Introduction
At present, breast cancer is the most common type of
cancer in women and one of the leading causes of cancer-associated
mortality in women worldwide, despite improvements in diagnostic
techniques and treatment modalities (1). The progression and metastasis of breast
cancer are the main causes of mortality. Based on cellular markers
reflecting the available targeted therapies, breast cancer is
characterised as oestrogen receptor (ER)- or progesterone receptor
(PR)-positive, human epidermal growth factor receptor 2
(HER2)-positive and triple-negative breast cancer (TNBC) (2). Targeted therapy can be implemented for
ER/PR-positive and HER2-positive breast cancer; however, since TNBC
is defined by the absence of the ER, PR and HER2 genes, there is no
standard treatment for TNBC at present (3). The lack of targeted therapies and the
poor prognosis in patients with TNBC has prompted major research
efforts to identify new molecular targets of TNBC.
Studies have shown that functional changes in the
immune system play an important role in the occurrence and
development of carcinoma, where immunotherapy was a breakthrough
point in cancer treatment (4). CD47,
also known as integrin-associated protein, is a transmembrane
protein that is highly expressed on the surface of various cancer
cells. It interacts with thrombospondin-1, signal-regulatory
protein-α (SIRP-α) and other proteins to regulate various cellular
functions, such as T cell activation and cell migration (5). Emerging evidence has indicated the
function of CD47 as a dominant anti-engulfment signal on tumour
cells, by binding with SIRP-α on phagocytic immune cells to prevent
engulfment (6–8). Given that the expression of CD47 was
inhibited in certain malignant tumours, the blockade of the CD47
anti-engulfment signal increased the phagocytosis of tumour cells
in solid tumours and haematological malignancies (6). Therefore, CD47 is a protective
therapeutic target on solid tumour cells.
Although breast cancer was not considered an
immunogenic malignant tumour, certain studies have revealed an
association between the intratumoural immune response and tumour
progression (9–11). Since TNBC and HER2-positive breast
cancer are highly proliferative, increased genomic instability and
mutational burden may result in the exposure of a large number of
tumour antigens and promote the anti-tumour immune response
(12). Thus, TNBC and HER2-positive
breast cancer were considered to be more immunogenic than other
types of breast cancer (12).
Although studies have shown the abnormal expression of CD47 in
breast cancer cell lines, breast cancer stem cells, bone marrow,
peripheral blood and the circulating tumour cells of breast cancer
(13–17), most of these studies focused on the
association between CD47 and breast cancer at the cytological
level. There is little direct evidence of the expression of CD47 in
breast cancer solid tumours, particularly in TNBC solid tumours,
and its association with metastasis, survival rate and prognosis
have rarely been reported.
Epithelial-mesenchymal transition (EMT) is a
favourable explanation for the distant metastasis of breast cancer
(18). The essential characteristics
of EMT include the destruction of tight junctions and the loss of
cell-to-cell contact, which leads to the loss of epithelial
characteristics (such as the loss of the epithelial cell adhesion
protein E-cadherin) and the acquisition of a mesenchymal morphology
(such as the gain of the mesenchyme-associated molecule N-cadherin)
(18). EMT can be induced by a
variety of stimulants, such as cytokines and growth factor
signalling molecules, and transforming growth factor-β (TGF-β) is
strongly involved in the induction of EMT in epithelial cells
(including breast cancer epithelial cells) (19). However, the association between the
expression of CD47 and EMT in breast cancer was rarely reported. In
the present study, the expression of CD47 and its prognostic
significance in TNBC solid tumours was evaluated, as well as the
association between CD47 and EMT, in order to explore novel
mechanisms for the development of TNBC therapies.
Materials and methods
Tissues
Formalin-fixed, paraffin-embedded tissue samples
from 57 patients with TNBC, who underwent surgery for primary
breast cancer, were randomly selected from the medical records of
the Department of Pathology, Renmin Hospital of Wuhan University
between August 2009-December 2010. The inclusion criteria were as
follows: i) Received no treatment prior to surgery; and ii) female.
Patient ages were in the range of 31–69 years (mean, 50.2 years;
median, 42 years). Major clinicopathological parameters, including
age, menopausal status, tumour-node-metastasis (TNM) stage,
histological grade, lymph node metastasis and recurrence, are
summarized in Table II. TNM stage
was grouped according to the American Joint Committee on Cancer 7th
Edition Cancer Staging Manual 2010 (20). The histological grade was classified
in accordance with the 4th edition of the WHO histological grading
(21). Each patient was followed up
for 5 years after surgery and the survival times were recorded. A
total of 40 cases of benign breast lesions were also randomly
selected from Department of Pathology, Renmin Hospital of Wuhan
University between August 2009 and December 2010 as controls. The
ages were in the range of 19–64 years, with a mean age of 35 years.
Written informed consent was obtained from the patients, and the
study was approved by the Ethics Committee of Renmin Hospital of
Wuhan University.
 | Table II.Association between CD47 protein
expression and clinicopathological parameters of patients with
triple-negative breast cancer. |
Table II.
