample sizes have low power to identify low penetric
genetic variants. On the other hand, the lack of reproducibility
might also be due to discrepant genetic or lifestyle backgrounds.
To solve the inconsistence of previous studies and verify the
association of XRCC3 Thr241Met polymorphisms and oral cancer
risk meta-analysis was conducted using the most recent published
datasets.
Materials and methods
Search strategy for identification of
associated studies
An extensive literature review was conducted by
using Embase, Medline, PubMed, Wanfang and CNKI online resources.
Related papers published from January 2000 to September 2017 were
selected. In order to include both English and Chinese-language
articles, the keywords ‘XRCC3 or X-ray repair
cross-complementing protein 3’, ‘polymorphism or SNP’, ‘oral cancer
or oral squamous cell carcinoma’ and their combinations were
utilized as well as the relevant Chinese characters were chosen in
searches of the Chinese database. To filter and include more
related articles, bibliography listed in the retrieved papers was
manually searched. If there were multiple articles from the same
authors or research groups, the most recent data or the largest
datasets were included.
Inclusion and exclusion criteria
All the selected studies had to be covered by the
following inclusion criteria: case-and-control studies that focused
on the association study of XRCC3 Thr241Met polymorphisms
regarding oral cancer risk. Distribution of genotypes and allele
information of patients or controls were accessible, or with the
odds ratio (OR) with its 95% confidence intervals (CIs). The
criteria of exclusion included: review articles or abstracts from
conferences; studies without control group or data were not
available to be extracted.
The study was approved by the Ethics Committee of
the Second Affiliated Hospital of Soochow University (Suzhou,
China). Patients or their guardians signed an informed consent.
