Introduction
Cancer is one of the most pivotal public health
concerns worldwide and has therefore been the focus of an
increasing amount of attention. Colorectal cancer is one of the
most common causes of cancer-associated mortality, and the USA
statistics for 2018 reported that the estimated number of new
colorectal cancer cases will reach 140,250 (1). With increased understanding and
improved treatment options, the 5- and 10-year survival rates for
colorectal cancer have reached 65 and 58%, respectively (2). The primary treatment for colorectal
cancer is surgery, accompanied by chemotherapy, immunotherapy and
radiation (3). Therefore, the
molecular detection of colorectal cancer provides a non-invasive
diagnosis, which has been widely accepted by the majority of
patients and clinicians (4).
Chloride channel accessory (CLCA) is a gene family
of Ca2+ activated chloride channels, which includes four
genes in humans and a minimum of six genes in mice (5,6). Each
member of this gene family map to a similar location on the
chromosome 1p31-p22, share a similar hypothesized structure and
share a similar homology; however, these genes substantially differ
in their tissue locations (7,8). The
human genome encodes three functional CLCA proteins, which are
encoded by CLCA1, CLCA2 and CLCA4. CLCA3 is considered a shortened
pseudogene, as it does not encode a protein due to the fact that it
possesses premature stop codons, and is therefore not likely to be
expressed at detectable levels (9).
CLCA1, which was the first reported human CLCA family member, is
primarily expressed in the large and small intestines, particularly
in the crypt cells (6). Previous
studies have demonstrated that increased expression of CLCA1
decreases the aggressiveness of colorectal cancer cells in
vitro and in vivo (10,11). A
high expression of CLCA2 has been observed in the trachea and
mammary glands, and has been reported to serve a role in breast
cancer by acting as a p53 family target (12,13).
Predominant expression of CLCA4 and CLCA1 has been observed in
colon tissue (14). Similar to
CLCA2, it has been identified that ectopic expression of CLCA4
results in the inhibition of breast cancer cell growth (15).
A previous study reported that CLCA1 inhibited the
growth of colon cancer cell lines (11). However, to the best of our knowledge,
few studies have investigated the prognostic value of CLCA family
expression in patients with colon cancer. Therefore, the present
study investigated the prognostic value of CLCA mRNA expression
levels using data from 438 patients with colon cancer in The Cancer
Genome Atlas (TCGA) database. Bioinformatics analysis demonstrated
that elevated expression levels of CLCA1 and CLCA2 were associated
with a favorable prognosis in colon cancer.
Materials and methods
Data preparation
TCGA (cancergenome.nih.gov) was employed for obtaining the
survival data, including sex, age, tumor-node-metastasis (TNM)
stage, events, survival time, death status, and the mRNA expression
levels of CLCA1, CLCA2 and CLCA4. All datasets contained clinical
and follow-up data. Datasets without prognostic outcome information
were excluded from the current study. The patients were divided
into low and high expression groups based on the median value of
gene expression of each CLCA and the patients' survival data were
extracted.
Association and bioinformatics
analysis
The present study investigated the functions and
associations of CLCA genes by multiple bioinformatics approaches.
The relative degrees of expression of CLCA genes in multiple normal
tissue samples were calculated using the GTEx Portal (www.gtexportal.org/home) (16). The Metabolic gEne RApid Visualizer
(MERAV) tool (merav.wi.mit.edu) (17) was searched for the purpose of
constructing a boxplot of the expression levels of CLCA genes in
normal tissues and primary tumors of colon cancer. Co-expression
analysis was performed using GeneMANIA (www.genemania.org) (18). The Database for Annotation,
Visualization, and Integrated Discovery (version 6.7; david.ncifcrf.gov/tools.jsp) (19,20) was
employed for analyzing the functional enrichment, which included
gene ontology (GO) functional analysis and Kyoto Encyclopedia of
Genes and Genomes (KEGG) pathway analysis. P<0.05 was considered
to indicate a statistically significant difference.
Survival analysis
With regard to each CLCA mRNA expression, division
of patients into high- and low-expression cohorts was performed
using median gene expression level as the cut-off value. Evaluation
of the prognosis of colon cancer was based on overall survival (OS)
time. Kaplan-Meier curves with a log-rank test were used for the
identification of associations between the mRNA expression levels
of the three CLCAs and patient survival. Adjustments for age, sex
and TNM stage were made using the Cox proportional hazards
regression framework.
Joint-effects analysis
The present study also performed a joint-effects
analysis for combining the genes identified as significant with the
help of the survival analysis. Groups were formulated through the
summarization of the selected expression of genes associated with
the improved OS time in one group (Group 1), a worse OS time in
another group (Group 3) and others in the last group (Group 2). The
grouping is presented in Table
III.
 | Table III.Grouping information for the
combination among CLCA genes. |
Table III.
Grouping information for the
combination among CLCA genes.
