Introduction
Apoptosis-inducing factor (AIF) is a mitochondrial
oxidoreductase that plays a role in oxidative phosphorylation and
redox control in normal cells (1).
AIF was originally cloned and identified as a mitochondrial
effector of caspase-independent apoptotic cell death (2). Programmed cell death is a fundamental
requirement for embryogenesis, organ metamorphosis and tissue
homeostasis (3). In mammals, the
release of mitochondrial cytochrome c results in the
cytosolic assembly of the apoptosome, a caspase activation complex
involving apoptotic protease-activating factor 1 (APAF1) and
caspase-9, which induce apoptosis (4). However, there are
mitochondria-regulated cell-death pathways that are independent of
APAF1/caspase-9. Similar to cytochrome c, under normal
conditions, AIF is usually confined to the mitochondrial
intermembrane space and released in response to death stimuli, and
following the induction of apoptosis, AIF is translocated to the
nucleus, where it influences chromosome condensation and
fragmentation (1,5). In addition, AIF can induce mitochondria
to release the apoptogenic proteins including cytochrome c
and caspase-9 (1). Joza et al
(3) showed that the genetic
inactivation of AIF renders embryonic stem cells resistant to cell
death after serum deprivation. Moreover, AIF is essential for
programmed cell death during the cavitation of embryoid bodies,
which is the very first wave of cell death indispensable for mouse
morphogenesis (3). AIF-dependent
cell death exhibits structural features similar to those of
apoptosis and can be genetically uncoupled from APAF1 and caspase-9
expression (5). Joza et al
(3) concluded that their data
provide genetic evidence for a caspase-independent pathway of
programmed cell death that controls early morphogenesis.
In addition to its role in cell death, Shen et
al (6) reported that AIF could
influence tumor invasion and metastasis by physically interacting
with and protecting phosphatase and tensin homolog on chromosome
ten (PTEN) from oxidation. PTEN is a tumor suppressor that is
susceptible to oxidation-mediated inactivation (7–10). A
study by Tsai et al (11)
demonstrated that an asthma medication, montelukast, induced lung
cancer cell death via the nuclear translocation of AIF. Ohyama
et al (12) observed a
similar phenomenon where phosphatidylinositol derivatives induced
caspase-independent apoptosis in gastric cancer cells through the
accumulation of AIF and AIF-homologous mitochondrion-associated
inducer of death in cancer cell nuclei. These findings indicated
that AIF is involved in cell homeostasis and tumor development.
The AIF gene encodes a mitochondrial flavin adenine
dinucleotide-dependent oxidoreductase that serves a role in
oxidative phosphorylation and redox control in healthy cells by
triggering chromatin condensation and DNA fragmentation (2). Xu et al (13) reported that owing to AIF deletion and
the methylation of its promoter, AIF was downregulated in a
majority of renal cell carcinomas (RCC) and that AIF overexpression
in RCC cell lines could considerably induce apoptosis. Further
investigations by Xu et al (13) demonstrated that AIF interacts with
STK3, a known apoptosis regulator, and enhances its phosphorylation
at Thr180. Despite these previous findings of the role of AIF in
cancer, further studies are warranted to investigate the
association between AIF expression and RCC grade.
RCC is a high-risk and high-mortality kidney cancer
originating in the lining of the proximal convoluted tubules, and
accounts for ~2–3% of human cancers worldwide (14,15). In
China, RCC is the second most frequent genitourinary malignant
cancer, demonstrating a steady increase in frequency (16). Since 2005, following a clearer
understanding of the molecular mechanisms underlying RCCs, targeted
therapies with small molecules have replaced cytokines in treating
metastatic RCC. The survival time has increased from 10–12 months
to 20–22 months (17,18). Considering the mortality rate
associated with RCC and the challenging therapy, the identification
of novel molecular markers for kidney cancer is vital for
decreasing mortality and enhancing quality of life for patients. In
addition, the identification of novel markers can facilitate the
evaluation of individual risk, prognosis and help to predict the
effects of therapy and advocacy for personalized treatment
(19).
Although AIF was reported to decrease apoptosis in
press kidney cells, the expression of AIF in RCC and the
association between AIF expression and prognosis of RCC required
further study. In the present study, AIF expression was
investigated in RCC using immunohistochemistry (IHC), and the
association between AIF expression and the prognosis of RCC was
evaluated.
Materials and methods
Patients and tissue samples
In total, 96 pairs (78 of clear cell carcinoma, 13
of papillary carcinoma and 5 of other tissue types) of RCC and
adjacent tissue specimens and a second set of 15 pairs of RCC and
adjacent fresh tissue were collected from patients who attended the
Haikou Municipal Hospital, which is affiliated to the Xiangya
School of Medicine of the Central South University (Haikou, China).