Statistical analysis
In the present meta-analysis, XRCC3 Thr241Met
polymorphism between patients and controls was compared by the
unadjusted ORs and 95% CIs. Significance of the pooled ORs is
indicated and tested via Z-test. The Mantel-Haenszel model using
the χ2 test was performed to estimate heterogeneity
between different groups. The following five genetic models were
performed to assess the association between the Thr241Met
polymorphism and oral cancer: allele model (Met vs. Thr),
homozygous model (Met/Met vs. Thr/Thr), dominant model (Met/Met +
Thr/Met vs. Thr/Thr), heterozygous model (Met/Thr vs. Thr/Thr) and
recessive model (Met/Met vs. Thr/Met + Thr/Thr). Between-study
heterogeneity was captured by the inconsistency index I2
test along with the statistic Q-test. Fixed effect model was
employed in case of homologous effects (P>0.01 and I2
<50%). Random-effect model was utilized when the effects were
heterogeneous (P<0.01 and I2 >50%). Egger
regression asymmetry test and funnel plots were utilized to
validate publication bias. Probability ≤0.05 was considered as
significant with the exception of the I2 statistic and
Egger's test, when a significant level of <0.1 was applied.
STATA version 11.0 for Windows was used for statistical analyses
and data management.
Data extraction and quality
assessment
Two independent investigators performed extraction
of data. Disagreements were solved by adjustments of the third
investigator through achieving a consensus. The following
information was included: year of publication, the first author,
sample size, country, genotyping methods, ethnicity, the
Hardy-Weinberg equilibrium (HWE) in controls, and allele and
genotype distribution.
Results
Study identification and
inclusion
In summary, 15 relevant studies were kept after the
above-mentioned searches. Using the inclusion and exclusion
criteria, finally 7 studies were selected, which include 1,615 oral