| Group | Patients
(n=438) | Composition |
|---|
| 1 | 170 | High CLCA1 + high
CLCA2 |
| 2 | 98 | High CLCA1 + low
CLCA2 or low CLCA1 + high CLCA2 |
| 3 | 170 | Low CLCA1 + low
CLCA2 |
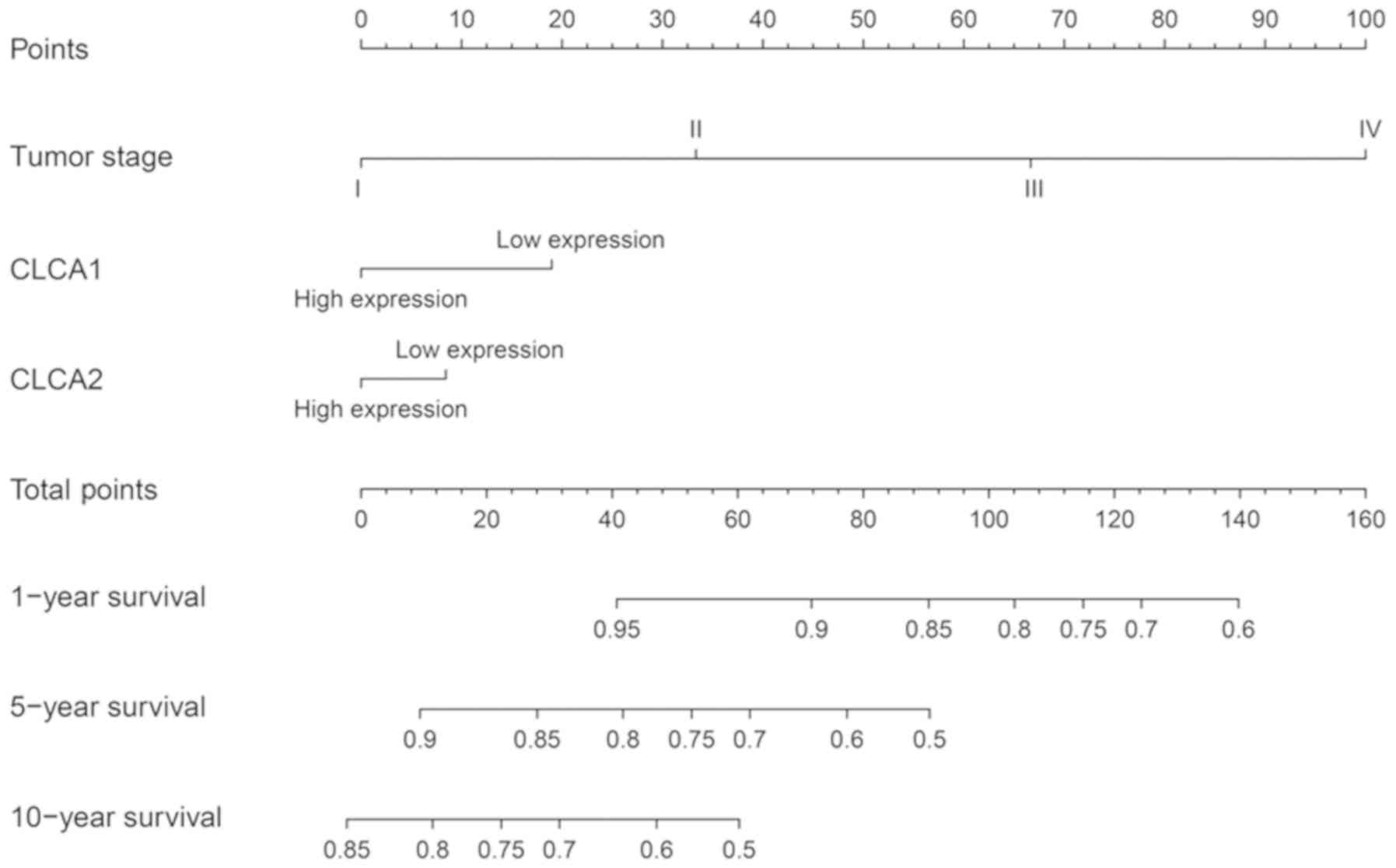
Nomogram
In accordance with the clinical information, coupled
with the findings of the survival analysis subsequent to adjusting
it with Cox proportional hazards regression model, a risk model for
tumor stage, CLCA1 and CLCA2 expression levels was
established. Nonetheless, the points against each factor were
scored and 1-, 5- and 10-year survival rates were computed.
Gene set enrichment analysis
(GSEA)
GSEA 3.0 (http://software.broadinstitute.org/gsea/msigdb/index.jsp)
was employed for the purpose of analyzing disparities in the levels
of the gene expression of the biological pathways in the low and
high expression groups for each gene with reference gene sets from
the Molecular Signatures Database of c2 (KEGG gene sets:
c2.all.v6.1.symbols.gmt), c5 (GO gene sets:
c5.all.v6.1.symbols.gmt) and c6 (oncogenic signatures gene sets:
c6.all.v6.2.symbols.gmt) (21). In
addition, the number of permutations was set at 1,000. Enrichment
findings that satisfied a typically significance cutoff of
P<0.05 with a false discovery rate (FDR)<0.25 were regarded
as statistically significant.
Statistical analysis
Kaplan-Meier survival analysis with a log-rank test
was used for the calculation of the OS time and P-values for all
associations. Cox proportional hazards regression analysis was also
employed to calculate the crude or attuned hazard ratio (HR) and
95% confidence interval (CI) in the univariate and multivariate
analyses. Statistical analyses of gene expressions in different
groups were performed using one-way analysis of variance followed
by Student-Newman-Keuls multiple comparison test. P<0.05 was
considered to indicate a statistically significant difference. The
Kaplan-Meier and scatter curves were generated using GraphPad
software (version 7.0; GraphPad Software, Inc., La Jolla, CA, USA).
Analysis of the data was performed with SPSS 20.0 software (IBM
Corp., Armonk, NY, USA). The correlation plots and nomogram were
produced by R platform (version 3.5.1; www.r-project.org).
Results
Clinical features and outcomes
An aggregate of 438 patients with colon cancer, who
had accomplished follow-up profiles, were recruited for the purpose
of investigation. The clinical characteristics of the patients have
been summarized in Table I. TNM
stage was significantly associated with the median survival time
(MST; P<0.001; Table I).
 | Table I.Demographic and clinical data for 438
patients with colon cancer. |
Table I.
Demographic and clinical data for 438
patients with colon cancer.
