Introduction
Gastrointestinal stromal tumor (GIST) is a rare
neoplasm with an unadjusted incidence estimated at around 1 in
100,000 per year, however it is the most common mesenchymal tumor
of the gastrointestinal tract (1).
GISTs arise mainly in the stomach and small intestine, but they can
be found anywhere in the gastrointestinal tract (2). Several genetic alterations are involved
in the pathogenesis of GISTs with the two most common
being-activating mutations of KIT gene and platelet-derived
growth factor receptor-α (PDGFRA) gene mutations (3,4). Surgery
remains the gold standard and only curative option in the treatment
of localized disease, but management of advanced stage tumors is
very challenging. Significant improvement in the treatment of
advanced GIST has been achieved due to better understanding of the
molecular background and introduction of tyrosine kinase inhibitors
(TKIs). The first TKI that revolutionized management of GIST was
imatinib-a small molecule selective inhibitor of KIT, PDGFRA and
BCR-ABL fusion protein. Imatinib is currently the standard of care
in the first-line treatment in advanced (metastatic and/or
inoperable) disease and the standard adjuvant therapy after
resection of primary tumors at high risk of relapse (5). Despite its spectacular improvements to
patient survival, response to imatinib therapy is limited by time
and the majority of patients develop resistance to this TKI.
Between 10 and 15% of GISTs are primary resistant to imatinib,
whereas during the first 2 years from start of imatinib,
approximately 50% develop secondary resistance (6). In the case of progression, the dose of
imatinib can be increased if it has not been used previously at
maximal dosage, or the patient can be switched to other drugs.
Sunitinib is the only approved drug for the
second-line treatment of GISTs (5,7), It is
an oral multitargeted receptor tyrosine kinase inhibitor, that
targets stem cell factor receptor KIT, PDGFRA/B, FMS-like tyrosine
kinase-3 receptor (FLT3), the vascular endothelial growth factor
receptors (VEGFR-1, VEGFR-2, VEGFR-3), and the glial cell-line
derived neurotrophic factor receptor (RET). Results from clinical
trials have shown significant efficacy of sunitinib in
imatinib-resistant patients with a median progression free survival
(PFS) of 6–8 months compared to a median PFS of 1–2 months on
placebo (7).
Prognostic and predictive factors are investigated
to identify the patients who benefit the most from sunitinib
therapy. The prognostic value of blood morphology-derived factors
was confirmed to correlate with survival in several cancers,
including GIST. Neutrophil-to lymphocyte ratio (NLR) was shown to
play a prognostic role in primary GIST after radical resection, in
patients receiving imatinib as adjuvant therapy or in the
first-line of treatment for advanced disease. However, the NLR has
not been evaluated in patients treated with sunitinib in the
second-line settings.
The important factor associated with patient
survival and response to treatment of GIST is the genetic profile
of the tumor. Primary tumor genotype was found to be associated
with PFS and OS in patients treated with imatinib. Patients with
KIT exon 11 mutations show greater benefit from imatinib
therapy than patients with other mutations (8), whereas exon 18 PDGFRA D842V
mutant GIST are not sensitive to imatinib and other tyrosine kinase
inhibitors (9). During treatment
with sunitinib, better outcomes were observed in patients harboring
primary mutations in KIT exon 9 than in exon 11 (10,11).
The aim of this study was to evaluate the prognostic
and predictive value of NLR in patients with advanced GIST treated
with sunitinib as a second line treatment after imatinib failure.
Additionally, we have investigated the impact of the baseline tumor
genotype on patient survival.
Materials and methods
Patients
Between September 1, 2005 and June 30, 2016, 232
patients with histologically confirmed unresectable and/or
metastatic CD117-positive GIST were treated with sunitinib as a
second-line treatment at a reference sarcoma center, Department of
Soft Tissue/Bone Sarcoma and Melanoma, Maria Sklodowska-Curie
Institute-Oncology Center in Warsaw, Poland. Of these, 146 patients
that had complete laboratory test results were included in this
retrospective study. Data were extracted from institutional medical
records with the ONKOSYS Medstreamer software. The local Bio-Ethics
Committee has approved the study, according to the guidelines on
good clinical practice and local bylaws. At the beginning of
treatment, each patient provided informed consent for use of their
data for future studies. Collected data included the following
clinicopathologic characteristics: Age, gender, tumor size, tumor
location, number of mitosis, mutational status and laboratory
findings, PFS, and overall survival.
NLR was calculated as the ratio of absolute
neutrophil count to absolute lymphocyte count obtained from the
complete blood count. To evaluate prognostic impact of the initial
status, the NLR was evaluated at baseline. Further assessments were
carried out after 3 months of treatment and upon progression or at
last observation.
Genotype status before TKI therapy for the presence
of mutations in the KIT (exons 9, 11, 13, 17) and
PDGFRA (exons 12, 14, 18) genes, including PDGFRA 18
D842V mutation, was available for 72 (49.3%) cases.