cancer cases and 1,897 related controls. The 7 articles were from
studies of five countries and regions, mainly involving two
ethnicities (3 groups of Caucasians and 4 groups of Asians). Sample
sizes of the data ranged from 92 to 1744. Polymerase chain reaction
restriction fragment length polymorphism (PCR-RFLP) method was
applied for SNP genotype. Detailed information is provided in
Table I.
 | Table I.Main characteristics of included
studies in this meta-analysis. |
Table I.
Main characteristics of included
studies in this meta-analysis.
|
|
|
|
|
|
Genotype
and allele information |
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
|
|
|
| Sample size | Cases | Controls |
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| First authors
(Refs.) | Year | Country | Cases | Controls | CC | CT | TT | C | T | CC | CT | TT | C | T | HWE | Gentotyping
methods |
|---|
| Avci et al
(14) | 2017 | Turkey | 111 | 148 | 34 | 25 | 52 | 93 | 129 | 28 | 81 | 39 |
137 | 159 | 0.22 | PCR-RFLP |
| Kietthubthew et
al (10) | 2006 | Thailand | 106 | 164 | 83 | 22 | 1 |
188 | 24 | 140 | 23 | 1 |
303 | 25 | 0.96 | PCR-RFLP |
| Majumder et
al (15) | 2005 | India | 310 | 348 | 201 | 97 | 12 |
499 | 121 | 220 | 120 | 8 |
560 | 136 | 0.07 | PCR-RFLP |
| Dos Reis et
al (13) | 2012 | Brazil | 144 | 144 | 63 | 72 | 9 |
198 | 90 | 52 | 78 | 14 |
182 | 106 | 0.05 | PCR-RFLP |
| dos Santos Pereira
et al (11) | 2015 | Brazil | 53 | 39 | 10 | 34 | 9 |
54 | 52 | 15 | 20 | 4 | 50 | 28 | 0.48 | PCR-RFLP |
| Tsai et al
(16) | 2014 | China | 788 | 956 | 684 | 92 | 12 | 1460 | 116 | 878 | 73 | 5 | 1829 | 83 | 0.01 | PCR-RFLP |
| Yang et al
(12) | 2015 | China | 103 | 98 | 96 | 7 | 0 |
199 |
7 | 89 |
9 | 0 |
187 |
9 | 0.63 | PCR-RFLP |
Association of XRCC3 Thr241Met SNP
regarding overall oral cancer risk
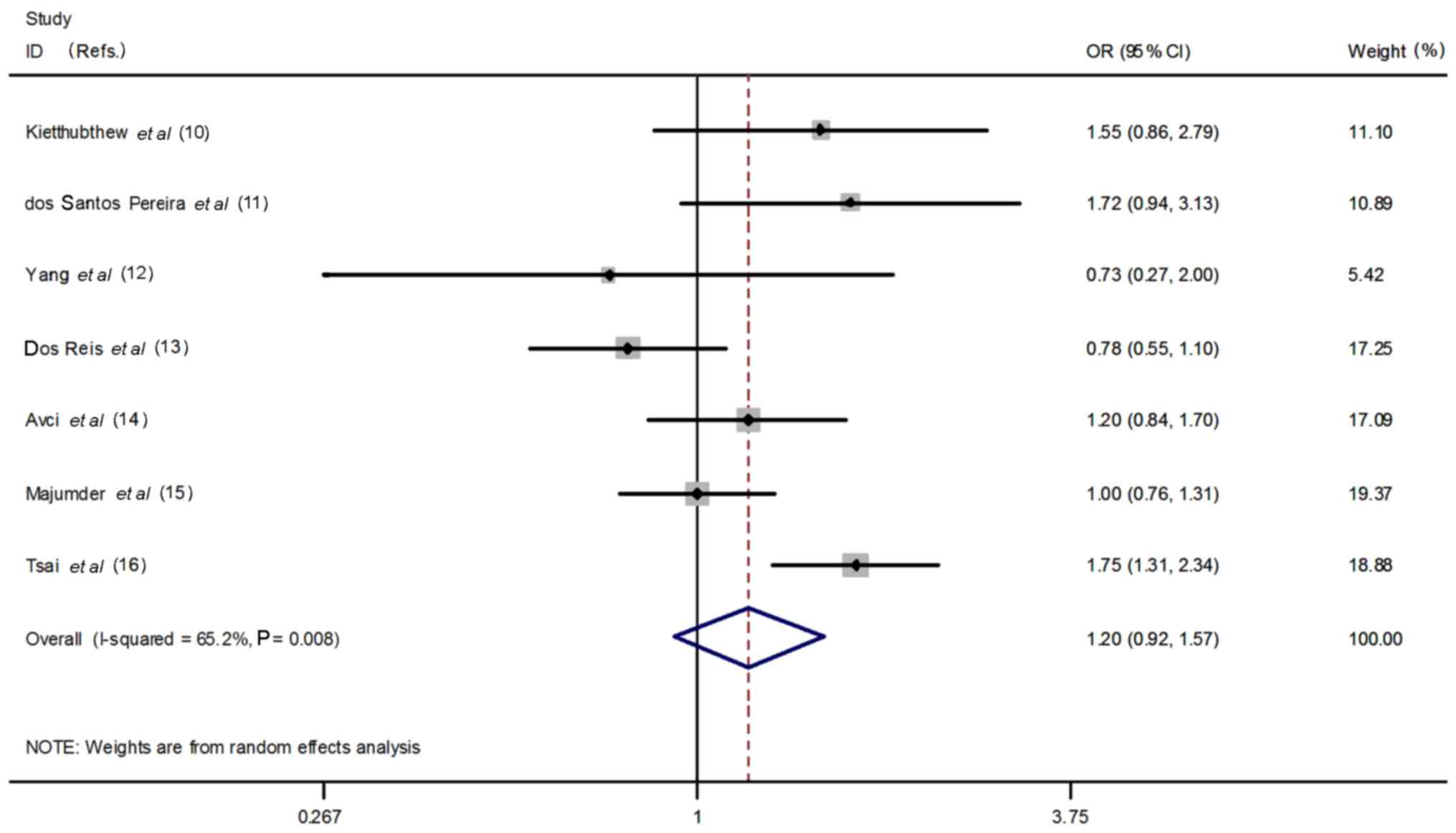
There are seven articles that contain 1,615 oral
cancer patients and 1,897 controls. Heterogeneity of the involved