| Variable | Patients
(n=438) | No. of mortalities,
% | MST, days | HR (95% CI) | Log-rank
P-value |
|---|
| Age, years |
|
|
|
| 0.398 |
|
<60 | 122 | 81.1 | 3,039 | Ref. |
|
|
≥60 | 316 | 76.3 | 2,535 | 1.223
(0.766–1.952) |
|
| Sex |
|
|
|
| 0.545 |
|
Female | 204 | 78.4 | 2,990 | Ref. |
|
|
Male | 234 | 76.9 | 2,320 | 1.131
(0.759–1.686) |
|
| TNM stage |
|
|
|
| <0.001 |
| I | 73 | 94.5 | 3,234 | Ref. |
|
| II | 167 | 83.8 | 2,838 | 2.24
(0.781–6.421) |
|
|
III | 126 | 75.4 | 2,856 | 4.068
(1.434–11.538) |
|
| IV | 61 | 49.2 | 1,114 | 11.291
(3.980–32.026) |
|
|
Unknown | 11 |
|
|
|
|
Bioinformatics analysis
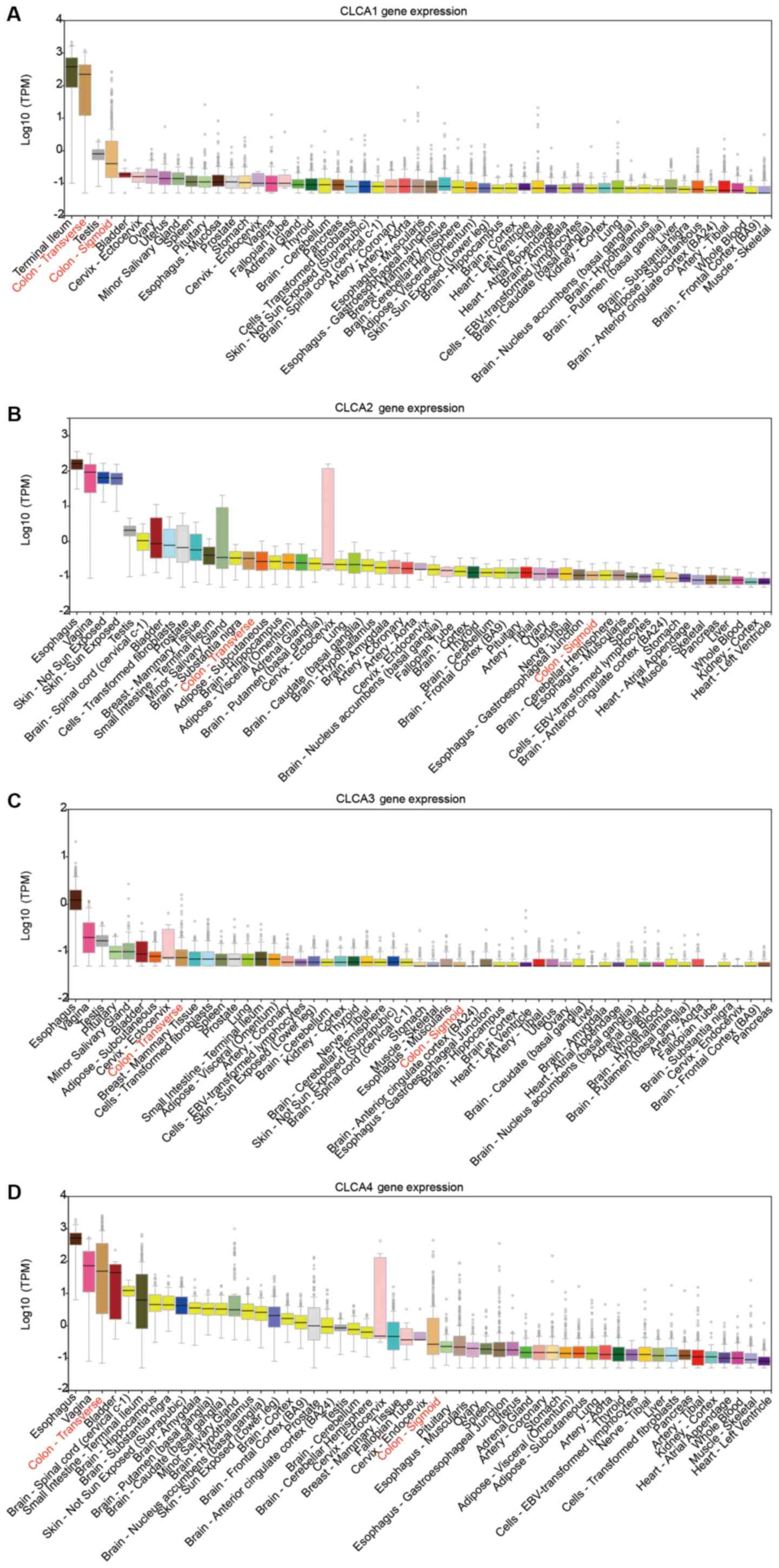
The CLCA family consists of four members (9). The bioinformatics analysis revealed
that CLCA1, CLCA2, CLCA3 and CLCA4 were expressed at high levels in
the human colon tissue. (Fig. 1A-D).
The expression levels of CLCA1, CLCA2, CLCA3 and CLCA4 in colon
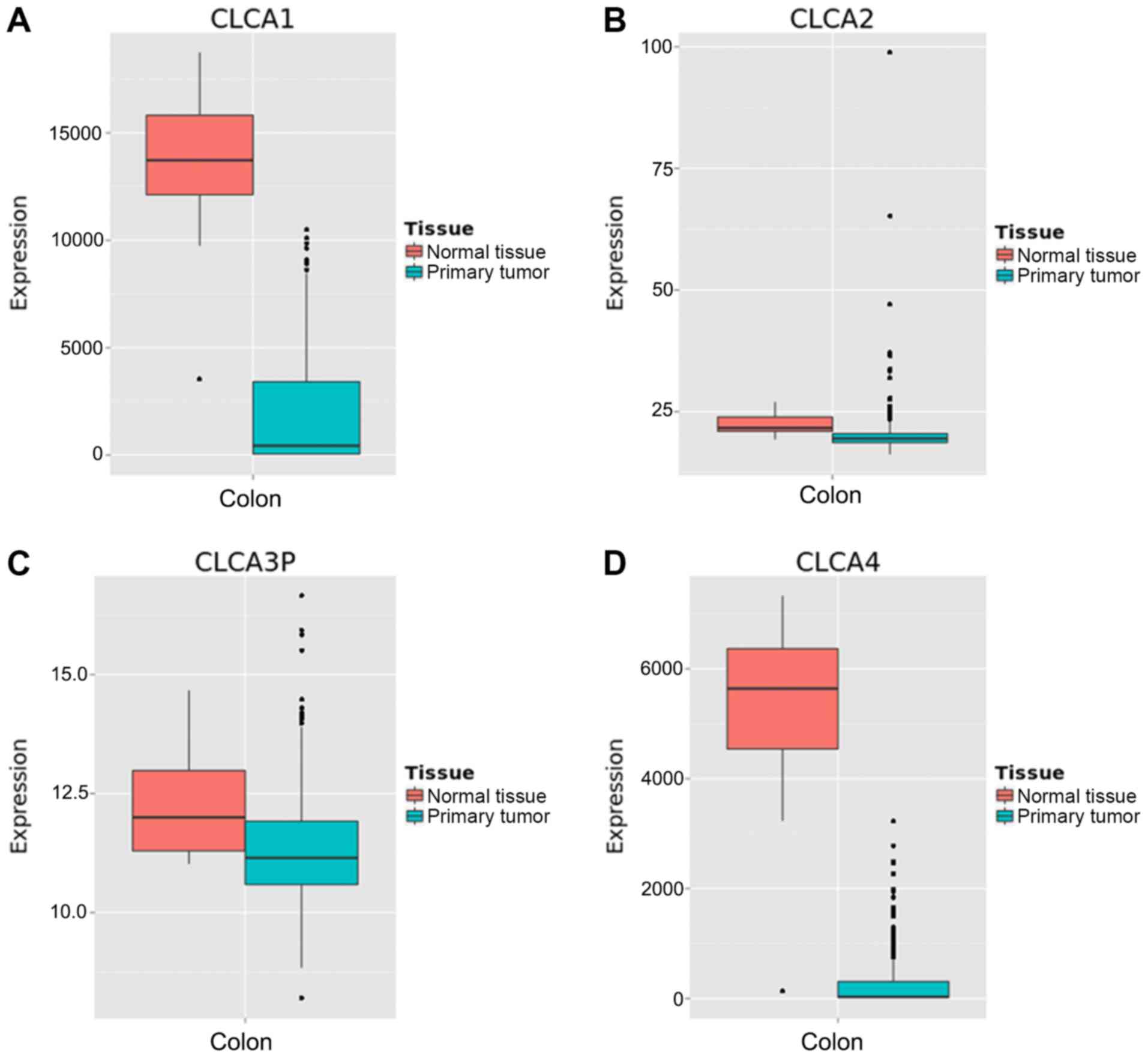
tissue were higher than the majority of the other organs (Fig. 1). Boxplots illustrating
dissimilarities in the expression of the four CLCA genes in normal
colon tissue compared with primary colon cancer tissue were
generated by MERAV (Fig. 2). The
