Introduction
Cancer plagues human beings since ancient era and
there is still no complete therapy until today. This plague is
usually removed from the patients by surgery or sometimes only by
radiation or drugs, although, these treatments not always succeed.
There are many reasons for these failures, one of them was thought
to be the small population of cells that can initiate cancer
development and consequently recurrence of cancer. Decades ago,
leukemic stem cell was obtained from mice injected with human acute
myeloid leukemia cells (1). This
finding concreted the existence of cancer stem cells (CSCs) and
researchers started to research on them. CSCs have normal stem cell
properties like self-renew and multipotency as well as they provide
their progenies as cancer cells (2).
They can make not only cancer tissue but also cancer
microenvironment (3) and contribute
to cancer proliferation, invasion, and metastasis. Moreover, due to
the resistance to chemo- and radiation-therapy, cancer can recur
from survived residual CSCs even after treatment. Therefore, the
complete cure of cancer is often difficult, so that the development
of effective therapy to treat CSCs should eagerly be expected. For
the development of the effective therapy, enough number of CSCs
should be required for the characterization. However, it is
difficult to obtain sufficient quantities of CSCs for analysis from
clinical specimens since only small population of CSCs is present
in a cancer tissue.
We have previously demonstrated that CSCs can be
artificially induced by culturing mouse induced pluripotent stem
cells (iPSCs) in the presence of culture supernatant of cancer cell
lines (3–9). In the current study, we induced CSCs
from mouse embryonic stem cells (mESCs). They form colony on coated
dish, spheroids in suspension culture, and tube structure with
appropriate growth factors after induction. They also induce tumor
in C57BL/6 mice and keep expressing stem cell markers during
induction. Taken together, mESCs can be converted into CSCs and
would be a touchstone as CSCs can be derived from normal stem
cells.
Materials and methods
Cell culture
Mouse ESCs [B6J-23URT cells
(RBRC-AES0143) and B6G-2 cells (RBRC-AES0003)] were purchased from
Riken Cell Bank (Tokyo, Japan) and maintained in DMEM
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) containing 15% FBS
(Nichirei, Tokyo, Japan), 0.1 mM NEAA (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 2 mM L-Glutamine
(Sigma-Aldrich; Merck KGaA), 0.1 mM 2-mercaptoethanol (Wako, Osaka,
Japan), 1×106 Unit/ml LIF (Wako), 50 U/ml penicillin and
50 µg/ml streptomycin (Wako) on feeder layers of
mitomycin-C-treated mouse embryonic fibroblast (MEF) cells
(RCHEFC003; Reprocell, Kanagawa, Japan). B6G-2 cells are derived
from a transgenic mouse, of which cells are introduced GFP gene
into the genome so that GFP should constitutively express (10). Mouse Lewis lung carcinoma (LLC) cells
(11,12) (JCRB1348; JCRB cell Bank, Osaka,
Japan) and mouse melanoma B16 cells (JCRB0202; JCRB cell Bank)
(13–15) were purchased from ATCC (Manassas, VA,
USA) and maintained in DMEM (Sigma-Aldrich; Merck KGaA) containing
10% FBS. Mouse melanoma B16 cells were maintained in RPMI1640
(Sigma-Aldrich; Merck KGaA) containing 10% FBS. The cells were
incubated at 37°C under the atmosphere of 5% CO2.
To prepare the conditioned medium (CM), cell culture
supernatant of the different mouse cancer cell lines was collected
from confluent dishes and filtered through 0.45 µm filter (EMD
Millipore, Billerica, MA, USA). For the CSCs conversion, mESCs
(without MEF feeder cells) were maintained in the medium equally
mixed from the CM and the fresh medium of mESCs without LIF. The
medium was changed every day. Mouse ESCs without the CM treatment
were used as controls. Namely, mouse ESCs were cultured in mESCs
medium without LIF or equal mixture of mESCs medium and 5% FBS/DMEM
without LIF. Mouse ESCs were passaged when they reached 80%
confluent. B6J-23URT cells cultured with the CM of LLC
cells and B16 cells are termed B6J-LLCcm and B6J-B16cm,
respectively. B6G-2 cells cultured with the CM of LLC cells were
termed B6G-LLCcm.
For primary culture, the tumors of mouse allografts
were cut into small pieces (approximately 1 mm3) in
HBSS. After washing two times, the tissues were transferred into a
15-ml tube with 0.25% trypsin of five to six-fold volume and
incubated at 37°C for 40 min. 5 ml of DMEM containing 10% FBS was
then added to terminate digestion. The cellular suspension was then
placed into a new tube and centrifuged at 1000 rpm for 10 min. The
cell pellet was resuspended in 5 ml HBSS, and centrifuged at 1000
rpm for 5 min. The cell pellet was then placed into an appropriate
volume of mES medium without LIF and the cells were seeded into a
0.1% gelatin coated 60 mm dish at a density of 5×105/ml.
Cells were passaged every 3 days and cellular morphology was
observed and photographed using Olympus IX81 microscope equipped
with a fluorescence device (Olympus, Tokyo, Japan) and analyzed by
MetaMorph (Molecular Devices, LLC, Sunnyvale, CA, USA).