The inclusion criteria were patients undergoing radical nephrectomy
or nephrosparing surgery. The exclusion criteria were patients
receiving chemotherapy or targeted drug therapy and patients with
distant RCC metastasis (T4) that did not undergo surgical treatment
patients with grade IV RCC were excluded due to the very small
number of patients, which was not suitable for statistical
analysis. The 96 pairs of RCC and adjacent tissue specimens were
collected by surgical removal between June 2004 and June 2014 from
63 males and 33 females aged 25–82 (mean, 64) years. All specimens
were fixed in 10% formalin at 4°C for 12 h within 1 h of surgery,
and then embedded in paraffin. Among these, 68 patients were
followed up for 6–118 months (mean follow-up time, 47 months). None
of the patients received chemotherapy or any other medications
prior to surgery. The present study was approved by the Ethics
Committee of Haikou Municipal Hospital, which is affiliated to the
Xiangya School of Medicine of the Central South University. Written
informed consent was obtained from all patients.
IHC
Paraffin-embedded tissue sections were dewaxed,
hydrated and placed in EDTA antigen-repair solution (pH 9.0) at
95°C for 10 min for antigen repair. Endogenous peroxidase was
blocked using 3% H2O2 for 10 min at room
temperature. Subsequently, anti-human AIF antibody (1:100 in PBS;
cat. no. 200-401-985; Rockland Immunochemicals Inc.) was added, and
the tissue sections were incubated at 4°C in a humidity chamber
overnight. The sections were then treated with horseradish
peroxidase-conjugated secondary antibodies (1:200 in PBS; cat. no.
GB23303; Servicebio) at 37°C for 30 min and diaminobenzidine
reagent according to the manufacturer's instructions (DAB
Horseradish Peroxidase Color Development Kit; cat. no. P0203;
Beyotime Institute of Biotechnology). For the negative control
group, PBS buffer without primary antibody was used. The results of
the IHC were imaged at 20× and 40× on an Olympus Corporation light
microscope and subjected to a double-blind analysis by two
pathologists. Each specimen was randomized to count the number of
positive cells in five high-power fields and was scored based on
the average percentage of positive cells: ≤5%, 0 points; 6–25%, 1
point; 26–50%, 2 points; 51–75%, 3 points; and >75%, 4 points.
In addition, the specimens were scored according to the intensity
of color: No coloring, 0 points; light yellow, 1 point; brown, 2
points; light brown, 3 points; and dark brown, 4 points. Finally,
the two scores were added and categorized as follows: 0–2,
negative; 3–5, weak positive; and 6–8, strong positive. To
facilitate statistical analysis, a score of ≥3 points was
classified as positive. To quantify AIF expression in Figs. 1B and 3B, Image Pro plus version 6.0 software
(Media Cybernetics, Inc.) was used to quantify the intensity of
staining.
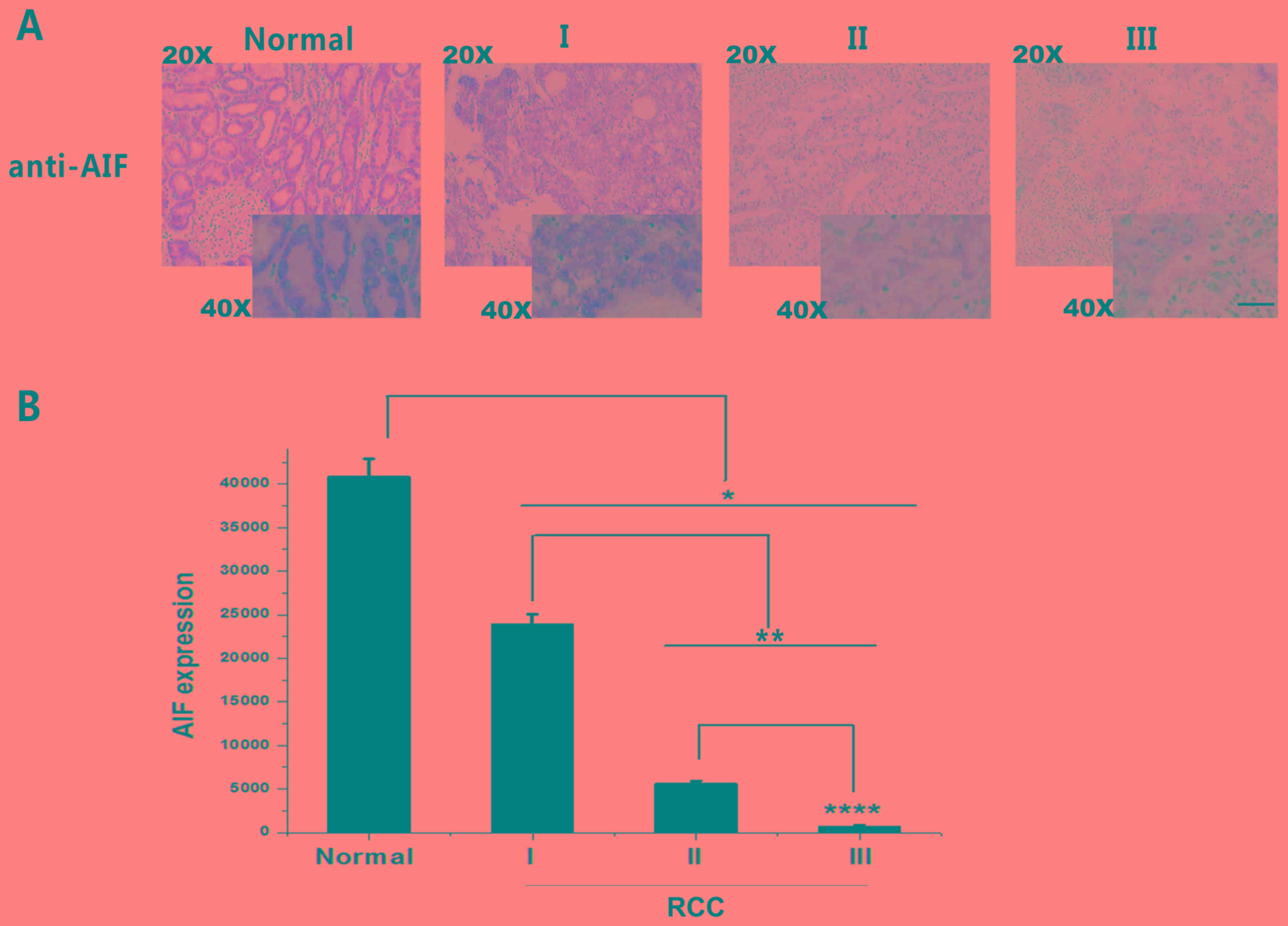 | Figure 3.AIF expression in RCC grades I, II and
III and adjacent normal tissue. (A) IHC staining of AIF (brown)
revealed a decrease in AIF expression with tumor grade. (B)
Quantification of AIF expression in RCC grades I, II and III. AIF