median expression levels of CLCA1, CLCA2, CLCA3 and CLCA4 were
higher in the normal colon tissue when compared with that in the
primary colon cancer tissue. Scatter plots for the expression of
CLCA1, CLCA2 and CLCA4 in accordance with the 50th percentile
cutoff are presented in Fig. 3A. The
expression level of CLCA3 is not provided because CLCA3 is a
truncated pseudogene and does not encode a protein (9). Scatter plots for the expression of the
three genes in different TNM stages have been presented in Fig. 3B. A gene co-expression interaction
analysis revealed that all the CLCA genes were co-expressed
(Fig. 3C), with the exclusion of
CLCA3 as it was not recognized by GeneMANIA. The assessment of
biological functions of the CLCA genes, excluding CLCA3, was
performed in accordance with the biological process, molecular
function and cellular component categories for the GO functional
analysis, and the results of KEGG pathway analysis are presented in
Fig. 3D.
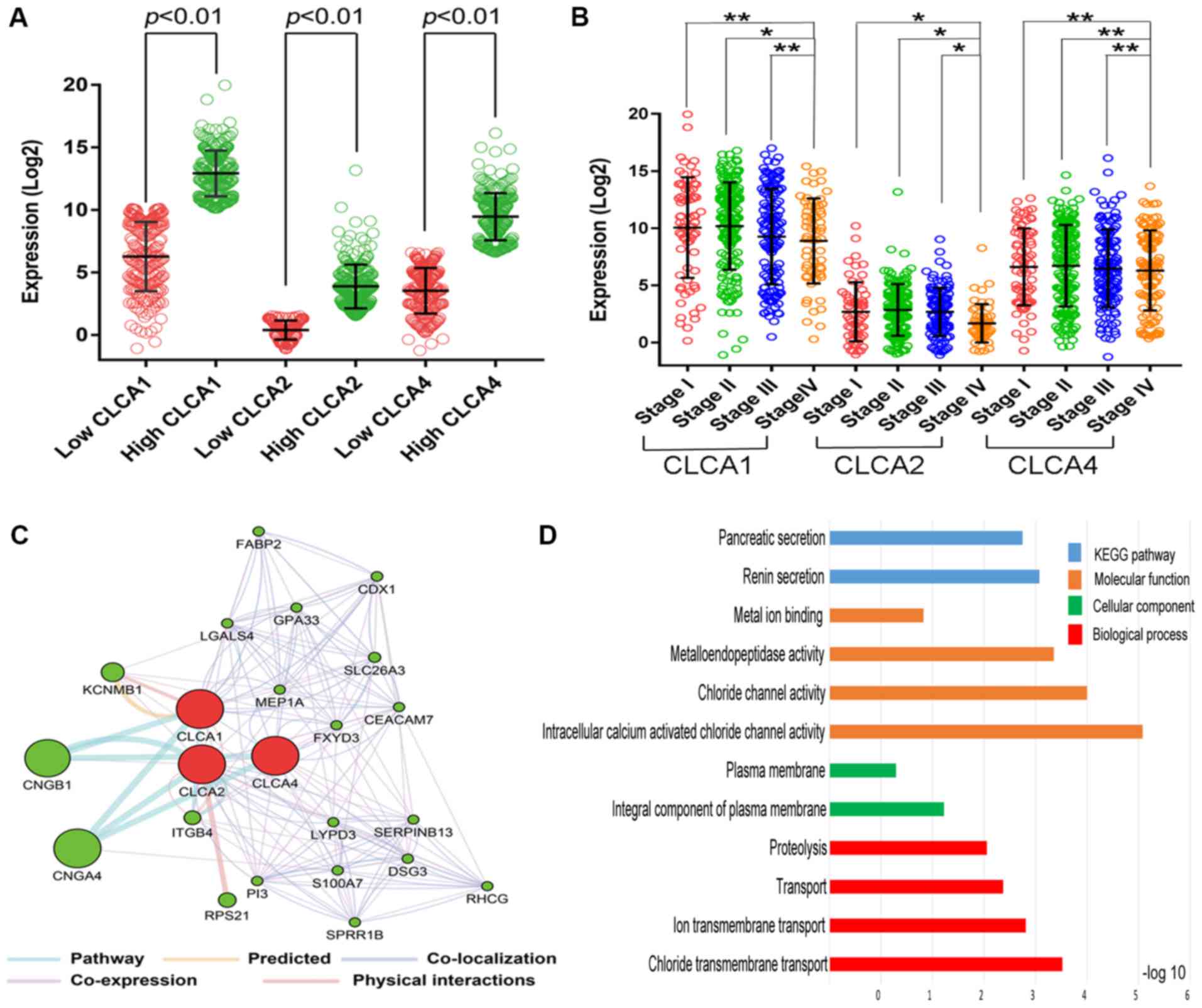 | Figure 3.Intergroup differences of gene
expression, gene interaction network, GO and KEGG enrichment
results. (A) Scatter plots for CLCA1, CLCA2 and CLCA4 gene
expression levels according to data from The Cancer Genome Atlas.