Association between CD47 protein
expression and clinicopathological parameters of patients with
triple-negative breast cancer.
|
|
| CD47 protein
expression |
|---|
|
|
|
|
|---|
| Clinicopathological
parameters | All cases, n | Negative, n | Positive, n
(%) | χ2 | P-value |
|---|
| Age, years |
|
|
| 0.888 | 0.346 |
|
<50 | 33 | 9 | 24 (72.7) |
|
|
|
>50 | 24 | 4 | 20 (83.3) |
|
|
| Menopause |
|
|
| 0.150 | 0.698 |
|
After | 28 | 7 | 21 (75.0) |
|
|
|
Before | 29 | 6 | 23 (79.3) |
|
|
| TNM |
|
|
| 7.241 | 0.027 |
| I | 1 | 1 | 0 (0) |
|
|
| II | 38 | 11 | 27 (71.1) |
|
|
|
III | 18 | 1 | 17 (94.4) |
|
|
| Histological
grade |
|
|
| 0.331 | 0.848 |
| G1 | 6 | 1 | 5 (83.3) |
|
|
| G2 | 27 | 7 | 20 (74.1) |
|
|
| G3 | 24 | 5 | 19 (79.2) |
|
|
| Lymph node
metastasis |
|
|
| 4.403 | 0.036 |
|
Yes | 32 | 4 | 28 (87.5) |
|
|
| No | 25 | 9 | 16 (64.0) |
|
|
| Recurrence |
|
|
| 5.900 | 0.015 |
|
Yes | 30 | 3 | 27 (83.3) |
|
|
| No | 27 | 10 | 17 (70.4) |
|
|
Immunohistochemistry (IHC)
IHC was performed to detect CD47, E-cadherin,
N-cadherin and TGF-β. Formalin-fixed, paraffin-embedded tissue
samples were cut into 4 µm sections and then deparaffinised with
xylene twice for 10 min and rehydrated with 100% ethanol twice for
5 min, 95% ethanol twice for 2 min and 85% ethanol for 2 min at
room temperature. Following that, the sections were blocked with 3%
H2O2 for 10 min at room temperature to block
endogenous peroxidase activity and then subjected to antigen
retrieval in citrate buffer (pH 6.0) at 98°C for 15 min. This was
followed by incubation with primary antibodies: Anti-CD47 (1:100;
cat. no. sc-12730; Santa Cruz Biotechnology, Inc.); anti-E-cadherin
(1:100; cat. no. ab1416; Abcam); anti-N-cadherin (1:500; cat. no.
ab18203; Abcam); or anti-TGF-β (1:100; sc-130348; Santa Cruz
Biotechnology, Inc.), for 1 h at 37°C. The slides were incubated
with a biotinylated secondary antibody using the Dako LSAB2
system-horseradish peroxidase (cat. no. K0672; Agilent
Technologies, Inc.) for 30 min at 37°C. The reaction products were
stained with 3,3′-diaminobenzidine at room temperature for 2–3 min
and lightly counterstained with haematoxylin at room temperature
for 2 min. Sections incubated in PBS without a primary antibody
were used as a blank control. The slides were examined under an
Olympus light microscope (Olympus Corporation) at ×200 and ×400
magnification.
Staining evaluation
Immunohistochemical staining of all sections were
evaluated by two independent pathologists (JY and HH). The
evaluating pathologists were blinded to the clinical data. In the
present study, the expression of CD47 was mainly localised to the
cytoplasm or cell membrane, which appeared yellow or brown. The
degree of CD47 reactivity was scored by applying a
semi-quantitative immunoreactivity scoring (IRS) system as
described by Baccelli et al (13). The staining intensity was categorized
into four grades: 0, no immunostaining; 1, weak staining; 2,
moderate staining; and 3, strong staining. The percentage of
positive cells was categorized into five grades: 0, 0%; 1, 1–10%;
2, 11–50%; 3, 51–80%; and 4, >80%. The staining intensity and
percentage of positive cells were multiplied to obtained an IRS, in
the range of 0–12 for each individual case. A case was scored as
positive for CD47 with an IRS between 7 and 12 and negative with an
IRS between 0–6 (13).
E-cadherin-positive cells contained yellow or dark
brown granules on the cell membrane. N-cadherin-positive cells
exhibited a yellow or dark brown colour in the cytoplasm or at the
cellular membrane. According to the method described by Nakajima
et al (22) and Liu et
al (23), E-cadherin expression
was considered to be normal if ≥90% of cancer cells exhibited a
staining pattern similar to that in normal epithelial cells, and
sections with <90% of the cancer cells stained or with a
complete absence of staining were classified as a reduced pattern.
Therefore, ≥90% indicated positivity and <90% indicated
negativity for E-cadherin expression. Similarly, according to the
method described by Liu et al (23), the proportion of positive cells ≥5%
indicated positivity, whereas <5% indicated negativity for
N-cadherin expression.
TGF-β was primarily located in the cytoplasm or
nucleus and was indicated by a yellow or brown colour. The
immunohistochemical staining of tumour cells was evaluated
semi-quantitatively, as described by Chen et al (24), based on: i) Staining intensity, where
0, No staining; 1, weak staining; 2, moderate staining; and 3,
strong staining; and ii) extent of the staining, where 0, ≤5%; 1,
6–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%. The final score was
obtained by multiplying the scores for both the staining intensity
and the extent of the staining; a final score ≤2 indicated negative
staining, and >2 indicated positive staining.