Suspension cultures to generate spheroids were
performed as described in Dontu et al (16). Briefly, single cell of mouse ESC
derived CSC or primary culture were plated on 6 cm ultra-low
attachment dishes (Corning Incorporated, Corning, NY, USA) with mES
medium containing CM without LIF. After they grew, medium was
changed to serum-free mESCs medium added
Insulin-Transferrin-Selenium-X (ITS-x) (Life Technologies, Grand
Island, NY, USA) without LIF. Spheroids cells were recognized after
about a week.
To assay tube formation, a 96-well plate was coated
with 50 µl/well of Matrigel (Corning Incorporated) by the
incubation at 37°C for 30 min. Then the trypsinized mouse ESC
derived CSC or primary culture cells were seeded at
5×104 cells/well with 50 µl of EGM-2 medium with growth
factors (Lonza, Basel, Switzerland) and cultured for 18 to 24
h.
Animal experiments
Healthy 4-week-old C57BL/6J mice were purchased from
Charles River Laboratories (Tokyo, Japan). 105 to
106 B6J-LLCcm or B6J-B16cm cells were subcutaneously or
intraperitoneally injected into two mice each-before 8 weeks of
age. B6J-23URT cells were also injected the same way as
a control of these cells. 105 to 106
B6G-LLCcm cells were subcutaneously and intraperitoneally injected
into three mice each. B6G-2 cells were also injected the same way
as a control. Mice were daily monitored. When size of the tumor
became large enough (around 15 mm), mice had been anesthesia with
isoflurane using simple inhalation anesthesia machine for small
animal experiments (NARCOBIT-E(II); KN-1071; Natsume Seisakusho
Co., Ltd, Japan), and flow meter (RK1710; KOFLOC, Japan) and
removed the tumor. Mice were sacrificed when tumors were
removed.
Histologic analysis
Tumors were fixed with 4%-paraformaldehyde in
phosphate buffered solution (Nacalai Tesque, Kyoto, Japan) and then
processed using a routine wax-embedding procedure for histologic
examination. 5-µm-thick sections were stained with hematoxylin and
eosin (HE).
RNA extraction, cDNA synthesis and
quantitative real time PCR
To test the stem cell marker gene expressions in
obtained CSCs or primary cultured cells, total RNA was isolated
from B6J-LLCcm, B6J-B16cm, and B6G-LLCcm cells with RNeasy Mini Kit
(QIAGEN, Hilden, Germany) and then treated with DNase I (Takara
Bio, Kusatsu, Japan). 2 µg of RNA was reverse transcribed with
SuperScript III First-strand Synthesis System (Invitrogen,
Carlsbad, CA, USA). Quantitative real-time PCR was performed with
LightCycler 480 SYBR Green I Master mix (Roche, Basel, Switzerland)
according to manufacturer's instructions. The sequences of forward
and reverse primers used for qPCR were as following: Sox2,
5′-TAGAGCTAGACTCCGGGCGATGA-3′ and 5′-TTGCCTTAAACAAGACCACGAAA-3′;
Pou5f1, 5′-TCTTTCCACCAGGCCCCCGGCTC-3′ and
5′-TGCGGGCGGACATGGGGAGATCC-3′; Nanog,
5′-AGGGTCTGCTACTGAGATGCTCTG-3′ and 5′-CAACCACTGGTTTTTCTGCCACCG-3′;
Gapdh, 5′-AACGGCACAGTCAAGGCCGA-3′ and
5′-ACCCTTTTGGCTCCACCCTT-3′. qPCR was performed by LightCycler 480
Instrument (Roche, Basel, Switzerland) with 400 nM of each primer.
Cycling conditions of the used genes were shown in Table I. Gene expression level was
normalized with that of Gapdh mRNA.
 | Table I.Reverse transcription-quantitative PCR
conditions of each primer. |
Table I.
Reverse transcription-quantitative PCR
conditions of each primer.
| Primer | Gapdh | Nanog | Pou5f1 | Sox2 |
|---|
| Initial
Denaturation | 95°C, 10 min | 95°C, 10 min | 95°C, 10 min | 95°C, 10 min |
| Denaturation | 95°C, 10 sec | 95°C, 10 sec | 95°C, 10 sec | 95°C, 10 sec |
| Annealing | 61°C, 10 sec | 61°C, 10 sec | 58°C, 10 sec | 58°C, 10 sec |
| Extension | 72°C, 10 sec | 72°C, 15 sec | 72°C, 10 sec | 72°C, 13 sec |
| No. of cycles | 45 | 45 | 45 | 45 |
Statistical analysis
The results were expressed as the mean ± standard
deviation. The statistical significance was assessed by one-way
analysis of variance followed by Tukey's post-hoc test for multiple
group comparisons. P-value lower than 0.05 was considered as
statistically significant.