(B) Scatter plots for CLCA1, CLCA2 and CLCA4 gene expression levels
at different TNM stages. (C) Gene interaction network among CLCA
genes produced by the GeneMANIA. (D) Analysis of the enriched GO
terms and KEGG pathways for CLCA genes using the Database for
Annotation, Visualization, and Integrated Discovery. *P<0.05,
**P>0.05. CLCA, chloride channel accessory; TNM,
tumor-node-metastasis; GO, gene ontology; KEGG, Kyoto Encyclopedia
of Genes and Genomes. |
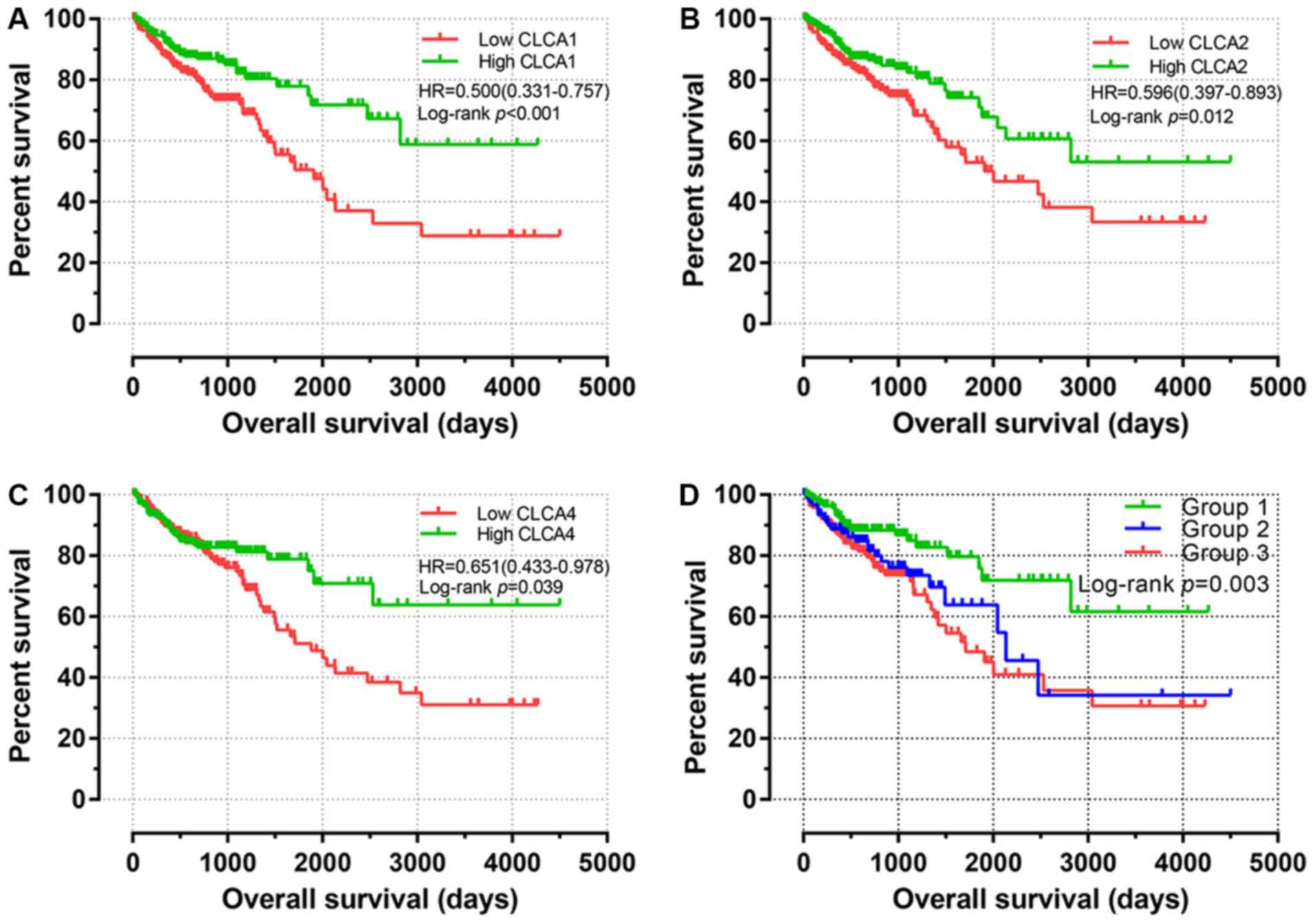
Survival influence of differential
CLCA gene expression
The key findings of the univariate survival analysis
are presented in Fig. 4A-C. The
results revealed that high expression levels of CLCA1, CLCA2 and
CLCA4 are significantly associated with an improved OS time for
patients with colon cancer (P<0.001, P=0.012 and P=0.039,
respectively). As identified by the multivariate Cox proportional
hazards regression analysis, there were associations of TNM stage
with the prognosis of patients with colon cancer (Table I). The multivariate survival analysis
suggested that, individually, elevated expression levels of CLCA1
(HR, 0.577; 95% CI, 0.376–0.885; adjusted P=0.012) and CLCA2 (HR,
0.647; 95% CI, 0.427–0.982; adjusted P=0.041) were associated with
a favorable OS time (Table II).
 | Table II.Prognostic survival analysis
according to high or low expression of CLCA family
genes. |
Table II.
Prognostic survival analysis
according to high or low expression of CLCA family
genes.
| Gene
expression | Patients
(n=438) | No. of events,
% | MST, days | Crude HR (95%
CI) | Crude P-value | Adjusted HR (95%
CI)a | Adjusted
P-valuea |
|---|
| CLCA1 |
|
|
|
| <0.001 |
| 0.012 |
|
Low | 219 | 71.2 | 2,236 | Ref. |
| Ref. |
|
|
High | 219 | 84.0 | 3,125 | 0.500
(0.331–0.757) |
| 0.577
(0.376–0.885) |
|
| CLCA2 |
|
|
|
| 0.012 |
| 0.041 |
|
Low | 219 | 73.1 | 2,301 | Ref. |
| Ref. |
|
|
High | 219 | 82.2 | 3,070 | 0.596
(0.397–0.893) |
| 0.647
(0.427–0.982) |
|
| CLCA4 |
|
|
|
| 0.039 |
| 0.161 |
|
Low | 219 | 72.6 | 2,274 | Ref. |
| Ref. |
|
|
High | 219 | 82.6 | 3,305 | 0.651
(0.433–0.978) |
| 0.743
(0.491–1.125) |
|
Subsequently, a joint-effects model was constructed
for the determination of the cumulative impacts of CLCA
genes on the OS time of patients with colon cancer. Furthermore,
different groups for this analysis were generated in accordance
with the expression of CLCA1 and CLCA2 (Table III). In the analysis, high CLCA1
and CLCA2 expression levels were observed to be significantly
associated with a favorable OS time (P=0.003; Fig. 4D). The nomogram prognostic evaluation
model was developed on the basis of the multivariate analysis
(Fig. 5).