All patients were treated with imatinib in the first
line and had objective disease progression diagnosed by computed
tomography (CT) scans before starting sunitinib treatment.
Sunitinib was prescribed as part of a standard scheme of therapy.
Patients received sunitinib orally at a starting dose of 50 mg once
daily in 6-week cycles: 4 weeks of treatment and 2 weeks without.
According to the standard guidelines, dose reduction (to 37.5 mg or
25 mg), treatment interruption or modulation to dosage of 37.5 mg
on a continuous schedule could be used to manage adverse events and
optimize the benefit-risk profile. Treatment was stopped when
disease progression was observed on scans, when the patient
presented with signs of clinical progression, when unacceptable
adverse events occurred, or in the case of death. Patient follow-up
consisted of regular physical examinations and laboratory
assessment every 4–6 weeks, and serial CT scans performed every 2–3
month according to the strict setting of the drug program
reimbursed by national health insurance in our country. The
evaluation of tumor response was carried out based on the Response
Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (12). In case of progression, patients were
treated with other TKI, or they received the best supportive care
only. If feasible, they were included in clinical trials with new
compounds.
Statistical analysis
All statistical analyses were performed using the R
language environment version 3.5.2. The Kaplan-Meier method with
the log-rank tests for bivariate comparisons was used for
calculation of survival. The aim of this study was to assess the
correlation of NLR value before treatment and at progression or
last follow-up with patient PFS and OS times in advanced GIST cases
treated with sunitinib in the second line. Overall survival was
defined as the time from initiation of sunitinib treatment until
the most recent follow-up or death of any cause. The Polish
National Death Registry was used to confirm the dates of all
patient deaths. PFS was defined as the time from initiation of
sunitinib treatment until disease progression, death of any cause,
or most recent follow-up. The following variables were included
univariate and multivariate analyses of factors influencing
survival: demographic data (sex, age at the start of sunitinib
therapy <55 or ≥55 years-a cutoff value of 55 was selected
because it is the most robust cut off closest to the median value
of 57), primary tumor genotype (KIT exon 11 mutations,
KIT exon 9 mutations, PDGFRA 18 D842V mutation, other
KIT mutations, other PDGFRA mutations, wild-type),
the maximal diameter of the largest tumor, primary tumor location
(gastric vs non-gastric), the mitotic index of the tumor [≤5/50
high power field on microscopy (HPF) vs. >5/50 HPF] and baseline
(1–7 days before start of sunitinib therapy) NLR (≤2.4 vs.
>2.4). All covariates significant at the 20% level in the
univariate model were included in a multivariate analysis, which
was performed with Cox proportional hazards models, applying the
stepwise model building procedure. P<0.05 was considered to
indicate a statistically significant difference.
An NLR cutoff of 2.4 was selected based on maximally
selected log-rank statistics implemented in the maxstat package
with inclusion of recent literature data. Based on the statistical
calculations a cutoff value of 2.4 resulted in the best
diversification of the population into subpopulations with varying
degrees of positive or negative prognosis.
Results
Patient characteristics
One hundred forty-six patients (81 males and 65
females) were included in the study. The median age at the start of
sunitinib therapy was 74 years (range 18–84 years). Thirty-six
(24.7%) patients had tumors located in the stomach, whereas 107
(73.3%) had tumors presenting in non-gastric locations. There were
no significant differences between the NLR≤2.4 and NLR>2.4
groups. The clinicopathological characteristics of the study
population are presented in Table
I.
 | Table I.Distribution of clinicopathological
characteristics of patients in overall population and stratified by
pre-treatment NLR. |
Table I.
Distribution of clinicopathological
characteristics of patients in overall population and stratified by
pre-treatment NLR.