Results
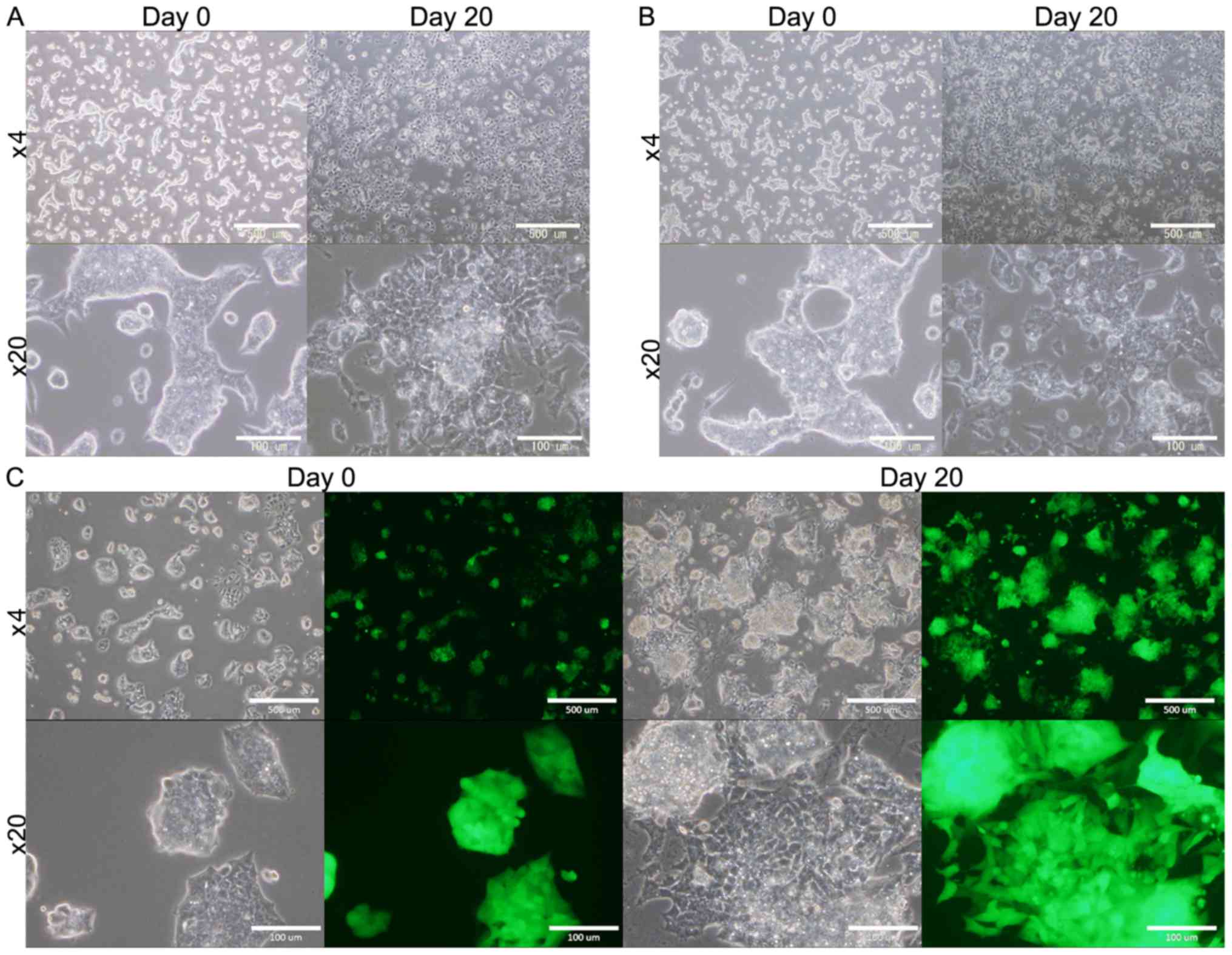
mESCs cultured in the CM
mESCs (B6J-23URT, B6G-2 cells) survived
for 4 weeks when they were cultured in the presence of CM, while
they ceased proliferating and then died in the absence of the CM
(Fig. 1). As the controls, mESCs
were cultured in mES medium without LIF or in DMEM containing 5%
FBS without LIF. However, in both condition the cells gradually
differentiated and died during 2 to 3 weeks (data not shown). This
expects that the presence of some factors in the CM that provide
some survival signals to the cells.