Statistical analysis
The χ2 test was used to compare the CD47
expression between TNBC tissues and benign breast lesions, analyse
the association between CD47 expression and the clinicopathological
parameters, and evaluate the association between CD47 expression
and EMT markers. Kaplan-Meier analysis and the log-rank test were
used for survival analysis. The prognostic value of CD47 was
estimated by univariate and multivariate Cox proportional hazard
regression analysis. All statistical analyses were performed with
SPSS software version 19.0 (IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference. Data are presented
as the mean ± standard error of the mean. The experiments were
repeated in triplicate.
Results
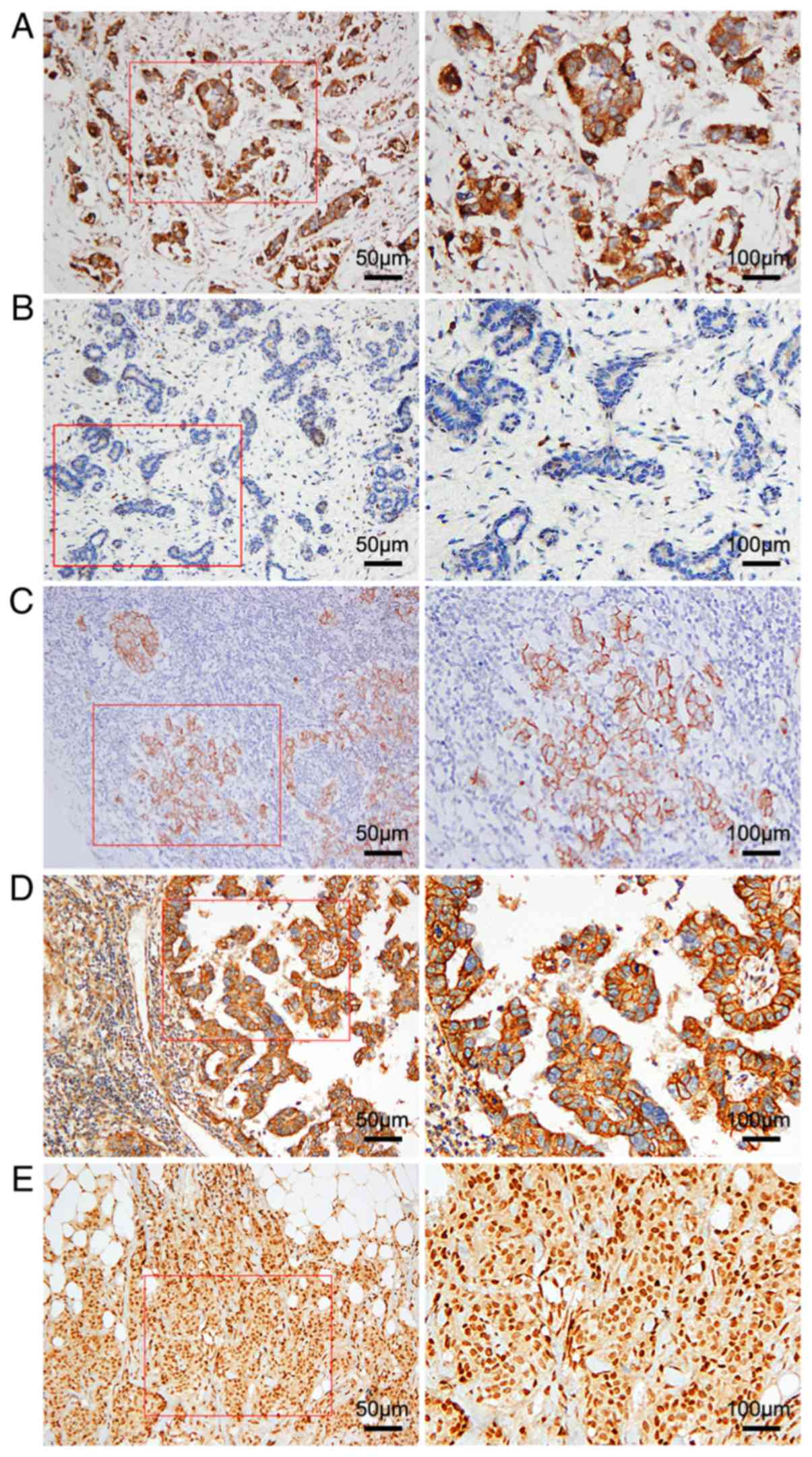
CD47 is highly expressed in TNBC
tissues
The expression of CD47 was detected in 40 benign
breast lesions and 57 TNBC tissues (Fig.
1A and B). In TNBC tissues, CD47 was mainly expressed in the
cytoplasm or at the cell membrane in brown granules (Fig. 1A), and positive immunohistochemical
expression of CD47 was found in 44/57 (77.2%) cases. The
immunoreactivity for CD47 in benign breast lesions was negative or
weakly positive in the cytoplasm (Fig.
1B). The CD47-positive rate in 40 cases of benign breast
lesions was 30% (12/40 cases). The CD47-positive rate was
significantly higher in TNBC tissues than in benign breast lesions
(χ2=21.453; P<0.001; Table
I).
 | Table I.Expression of CD47 protein in breast
benign lesions and triple-negative breast cancer tissues. |
Table I.