|
Characteristics | Overall patients n
(%) | NLR≤2.4 n (%) | NLR>2.4 n
(%) | P-value |
|---|
| Sex |
|
|
|
|
|
Male | 81 (55.5) | 36 (24.7) | 45 (30.8) | 0.335 |
|
Female | 65 (44.5) | 35 (24.0) | 30 (20.5) |
|
| Age, y |
|
|
|
|
|
<55 | 58 (39.7) | 42 (28.8) | 46 (31.5) | 0.921 |
|
≥55 | 88 (60.2) | 29 (19.9) | 29 (19.9) |
|
| Tumor location |
|
|
|
|
|
Gastric | 36 (24.7) | 21 (14.7) | 15 (10.4) | 0.312 |
|
Nongastric | 107 (73.4) | 50 (35.0) | 57 (39.9) |
|
| Tumor size, cm |
|
|
|
|
| <10
cm | 43 (29.5) | 21 (14.4) | 22 (15.1) | 0.447 |
| >10
cm | 72 (49.3) | 32 | 40 (27.4) |
|
|
Unknown | 31 (21.2) | 18 (12.3) | 13 (8.9) |
|
| Mitotic index |
|
|
|
|
|
<5/HPF | 24 (16.4) | 9 (6.1) | 15 (10.2) | 0.485 |
|
≥5/HPF | 70 (48.0) | 36 (24.7) | 34 (23.3) |
|
|
Unknown | 52 (35.6) | 26 (17.8) | 26 (17.8) |
|
| Pre-treatment
NLR |
|
|
|
|
|
<2.4 | 71 (48.6) | NA | NA |
|
|
≥2.4 | 75 (51.4) | NA | NA |
|
| Mutational
status |
|
|
|
|
|
KIT 11 | 43 (29.5) | 21 | 22 | 0.999 |
|
KIT 9 | 15 (10.3) | 8 | 7 |
|
|
PDGFRA 18 D842V | 4 (2.7) | 1 | 3 |
|
| Other
PDGFRA | 0 (0) | 0 | 0 |
|
| Other
KIT | 1 (0.7) | 1 (0.7) | 0 |
|
|
Wild-type | 9 (6.2) | 4 (2.7) | 5 (3.4) |
|
|
Unknown | 74 (50,7) | 36 | 38 |
|
In the group of 72 patients with available tumor
genotype data, 59.7% (n=43) of GIST cases exhibited KIT exon
11 mutations, 20.8% (n=15) exhibited KIT exon 9 mutations,
5.5% (n=5) PDGFRA 18 D842V mutation, 1 patient had other
KIT mutations, and 12.5% (n=9) of tumors were of the
wild-type with no mutations detected.
The median NLR at baseline was 2.47 (range 0.6–27.6,
mean 3.4±3.5) whereas the median NLR at progression (or last
available follow-up) was 1.87 (range, 0.3–23.3, mean 2.6±2.8).
Seventy-one patients (48.6%) had a baseline NLR≤2.4 and 75 patients
(51.4%) had a baseline NLR>2.4.
Sunitinib treatment outcomes
The median follow-up time was 70.1 months (range:
64–104.5). One hundred and fifteen patients (78.8%) progressed
during sunitinib therapy. At the time of the analysis 28 patients
(26%) were alive. Eight patients were lost during follow-up. The
median PFS on sunitinib treatment was 12.4 months (95% CI 9.6–16),
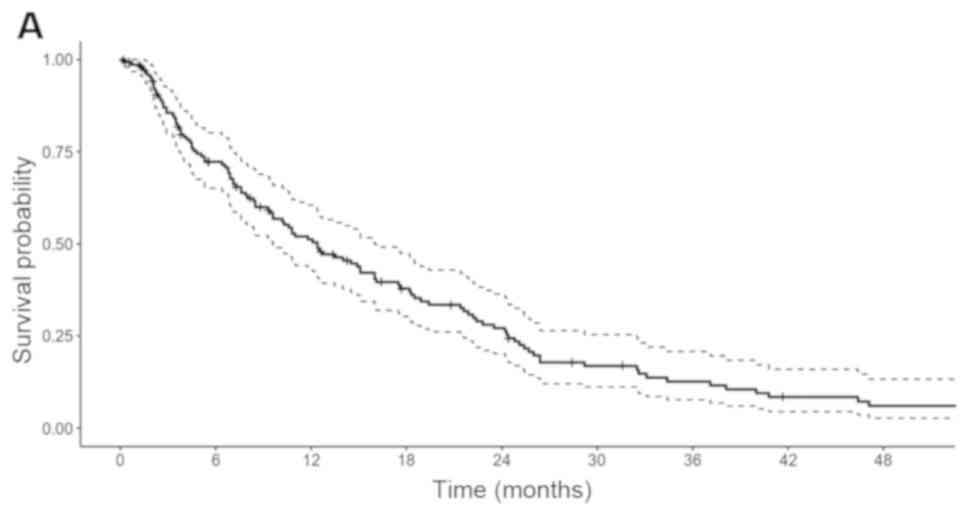
the 2-year rate was 27.1% and the 5-year rate was 4.8% (Fig. 1A). The median OS was 22.8 months (95%
CI 18.5–28.9), 2-year rate was 47.8% and 5-year rate 13.8%
(Fig. 1B).