expression was significantly decreased in RCC tissues compared with
that in adjacent normal tissues. *P<0.05, **P<0.01,
****P<0.0001. Scale bar, 400 µm. Normal, n=96; grade I, n=36;
grade II, n=24; and grade II, n=36). AIF, apoptosis-inducing
factor; RCC, renal cell carcinoma. |
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
A second set of 15 pairs of RCC and adjacent fresh
tissue specimens were collected by surgical removal between April
2015 and April 2017 including 11 males and 4 females aged 25–82
(mean, 63±8.7) years. Total RNA from 15 pairs of RCC and adjacent
tissues, which were stored in liquid nitrogen, was extracted using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Briefly, 2 µg of RNA was
added to a solution of random primers (2 ml; Beijing ComWin Biotech
Co., Ltd.) and water to a final volume of 15 µl. The mix was
incubated at 70°C for 10 min, and then placed on ice immediately
for 5 min. Subsequently dNTPs (0.25 µl; Takara Bio, Inc.), M-MLV
Reverse Transcriptase (1 ml; Promega Corporation) and RNase
inhibitor (0.5 ml; Promega Corporation) were added to the 15-µl
mix. Finally, water was added to a final volume of 25 µl and the
solution was incubated at 37°C for 1 h to synthesize cDNA. qPCR was
performed using 10 µl SYBR® Green PCR Master mix (Roche
Diagnostics), with specific forward and reverse primers and cDNA on
an ABI QuantStudio version 5 (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling conditions for the qPCR were:
95°C For 10 min followed by 40 cycles of 95°C for 15 sec and 60°C
for 30 sec. The following primers were obtained from Tsingke
Biological Technology: AIF forward, 5′-AAGAAGTGGTCTGACCTCAAGA-3′
and reverse, 5′-AGGTTGCAGATACGTTGTTGC-3′; and GAPDH forward,
5′-ATGGGGAAGGTGAAGGTCG-3′ and reverse,
5′-GGGGTCATTGATGGCAACAATA-3′. GAPDH was used as the reference gene.
The results were quantified using the 2−ΔΔCq as
described previously (20).
Immunofluorescence assay
The 96 pairs of RCC and adjacent tissues (grades I,
II and III) were incubated with a primary AIF monoclonal antibody
(1:50; cat. no. MA5-15880; Invitrogen; Thermo Fisher Scientific,
Inc.). The tissues were incubated with a voltage-dependent
anion-selective channel 1 (VDAC1) rabbit polyclonal antibody (1:50;
cat. no. 10866-1-AP; Proteintech Group, Inc.) at 4°C overnight,
which served as a mitochondrial marker. The tissues were
subsequently incubated at 37°C for 1 h with an Alexa Fluor 488 goat
anti-mouse immunoglobulin G (IgG) (H+L) antibody (1:20,000; cat.
no. A32723; Thermo Fisher Scientific, Inc.) and an Alexa Fluor 555
goat anti-rabbit IgG (H+L) antibody (1:500; cat. no. A21428; Thermo
Fisher Scientific, Inc.). Nuclei were counterstained using DAPI
(Invitrogen; Thermo Fisher Scientific, Inc.) at room temperature
for 10 min, and images were obtained using a FV1000 confocal
microscope at 60× magnification. The signal values of AIF (green),
VDAC1 (red), Merge (yellow) and DAPI (blue) were counted from 6
randomly selected with Image Pro Plus6.0 for quantification. The
ratio of Merge/AIF was the percentage of mitochondria, and the rest
were considered nuclei.