Expression of CD47 protein in breast
benign lesions and triple-negative breast cancer tissues.
|
|
| CD47 protein
expression |
|---|
|
|
|
|
|---|
| Groups | All cases, n | Positive, n
(%) | χ2 | P-value |
|---|
| Breast benign
lesions | 40 | 12 (30.0) | 21.453 | <0.001 |
| Triple-negative
breast cancer tissues | 57 | 44 (77.2) |
|
|
Association between CD47 expression
and clinicopathological parameters of TNBC
The association between CD47 expression and
clinicopathological parameters were further analysed in 57 TNBC
tissues (Table II). Positive
staining for CD47 was significantly associated with advanced TNM
stage (χ2=7.241; P=0.027), lymph node involvement
(χ2=4.403; P=0.036) and recurrence (χ2=5.900;
P=0.015). The CD47-positive rate increased with increasing TNM
stage (0, 71.1 and 94.4%). The CD47-positive rate in patients with
lymph node metastasis or recurrence was significantly higher than
that in patients without lymph node metastasis or recurrence. There
was no significant association between positive staining for CD47
and other clinicopathological parameters, such as patient age,
menopause and histological grade (P>0.05).
Association between CD47 and EMT
markers
Currently, EMT is a favourable explanation for the
distant metastasis of epithelial cancers, including TNBC. The loss
of E-cadherin and the gain of N-cadherin expression are markers of
EMT. Consistent with previous reports, the expression of E-cadherin
was decreased (Fig. 1C), whereas the
expression of N-cadherin was increased (Fig. 1D) in TNBC tissues. The TGF-β pathway
has been strongly implicated in inducing EMT in epithelial cells
(17). Thus, the expression of TGF-β
was also examined in TNBC tissues (Fig.
1E).
The association between the expression of CD47 and
EMT markers was investigated (Table
III). The expression of CD47 was associated with decreased
expression of E-cadherin (χ2=4.414; P=0.036) and
increased expression of N-cadherin (χ2=9.216; P=0.002).
CD47 expression was associated with increased expression of TGF-β
(χ2=8.093; P=0.004). These results suggested that the
expression of CD47 may be involved in the process of EMT in
TNBC.
 | Table III.Association between the expression of
CD47 and other proteins. |
Table III.
Association between the expression of
CD47 and other proteins.
|
| CD47 protein
expression |
|
|
|---|
|
|
|
|
|
|---|
| Protein | Positive | Negative | χ2 |
P-valuea |
|---|
| E-cadherin |
|
| 4.414 | 0.036 |
|
Positive, n | 13 | 8 |
|
|
|
Negative, n | 31 | 5 |
|
|
|
Positive rate, % | 29.55 | 61.54 |
|
|
| N-cadherin |
|
| 9.216 | 0.002 |
|
Positive, n | 38 | 6 |
|
|
|
Negative, n | 6 | 7 |
|
|
|
Positive rate, % | 86.36 | 46.15 |
|
|
| TGF-β |
|
| 8.093 | 0.004 |
|
Positive, n | 35 | 5 |
|
|
|
Negative, n | 9 | 8 |
|
|
|
Positive rate, % | 79.55 | 38.46 |
|
|
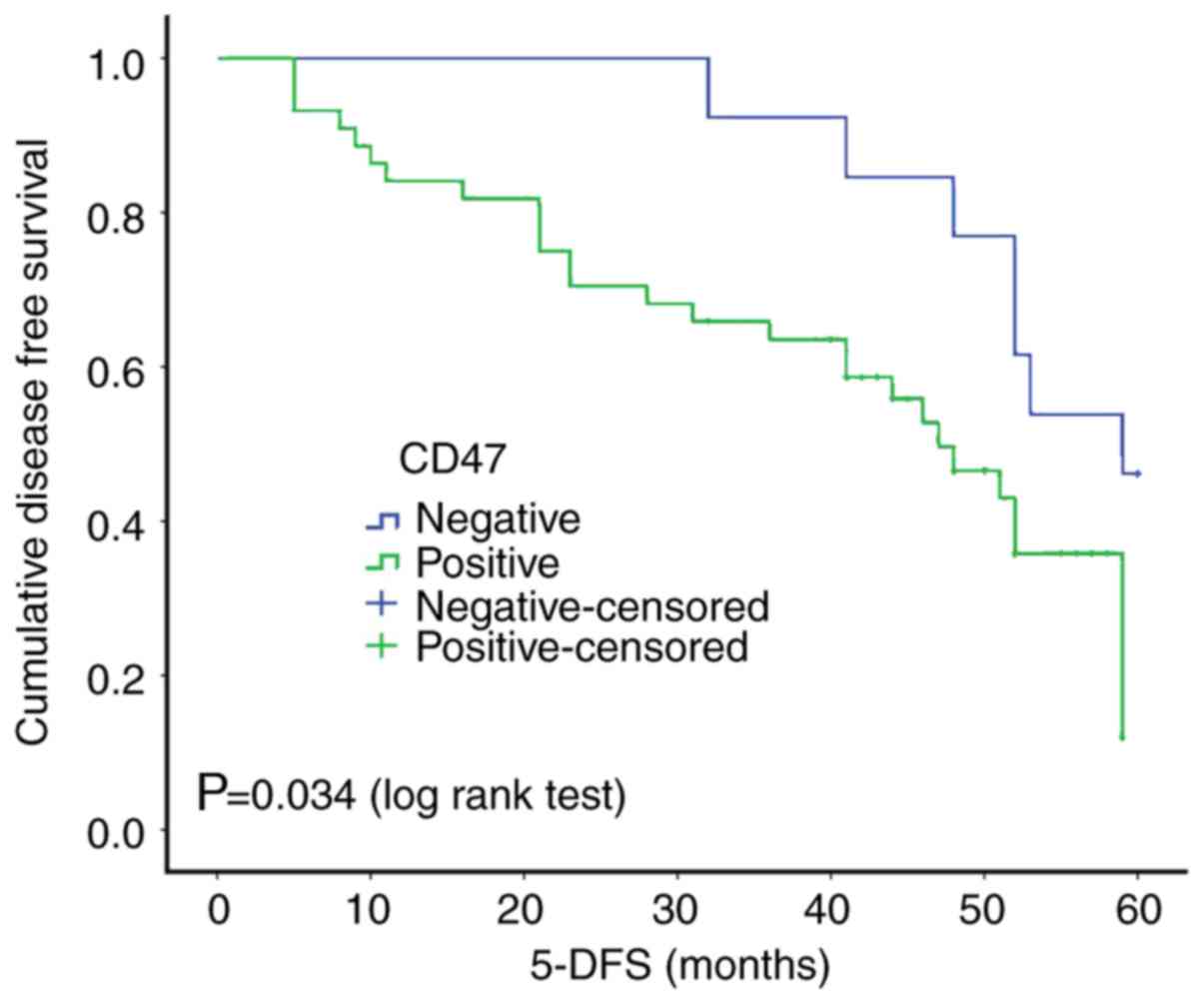
Expression of CD47 is a marker of poor
prognosis in TNBC
The prognostic value of CD47 was determined in 57
TNBC cases, following a 5-year follow-up. Kaplan-Meier analysis and
the log-rank test showed a statistically significant association