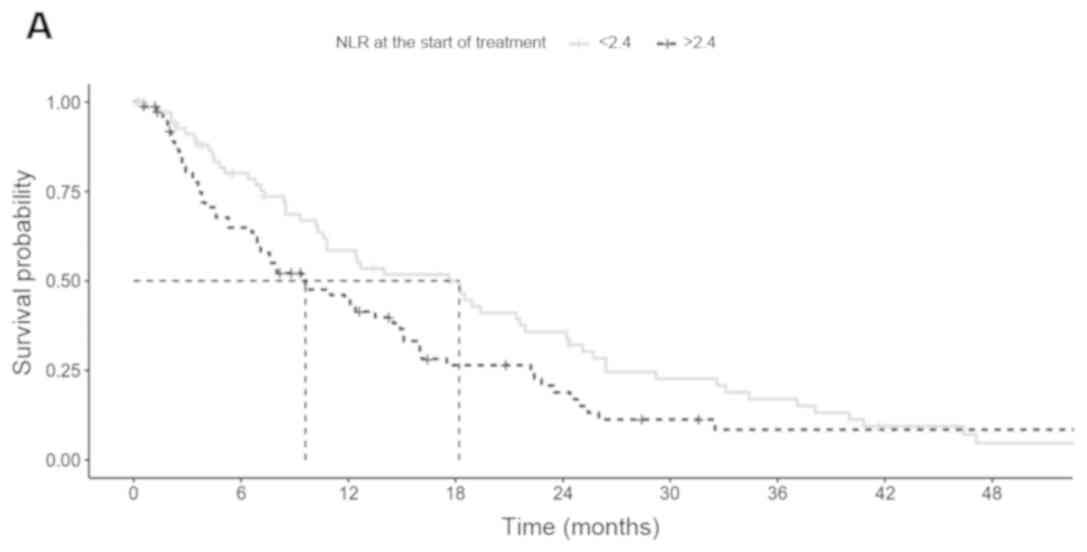
The median PFS in patients with a baseline NLR≤2.4
and NLR>2.4 were 18.2 and 9.6 months (P=0.075; Fig. 2A) respectively, whereas the median OS
were 30 and 16.4 months (P=0.002; Fig.
2B), respectively.
Univariate analysis of factors
associated with PFS and OS
Male sex (HR 1.65, 95% CI 1.13–2.4, P=0.009) and
mutations in KIT exon 11 (HR 2.23, 95% CI 1.29–3.84,
P=0.004) were significantly associated with PFS in the univariate
analysis (Table II). NLR was
significantly associated with PFS when treated as a continuous
variable (HR 1.06 per unit change, 95% CI: 1.001–1.123, p=0.045).
Male sex (HR 1.55, 95% CI 1.05–2.28, p=0.026), age ≥55 (HR 1.65,
95% CI 1.1–2.48, P=0.015), mutations in KIT exon 11 (HR
2.64, 95% CI 1.47–4.73, P=0.001), unknown mutational status (HR
2.02, 95%CI 1.15–3.54), and baseline NLR >2.4 (HR 1.85, 95% CI
1.26–2.72, P=0.002), were significantly associated with shorter OS
in the univariate analysis (Table
III).
 | Table II.Univariate and multivariate analysis
of association of clinicopathological factors with progression-free
survival. |
Table II.
Univariate and multivariate analysis
of association of clinicopathological factors with progression-free
survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Female | 1 |
|
| 1 |
|
|
|
Male | 1.65 | 1.13–2.4 | 0.009 | 1.35 | 0.9–2.03 | 0.15 |
| Age |
|
|
|
|
|
|
|
<55 | 1 |
|
| 1 |
|
|
|
≥55 | 1.23 | 0.84–1.8 | 0.299 | 1.26 | 0.84–1.9 | 0.27 |
| Tumor location |
|
|
|
|
|
|
|
Nongastric | 1 |
|
| 1 |
|
|
|
Gastric | 1.32 | 0.84–2.07 | 0.23 | 1.29 | 0.78–2.11 | 0.32 |
| Tumor size |
|
|
|
|
|
|
| 1 | 1 |
|
| 1 |
|
|
| 2 | 1.33 | 0.87–2.05 | 0.19 | 1.25 | 0.80–1.96 | 0.32 |
|
Unknown | 1.24 | 0.7–2.15 | 0.45 | 1.16 | 0.57–2.38 | 0.68 |
| Mitotic index |
|
|
|
|
|
|
|
<5/HPF | 1 |
|
| 1 |
|
|
|
≥5/HPF | 1.16 | 0.69–1.97 | 0.58 | 0.97 | 0.56–1.69 | 0.92 |
|
Unknown | 1.22 | 0.7–2.15 | 0.48 | 1.02 | 0.5–2.07 | 0.96 |
| Pre-treatment
NLR |
|
|
|
|
|
|
|
≤2.4 | 1 |
|
| 1 |
|
|
|
>2.4 | 1.4 | 0.97–2.03 | 0.076 | 1.31 | 0.89–1.93 | 0.17 |
| Mutational
status |
|
|
|
|
|
|
| KIT
11 | 2.23 | 1.29–3.84 | 0.004 | 2.38 | 1.27–4.47 | 0.007 |
|
Other | 1 |
|
| 1 |
|
|
|
Unknown | 1.58 | 0.95–2.63 | 0.08 | 1.64 | 0.9–2.97 | 0.11 |
 | Table III.Univariate and multivariate analysis
of association of clinicopathological factors with progression-free
survival. |
Table III.