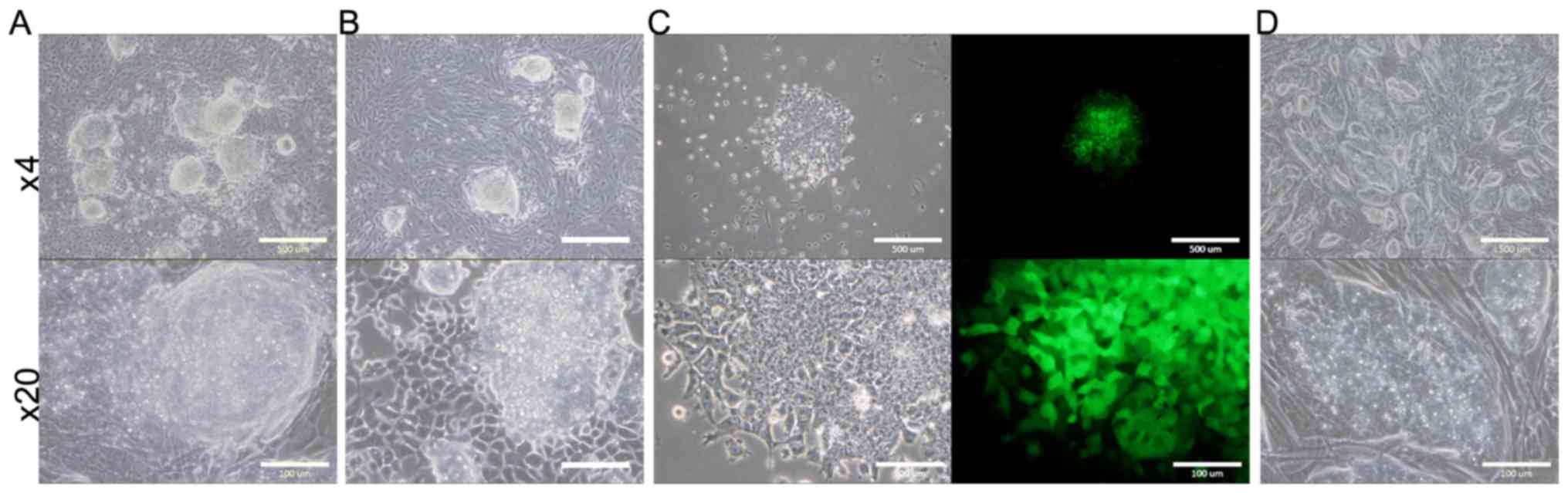
Evaluation of tumorigenic ability
The survived B6J-LLCcm, B6J-B16cm, and B6G-LLCcm
cells, cultured in CM for 30 days, were injected into C57BL/6J
mice. The results are summarized in Table II. (Only the total number could be
shown, but the both interperitoneally (IP) or subcutaneously (SC)
injected mice developed tumor.) The mice transplanted with B6J
derived cells developed tumor in about 40 to 60 days and those with
B6G derived cells in about 30 days. In each section of the tumors,
there were lots of cells infiltrated into the tumor tissue
(Fig. 2). Primary cultures of these
tumor cells are also shown in Fig.
3. In the primary culture of B6G-LLCcm cells that transplanted
in mice, GFP expression was observed (Fig. 3C). This means that the formed tumors
are derived from the transplanted cells. When these cultured cells
were transplanted into healthy C57BL/6J mice, the tumor appeared
again and the stem cell colonies were observed among the primary
culture of the tumor cells (Fig.
3D). Moreover, when serial transplantations were done with 5-mm
cube of tumors developed from B6J-LLCcm or B6J-B16cm cells, tumors
size became larger (data not shown). These results revealed that,
the tumors were concluded malignant and they included the
population of CSCs.
 | Table II.A summary of tumor formation by the
transplantation of mouse embryonic stem cells treated with
conditioned medium. |
Table II.
A summary of tumor formation by the
transplantation of mouse embryonic stem cells treated with
conditioned medium.
| Cell type | No. of mice that
developed tumors out of total n |
|---|
| B6J-LLCcm | 4/4 |
| B6J-B16cm | 3/4 |
| B6G-LLCcm | 2/6 |
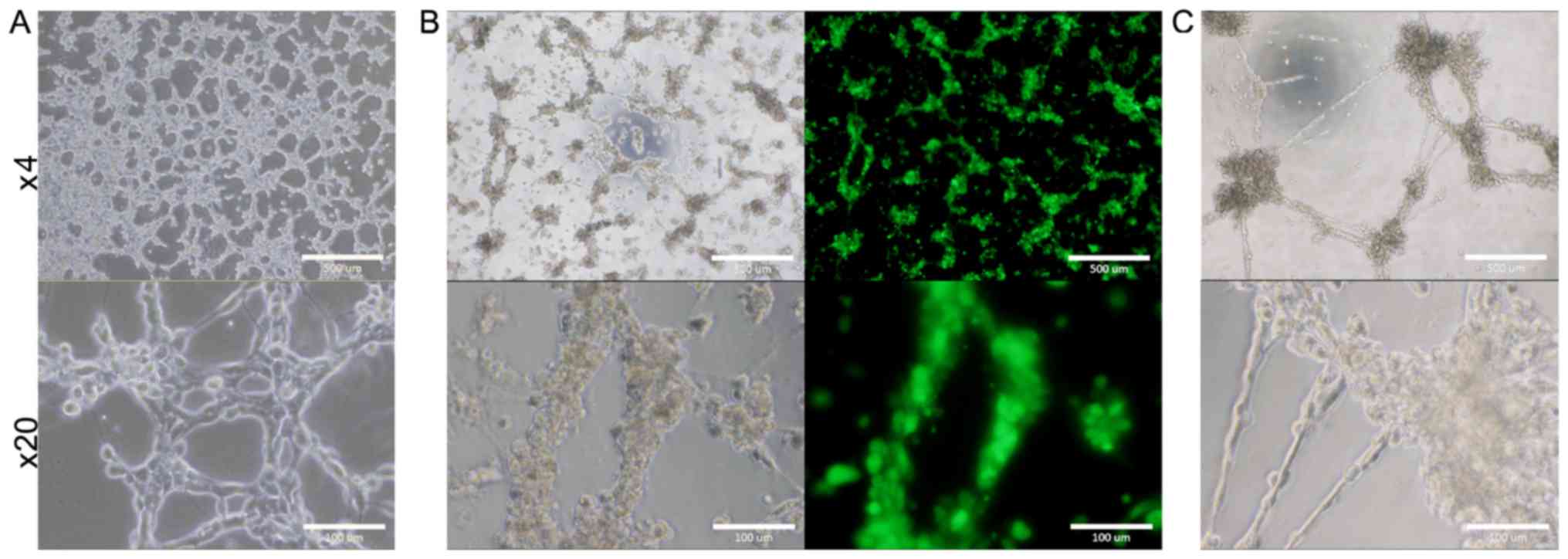
Potential of tube and sphere
formation
The ability to form tube in the presence of type IV
collagen was assessed with B6J-LLCcm and B6G-LLCcm cells. The cells
being seeded onto the wells coated with Matrigel, the medium was
changed to EGM-2 medium after 24 h. All of cells as well as primary
cells from the tumors derived from B6J-LLCcm cells formed
vessel-like luminal structures (Fig.