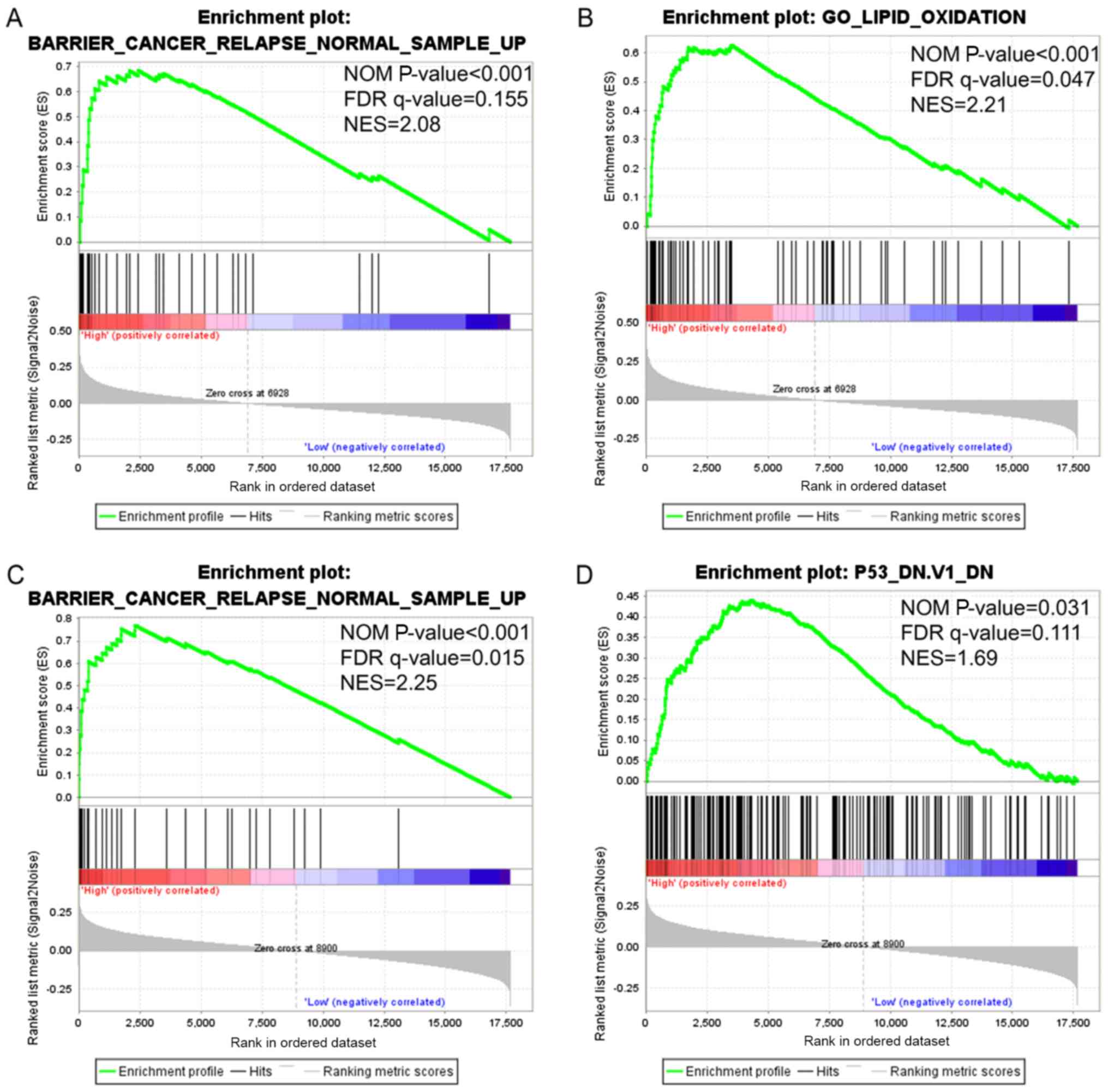
Gene set enrichment analysis
(GSEA)
GSEA analysis was performed in order to investigate
the potential biological processes that a high expression of CLCA1
and CLCA2 is likely to exert an impact on. The findings obtained
from GSEA demonstrated that gene set differences in patients with
high vs. low CLCA1 and CLCA2 expression levels indicated that CLCA1
and CLCA2-regulated gene sets were primarily associated with colon
cancer relapse (P<0.001, FDR=0.155, Fig. 6A; P<0.001, FDR=0.015, Fig. 6C; respectively). The data also
revealed that the elevated expression of CLCA1 was associated with
lipid oxidation (P<0.001, FDR=0.047, Fig. 6B), while CLCA2 was associated with
the p53 pathway (P=0.031, FDR=0.111, Fig. 6D). In summary, the data suggest that
CLCA1 and CLCA2 are likely to be associated with the prognosis of
colon cancer.
Discussion
Recently, an increasing number of studies have
investigated the function of ion channels in the pathogenesis of
various cancer types (22,23). The chloride channel serves important
roles in tumorigenesis (24). In
addition, the CLCA proteins were primarily isolated in the year
1991 and have subsequently been revealed to be an intricate family
(9). Each human CLCA family member
identified thus far has been located on the short arm of the
chromosome 1 (1p22-31), in addition each CLCA protein shares a
similar homology and hypothesized structure; however, significant
differences exist in their tissue location (8). Numerous functions of the CLCA family
have been identified, including involvement in mucus secretion and
tumor metastasis, and regulation of apoptosis, cell cycle and blood
pressure (10,25,26).
The bioinformatics analysis of the present study
demonstrated that the most notable molecular functions of CLCA were
intracellular calcium activated chloride channel activity, chloride
channel activity and chloride transmembrane transport. An
increasing number of studies have suggested that CLCA proteins,
acting on calcium-dependent chloride channels and facilitating
chloride conductance, are closely associated with tumor progression
(12,27). With regard to colon cancer, CLCA
family members exert a pivotal function in the regulation of cell
development, invasion and metastasis. Note that CLCA4 was
misidentified as CLCA2 in a study conducted by Bustin et al
(28).