Statistical analysis
SPSS version 17.0.1 (SPSS, Inc.) and GraphPad Prism
6 (GraphPad Software, Inc.) were used for statistical analyses. The
differences between two groups were compared using a paired
Student's t-test, and ≥3 groups were compared using one-way ANOVA
with a Tukey's post hoc test. Survival rates were compared using
the Kaplan-Meier method and a log rank test was used to compare the
survival curves of patients in the two groups. The association
between clinicopathological features and AIF expression was
analyzed using a Pearson's χ2 test. P<0.05 was
considered to indicate a statistically significant difference.
Results
AIF staining is decreased in RCC
tissues
To investigate the role of AIF in RCC, 96 pairs of
RCC and adjacent normal tissues were collected and AIF expression
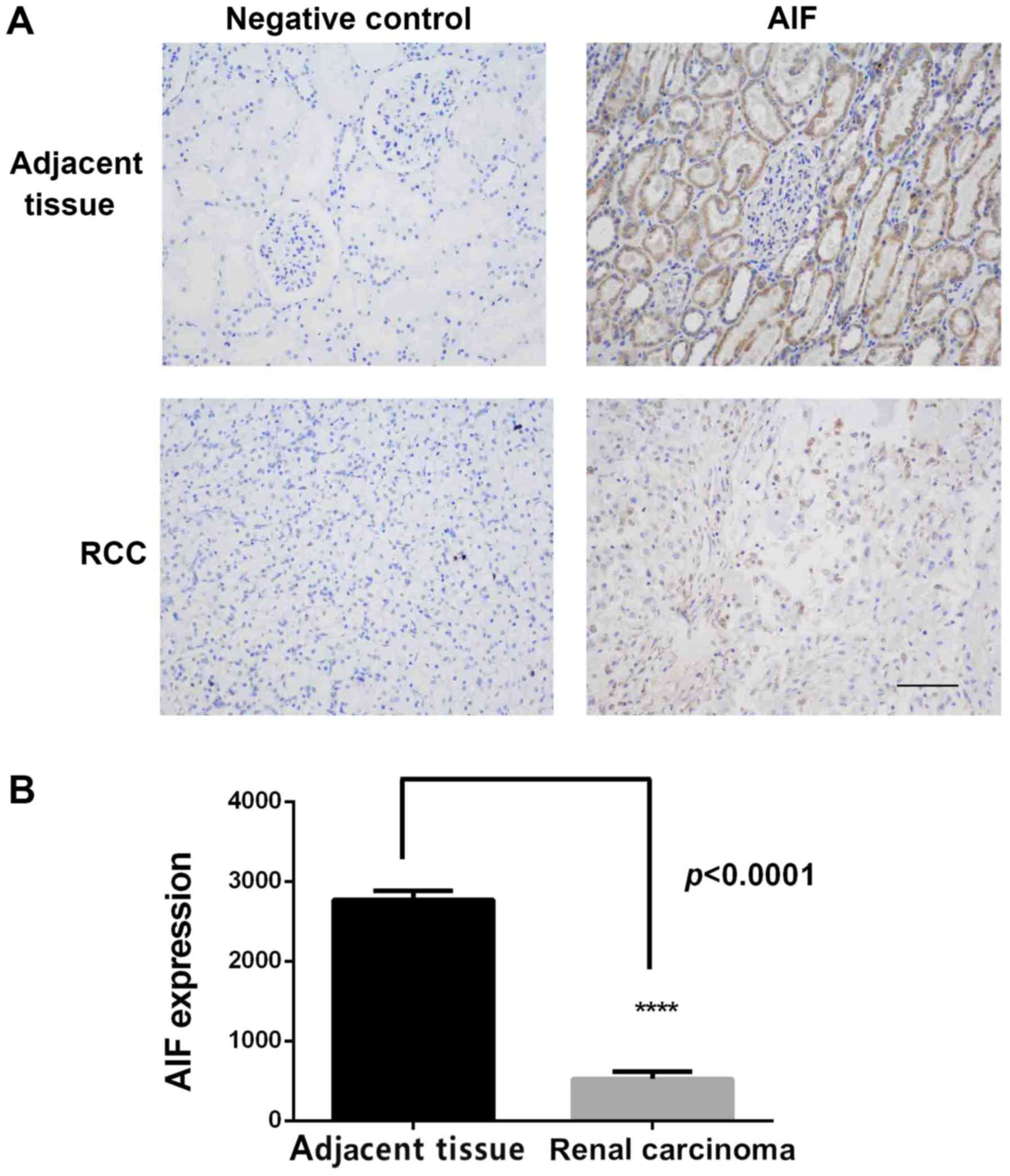
was determined using IHC. As shown in Fig. 1A, AIF was highly expressed in
adjacent normal tissues, primarily in renal tubular epithelial
cells with less expression in glomerular membrane cells. AIF
expression in RCC tissue was low. Quantification of staining
confirmed that AIF expression was significantly lower in RCC
tissues compared with normal renal tissue (Fig. 1B and Table
I).
 | Table I.Association of AIF expression in renal
cell carcinoma and adjacent normal tissue. |
Table I.