literature was analyzed. The fixed effect and random effect models
were respectively utilized. Despite the fact that T allele
frequency is rather high in patients comparing with controls (16.7%
vs. 14.4%), our analyses did not infer a significant association
between the T allele and oral cancer susceptibility (T vs. C:
OR=1.20, 95% CI=0.92–1.57, P=0.18) by applying the random effect
model (Fig. 1). Neither was
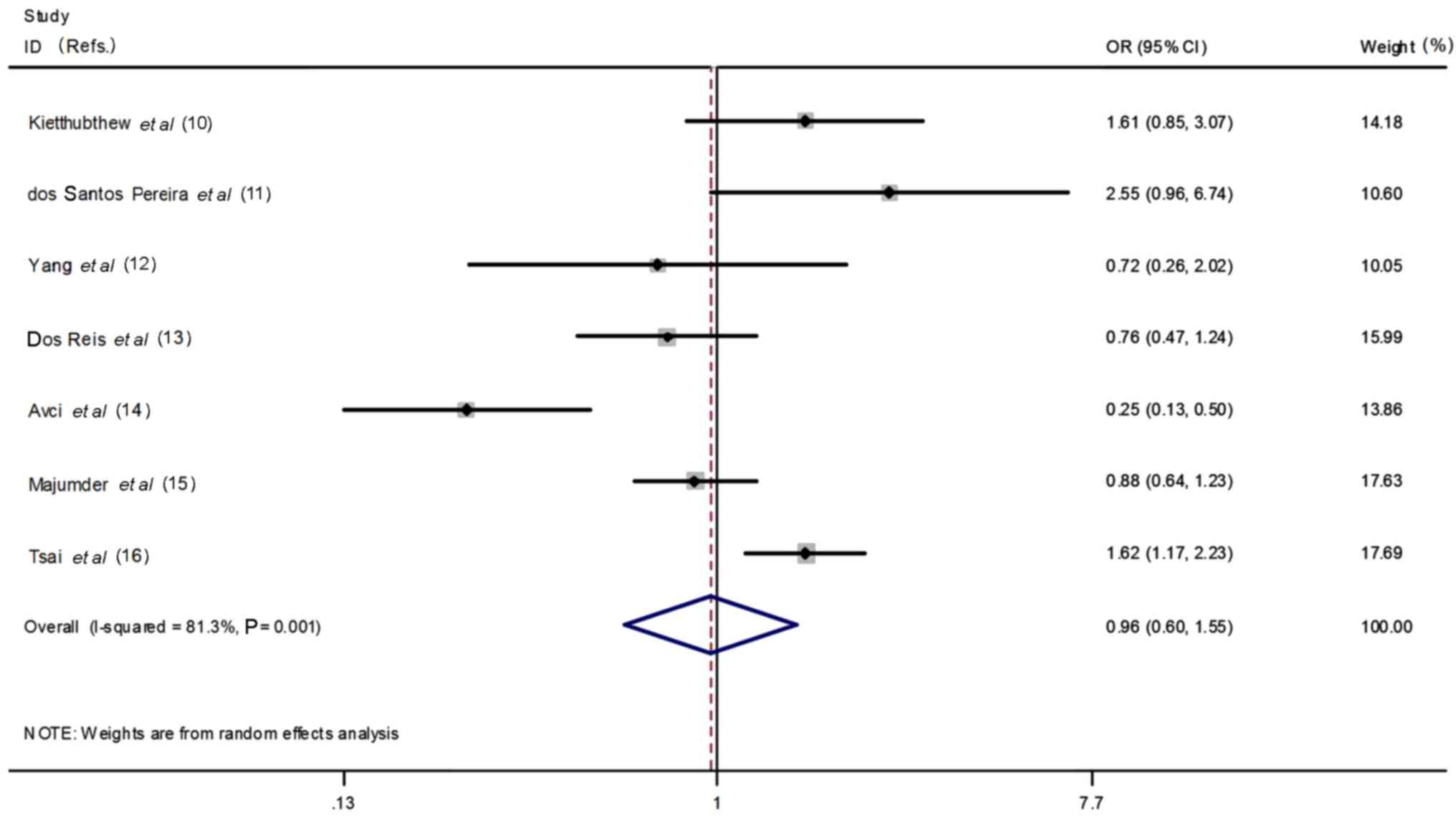
significance identified by using heterozygous model (CT vs. CC:
OR=0.96, 95% CI=0.60–1.55, heterogeneity P=0.001,
I2=81.3%, Fig. 2),
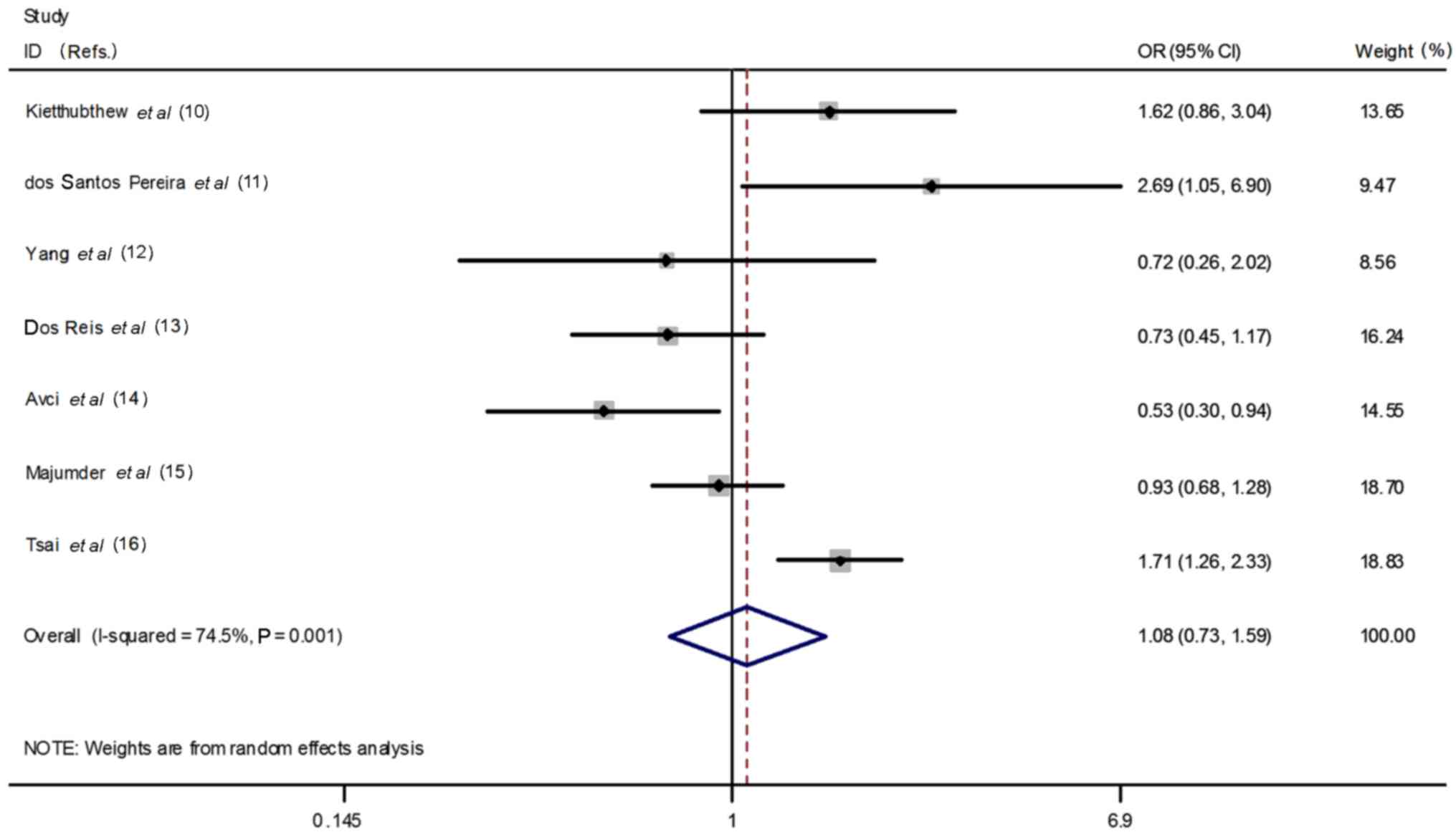
dominant model (CT + TT vs. CC: OR=1.08, 95% CI=0.73–1.59,
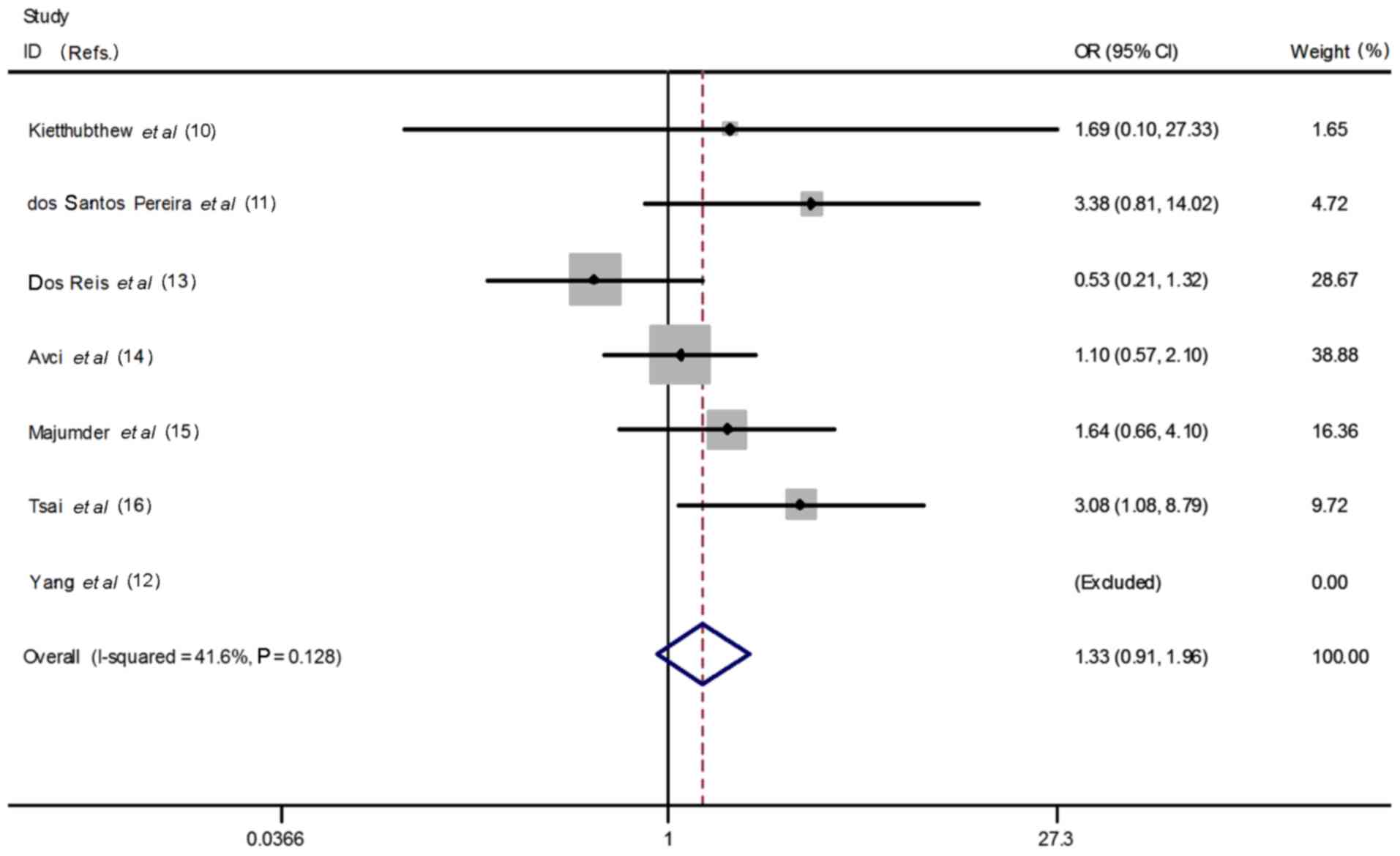
heterogeneity P=0.001, I2=74.5%, Fig. 3); homozygous model (CC vs. TT:
OR=1.33, 95% CI=0.91–1.96, heterogeneity P=0.13,
I2=41.6%, Fig. 4);
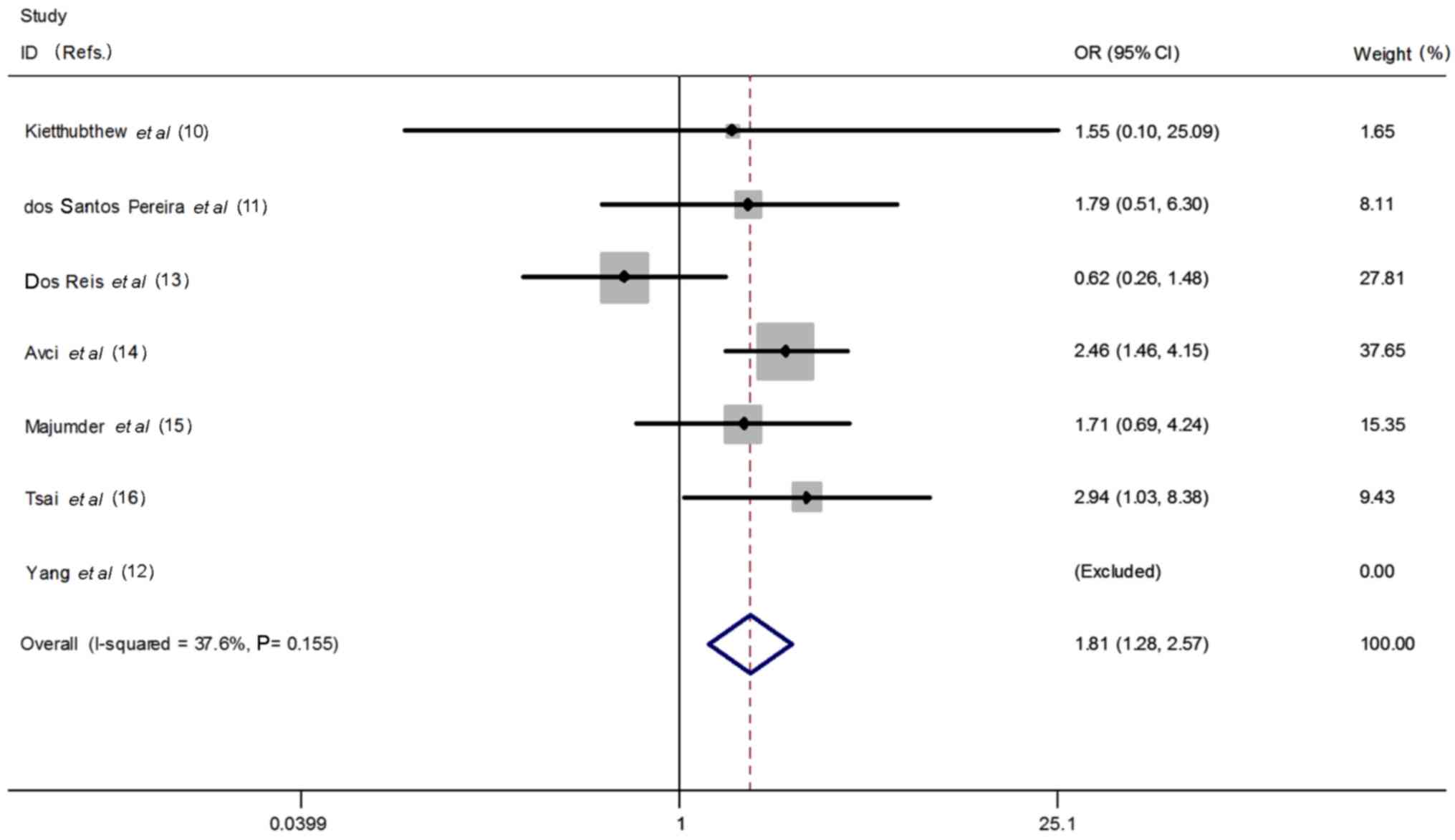
However, as shown in Fig. 5, a
significant relationship was identified for the recessive model. TT
genotype was positively associated with oral cancer risk (TT vs. CT
+ CC: OR=1.81, P=0.001, 95% CI=1.28–2.57, heterogeneity P=0.155,
I2=37.6%).
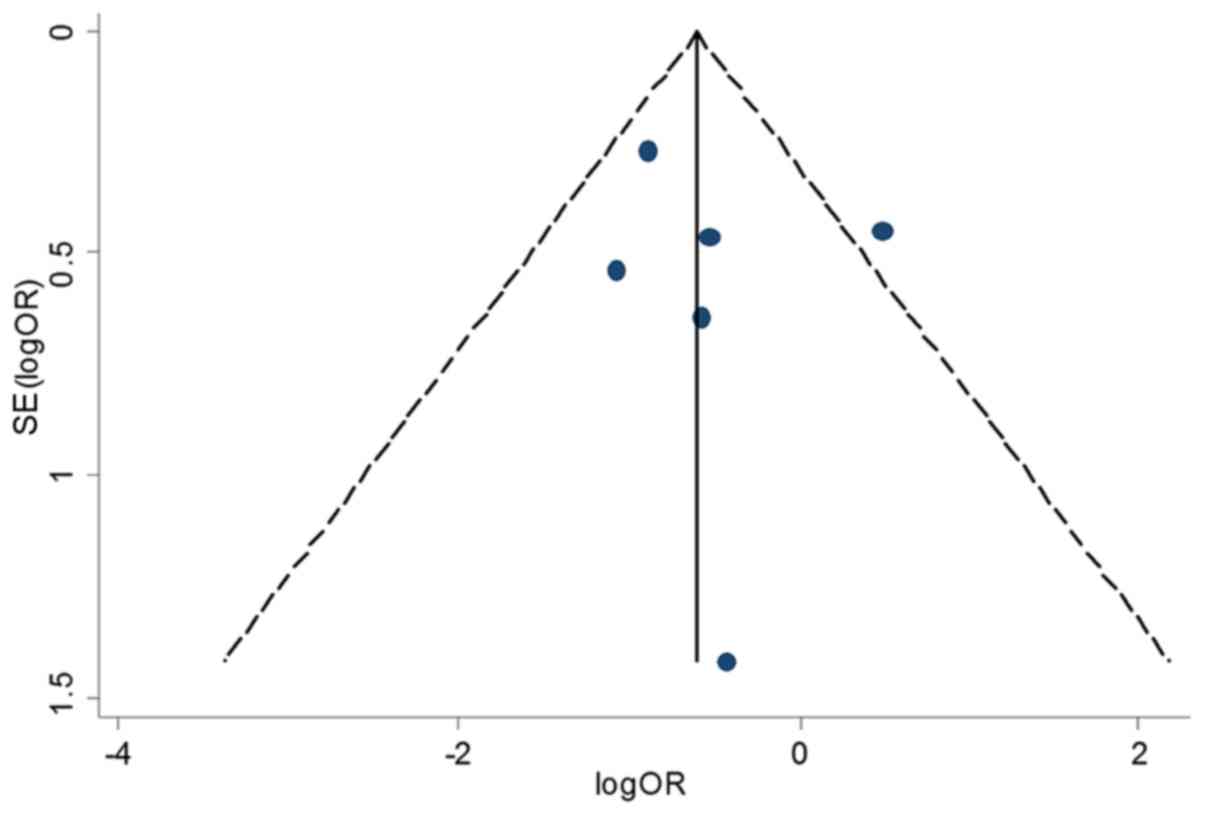
Publication bias and sensitivity
analysis
As there was substantial heterogeneity among studies
during the process of comprehensive comparisons, sensitivity
analyses were performed to indicate the effect of each single data
against the aggregated ORs. The recalculated ORs are not
substantially influenced, suggesting that our results are reliable
and they verify the hardiness of the present investigation. The
Egger's test and funnel plots were applied to validate potential
bias from the published literature. Shape of the funnel plots is
approximately symmetrical. Egger's test result shows no statistical
evidence for bias (P>0.05), indicating that there is no obvious
bias in this meta-analysis based on the searched publications and
the results are plausible (Fig.