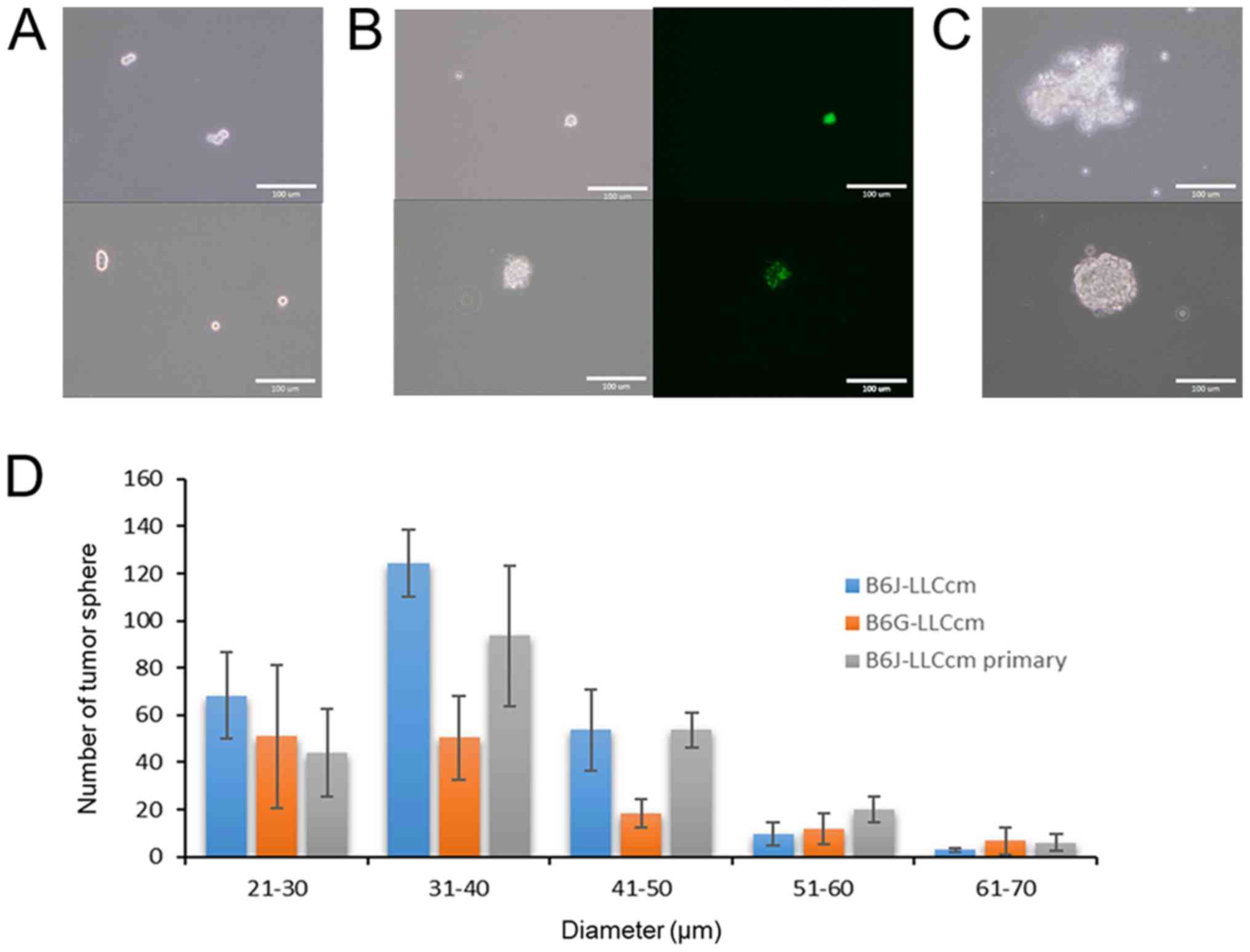
4). Sphere formation was also assessed with B6J-LLCcm and
B6G-LLCcm cells. Both cells exhibited spheroids in the non-adherent
condition (Fig. 5A and B). The
primary cultured cells derived from the tumor of B6J-LLCcm cells
also formed sphere structure (Fig.