Association of AIF expression in renal
cell carcinoma and adjacent normal tissue.
|
| AIF expression |
|
|
|---|
|
|
|
|
|
|---|
| Tissue type | Positive, n | Negative, n | χ2
value | P-value |
|---|
| Renal cell
carcinoma | 16 | 80 | 96.5 | <0.001 |
| Adjacent | 84 | 12 |
|
|
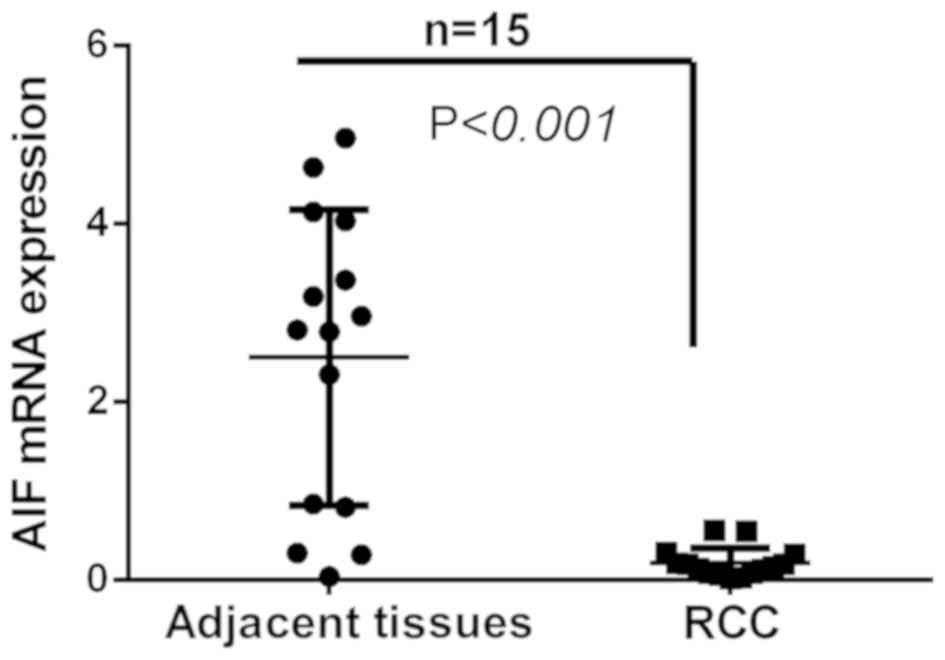
AIF mRNA expression is decreased in
RCC tissues
To further compare AIF expression in RCC and
adjacent normal tissues, AIF mRNA expression was determined in 15
pairs of RCC and adjacent tissues. The results revealed that AIF
expression was significantly decreased in RCC tissues compared with
that in adjacent normal tissues (Fig.
2).
AIF expression decreases with RCC
progression
IHC findings demonstrated that reduced AIF
expression was associated with increasing tumor grade. As shown in
Table II, 87.5% of the adjacent
normal tissue exhibited positive AIF expression. Conversely, 27.8,
16.7 and 5.6% of Grades I, II and III RCC samples exhibited
positive AIF expression, respectively (Table II). The results revealed that AIF
expression was associated with RCC progression (Table II). Quantification of AIF expression
based on IHC demonstrated that AIF expression was significantly
decreased in Grade I, II and III RCC compared with that in adjacent
normal tissues (Fig. 3).
Additionally, AIF expression in Group III was significantly lower
than that in Group II, which was also lower than that in Group I.
These results suggest that AIF expression is negatively associated
with RCC grade. Thus, AIF downregulation may be a useful biomarker
for diagnosing RCC and distinguishing tumor grades.
 | Table II.Apoptosis-inducing factor expression
in renal cell carcinoma grades I, II and III and adjacent normal
tissue. |
Table II.
Apoptosis-inducing factor expression
in renal cell carcinoma grades I, II and III and adjacent normal
tissue.