6).
Stratified analyses by ethnicity and
areca nut chewing
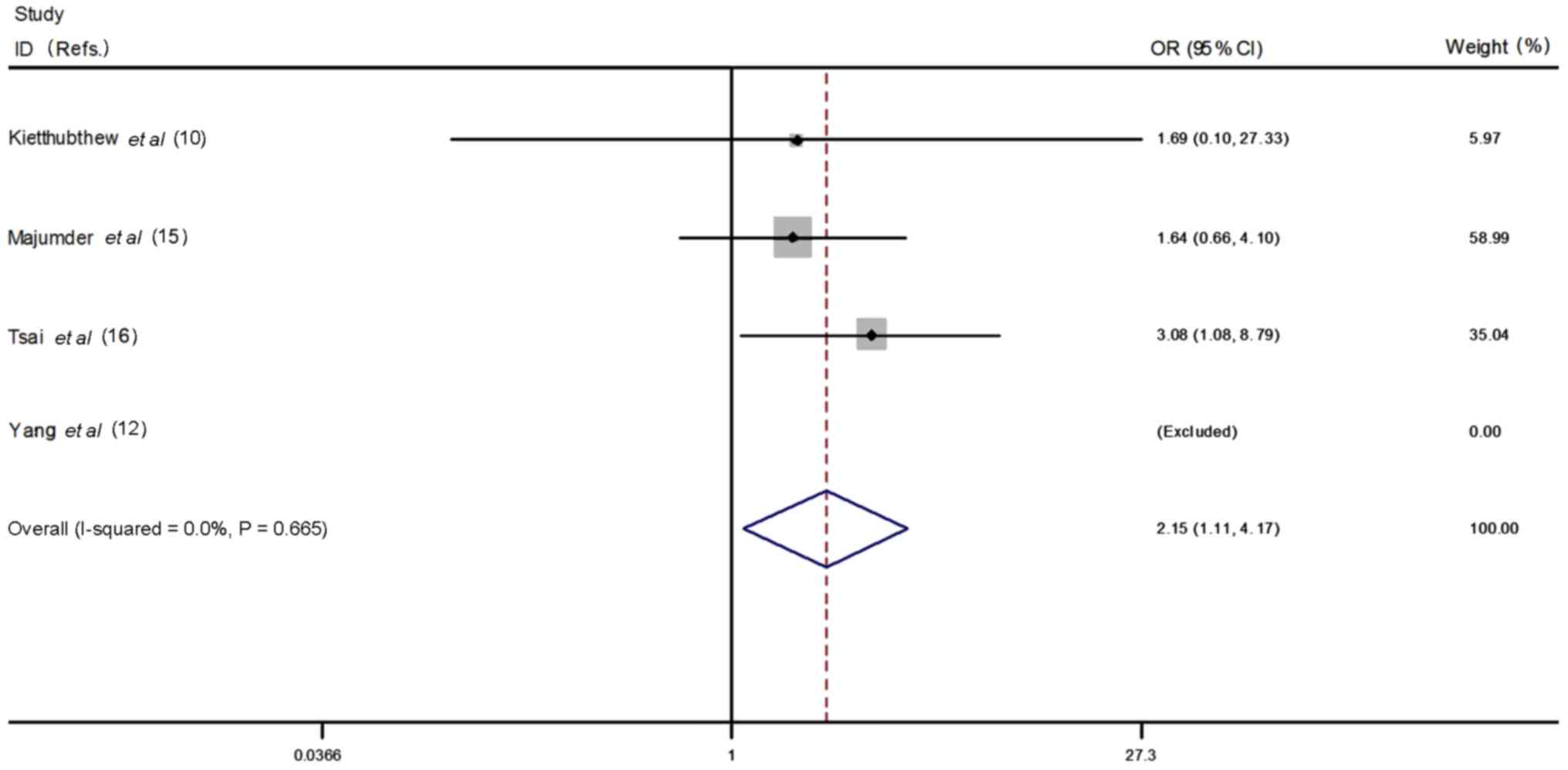
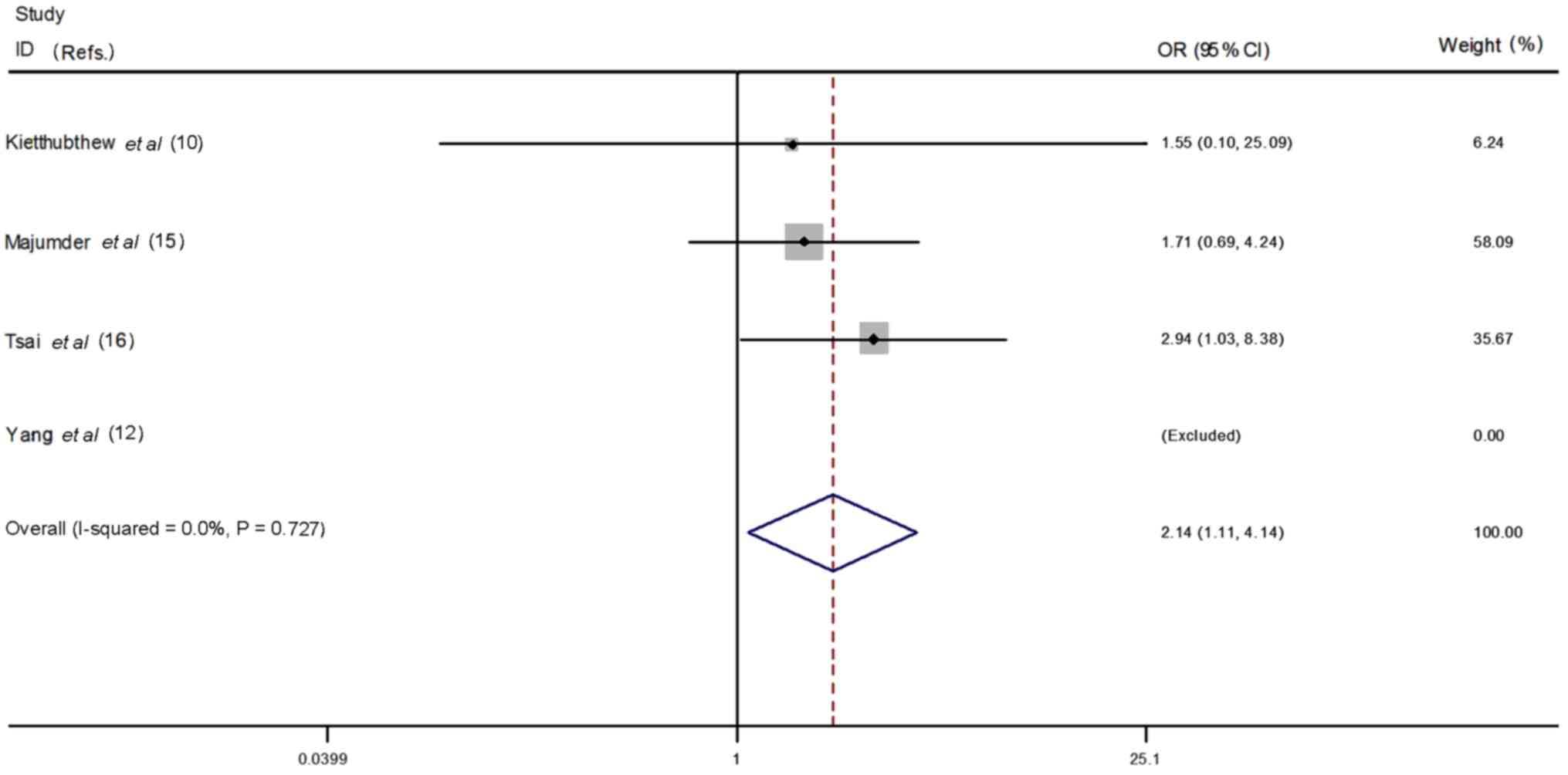
In the ethnicity stratified analysis, more
significant associations were detected in Asian population.