In the present study, elevated expression levels of
CLCA1, CLCA2 and CLCA4 in normal tissue were observed. Kaplan-Meier
curves from univariate survival analysis revealed that elevated
expression levels of CLCA1, CLCA2 and CLCA4 in tumor tissues were
associated with a favorable OS time in all patients with colon
cancer, which suggests that CLCA1, CLCA2 and CLCA4 act as tumor
suppressors in colon cancer. In addition, the multivariate survival
analysis validated the results of the univariate survival analysis,
except for CLCA4. The univariate survival analysis revealed that
the elevated expression of CLCA4 was associated with a favorable
prognosis, whereas, in the multivariate survival analysis, neither
low nor elevated expression of CLCA4 was observed to be associated
with OS time. This is likely due to the adjustment in the Cox
proportional hazards regression model, which suggested that CLCA4
is not likely to be an independent risk factor.
CLCA1 is primarily expressed in the large and small
intestines, in particular in the crypt cells (6). CLCA1 has also been reported to be
involved in the formation of the mucus and mucins of the goblet
cells, providing the foremost protection line of the
gastrointestinal tract, together with interacting with the immune
system (29,30). There have been several studies to
date, which have hypothesized that CLCA1 functions a tumor
suppressor in colorectal cancer (10,11,28). For
example, it has been reported that the elevated expression level of
CLCA1 has the potential to suppress the incidence of colorectal
cancer not only in vitro but also with in vivo
experiments, as it was associated with inhibiting the Wnt signaling
pathway, as well as EMT (10). In
addition, CLCA1 is likely to control the proliferation to the
differentiation transition of enterocytes through the regulation of
the Wnt/β-catenin signaling pathway, accordingly acting as a tumor
suppressor in colorectal tumorigenesis (11,31).
Similar to CLCA1, CLCA2 has also been confirmed to suppress
migration and invasiveness in breast and colorectal cancer cell
lines (13,32). Despite the fact that CLCA1 and CLCA2
have a similar domain structure, their amino acid conservation is
only almost 40%, and CLCA2 is primarily expressed in the trachea
(9,33). The tumor suppressor protein p53
substantially contributes to the prevention of human cancer and to
a transcription element activating and/or repressing its target
genes (34). As reported in the
present study, CLCA2 is a p53 target gene that regulates the
p53-induced apoptotic pathway (27).
CLCA2 has also been hypothesized to function as a p53-inducible
senescence mediator in regulating the senescence pathway, together
with carcinogenesis (35). Tumor
relapse is regarded as a pivotal prognostic factor for patients
with cancer (36). Early relapse
substantially reduced OS rates when compared with the non-early
recurrence cases in colon cancer (37). In the present study, patients in the
GSEA dataset with colon cancer revealed that high CLCA1 and CLCA2
expressions were associated with cancer relapse, and that CLCA2 had
a significant association with the p53 pathway, which was in line
with previous research (21,38). Meanwhile, GSEA will help in linking
prior knowledge to the newly generated data. This will ultimately
aid in elucidating the collective behavior of genes in states of
health and disease (21). In
addition, the GSEA dataset demonstrated that high expression levels
of CLCA1 and CLCA2 were associated with colon cancer relapse and
not survival. This may be associated with other prior studies
conducted on CLCA genes and relapse (15,31).
With the increase of newly generated data, the association between
CLCA and prognosis will appear in GSEA. The joint-effects analysis
in the present study revealed that high expression levels of CLCA1
and CLCA2 were associated with a favorable OS time in patients with
colon cancer. The nomogram prognostic evaluation framework was
capable of assessing the patient survival time that was
advantageous to the individualized therapy. In addition, the
expression of CLCA1 and CLCA2 was previously observed in colon
cancer, which indicated that the upregulated expression of these
two genes is likely to indicate a favorable prognosis in colon
cancer.
However, there were limitations to the present
investigation. Firstly, the medical information in the public
databases was not detailed, as not all of the 438 cases included
data on the specific tumor size and type. Therefore, confounding
factors that may impact the prognosis of patients were not factored
into the Cox proportional hazards regression model. Secondly, the
patient data used in the present study were entirely obtained from
a single source. Therefore, it is imperative to validate the
prognostic value of these genes in colon cancer with the help of
the independent external validation datasets that contain the
complete clinical information. Despite these limitations, the
present study, to the best of our knowledge, is the first to report
that the upregulation of CLCA1 and CLCA2 in colon cancer is
associated with a favorable prognosis, and that CLCA1 and CLCA2 are
potential prognostic biomarkers for patients with colon cancer.
In conclusion, the present study demonstrated that
high expression levels of CLCA1 and CLCA2 were individually and
mutually associated with a favorable prognosis for colon cancer.
CLCA1 and CLCA2 exhibit potential as prognostic
biomarkers for patients with colon cancer. However, these results
require confirmation in further investigations.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science
Universities Foundation (Guangxi, China; grant no. 2013ZD015).
Availability of data and materials
The datasets analyzed in the present study are all
available in TCGA (cancergenome.nih.gov).
Authors' contributions
XP and JG conceived and designed the study. XP and
QW processed the data and performed the statistical analysis. CX,
LY and SP wrote and revised the manuscript and helped to perform
the analysis and interpretation of data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CLCA
|
chloride channel accessory
|
|
OS
|
overall survival
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feo L, Polcino M and Nash GM: Resection of
the primary tumor in stage IV colorectal cancer: When is it
necessary? Surg Clin North Am. 97:657–669. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuipers EJ, Grady WM, Lieberman D,
Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ and Watanabe T:
Colorectal cancer. Nat Rev Dis Primers. 1:150652015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gruber AD, Elble RC, Ji HL, Schreur KD,
Fuller CM and Pauli BU: Genomic cloning, molecular
characterization, and functional analysis of human CLCA1, the first
human member of the family of Ca2+-activated Cl-channel proteins.