5C). The size and number of spheres was analyzed; however, no
significant differences were observed (Fig. 5D). Our results indicated that, the
converted cells exhibited CSC properties of differentiation and
self-renewal potentials.
Stemness markers expression
To investigate the stemness characteristics of
mESCs, the expression of stem cell markers, Nanog, Pou5f1,
and Sox2 was assessed by RT-qPCR in B6J-LLCcm, B6J-B16cm,
and B6G-LLCcm cells (either at day 0 or after treatment with the
CM) as well as in the primary cultured cells of B6J-LLCcm and
B6JB16cm. Nanog was highly expressed in the cells treated
with the CM while the other two genes were expressed as much as in
mESCs at day 0. Moreover, the expression levels of the genes in the
primary culture cells were similar to those in mESCs at day 0
(Fig. 6A and B). Since Nanog
is thought to have a key role in maintaining pluripotency (17,18),
these results indicate that induced CSCs should keep the potential
of differentiation through tumor formation. In B6G-LLCcm cells, the
expression of Nanog and Pou5f1 at day 0 was similar.
In contrast, the expression of Sox2 was highly kept during
the treatment with the CM (Fig. 6C).
This observation may indicate the undifferentiated state of
B6G-LLCcm cells as CSCs when the report that high expression of
Sox2 was attributed to poor prognosis in carcinoma (19) is taken into consideration. Meanwhile,
the expression of Nanog gene in B6G-LLCcm cells is lower
than those of B6J-LLCcm and B6J-B16cm cells. This might be the
reason of lower rate of oncogenesis in the mice (Table II) as previously found in squamous
cell carcinomas (20,21). The expression of those genes might be
involved in the progression of cancer but further study is
needed.
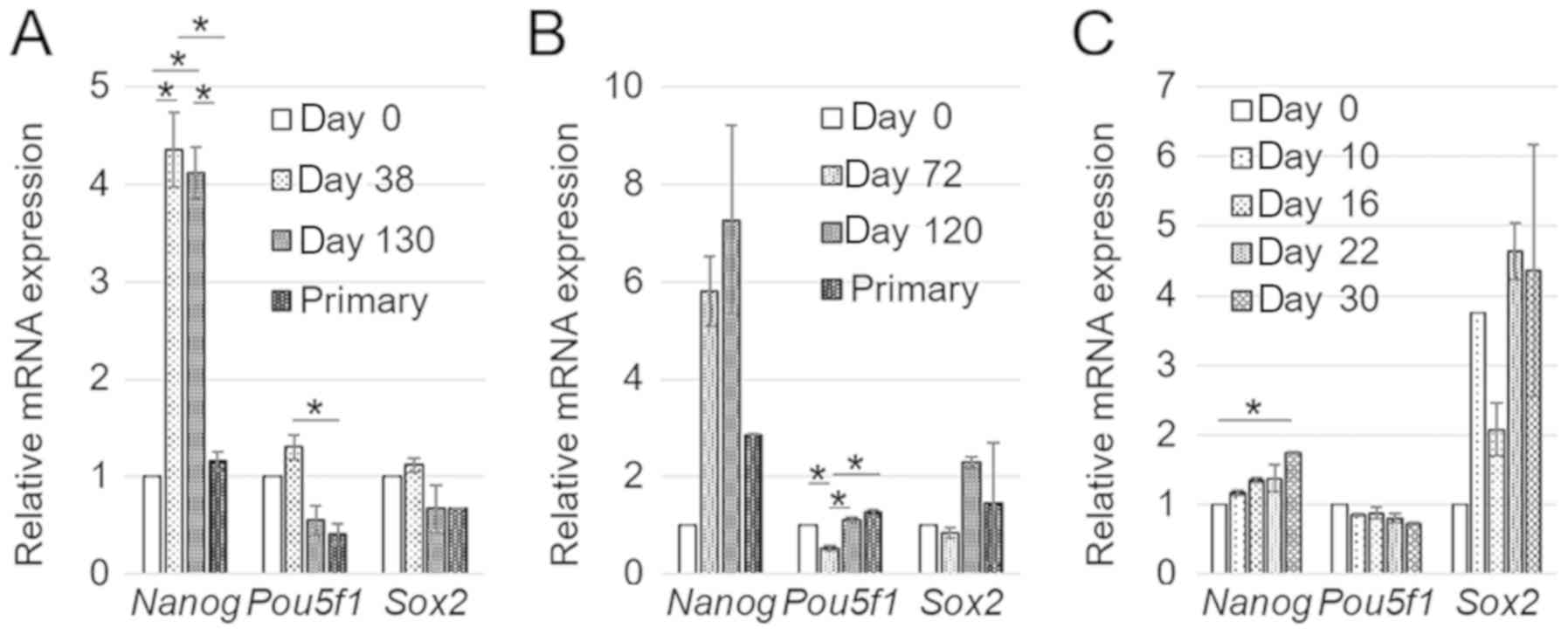 | Figure 6.The comparison mRNA expression levels
of stem cell markers in mESCs treated with during the treatment
with the CM or in the primary cultured cells. The data were
expressed as mean ± SD of three independent experiments. (A)
B6J-LLCcm (P-values for Nanog, Pou5f1, and Sox2 were
5.63×10−7, 0.000104, and 0.000118, respectively), (B)
B6J-B16cm (P-values for Nanog, Pou5f1, and Sox2 were
0.0101, 0.000234, 0.169, respectively), and (C) B6G-LLCcm cells
(P-values for Nanog, Pou5f1, and Sox2 were 0.00322,
0.0240, and 0.0291, respectively). *P<0.05, as indicated
(Tukey's post-hoc test). |
Discussion
In the present study, mESCs were successfully
demonstrated to be converted into CSCs exhibiting the potential of
differentiation and self-renewal together with malignant
tumorigenicity followed by a number of infiltrated cells. The GFP
expressing cells were found in the developed tumors referring to
the converted mESCs is the source cells that formed the tumor.