between a high expression level of CD47 and a low 5-year
disease-free survival (5-DFS) time (P=0.034; Fig. 2), indicating that a high expression
level of CD47 may serve as a novel marker of TNBC with a poor
clinical outcome. To further validate the prognostic significance
of this new parameter, CD47 and all clinicopathological features,
including age, menopausal status, TNM stage, histological grade,
lymph node metastasis and recurrence, were subjected to univariate
and multivariate Cox proportional hazard regression analysis. As
presented in Table IV, univariate
Cox proportional hazard regression analysis confirmed that an
advanced TNM stage (P<0.001; HR=5.964; 95% CI=2.709–13.129), the
presence of lymph node metastasis (P<0.001; HR=4.596; 95%
CI=2.096–10.079), recurrence (P<0.001; HR=64.448; 95%
CI=9.512–436.670), and positive CD47 expression (P=0.042; HR=2.464;
95% CI=1.032–5.882) were significantly associated with a poor 5-DFS
time in patients with TNBC. Multivariate statistical analysis
confirmed that recurrence (P<0.001; HR=91.009; 95%
CI=9.950–832.400) and positive CD47 expression (P=0.037; HR=3.432;
95% CI=1.079–10.922) were significant independent predictors of
poor 5-DFS time.
 | Table IV.Univariate and multivariate Cox
proportional hazard regression analysis of 5-year disease-free
survival times of patients with triple-negative breast cancer. |
Table IV.
Univariate and multivariate Cox
proportional hazard regression analysis of 5-year disease-free
survival times of patients with triple-negative breast cancer.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
|
|
| 95% CI |
|
| 95% CI |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Variable | HR | Lower | Upper | P-value | HR | Lower | Upper | P-value |
|---|
| Age (≤50 vs.
>50) | 1.113 | 0.561 | 2.207 | 0.760 | 0.372 | 0.098 | 1.414 | 0.147 |
| Menopause (after
vs. before) | 0.698 | 0.355 | 1.373 | 0.298 | 0.386 | 0.111 | 1.340 | 0.134 |
| TNM stage (I and II
vs. III) | 5.964 | 2.709 | 13.129 | <0.001 | 1.361 | 0.524 | 3.531 | 0.527 |
| Histological grade
(grade 1 and 2 vs. grade 3) | 1.551 | 0.790 | 3.048 | 0.203 | 1.594 | 0.760 | 3.340 | 0.217 |
| Lymph node
metastasis (no vs. yes) | 4.596 | 2.096 | 10.079 | <0.001 | 2.255 | 0.814 | 6.243 | 0.118 |
| Recurrence (no vs.
yes) | 64.448 | 9.512 | 436.670 | <0.001 | 91.009 | 9.950 | 832.400 | <0.001 |
| CD47 (negative vs.
positive) | 2.464 | 1.032 | 5.882 | 0.042 | 3.432 | 1.079 | 10.922 | 0.037 |
Discussion
TNBC accounts for 15% of all breast cancer cases and
is characterised by early relapse and metastasis (25). The ability of cancer cells to escape
the innate and adaptive immune systems plays a key role in the
formation of secondary (recurrent and/or metastatic) tumours
(26,27). Lehmann et al (28) reported that the loss of immune
infiltration in tumours was associated with mortality in patients
with TNBC. One of the main mechanisms regulating the escape of
cancer cells from innate immunity is the expression of CD47, which
interacts with SIRP-α on macrophages to prevent phagocytosis.