(Asians: homozygous model CC vs. TT: OR=2.15, 95% CI=1.11–4.17,
P=0.02, heterogeneity P=0.67, I2=0.0%, Fig. 7; recessive model CT + CC vs. TT:
OR=2.14, 95% CI=1.11–4.14, P=0.02, heterogeneity P=0.78,
I2=0.0%, Fig. 8;
heterozygous model CC vs. CT: OR=1.20, 95% CI=0.80–1.81, P=0.38,
heterogeneity P=0.04, I2=63.9%; dominant model CC vs. TT
+ CT: OR=1.25, P=0.29, 95% CI=0.83–1.88, heterogeneity P=0.03,
I2=66.2%; allele C vs. T: OR=1.28, 95% CI=0.87–1.88,
P=0.21, heterogeneity P=0.03, I2=67.5%). No significant
association were identified in Caucasians: (homozygous model CC vs.
TT: OR=1.09, 95% CI=0.47–2.53, P=0.84, heterogeneity P=0.09,
I2=57.7%; recessive model CT + CC vs. TT: OR=1.43, 95%
CI=0.57–3.61, P=0.45, heterozygous model CC vs. CT: OR=0.75, 95%
CI=0.25 - 2.27, P=0.61, heterogeneity P=0.001, I2=87.1%;
dominant model CC vs. TT + CT: OR=0.92, P=0.82, 95% CI=0.92–1.95,
heterogeneity P=0.02, I2=66.2%; heterogeneity P=0.03,
I2=71.9%; allele C vs. T: OR=1.11, 95% CI=0.74–1.69,
P=0.61, heterogeneity P=0.05, I2=66.4%). Three studies
included areca nut chewing for stratified analysis, which allowed
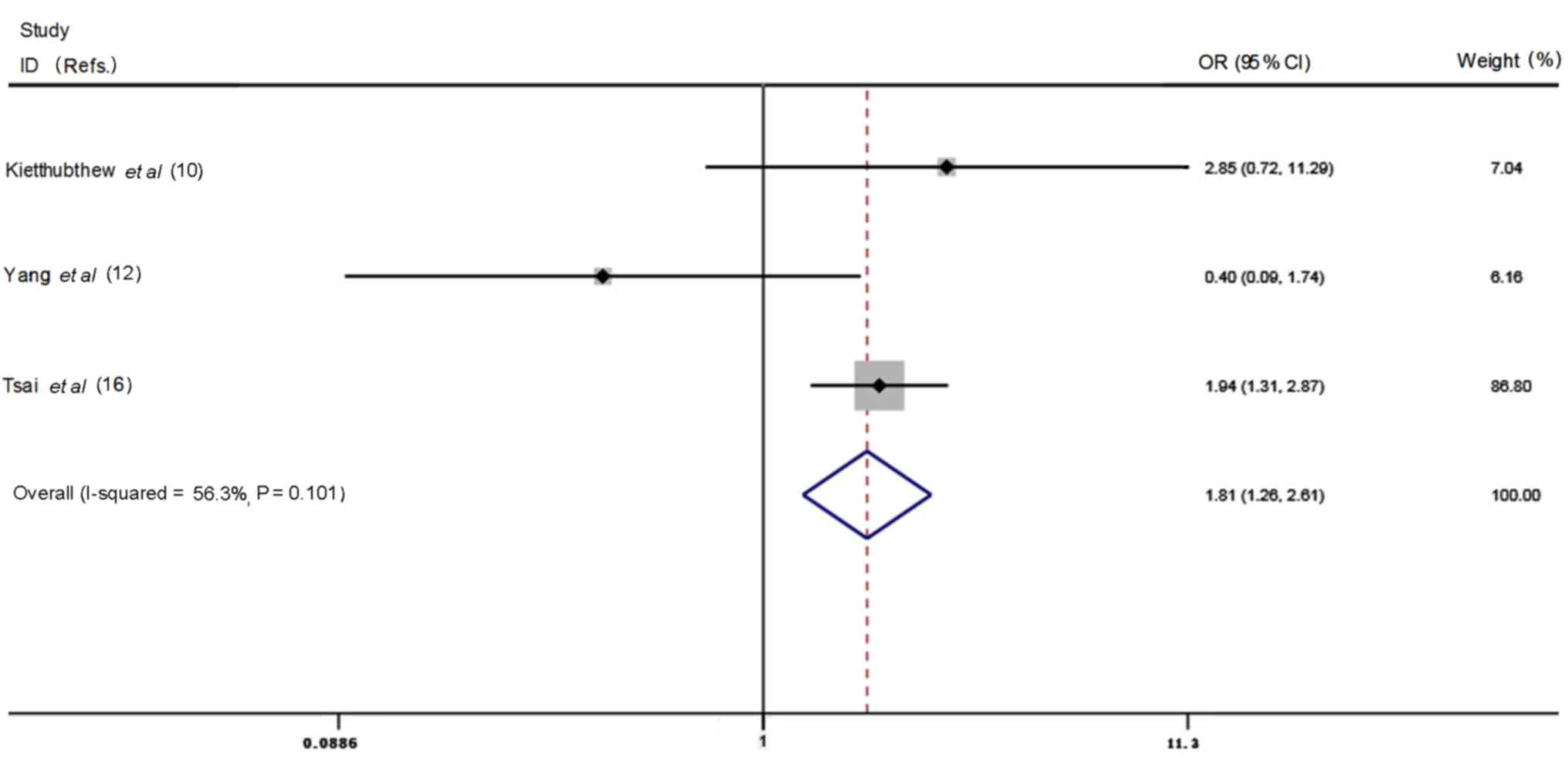
for further analysis (Table II).
There was no significant association between the T allele carrier
and oral cancer risk in people without areca nut chewing habit
(OR=1.40, 95% CI=0.81–2.43, p=0.23, heterogeneity P=0.30,
I2=16.9%). In the areca nut chewers, T allele carrier
had significantly higher risk of oral cancer (OR=1.81, 95%
CI=1.26–2.61, P=0.001, heterogeneity P=0.10, I2=56.3%,
Fig. 9).
 | Table II.Association of areca nut chewing,
XRCC3 Thr241Met polymorphism and oral cancer risk. |
Table II.