| Group | Samples, n | Positive, % | Negative, % |
|---|
| Normal tissue | 96 | 87.5 | 12.5 |
| RCC (total) | 96 | 16.7 | 83.3 |
| Grade I | 36 | 27.8 | 72.2 |
| Grade II | 24 | 16.7 | 83.3 |
| Grade III | 36 | 5.6 | 94.4 |
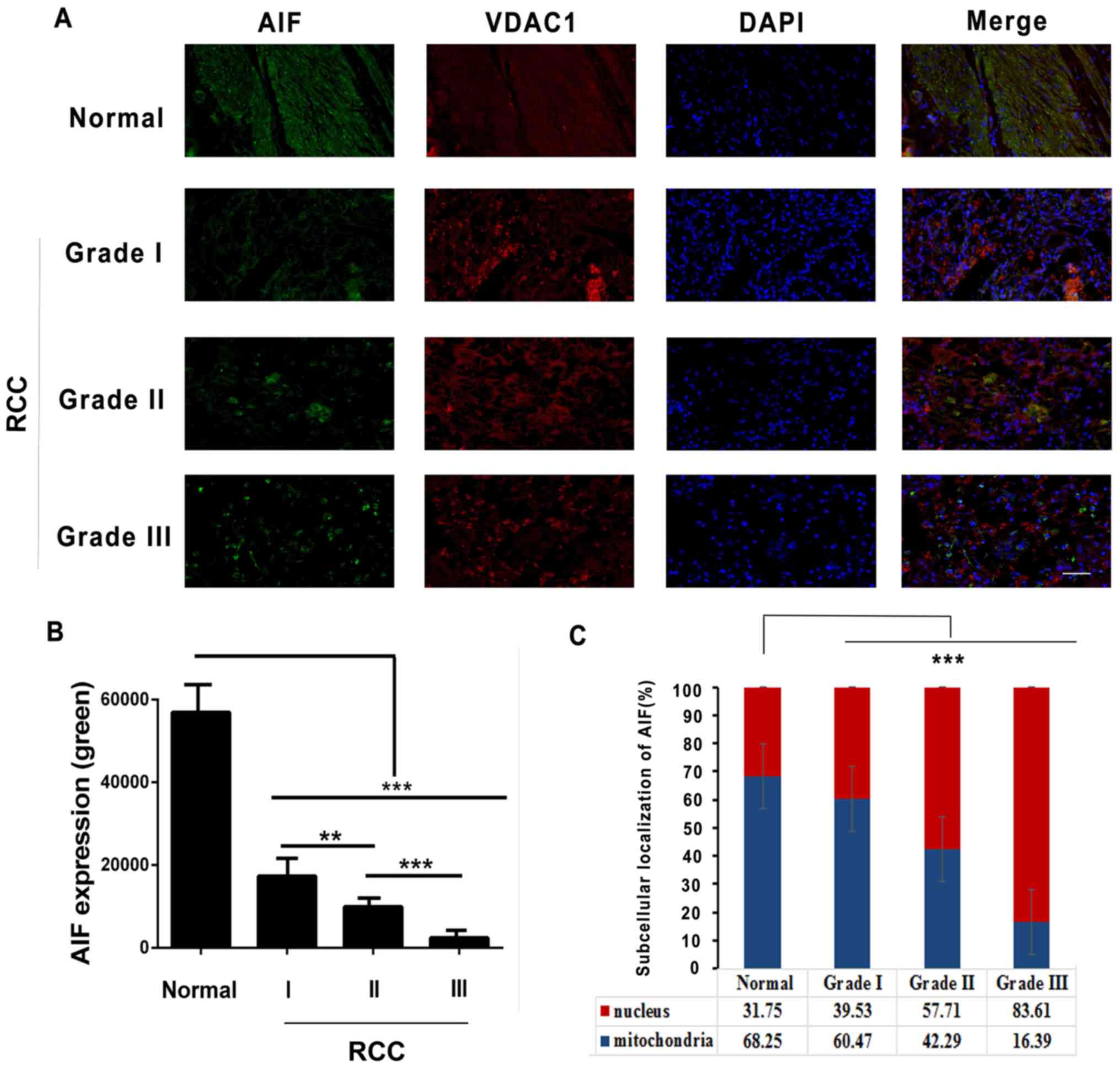
Reportedly, translocation of AIF from the
mitochondrial intermembrane space in normal embryonic stem cells to
the nucleus induces apoptosis (3).
To examine the subcellular localization of AIF in RCCs,
immunofluorescence staining was performed. As presented in Fig. 4A and B, AIF was found to be
downregulated in RCC tissues according to tumor grade.
Additionally, it was observed that AIF was primarily present in
mitochondria (62.25%) in adjacent tissues, and less AIF was present
in the nuclei (37.75%). In RCC tissues, more AIF was present in
nuclei compared with mitochondria (Grade I, 39.53%; Grade II,
57.71% and Grade III, 83.61%) (Fig. 4A
and C). These results suggest that AIF may translocate from the
mitochondria to nucleus in RCC tissues.
Association between AIF expression and
clinicopathological features in patients with RCC
Positive AIF expression rate in RCC was associated
with age, tumor (T) stage and grade (P<0.05; Table III). The incidence of positive AIF
expression in individuals aged <60 years was higher than that in
individuals aged ≥60 years. Based on clinical T stage, the
frequency of AIF-positive expression in stage T3 RCCs was
significantly lower than that in stage T1 RCCs. Histopathological
grade also indicated that the AIF-positive expression rate in
patients with G3 grade RCCs was significantly lower than that in
patients with G1 grade RCCs. These results suggest that decreased
AIF expression is associated with RCC progression and grade. More
severe malignancy was associated with decreased AIF-positive
expression. AIF expression did not significantly differ between
patients with lymph node metastasis and those without lymph node
metastasis (P>0.05).
 | Table III.Association between AIF expression
and clinicopathological data of patients with renal cell
carcinoma. |
Table III.