Shuptrine et al (29)
identified 709 genes that selectively regulated adaptive
anti-tumour immunity in a syngeneic TNBC model, by developing an
unbiased, in vivo, genome-wide RNA interference screening
platform. Of the five genes with the greatest impact identified by
the screening, CD47 had the greatest impact on the immune
regulatory pathway (29).
CD47 was first described as a tumour antigen in
human ovarian cancer in the 1980s (30). Since then, CD47 has been found to be
highly expressed in various types of human cancer, including
non-Hodgkin's lymphoma (31), acute
myeloid leukaemia (32),
hepatocellular carcinoma (33,34) and
bladder cancer (35), and its
expression is closely associated with the differentiation,
metastasis, survival and prognosis of tumours. Accordingly, CD47 is
considered a biomarker of cancer, and its high expression is an
indicator of poor clinical prognosis. In breast cancer, the
abnormal expression of CD47 has been detected in various breast
cancer-associated cells, including in breast cancer cell lines
(36), breast cancer stem cells
(17), bone marrow (14), peripheral blood (14) and circulating tumour cells (13,16) of
breast cancer. However, most studies have evaluated the association
between CD47 and breast cancer at the cytological level, and few
have reported on the expression of CD47 in solid breast cancer
tumours, particularly TNBC. The present study was the first, to the
best of our knowledge, to reveal the overexpression of CD47 in TNBC
solid tumour tissues compared with its expression in benign breast
lesions.
CD47 is a novel prognostic biomarker of certain
malignant tumours. In circulating colorectal cancer cells, the
upregulation of the CD47 gene was associated with distant
metastasis as a potential immune escape mechanism (37). In non-small cell lung cancer,
increased CD47 expression was associated with clinical staging, T
classification, lymph node metastasis and distant metastasis
(38). Consistent with the above
reports, the overexpression of CD47 in TNBC was significantly
associated with TNM stage, lymph node involvement and recurrence in
the present study. In addition, high CD47 expression levels have
been reported to be an unfavourable independent prognostic factor
for 5-DFS time (14). A previous
study on patients with breast cancer indicated the low survival
rate of patients with high CD47 expression in the bone marrow and
circulating tumour cells than that of patients with low CD47
expression (14). In the present
study, Kaplan-Meier analysis and the log-rank test demonstrated a
poor 5-DFS time in patients with TNBC and high expression levels of
CD47. A study by Baccelli et al (13) demonstrated the co-expression of
MET-CD47 as an independent prognostic factor for the overall
survival (OS) of patients with breast cancer, and that this was
associated with lymph node metastasis. It was found that the OS of
patients with MET-CD47 double-positive luminal breast cancer was
10.3 years lower than in those with MET-CD47 double-negative
expression. Further investigation in the present study, through
multivariate statistical analysis, indicated the expression of CD47
in TNBC as an independent significant predictor of poor 5-DFS time.
Overall, these findings revealed the potential of CD47 as a
prognostic marker in TNBC.
High expression of CD47 in various tumours indicates
poor prognosis; however, the underlying mechanism of the
transcriptional regulation of CD47 remains unclear. EMT is
regulated by a series of EMT-induced transcription factors that
promote immune escape and drug resistance (39). Noman et al (40) reported the upregulation of CD47 in
various EMT-activated human breast cancer cells and in the
inhibition of phagocytosis. Li et al (41) demonstrated the induction of EMT by
CD47, through the modulation of E-cadherin and N-cadherin, which
contribute to the invasion of high-grade serous ovarian carcinoma.
This suggests a potential association between CD47 expression and
EMT. Consequently, the association between CD47 expression in TNBC
and several markers of EMT was evaluated in the present study. The
expression of CD47 was associated with high expression of
N-cadherin and TGF-β, in contrast with decreased expression of
E-cadherin. Thus, the expression of CD47 may be involved in the
process of EMT in TNBC. Mechanistically, SNAI1 and ZEB1 have been
reported to be major regulators of CD47 (40). The overexpression of SNAI1 or ZEB1 in
human breast cancer epithelial cells results in the activation of
EMT and the upregulation of CD47, by binding directly to the
E-box-2 and E-box-3 motifs of the human CD47 proximal promoter
(40). This indicates an
EMT-dependent upregulation of CD47 in breast cancer. On the other
hand, Shinohara et al (42)
demonstrated the co-localization of CD47 with E-cadherin at
cell-cell adhesion sites in mouse breast cancer-derived epithelial
cells. Since E-cadherin was known to be involved in the actin
cytoskeleton of epithelial cells, CD47 was found to reorganize the
actin cytoskeleton to participate in the regulation of cell-cell
adhesion and cell migration (42).
Based on the findings of the present study and those from the
literature, the involvement of CD47 in the process of EMT in TNBC
is speculated. Whether CD47 promotes EMT in epithelial cells, or
whether EMT-associated transcription factors or proteins regulate
the expression of CD47, is yet to be determined.
At present, there are few studies on the expression
of CD47 in TNBC solid tumours and its association with EMT. In the
present study, the overexpression of CD47 was demonstrated in TNBC,
which was associated with advanced TNM stage, lymph node
involvement, recurrence and a reduced 5-DFS time. In addition, the
expression of CD47 was associated with several EMT markers in TNBC.