Moreover, CSCs were found expressing the markers of
undifferentiated cells implying they kept stemness. As the results,
mouse ESCs have been found to be converted into CSCs in a short
period when affected by some factors derived from the cancerous
microenvironment. In this context, the induction of CSCs should not
always depend on gene mutations/translocations. This implies normal
stem cells might have a differentiation potential to become a
cancer origin. This is not a new concept and have been discussed
for a while (22–24). Although most of the people focused on
the gene mutation, our results shed light on the initial
microenvironment of stem cells. Further investigations are still
needed to study the mechanism(s) and factor(s), that responsible
for the induction of CSCs. mECSs would be useful for obtaining
enough number of CSCs for clarification of the mechanisms that
involved in cancer development.
Although mouse and human iPSCs are continuously
converted into CSCs (3–6,8,9,25,26), the
exact inducing factors are not unknown yet. To find them,
microarray gene analysis was performed on these CSCs (25,27) and
analysis of CM is also ongoing (data not shown). This induction
process might be complicated and could be clarify little by little.
Additionally, there are only two cancer cell lines used to obtain
CM to induce CSCs. Various kinds of cancer cell lines were already
used for the induction but there are still several cancer cell
lines. It should be cleared all or not all cell lines are could be
used for this. Moreover, both SC and IP injected CSCs are developed
tumor in this research. This might be because they are stem-like
cells rather than cancer cells. Consequently, tissue-specific
cancer could be induced only if they were injected into organ or
tissue induction might be needed for the tissue-specific tumor
induction.
Immune system is also important for tumor exclusion.
Currently, we used C57BL/6J mice with normal immune system is
normal, therefore, they don't develop tumors without gene mutation.
However, with more than 106 induced CSCs, more than half
of them developed malignant tumor. Proven that CSCs were not
completely excluded by their immune system and there might be a
clue for cancer immune evasion.
Acknowledgements
Not applicable.
Funding
The present study was partly supported by the
Grant-in-Aid for Scientific Research (A) (grant no. 25242045; MS),
Grant-in-Aid for Scientific Research (C) (grant no. 16K07116; YI)
and the Grant-in-Aid for Challenging Exploratory Research (grant
no. 26640079; MS).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
All authors contributed, revised all data, and
approved the current work. CM and BEA performed all of the
experiments. YI and TO performed the pathological assessments on
the tumors. AS and MS conceived and designed the research,
supervised the study and gave final approval of the version to be
published.
Ethics approval and consent to
participate
The plan of animal experiments was reviewed and
approved by the Ethics Committee for Animal Experiments of Okayama
University under the IDs OKU-2012482 and OKU-2013252.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CSC
|
cancer stem cell
|
|
mESCs
|
mouse embryonic stem cells
|
References
|
1
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Hajj M and Clarke MF: Self-renewal and
solid tumor stem cells. Oncogene. 23:7274–7282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prieto-Vila M, Yan T, Calle AS, Nair N,
Hurley L, Kasai T, Kakuta H, Masuda J, Murakami H, Mizutani A and
Seno M: iPSC-derived cancer stem cells provide a model of tumor
vasculature. Am J Cancer Res. 6:1906–1921. 2016.PubMed/NCBI
|
|
4
|
Oo AKK, Calle AS, Nair N, Mahmud H,
Vaidyanath A, Yamauchi J, Khayrani AC, Du J, Alam MJ, Seno A, et
al: Up-regulation of PI 3-kinases and the activation of PI3K-Akt
signaling pathway in cancer stem-like cells through DNA
hypomethylation mediated by the cancer microenvironment. Transl
Oncol. 11:653–663. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nair N, Calle AS, Zahra MH, Prieto-Vila M,
Oo AKK, Hurley L, Vaidyanath A, Seno A, Masuda J, Iwasaki Y, et al:
A cancer stem cell model as the point of origin of
cancer-associated fibroblasts in tumor microenvironment. Sci Rep.