Association between AIF expression
and clinicopathological data of patients with renal cell
carcinoma.
|
| AIF expression |
|
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | Positive, n | Negative, n | χ2
value | P-value |
|---|
| Age, years |
|
|
|
|
|
<60 | 10 | 24 | 6.157 | 0.013a |
|
≥60 | 6 | 56 |
|
|
| Sex |
|
|
|
|
|
Male | 10 | 53 | 0.083 | 0.773 |
|
Female | 6 | 27 |
|
|
| T stage |
|
|
|
|
| T1 | 9 | 21 | 6.583 | 0.037a |
| T2 | 3 | 39 |
|
|
| T3 | 4 | 20 |
|
|
| Grade |
|
|
|
|
| I | 10 | 26 | 6.400 | 0.041a |
| II | 4 | 20 |
|
|
|
III | 2 | 34 |
|
|
| Metastasis |
|
|
|
|
|
With | 2 | 19 | 0.987 | 0.32 |
|
Without | 14 | 61 |
|
|
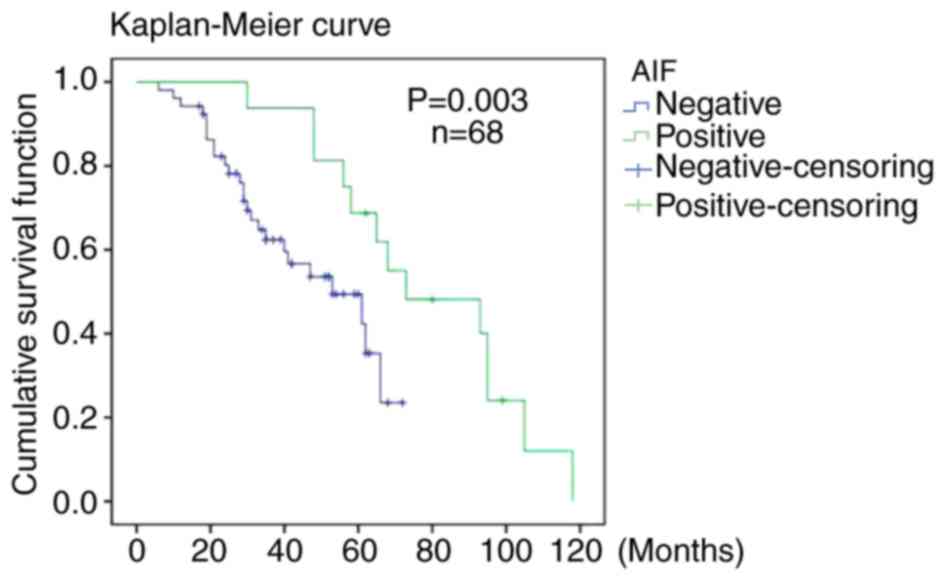
Negative AIF expression is associated
with a worse survival rate
Of the 96 patients who underwent surgery, 68 were
followed up for 6–118 months (mean follow-up time, 47 months). As
presented in Fig. 5, the overall
postoperative survival (OS) rate of the patients with negative AIF
expression was significantly lower compared with that of patients
with positive AIF expression (P=0.003). However, as the number of
patients with positive AIF expression in the group was low,
progression-free survival was not comparable between the two
groups.
Discussion
AIF is thought to be a tumor suppressor and serves a
critical role in caspase-independent cell apoptosis by triggering
chromatin condensation and DNA fragmentation (21,22).
Following an apoptotic signal, AIF is translocated from
mitochondria to the nucleus where it initiates caspase-independent
programmed cell death (3,23). However, AIF exhibits cell-type
specificity in the induction of apoptosis. For example, in colon,
breast and lung cancer cell lines, the mitochondria-to-nuclear
localization of AIF was induced by peroxides and certain drugs used
to treat cancer (24,25). Of note, in several colon and squamous
carcinomas, AIF expression was significantly upregulated (26,27).
In the present study, 96 pairs of RCC and adjacent
tissues were collected. Using IHC, it was demonstrated that AIF
expression was significantly lower in RCC tissues compared with
that in adjacent tissues. Although the IHC images in Fig. 3 appear notably different from the
images in Fig. 1, this is due to the
difference in the graphed background, and, as such, the
quantification of AIF expression in Figs. 1B and 3B are very different due to the difference
in staining color. In addition, immunofluorescent localization of
the protein suggested that AIF translocated from mitochondria to
the nucleus in RCC tissues (Fig. 4).
AIF mRNA expression in RCC tissues, analyzed using RT-qPCR, was
lower compared with that in normal renal tissues (Fig. 1). These results suggested that AIF
downregulation in RCC tissue is associated with kidney
tumorigenesis. Previous studies have reported that AIF was
downregulated by factors such as hypoxia, microRNAs and epigenetic
effectors (28–30). In addition, after 6–118 months of
follow-up, the OS rates of patients with negative AIF expression
were significantly worse compared with those of patients with
positive AIF expression. Notably, a study by Xu et al
(13) reported that AIF was
significantly downregulated in kidney tumor specimens and that AIF
overexpression induced apoptosis in RCC cells. The results of the
present study are consistent with these findings reported by Xu
et al (13).