Univariate and multivariate analysis
of association of clinicopathological factors with progression-free
survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Female | 1 |
|
| 1 |
|
|
|
Male | 1.55 | 1.05–2.28 | 0.026 | 1.24 | 0.83–1.87 | 0.3 |
| Age |
|
|
|
|
|
|
|
<55 | 1 |
|
| 1 |
|
|
|
≥55 | 1.65 | 1.1–2.48 | 0.015 | 1.63 | 1.06–2.53 | 0.028 |
| Tumor location |
|
|
|
|
|
|
|
Nongastric | 1 |
|
| 1 |
|
|
|
Gastric | 1.26 | 0.8–2.02 | 0.32 | 1.35 | 0.81–2.24 | 0.25 |
| Tumor size |
|
|
|
|
|
|
| 1 | 1 |
|
| 1 |
|
|
| 2 | 1.3 | 0.84–2.01 | 0.25 | 1.15 | 0.72–1.83 | 0.55 |
|
Unknown | 1.49 | 0.84–2.64 | 0.17 | 1.44 | 0.66–3.14 | 0.36 |
| Mitotic index |
|
|
|
|
|
|
|
<5/HPF | 1 |
|
| 1 |
|
|
|
≥5/HPF | 1.26 | 0.74–2.14 | 0.4 | 1.15 | 0.66–2.0 | 0.64 |
|
Unknown | 1.54 | 0.86–2.77 | 0.15 | 1.24 | 0.58–2.64 | 0.58 |
| Pre-treatment
NLR |
|
|
|
|
|
|
|
≤2.4 | 1 |
|
| 1 |
|
|
|
>2.4 | 1.85 | 1.26–2.72 | 0.002 | 1.92 | 1.27–2.91 | 0.002 |
| Mutational
status |
|
|
|
|
|
|
| KIT
11 | 2.64 | 1.47–4.73 | 0.001 | 3.39 | 1.73–6.63 | <0.001 |
|
Other | 1 |
|
| 1 |
|
|
|
Unknown | 2.02 | 1.15–3.54 | 0.014 | 2.29 | 1.19–4.39 | 0.013 |
Multivariate analysis of factors
associated with PFS and OS
The multivariate analysis revealed that only
mutations in KIT exon 11 (HR 2.38, 95% CI 1.27–4.47,
P=0.007) were associated with shorter PFS on sunitinib (Table II). NLR>2.4 was not associated
with PFS in the multivariate analysis (HR 1.31, 95%CI 0.89–1.93,
P=0.17). Mutations in KIT exon 11 (HR 3.39, 95% CI
1.73–6.63, P<0.001), unknown mutational status (HR 2.29, 95% CI
1.19–4.39), age ≥55 (HR 1.63, 95% CI 1.06–2.53, P=0.028) and
baseline NLR >2.4 (HR 1.92, 95% CI 1.27–2.9, P=0.002), were
independently associated with shorter OS in the multivariate
analysis (Table III).
Discussion
The prognostic value of NLR was previously shown for
a variety of malignancies, including soft tissue sarcomas (13), colorectal, renal, lung and pancreatic
cancers (14–16). There is a growing body of data
supporting the usefulness of NLR in prognostication in GISTs. Its
role as an independent prognostic factor of RFS in localized
primary GISTs has already been presented (17–19).
Moreover, in this group of patients increased NLR was correlated
with shorter OS (18,20). However, some authors present opposite
results (21). High NLR was also
associated with the characteristic features of high-risk tumors,
what suggests that high risk GISTs can promote systemic
inflammation (20). Much less is
known about the role of NLR in patients treated systemically due to
metastatic/unresectable GIST. In our previous study we reported
that NLR>2.7 at baseline was significantly associated with poor
OS and PFS in patients receiving imatinib in the first line of
treatment of advanced GIST (10). To
our knowledge, this is the first study showing that pretreatment
NLR may be associated with OS in advanced GIST treated with
sunitinib as a second-line therapy. In this study, we have found
that baseline NLR>2.4 is a negative independent prognostic
factor for OS.
Direct mechanisms that clarify the poor survival
outcomes in patients with high blood NLR are poorly understood. One
potential explanation of this phenomenon could be connection
between high NLR and the systemic inflammation induced by tumor
cells and associated host cells. There is increasing evidence of an
association between cancer and inflammation; in fact, inflammation
is now considered a hallmark of cancer (22). Moreover, the tumor niche and
tumor-associated inflammation play crucial roles in carcinogenesis,
progression, and the development of metastasis. Inflammatory
processes in the tumor are reflected by changes in leucocyte blood
count or increased levels of circulating cytokines (23). Perez et al (19) suggested that NLR in GIST is mainly
determined by the absolute neutrophil count because there is no
established link between blood lymphocytes and prognosis in solid
tumors. Rather, the lymphocyte count alone can be considered a
measure of patient-specific immune response against cancer with
limited prognostic value. Neutrophilia can inhibit the immune
system by suppressing the cytolytic activity of lymphocytes,
natural killer cells, or activated T cells (24,25).