7:68382017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calle AS, Nair N, Oo AK, Prieto-Vila M,
Koga M, Khayrani AC, Hussein M, Hurley L, Vaidyanath A, Seno A, et
al: A new PDAC mouse model originated from iPSCs-converted
pancreatic cancer stem cells (CSCcm). Am J Cancer Res. 6:2799–2815.
2016.PubMed/NCBI
|
|
7
|
Matsuda S, Yan T, Mizutani A, Sota T,
Hiramoto Y, Prieto-Vila M, Chen L, Satoh A, Kudoh T, Kasai T, et
al: Cancer stem cells maintain a hierarchy of differentiation by
creating their niche. Int J Cancer. 135:27–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen L, Kasai T, Li Y, Sugii Y, Jin G,
Okada M, Vaidyanath A, Mizutani A, Satoh A, Kudoh T, et al: A model
of cancer stem cells derived from mouse induced pluripotent stem
cells. PLoS One. 7:e335442012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L, Mizutani A, Kasai T, Yan T, Jin G,
Vaidyanath A, El-Aarag BY, Liu Y, Kudoh T, Salomon DS, et al: Mouse
induced pluripotent stem cell microenvironment generates
epithelial-mesenchymal transition in mouse Lewis lung cancer cells.
Am J Cancer Res. 4:80–88. 2014.PubMed/NCBI
|
|
10
|
Shimizukawa R, Sakata A, Hirose M,
Takahashi A, Iseki H, Liu Y, Kunita S, Sugiyama F and Yagami K:
Establishment of a new embryonic stem cell line derived from
C57BL/6 mouse expressing EGFP ubiquitously. Genesis. 42:47–52.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bertram JS and Janik P: Establishment of a
cloned line of lewis lung carcinoma cells adapted to cell culture.
Cancer Lett. 11:63–73. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharma S, Stolina M, Lin Y, Gardner B,
Miller PW, Kronenberg M and Dubinett SM: T cell-derived IL-10
promotes lung cancer growth by suppressing both T cell and APC
function. J Immunol. 163:5020–5028. 1999.PubMed/NCBI
|
|
13
|
Fidler IJ: Selection of successive tumour
lines for metastasis. Nat New Biol. 242:148–149. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fidler IJ: Biological behavior of
malignant melanoma cells correlated to their survival in vivo.
Cancer Res. 35:218–224. 1975.PubMed/NCBI
|
|
15
|
Fidler IJ, Darnell JH and Budmen MB:
Tumoricidal properties of mouse macrophages activated with
mediators from rat lymphocytes stimulated with concanavalin A.
Cancer Res. 36:3608–3615. 1976.PubMed/NCBI
|
|
16
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mitsui K, Tokuzawa Y, Itoh H, Segawa K,
Murakami M, Takahashi K, Maruyama M, Maeda M and Yamanaka S: The
homeoprotein Nanog is required for maintenance of pluripotency in
mouse epiblast and ES cells. Cell. 113:631–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Torres J and Watt FM: Nanog maintains
pluripotency of mouse embryonic stem cells by inhibiting NFkappaB
and cooperating with Stat3. Nat Cell Biol. 10:194–201. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dai W, Tan X, Sun C and Zhou Q: High
expression of SOX2 is associated with poor prognosis in patients
with salivary gland adenoid cystic carcinoma. Int J Mol Sci.
15:8393–8406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Palla AR, Piazzolla D, Alcazar N, Cañamero
M, Graña O, Gómez-López G, Dominguez O, Dueñas M, Paramio JM and
Serrano M: The pluripotency factor NANOG promotes the formation of
squamous cell carcinomas. Sci Rep. 5:102052015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim J, Liu Y, Qiu M and Xu Y: Pluripotency
factor Nanog is tumorigenic by deregulating DNA damage response in
somatic cells. Oncogene. 35:1334–1340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brücher BL and Jamall IS: Epistemology of
the origin of cancer: A new paradigm. BMC Cancer. 14:3312014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bjerkvig R, Tysnes BB, Aboody KS, Najbauer
J and Terzis AJ: Opinion: The origin of the cancer stem cell:
Current controversies and new insights. Nat Rev Cancer. 5:899–904.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Visvader JE: Cells of origin in cancer.
Nature. 469:314–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seno A, Kasai T, Ikeda M, Vaidyanath A,
Masuda J, Mizutani A, Murakami H, Ishikawa T and Seno M:
Characterization of gene expression patterns among artificially
developed cancer stem cells using spherical self-organizing Map.
Cancer Inform. 15:163–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shigehiro T, Masuda J, Saito S, Khayrani
AC, Jinno K, Seno A, Vaidyanath A, Mizutani A, Kasai T, Murakami H,
et al: Practical liposomal formulation for taxanes with
polyethoxylated castor oil and ethanol with complete encapsulation
efficiency and high loading efficiency. Nanomaterials (Basel).
7(pii): E2902017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seno A and Seno M: Commonly expressed
genes among cancer stem cells induced from hiPSCs and obtained from
cancer tissues or cell lines. Tumor Microenvironment. 2018.doi:
10.4103/tme.tme_1_18. View Article : Google Scholar
|