Association of areca nut chewing,
XRCC3 Thr241Met polymorphism and oral cancer risk.
| Life style First
authors (Refs.) | Genotypes | Adjusted OR | 95% CI | P-value |
|---|
| No areca nut
chewing |
|
|
|
|
|
Kietthubthew et al
(10) | CT/TT |
2.61 | 0.97–7.11 | 0.06 |
| Yang
et al (12) | CT/TT | 0.5 | 0.0–11.5 | N/A |
| Tsai
et al (16) | CT/TT | 1.1 | 0.56–2.17 | 0.86 |
| Areca nut
chewing |
|
|
|
|
|
Kietthubthew et al
(10) | CT/TT |
2.85 | 0.72–11.3 | 0.14 |
| Yang
et al (12) | CT/TT | 0.4 | 0.1–1.9 | N/A |
| Tsai
et al (16) | CT/TT |
1.94 | 1.31–2.87 |
0.001 |
Discussion
DNA repairing systems play an indispensable role in
protecting cells against carcinogenic agents. It is largely
accepted that environmental carcinogens would stimulate DNA damage,
which would then induct genomic instability (17). In this manner, alternations in DNA
repairing genes may potentially influence the susceptibility of
each disease, which would affect therapy as well as prognosis
(18–20). In the current study, we verified the
association between XRCC3 Thr241Met polymorphism and oral
cancer risk. Results illustrated that TT (Met/Met) genotype was
associated with high risk of oral cancer through the recessive
model (P=0.001). Further stratification analyses demonstrated that
the Met/Met genotype was significantly associated with oral cancer
under both recessive and homozygous models in Asians (P<0.05).
In contrast, no association was detected regarding XRCC3
Thr241Met polymorphisms to oral cancer risk in non-Asians.
XRCC3, a paralog of RAD51 protein, functions
in homologous recombination repair (HRR) pathway and maintaining
genomic stability in mammalian cells. The Thr241Met polymorphism
resides in an evolutionary conserved region, the adenosine
triphosphate-binding domain, which is the exclusive region with
certain functional activity of XRCC3 protein (21). The Thr241Met polymorphism is
suggested to have a possible mechanic function in the protein
structure (22). For instance,
XRCC3 241Met has been shown to be functionally deficient in
repairing X-ray inducted chromosome aberrations, which infer that
the genotyping of the variant is defective in the base excision
repair (BER) (23). Several
epidemiological studies have indicated that there was an
association between XRCC3 polymorphisms and oral cancer
susceptibility, whereas a large amount of results remained
contradictory. In the current study, the Met/Met genotype of
Thr241Met polymorphism associated with high risk oral cancer was
validated, which is consistent with previous functional studies.
People with TT(Met/Met) genotype may have a defect in DNA repair
function thus they are more susceptible to cancer, while those who
have one and/or two Thr allele may have appropriate function which
protects against cancer (23).
People with homozygous of Met allele and defect in HRR function may
be more susceptible to cancer, while those who have one and/or two
Thr allele may have normal function which protects them against
cancer. Moreover, by analysis of gene-environment interactions,
comparison of the CC genotype demonstrated that the Met allele
carriers had significantly increased risk of oral cancer in areca
nut chewers, but not in those without this habit. As areca nut
chewing may induce mitochondrial DNA mutation and oxidative DNA
damage (24,25), and people with the XRCC3 241Met
allele and functional deficient in repair of DNA mutations may be
susceptible to development of cancer. However, we were not able to
perform further analysis with limited information regarding
smoking, alcohol drinking and genotyping.
So far, there is only one meta-analysis report
regarding the association of XRCC3 Thr241Met polymorphism to
oral cancer risk reporting that XRCC3 Thr241Met polymorphism
is irrelevant to the risk of oral cancer (26). However, the authors included upper
aero-digestive cancer and leukoplakia, a common precancer lesion,
in their association analysis. Biology may differ from tumors of
different origins, for example, Benhamou et al (27) found that there is no significant
association between the XRCC3 polymorphism and cancer of the
upper aerodigestive tract. Analysis of widely determined cancer
sites may lead to inconclusive results. In our analysis, more
focused research on homogeneous cancer sites have reduced
variations and are able to find a moderate association between
XRCC3 Thr241Met polymorphism and oral cancer risk.
It is also important to note that the distributions
of SNPs are highly different in various ethnic populations.
Therefore, ethnicity may be a confounder. Previous association
studies were performed in different populations, which may raise
the discrepancy. For instance, the distribution of the XRCC3
Thr241Met variant genotypes (Thr/Met and Met/Met) was 14.0 and
0.6%, respectively, in the Thai population (10). The distribution was 20.0 and 4.0%,
respectively, in Brazil population (11); 34.5 and 2.3% in Indian population
(15); 54.7 and 26.4% in Turkish
population (14); 7.6 and 0.5% in
Taiwan Chinese (16). From these
results, it could be speculated that Chinese population may be more
resistant to environmental carcinogens due to the fact that the
population carry the Thr allele more frequently comparing with
other populations. On the other hand, both Brazilian and Turkish
populations are highly heterogeneous populations, with a mixture of
European, Asian and African populations. In our study,
stratification analysis illustrated that there was no significant
association regarding XRCC3 Thr241Met polymorphism to oral
cancer risk in these populations, indicating ethnic heterogeneity
may affect the overall result of association studies.
Preliminary data show that the variant Met allele
affects the development of oral cancer. The Met/Met genotype is
also associated with at least 8-fold increase of the risk for
developing metastases. With respect to clinical diagnosis and
practice, the Met/Met genotype was associated with a fairly high
risk of developing oral cancer at either stage III or IV (11). In addition, the genotype of
XRCC3 Thr241Met polymorphism was reported to be associated
with tumor size (14). However,
further studies using large populations are required to confirm
these findings.
Finally, the potential of a polymorphism to impair
DNA repair activity may be affected by other polymorphic alleles
interfering in the process. For example, those nucleotide excision
repairing genes for repair of smoking-induced DNA damage were not
included in this study. We could not rule out a possibility that
the Thr241Met polymorphism is within linkage disequilibrium regions
with polymorphic genotype of other DNA repair genes. Therefore,
further investigations exploring main contribution factors will be
performed in the future to better understand the susceptibility
phenomenon regarding oral cancer.
We suggest that the homozygote variants of Thr241Met
polymorphism regarding XRCC3 possibly act as factors for
oral cancer risk. Moreover, the increased oral cancer risk was more
obviously enhanced in Asians, but not in other populations. This
study provides insight into whether DNA repair gene polymorphisms
play an indispensable role in changing individual susceptibility to
oral cancer. Further validations of gene-environment and gene-gene
connections are necessary to explore the effects of XRCC3
Thr241Met polymorphisms on oral cancer.
Acknowledgements
We sincerely thank Mr. Jianxin Yang from the Second
Affiliated Hospital of Soochow University (Suzhou, China), for his
support with respect to the drafting of the manuscript.
Funding
This work was partly supported by funding of the
China National Natural Science Foundation (no. 31100944) and the
funding from Natural Science Foundation, Jiangsu Province, China
(no. BK2009107).