High NLR was found to be related to an increase in macrophage
infiltration of peritumoral tissue and elevated systemic
concentrations of IL-17, (26),
IL-12, IL-8, IL-7, IL-6, IL-1ra, MCP-1 and PDGFBB (27). Moreover, neutrophils can secrete
tumor growth promoting factors, including HGF, VEGF, IL-8, IL-6,
MMPs and elastases and thus lead to the formation of tumor
stimulating microenvironment, proliferation, migration, and
invasiveness of tumor cells (25).
Elevated NLR may indicate elevated concentrations of circulating
cytokines, including some factors promoting tumor growth such as
transforming growth factor-beta (TGF-beta) (28). Moreover, TGF-beta can promote an
increase in the number, activation, and survival of neutrophils and
reduce the number of lymphocytes (29). Altogether, NLR and circulating
inflammatory cytokines can perpetuate a tumor microenvironment and
reflect aggressive behavior.
Despite an almost 9-month difference in PFS between
patients with NLR≤2.4 and NLR>2.4, we have not found NLR to be
an independent predictive factor for PFS, what was also shown in
the population of patients receiving imatinib in the first-line
(10). The lack of a statistical
correlation between NLR and PFS in this study may have resulted
from the study's small sample size.
In addition to NLR, we found that primary mutations
in exon 11 KIT were associated with worse PFS and OS in
patients treated with sunitinib in the second line, with a 2.4- and
3.4-fold increase in hazard ratios for PFS and OS, respectively.
This finding is in alignment with our previous observation where we
have reported that tumors initially bearing KIT exon 9
mutations and wild-type tumors had significantly higher 1-year PFS
rates than those carrying KIT exon 11 or PDGFRA mutations
(68 and 57% vs. 34 and 15%, respectively) (10). Moreover, a retrospective study on
samples obtained from patients included in a phase I/II trial with
sunitinib also showed that patients with KIT exon 9
mutations had significantly better objective response rates, PFS
and OS than patients harboring KIT exon 11 mutations
(11). Previously, we found
PDGFRA mutations to be an independent negative prognostic
factor for PFS and OS (10);
however, this observation was not confirmed in the current study,
probably because of the small sample size of patients with
available genetic data and the low incidence of PDGFRA
mutations in the study population. Moreover, these results have
some limitations. We only analyzed the mutational status of primary
tumors and did not conduct screenings for secondary mutations
acquired during imatinib therapy. Although patients carrying
secondary mutations in exon 13 or 14 KIT had longer PFS on
sunitinib than patients with exon 17 or 18 KIT mutations
(11), the analysis of secondary
mutations can also be biased due to high heterogeneity and multiple
cell clones having different mutational patterns (30).
This study had some limitations, including its
retrospective nature, the presentation of a single institution
experience and a moderate sample size. Moreover, the relatively
long period of the treatment could have led to numerous biases,
including changes in side effect management. Nonetheless, our study
is the first to illuminate the role of NLR as a prognostic factor
in the second line treatment with sunitinib in patients with GIST.
A variety of concurrent conditions, including inflammation,
infections, and concomitant medication, may influence neutrophil
and lymphocyte counts independently from the tumor. The bias
associated with active infection is minimalized, because in such
conditions treatment is usually delayed; however, a confounding
effect of inflammation cannot be ruled out completely. Moreover,
the results of this study cannot be easily compared with other
analyses of patients with GIST or other solid malignancies due to
the high variability of the cutoff levels used for NLR, which
ranged from 2.04 (21) through 2.7
(19), 3.0 (17) to even 5 (31). Further investigations, preferable
randomized trials with larger cohorts of patients, are required to
confirm the prognostic role of NLR in patients with advanced
GIST.
Therefore, until now, only primary tumor mutational
status and sunitinib-induced hypertension were confirmed as
predictive and prognostic factors in patients with GIST treated
with sunitinib in the second-line setting (10). In the current study, we showed that
NLR is an independent prognostic factor for OS in this group of
patients. Moreover, we confirmed an earlier observation that the
KIT genotype is independently correlated with OS and PFS.
The an identification of NLR as independent prognostic factor of
GIST may be very useful in clinical practice because, it is an
easily measured, reproducible, widely available, and cost-effective
marker of systemic inflammation. Blood NLR can serve as a useful
parameter in the selection of appropriate and effective treatment
for patients with GIST who have progressed on imatinib. We do not
currently have reliable biomarkers within this group of patients.
Patients with high NLR and poorer prognoses on sunitinib are likely
good candidates for clinical trials with new compounds, especially
given that agents such as the new tyrosine kinase inhibitors
DCC-2618 and avapritinib are planned to be tested in GIST patients
after imatinib failure. Moreover, NLR may be taken into account in
the trials including immunotherapy; the first trial including a
combination of axitinib and avelumab is ongoing.