Overall, these findings suggest the potential of CD47 as a
prognostic marker and therapeutic target for TNBC.
However, some limitations of the present study
should be acknowledged. Firstly, the sample size is small. The
sample number will be increased in order to explore the role and
mechanism of CD47 in the progression of breast cancer in the
future. Secondly, the association between CD47 expression and
prognosis was evaluated using 5-DFS data, rather than OS. Since DFS
is not directly associated with OS, the effect of CD47 expression
on OS remains a topic for future studies. Therefore, the results of
the study should be interpreted with caution, and further
validation is warranted with a larger sample size and longer
clinical follow-up time.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from: The National
Natural Science Foundation of China (grant no. 31600866); the
Science and Technology Planning Project of Wuhan (grant no.
2017060201010172); and the Guidance Foundation of Renmin Hospital
of Wuhan University (grant no. RMYD2018M27).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CC, LL and JW collected the samples and performed
the statistical analysis; JY and HH evaluated the
immunohistochemical staining of all sections; JY, XS and HY
designed the study and wrote the manuscript; HY revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Renmin Hospital of Wuhan University (Wuhan, China)
(approval no. WDRY2019-K010). Patients provided written informed
consent for the use of their tissues in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hortobagyi GN, de la Garza Salazar J,
Pritchard K, Amadori D, Haidinger R, Hudis CA, Khaled H, Liu MC,
Martin M, Namer M, et al: The global breast cancer burden:
Variations in epidemiology and survival. Clin Breast Cancer.
6:391–401. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kumar P and Aggarwal R: An overview of
triple-negative breast cancer. Arch Gynecol Obstet. 293:247–269.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Engebraaten O, Vollan HKM and
Borresen-Dale AL: Triple-negative breast cancer and the need for
new therapeutic targets. Am J Pathol. 183:1064–1074. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Couzin-Frankel J: Breakthrough of the year
2013. Cancer immunotherapy. Science. 342:1432–1433. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu XJ, Kwon H, Li ZH and Fu YX: Is CD47
an innate immune checkpoint for tumor evasion? J Hematol Oncol.
10:122017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McCracken MN, Cha AC and Weissman IL:
Molecular pathways: Activating T cells after cancer cell
phagocytosis from blockade of CD47 ‘don't eat me’ signals. Clin
Cancer Res. 21:3597–3601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Willingham SB, Volkmer JP, Gentles AJ,
Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin
R, Cohen JD, et al: The CD47-signal regulatory protein alpha
(SIRPa) interaction is a therapeutic target for human solid tumors.
Proc Natl Acad Sci USA. 109:6662–6667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vonderheide RH: CD47 blockade as another
immune checkpoint therapy for cancer. Nat Med. 21:1122–1123. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gingras I, Azim HA Jr, Ignatiadis M and
Sotiriou C: Immunology and breast cancer: Toward a new way of
understanding breast cancer and developing novel therapeutic
strategies. Clin Adv Hematol Oncol. 13:372–382. 2015.PubMed/NCBI
|
|
10
|
Vonderheide RH, Domchek SM and Clark AS:
Immunotherapy for breast cancer: What are we missing? Clin Cancer
Res. 23:2640–2646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santa-Maria CA, Park SJ, Jain S and
Gradishar WJ: Breast cancer and immunology: Biomarker and
therapeutic developments. Expert Rev Anticancer Ther. 15:1215–1222.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bianchini G, Balko JM, Mayer IA, Sanders
ME and Gianni L: Triple-negative breast cancer: Challenges and
opportunities of a heterogeneous disease. Nat Rev Clin Oncol.
13:674–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baccelli I, Stenzinger A, Vogel V,
Pfitzner BM, Klein C, Wallwiener M, Scharpff M, Saini M,
Holland-Letz T, Sinn HP, et al: Co-expression of MET and CD47 is a
novel prognosticator for survival of luminal breast cancer
patients. Oncotarget. 5:8147–8160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagahara M, Mimori K, Kataoka A, Ishii H,
Tanaka F, Nakagawa T, Sato T, Ono S, Sugihara K and Mori M:
Correlated expression of CD47 and SIRPA in bone marrow and in
peripheral blood predicts recurrence in breast cancer patients.
Clin Cancer Res. 16:4625–4635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bener G, J Félix A, Sánchez de Diego C,
Pascual Fabregat I, Ciudad CJ and Noé V: Silencing of CD47 and
SIRPα by Polypurine reverse hoogsteen hairpins to promote MCF-7
breast cancer cells death by PMA-differentiated THP-1 cells. BMC
Immunol. 17:322016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baccelli I, Schneeweiss A, Riethdorf S,
Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bauerle T,
Wallwiener M, et al: Identification of a population of blood
circulating tumor cells from breast cancer patients that initiates
metastasis in a xenograft assay. Nat Biotechnol. 31:539–544. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kaur S, Elkahloun AG, Singh SP, Chen QR,
Meerzaman DM, Song T, Manu N, Wu W, Mannan P, Garfield SH and
Roberts DD: A function-blocking CD47 antibody suppresses stem cell
and EGF signaling in triple-negative breast cancer. Oncotarget.
7:10133–10152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Sarkissyan M and Vadgama JV:
Epithelial-mesenchymal transition and breast cancer. J Clin Med.
5:E132016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Massagué J: TGFβ signalling in context.