Availability of data and materials
The data generated and/or analyzed in the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JF drafted the manuscript. JF and WL performed
quality assessment and data classification. MZ and CX conducted
statistical analysis. All the authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Second Affiliated Hospital of Soochow University (Suzhou,
China). Patients or their guardians signed an informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
XRCC3
|
X-ray repair cross complementing group
3
|
|
SNPs
|
Single-nucleotide polymorphisms
|
|
CNKI
|
China National Knowledge
Infrastructure
|
|
OSCC
|
oral squamous cell carcinoma
|
|
OR
|
odds ratio
|
|
CI
|
confidence interval
|
|
PCR-RFLP
|
polymerase chain reaction restriction
fragment length polymorphism
|
|
HRR
|
homologous recombination repair
|
|
HWE
|
Hardy-Weinberg equilibrium
|
|
BER
|
base excision repair
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chaturvedi AK, Anderson WF,
Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, Rosenberg PS,
Bray F and Gillison ML: Worldwide trends in incidence rates for
oral cavity and oropharyngeal cancers. J Clin Oncol. 31:4550–4559.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
GBD 2013 Mortality and Causes of Death
Collaborators: Global, regional, and national age-sex specific
all-cause and cause-specific mortality for 240 causes of death,
1990–2013: A systematic analysis for the Global Burden of Disease
Study 2013. Lancet. 385:117–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen MK, Chiou HL, Su SC, Chung TT, Tseng
HC, Tsai HT and Yang SF: The association between hypoxia inducible
factor-1alpha gene polymorphisms and increased susceptibility to
oral cancer. Oral Oncol. 45:e222–e226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugimura T, Kumimoto H, Tohnai I, Fukui T,
Matsuo K, Tsurusako S, Mitsudo K, Ueda M, Tajima K and Ishizaki K:
Gene-environment interaction involved in oral carcinogenesis:
Molecular epidemiological study for metabolic and DNA repair gene
polymorphisms. J Oral Pathol Med. 35:11–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Risch N and Merikangas K: The future of
genetic studies of complex human diseases. Science. 273:1516–1517.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bănescu C, Tilinca M, Benedek EL, Demian
S, Macarie I, Duicu C and Dobreanu M: XRCC3 Thr241Met polymorphism
and risk of acute myeloid leukemia in a Romanian population. Gene.
526:478–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang L, An Y, Wang G, Lu T and Yang S:
Association between XRCC3 Thr241Met polymorphism and risk of
osteosarcoma in a Chinese population. Int J Clin Exp Pathol.
8:11670–11674. 2015.PubMed/NCBI
|
|
9
|
Michalska MM, Samulak D, Romanowicz H,
Jabłoński F and Smolarz B: Association between single nucleotide
polymorphisms (SNPs) of XRCC2 and XRCC3 homologous recombination
repair genes and ovarian cancer in Polish women. Exp Mol Pathol.
100:243–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kietthubthew S, Sriplung H, Au WW and
Ishida T: Polymorphism in DNA repair genes and oral squamous cell
carcinoma in Thailand. Int J Hyg Environ Health. 209:21–29. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
dos Santos Pereira J, Fontes FL, de
Medeiros SR, de Almeida Freitas R, de Souza LB and da Costa Miguel
MC: Association of the XPD and XRCC3 gene polymorphisms with oral
squamous cell carcinoma in a Northeastern Brazilian population: A
pilot study. Arch Oral Biol. 64:19–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang CH, Lin YD, Yen CY, Chuang LY and
Chang HW: A systematic gene-gene and gene-environment interaction
analysis of DNA repair genes XRCC1, XRCC2, XRCC3, XRCC4, and oral
cancer risk. OMICS. 19:238–247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dos Reis MB, Losi-Guembarovski R, de Souza
Fonseca Ribeiro EM, Cavalli IJ, Morita MC, Ramos GH, de Oliveira
BV, Mizuno LT, Rogatto SR and de Syllos Cólus IM: Allelic variants
of XRCC1 and XRCC3 repair genes and susceptibility of oral cancer
in Brazilian patients. J Oral Pathol Med. 42:180–185. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Avci H, Ergen A, Bireller ES, Ertugrul B
and Cakmakoglu B: A strong relationship between oral squamous cell
carcinoma and DNA repair genes. Biochem Genet. 55:378–386. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Majumder M, Sikdar N, Paul RR and Roy B:
Increased risk of oral leukoplakia and cancer among mixed tobacco
users carrying XRCC1 variant haplotypes and cancer among smokers
carrying two risk genotypes: One on each of two loci, GSTM3 and
XRCC1 (Codon 280). Cancer Epidemiol Biomarkers Prev. 14:2106–2112.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsai CW, Chang WS, Liu JC, Tsai MH, Lin CC
and Bau DT: Contribution of DNA double-strand break repair gene
XRCC3 genotypes to oral cancer susceptibility in Taiwan. Anticancer
Res. 34:2951–2956. 2014.PubMed/NCBI
|
|
17
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Werbrouck J, De Ruyck K, Duprez F,
Veldeman L, Claes K, Van Eijkeren M, Boterberg T, Willems P, Vral
A, De Neve W, et al: Acute normal tissue reactions in head-and-neck
cancer patients treated with IMRT: Influence of dose and
association with genetic polymorphisms in DNA DSB repair genes. Int
J Radiat Oncol Biol Phys. 73:1187–1195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
da Silva BS, Rovaris DL, Bonotto RM, Meyer
JB, Grohe RE, Perassolo MS, Palazzo RP, Maluf SW, Linden R and de
Andrade FM: The influence on DNA damage of glycaemic parameters,
oral antidiabetic drugs and polymorphisms of genes involved in the
DNA repair system. Mutagenesis. 28:525–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang WS, Tsai CW, Wang JY, Ying TH, Hsiao
TS, Chuang CL, Yueh TC, Liao CH, Hsu CM, Liu SP, et al:
Contribution of X-ray repair complementing defective repair in
Chinese hamster cells 3 (XRCC3) genotype to leiomyoma risk.
Anticancer Res. 35:4691–4696. 2015.PubMed/NCBI
|
|
21
|
Manuguerra M, Saletta F, Karagas MR,
Berwick M, Veglia F, Vineis P and Matullo G: XRCC3 and XPD/ERCC2
single nucleotide polymorphisms and the risk of cancer: A HuGE
review. Am J Epidemiol. 164:297–302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Werbrouck J, De Ruyck K, Duprez F, Van
Eijkeren M, Rietzschel E, Bekaert S, Vral A, De Neve W and Thierens
H: Single-nucleotide polymorphisms in DNA double-strand break
repair genes: Association with head and neck cancer and interaction
with tobacco use and alcohol consumption. Mutat Res. 656:74–81.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Au WW, Salama SA and Sierra-Torres CH:
Functional characterization of polymorphisms in DNA repair genes
using cytogenetic challenge assays. Environ Health Perspect.
111:1843–1850. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan DJ, Chang J, Chen WL, Agress LJ, Yeh
KT, Wang B and Wong LJ: Novel heteroplasmic frameshift and missense
somatic mitochondrial DNA mutations in oral cancer of betel quid
chewers. Genes Chromosomes Cancer. 37:186–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen CL, Chi CW and Liu TY: Hydroxyl
radical formation and oxidative DNA damage induced by areca quid in
vivo. J Toxicol Environ Health A. 65:327–336. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang EJ, Cui ZG, Xu ZF, Duan WY, Huang
SH, Tan XX, Yin ZH, Sun CF and Lu L: Lack of influence of an XRCC3
gene polymorphism on oral cancer susceptibility: Meta-analysis.
Asian Pac J Cancer Prev. 15:10329–10334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Benhamou S, Tuimala J, Bouchardy C, Dayer
P, Sarasin A and Hirvonen A: DNA repair gene XRCC2 and XRCC3
polymorphisms and susceptibility to cancers of the upper
aerodigestive tract. Int J Cancer. 112:901–904. 2004. View Article : Google Scholar : PubMed/NCBI
|