Acknowledgements
The preliminary version of this study was presented
as a poster during Annual American Society of Clinical Oncology
Congress in 2018 in Chicago, June 1st-5th, 2018, abstract number
11531 (https://ascopubs.org/doi/abs/10.1200/JCO.2018.36.15_suppl.11531).
Funding
No funding was received.
Authors' contributions
PS, PT, IL, AMC and PR were involved in conceiving
the study. PT, IL, AK, EB, HKP, CO, JS and PR were involved in the
data acquisition. PS, PT, IL AMC and PR were involved in the data
analysis and interpretation. PS, PT, AMC and PR prepared the
manuscript. All authors read and approved the final manuscript.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Ethics approval and consent to
participate
The study has been approved by the local Bio-Ethics
Committee (Bio-Ethics Committee at the Maria Sklodowska-Curie
Institute-Oncology Center, Warsaw, Poland; approval number
KB/9/2011) according to good clinical practice guidelines. Each
patient, at the beginning of treatment provided informed consent
for use of their data for future studies.
Patient consent for publication
Not applicable.
Competing interests
PR has received for lectures from Novartis, Roche,
Pfizer, BMS, Eli Lilly and MSD, and served as a member of the
advisory board for Novartis, Merck, Amgen, Blueprint Medicine,
Roche, BMS, and MSD. IL has received honoraria for lectures from
Novartis, Roche, Pfizer, BMS, and MSD. PT has received honoraria
and travel grants from Roche, Eli Lilly, Bayer, and Novartis. PS
has received travel grants from Roche and Pierre Fabre.
References
|
1
|
Nilsson B, Bümming P, Meis-Kindblom JM,
Odén A, Dortok A, Gustavsson B, Sablinska K and Kindblom LG:
Gastrointestinal stromal tumors: The incidence, prevalence,
clinical course, and prognostication in the preimatinib mesylate
era-a population-based study in western Sweden. Cancer.
103:821–829. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors: Pathology and prognosis at different sites. Semin
Diagn Pathol. 23:70–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirota S, Isozaki K, Moriyama Y, Hashimoto
K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M,
et al: Gain-of-function mutations of c-kit in human
gastrointestinal stromal tumors. Science. 279:577–580. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heinrich MC, Corless CL, Duensing A,
McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A,
Town A, et al: PDGFRA activating mutations in gastrointestinal
stromal tumors. Science. 299:708–710. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Casali PG, Abecassis N, Bauer S, Biagini
R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T,
Broto JM, et al: Gastrointestinal stromal tumours: ESMO-EURACAN
clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 29:iv68–iv78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Glabbeke M, Verweij J, Casali PG, Le
Cesne A, Hohenberger P, Ray-Coquard I, Schlemmer M, van Oosterom
AT, Goldstein D, Sciot R, et al: Initial and late resistance to
imatinib in advanced gastrointestinal stromal tumors are predicted
by different prognostic factors: A european organisation for
research and treatment of cancer-italian sarcoma group-australasian
gastrointestinal trials group study. J Clin Oncol. 23:5795–5804.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Demetri GD, van Oosterom AT, Garrett CR,
Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich
MC, Morgan JA, et al: Efficacy and safety of sunitinib in patients
with advanced gastrointestinal stromal tumour after failure of
imatinib: A randomised controlled trial. Lancet. 368:1329–1338.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Joensuu H, Wardelmann E, Sihto H, Eriksson
M, Sundby Hall K, Reichardt A, Hartmann JT, Pink D, Cameron S,
Hohenberger P, et al: Effect of KIT and PDGFRA mutations on
survival in patients with gastrointestinal stromal tumors treated
with adjuvant imatinib: An exploratory analysis of a randomized
clinical trial. JAMA Oncol. 3:602–609. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Farag S, Somaiah N, Choi H, Heeres B, Wang
WL, van Boven H, Nederlof P, Benjamin R, van der Graaf W, Grunhagen
D, et al: Clinical characteristics and treatment outcome in a large
multicentre observational cohort of PDGFRA exon 18 mutated
gastrointestinal stromal tumour patients. Eur J Cancer. 76:76–83.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rutkowski P, Bylina E, Klimczak A, Switaj
T, Falkowski S, Kroc J, Lugowska I, Brzeskwiniewicz M, Melerowicz
W, Osuch C, et al: The outcome and predictive factors of sunitinib
therapy in advanced gastrointestinal stromal tumors (GIST) after
imatinib failure-one institution study. BMC Cancer. 12:1072012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heinrich MC, Maki RG, Corless CL,
Antonescu CR, Harlow A, Griffith D, Town A, McKinley A, Ou WB,
Fletcher JA, et al: Primary and secondary kinase genotypes
correlate with the biological and clinical activity of sunitinib in
imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol.
26:5352–5359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu G, Ke LC and Sun SR: Prognostic value
of pretreatment neutrophil-to-lymphocyte ratio in patients with
soft tissue sarcoma: A meta-analysis. Medicine (Baltimore).