Genomics. 54:200–214. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gandhi R, Elble RC, Gruber AD, Schreur KD,
Ji HL, Fuller CM and Pauli BU: Molecular and functional
characterization of a calcium-sensitive chloride channel from mouse
lung. J Biol Chem. 273:32096–32101. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pauli BU, Abdel-Ghany M, Cheng HC, Gruber
AD, Archibald HA and Elble RC: Molecular characteristics and
functional diversity of CLCA family members. Clin Exp Pharmacol
Physiol. 27:901–905. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loewen ME and Forsyth GW: Structure and
function of CLCA proteins. Physiol Rev. 85:1061–1092. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel AC, Brett TJ and Holtzman MJ: The
role of CLCA proteins in inflammatory airway disease. Annu Rev
Physiol. 71:425–449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Hu W, Zhou J, Huang Y, Peng J, Yuan
Y, Yu J and Zheng S: CLCA1 suppresses colorectal cancer
aggressiveness via inhibition of the Wnt/beta-catenin signaling
pathway. Cell Commun Signal. 15:382017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang B, Cao L, Liu B, McCaig CD and Pu J:
The transition from proliferation to differentiation in colorectal
cancer is regulated by the calcium activated chloride channel A1.
PLoS One. 8:e608612013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gruber AD and Pauli BU: Tumorigenicity of
human breast cancer is associated with loss of the Ca2+-activated
chloride channel CLCA2. Cancer Res. 59:5488–5491. 1999.PubMed/NCBI
|
|
13
|
Sasaki Y, Koyama R, Maruyama R, Hirano T,
Tamura M, Sugisaka J, Suzuki H, Idogawa M, Shinomura Y and Tokino
T: CLCA2, a target of the p53 family, negatively regulates cancer
cell migration and invasion. Cancer Biol Ther. 13:1512–1521. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agnel M, Vermat T and Culouscou JM:
Identification of three novel members of the calcium-dependent
chloride channel (CaCC) family predominantly expressed in the
digestive tract and trachea. FEBS Lett. 455:295–301. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu Y, Walia V and Elble RC: Loss of CLCA4
promotes epithelial-to-mesenchymal transition in breast cancer
cells. PLoS One. 8:e839432013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carithers LJ, Ardlie K, Barcus M, Branton
PA, Britton A, Buia SA, Compton CC, DeLuca DS, Peter-Demchok J,
Gelfand ET, et al: A novel approach to high-quality postmortem
tissue procurement: The GTEx project. Biopreserv Biobank.
13:311–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shaul YD, Yuan B, Thiru P, Nutter-Upham A,
McCallum S, Lanzkron C, Bell GW and Sabatini DM: MERAV: A tool for
comparing gene expression across human tissues and cell types.
Nucleic Acids Res. 44(D1): D560–D566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Warde-Farley D, Donaldson SL, Comes O,
Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT,
et al: The GeneMANIA prediction server: Biological network
integration for gene prioritization and predicting gene function.
Nucleic Acids Res 38 (Web Server Issue). W214–W220. 2010.
View Article : Google Scholar
|
|
19
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pardo LA and Stühmer W: The roles of K(+)
channels in cancer. Nat Rev Cancer. 14:39–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cuddapah VA and Sontheimer H: Ion channels
and transporters [corrected] in cancer. 2. Ion channels and the
control of cancer cell migration. Am J Physiol Cell Physiol.
301:C541–C549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peretti M, Angelini M, Savalli N, Florio
T, Yuspa SH and Mazzanti M: Chloride channels in cancer: Focus on
chloride intracellular channel 1 and 4 (CLIC1 AND CLIC4) proteins
in tumor development and as novel therapeutic targets. Biochim
Biophys Acta. 1848:2523–2531. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoshino M, Morita S, Iwashita H, Sagiya Y,
Nagi T, Nakanishi A, Ashida Y, Nishimura O, Fujisawa Y and Fujino
M: Increased expression of the human Ca2+-activated Cl- channel 1
(CaCC1) gene in the asthmatic airway. Am J Respir Crit Care Med.
165:1132–1136. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim JA, Kang YS and Lee YS: Role of
Ca2+-activated Cl-channels in the mechanism of apoptosis induced by
cyclosporin A in a human hepatoma cell line. Biochem Biophys Res
Commun. 309:291–297. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Walia V, Ding M, Kumar S, Nie D, Premkumar
LS and Elble RC: hCLCA2 Is a p53-inducible inhibitor of breast
cancer cell proliferation. Cancer Res. 69:6624–6632. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bustin SA, Li SR and Dorudi S: Expression
of the Ca2+-activated chloride channel genes CLCA1 and CLCA2 is
downregulated in human colorectal cancer. DNA Cell Biol.
20:331–338. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pelaseyed T, Bergström JH, Gustafsson JK,
Ermund A, Birchenough GM, Schütte A, van der Post S, Svensson F,
Rodríguez-Piñeiro AM, Nyström EE, et al: The mucus and mucins of
the goblet cells and enterocytes provide the first defense line of
the gastrointestinal tract and interact with the immune system.
Immunol Rev. 260:8–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nyström EEL, Birchenough GMH, van der Post
S, Arike L, Gruber AD, Hansson GC and Johansson MEV:
Calcium-activated chloride channel regulator 1 (CLCA1) controls
mucus expansion in colon by proteolytic activity. EBioMedicine.
33:134–143. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang B, Cao L, Liu J, Xu Y, Milne G, Chan
W, Heys SD, McCaig CD and Pu J: Low expression of chloride channel
accessory 1 predicts a poor prognosis in colorectal cancer. Cancer.
121:1570–1580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ramena G, Yin Y, Yu Y, Walia V and Elble
RC: CLCA2 interactor EVA1 is required for mammary epithelial cell
differentiation. PLoS One. 11:e01474892016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sharma A, Ramena G, Yin Y, Premkumar L and
Elble RC: CLCA2 is a positive regulator of store-operated calcium
entry and TMEM16A. PLoS One. 13:e01965122018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kruse JP and Gu W: Modes of p53
regulation. Cell. 137:609–622. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanikawa C, Nakagawa H, Furukawa Y,
Nakamura Y and Matsuda K: CLCA2 as a p53-inducible senescence
mediator. Neoplasia. 14:141–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Herr HW and Donat SM: Prostatic tumor
relapse in patients with superficial bladder tumors: 15-year
outcome. J Urol. 161:1854–1857. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu CY, Uen YH, Tsai HL, Chuang SC, Hou MF,
Wu DC, Juo SH, Lin SR and Wang JY: Molecular detection of
persistent postoperative circulating tumour cells in stages II and
III colon cancer patients via multiple blood sampling: Prognostic
significance of detection for early relapse. Br J Cancer.
104:1178–1184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Barrier A, Lemoine A, Boelle PY, Tse C,
Brault D, Chiappini F, Breittschneider J, Lacaine F, Houry S,
Huguier M, et al: Colon cancer prognosis prediction by gene
expression profiling. Oncogene. 24:6155–6164. 2005. View Article : Google Scholar : PubMed/NCBI
|