Nat Rev Mol Cell Bio. 13:616–630. 2012. View Article : Google Scholar
|
|
20
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual (7th). Springer.
New York, NY: 2010.
|
|
21
|
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH
and Vijver MJ: WHO Classification of Tumours of the Breast. WHO
Classification of Tumours (4th). IARC Press. (Lyon, France).
2012.
|
|
22
|
Nakajima S, Doi R, Toyoda E, Tsuji S, Wada
M, Koizumi M, Tulachan SS, Ito D, Kami K, Mori T, et al: N-cadherin
expression and epithelial-mesenchymal transition in pancreatic
carcinoma. Clin Cancer Res. 10:4125–4133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu GL, Yang HJ, Liu T and Lin YZ:
Expression and significance of E-cadherin, N-cadherin, transforming
growth factor-β1 and twist in prostate cancer. Asian Pac J Trop
Med. 7:76–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen K, Wei H, Ling S and Yi C: Expression
and significance of transforming growth factor-β1 in epithelial
ovarian cancer and its extracellular matrix. Oncol Lett.
8:2171–2174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Newman LA, Reis-Filho JS, Morrow M, Carey
LA and King TA: The 2014 society of surgical oncology Susan G.
Komen for the cure symposium: Triple-negative breast cancer. Ann
Surg Oncol. 22:874–882. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gajewski TF, Schreiber H and Fu YX: Innate
and adaptive immune cells in the tumor microenvironment. Nat
Immunol. 14:1014–1022. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shuptrine CW, Ajina R, Fertig EJ,
Jablonski SA, Kim Lyerly H, Hartman ZC and Weiner LM: An unbiased
in vivo functional genomics screening approach in mice identifies
novel tumor cell-based regulators of immune rejection. Cancer
Immunol Immunother. 66:1529–1544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Knauf S, Kalwas J, Helmkamp BF, Harwell
LW, Beecham J and Lord EM: Monoclonal antibodies against human
ovarian tumor associated antigen NB/70K: Preparation and use in a
radioimmunoassay for measuring NB/70K in serum. Cancer Immunol
Immunother. 21:217–225. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chao MP, Alizadeh AA, Tang C, Myklebust
JH, Varghese B, Gill S, Jan M, Cha AC, Chan CK, Tan BT, et al:
Anti-CD47 antibody synergizes with rituximab to promote
phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 142:699–713.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Galli S, Zlobec I, Schurch C, Perren A,
Ochsenbein AF and Banz Y: CD47 protein expression in acute myeloid
leukemia: A tissue microarray-based analysis. Leuk Res. 39:749–756.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee TK, Cheung VC, Lu P, Lau EY, Ma S,
Tang KH, Tong M, Lo J and Ng IO: Blockade of CD47-mediated
cathepsin S/protease-activated receptor 2 signaling provides a
therapeutic target for hepatocellular carcinoma. Hepatology.
60:179–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiao Z, Chung H, Banan B, Manning PT, Ott
KC, Lin S, Capoccia BJ, Subramanian V, Hiebsch RR, Upadhya GA, et
al: Antibody mediated therapy targeting CD47 inhibits tumor
progression of hepatocellular carcinoma. Cancer Lett. 360:302–309.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chan KS, Espinosa I, Chao M, Wong D,
Ailles L, Diehn M, Gill H, Presti J Jr, Chang HY, van de Rijn M, et
al: Identification, molecular characterization, clinical prognosis,
and therapeutic targeting of human bladder tumor-initiating cells.
P Natl Acad Sci USA. 106:14016–14021. 2009. View Article : Google Scholar
|
|
36
|
Manna PP and Frazier WA: CD47 mediates
killing of breast tumor cells via Gi-dependent inhibition of
protein kinase A. Cancer Res. 64:1026–1036. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Steinert G, Schölch S, Niemietz T, Iwata
N, García SA, Behrens B, Voigt A, Kloor M, Benner A, Bork U, et al:
Immune escape and survival mechanisms in circulating tumor cells of
colorectal cancer. Cancer Res. 74:1694–1704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao H, Wang J, Kong X, Li E, Liu Y, Du X,
Kang Z, Tang Y, Kuang Y, Yang Z, et al: CD47 promotes tumor
invasion and metastasis in non-small cell lung cancer. Sci Rep.
6:297192016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: Emt: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Noman MZ, Van Moer K, Marani V, Gemmill
RM, Tranchevent LC, Azuaje F, Muller A, Chouaib S, Thiery JP,
Berchem G and Janji B: CD47 is a direct target of SNAI1 and ZEB1
and its blockade activates the phagocytosis of breast cancer cells
undergoing EMT. Oncoimmunology. 7:e13454152018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Y, Lu S, Xu Y, Qiu C, Jin C, Wang Y,
Liu Z and Kong B: Overexpression of CD47 predicts poor prognosis
and promotes cancer cell invasion in high-grade serous ovarian
carcinoma. Am J Transl Res. 9:2901–2910. 2017.PubMed/NCBI
|
|
42
|
Shinohara M, Ohyama N, Murata Y, Okazawa
H, Ohnishi H, Ishikawa O and Matozaki T: CD47 regulation of
epithelial cell spreading and migration, and its signal
transduction. Cancer Sci. 97:889–895. 2006. View Article : Google Scholar : PubMed/NCBI
|