97:e121762018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mei Z, Shi L, Wang B, Yang J, Xiao Z, Du
P, Wang Q and Yang W: Prognostic role of pretreatment blood
neutrophil-to-lymphocyte ratio in advanced cancer survivors: A
systematic review and meta-analysis of 66 cohort studies. Cancer
Treat Rev. 58:1–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106:dju1242014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guthrie GJ, Charles KA, Roxburgh CS,
Horgan PG, McMillan DC and Clarke SJ: The systemic
inflammation-based neutrophil-lymphocyte ratio: Experience in
patients with cancer. Crit Rev Oncol Hematol. 88:218–230. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goh BK, Chok AY, Allen JC Jr, Quek R, Teo
MC, Chow PK, Chung AY, Ong HS and Wong WK: Blood neutrophil-to-
lymphocyte and platelet-to-lymphocyte ratios are independent
prognostic factors for surgically resected gastrointestinal stromal
tumors. Surgery. 159:1146–1156. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stotz M, Liegl-Atzwanger B, Posch F, Mrsic
E, Thalhammer M, Stojakovic T, Bezan A, Pichler M, Gerger A and
Szkandera J: Blood-based biomarkers are associated with disease
recurrence and survival in gastrointestinal stroma tumor patients
after surgical resection. PLoS One. 11:e01594482016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perez DR, Baser RE, Cavnar MJ,
Balachandran VP, Antonescu CR, Tap WD, Strong VE, Brennan MF, Coit
DG, Singer S and Dematteo RP: Blood neutrophil-to-lymphocyte ratio
is prognostic in gastrointestinal stromal tumor. Ann Surg Oncol.
20:593–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang C, Hu WM, Liao FX, Yang Q, Chen P,
Rong YM, Guo GF, Yin CX, Zhang B, He WZ and Xia LP: Elevated
preoperative neutrophil-to-lymphocyte ratio is associated with poor
prognosis in gastrointestinal stromal tumor patients. OncoTargets
Ther. 9:877–883. 2016. View Article : Google Scholar
|
|
21
|
Racz JM, Cleghorn MC, Jimenez MC, Atenafu
EG, Jackson TD, Okrainec A, Venkat Raghavan L and Quereshy FA:
Predictive ability of blood neutrophil-to-lymphocyte and
platelet-to-lymphocyte ratios in gastrointestinal stromal tumors.
Ann Surg Oncol. 22:2343–2350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Galdiero MR, Bonavita E, Barajon I,
Garlanda C, Mantovani A and Jaillon S: Tumor associated macrophages
and neutrophils in cancer. Immunobiology. 218:1402–1410. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Petrie HT, Klassen LW and Kay HD:
Inhibition of human cytotoxic T lymphocyte activity in vitro by
autologous peripheral blood granulocytes. J Immunol. 134:230–234.
1985.PubMed/NCBI
|
|
25
|
Dumitru CA, Lang S and Brandau S:
Modulation of neutrophil granulocytes in the tumor
microenvironment: Mechanisms and consequences for tumor
progression. Semin Cancer Biol. 23:141–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Motomura T, Shirabe K, Mano Y, Muto J,
Toshima T, Umemoto Y, Fukuhara T, Uchiyama H, Ikegami T, Yoshizumi
T, et al: Neutrophil-lymphocyte ratio reflects hepatocellular
carcinoma recurrence after liver transplantation via inflammatory
microenvironment. J Hepatol. 58:58–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kantola T, Klintrup K, Väyrynen JP,
Vornanen J, Bloigu R, Karhu T, Herzig KH, Näpänkangas J, Mäkelä J,
Karttunen TJ, et al: Stage-dependent alterations of the serum
cytokine pattern in colorectal carcinoma. Br J Cancer.
107:1729–1736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elliott RL and Blobe GC: Role of
transforming growth factor Beta in human cancer. J Clin Oncol.
23:2078–2093. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang L, Pang Y and Moses HL: TGF-beta and
immune cells: An important regulatory axis in the tumor
microenvironment and progression. Trends Immunology. 31:220–227.
2010. View Article : Google Scholar
|
|
30
|
Wardelmann E, Merkelbach-Bruse S, Pauls K,
Thomas N, Schildhaus HU, Heinicke T, Speidel N, Pietsch T, Buettner
R, Pink D, et al: Polyclonal evolution of multiple secondary KIT
mutations in gastrointestinal stromal tumors under treatment with
imatinib mesylate. Clin Cancer Res. 12:1743–1749. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, Fletcher CD, O'Reilly DS, Foulis AK, Horgan PG and McMillan DC:
A comparison of inflammation-based prognostic scores in patients
with cancer. A glasgow inflammation outcome study. Eur J Cancer.
47:2633–2641. 2011. View Article : Google Scholar : PubMed/NCBI
|