In conclusion, Xu et al (13) suggested that AIF served a role in
cell survival and kidney tumorigenesis, whilst the present study
demonstrated an association between AIF expression and the
progression of RCC. Therefore, AIF may serve as biomarker for RCC
and determining AIF expression levels may have diagnostic value in
patients with kidney tumors. Targeting AIF may be an effective
treatment strategy for patients with RCC.
Acknowledgements
Not applicable.
Funding
This study was supported by the Hubei Polytechnic
University School Talent Introduction Project (grant no. 180104),
General project of Hainan Natural Science Foundation (grant no.
819MS136), Hubei Natural Science Foundation (grant no.
2014CFC1094), Huangshi Science and Technology Plan Project (grant
no. 2014A069-1), Key Laboratory of Hubei Province 2014 Open Fund
(grant no. SB201402) and Hubei Polytechnic University School
Innovation Research Project (grant no. 14×jz05Q).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
ZW designed and performed experiments. CY designed
and performed experiments, analyzed data and drafted the
manuscript. YH and ZL performed certain experiments. XY and CL
analyzed the data, provided valuable assistance and critically
revised the manuscript. ZS designed and supervised the project,
assisted with data analysis and interpretation, and critically
revised the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committees of the Hubei Polytechnic University School (Huangshi,
China) and that of the Haikou Hospital of Xiangya Medical School of
Central South University (Haikou, China). Written informed consent
was obtained from all patients.
Patient consent for publication
Written informed consent was obtained from all
examined patients for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AIF
|
apoptosis-inducing factor
|
|
RCC
|
renal cell carcinoma
|
|
OS
|
overall postoperative survival
|
|
RT-qPCR
|
reverse transcription quantitative
polymerase chain reaction
|
References
|
1
|
Ghezzi D, Sevrioukova I, Invernizzi F,
Lamperti C, Mora M, D'Adamo P, Novara F, Zuffardi O, Uziel G and
Zeviani M: Severe X-linked mitochondrial encephalomyopathy
associated with a mutation in apoptosis inducing factor. Am J Hum
Genet. 86:639–649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Susin SA, Lorenzo HK, Zamzami N, Marzo I,
Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler
M, et al: Molecular characterization of mitochondrial
apoptosis-inducing factor. Nature. 397:441–446. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Joza N, Susin SA, Daugas E, Stanford WL,
Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, et al:
Essential role of the mitochondrial apoptosis-inducing factor in
programmed cell death. Nature. 410:549–554. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Joza N, Pospisilik JA, Hangen E, Hanada T,
Modjtahedi N, Penninger JM and Kroemer G: AIF: Not just an
apoptosis-inducing factor. Ann N Y Acad Sci. 1171:2–11. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen SM, Guo M, Xiong Z, Yu Y, Zhao XY,
Zhang FF and Chen GQ: AIF inhibits tumor metastasis by protecting
PTEN from oxidation. EMBO Rep. 16:1563–1580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu SW, Wang H, Poitras MF, Coombs C,
Bowers WJ, Federoff HJ, Poirier GG, Dawson TM and Dawson VL:
Mediation of Poly (ADP-ribose) polymerase-1-dependent cell death by
apoptosis-inducing factor. Science. 297:259–263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leslie NR, Yang X, Downes CP and Weijer
CJ: PtdIns(3,4,5)P(3)-dependent and independent roles for PTEN in
the control of cell migration. Curr Biol. 17:115–125. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu CX, Li S and Whorton AR: Redox
regulation of PTEN by S-nitrosothiols. Mol Pharmacol. 68:847–854.
2015.
|
|
10
|
Kim DH, Suh J, Surh YJ and Na HK:
Regulation of the tumor suppressor PTEN by natural anticancer
compounds. Ann N Y Acad Sci. 1401:136–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsai MJ, Chang WA, Tsai PH, Wu CY, Ho YW,
Yen MC, Lin YS, Kuo PL and Hsu YL: Montelukast induces
apoptosis-inducing factor-mediated cell death of lung cancer cells.
Int J Mol Sci. 18:E13532017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohyama M, Tsuchiya A, Kaku Y, Kanno T,
Shimizu T, Tanaka A and Nishizaki T: Phosphatidylinositol
derivatives induce gastric cancer cell apoptosis by accumulating
AIF and AMID in the nucleus. Anticancer Res. 35:6563–6571.
2015.PubMed/NCBI
|
|
13
|
Xu S, Wu H, Nie H, Yue L, Jiang H, Xiao S
and Li Y: AIF downregulation and its interaction with STK3 in renal
cell carcinoma. PLoS One. 9:e1008242014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo H, German P, Bai S, Barnes S, Guo W,
Qi X, Lou H, Liang J, Jonasch E, Mills GB and Ding Z: The PI3K/AKT
pathway and renal cell carcinoma. J Genet Genomics. 42:343–353.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuusk T, Grivas N, de Bruijn R and Bex A:
The current management of renal cell carcinoma. Minerva Med.
108:357–369. 2017.PubMed/NCBI
|
|
16
|
Ye DW and Zhang HL: Critical appraisal of
sorafenib in the treatment of Chinese patients with renal cell
carcinoma. Onco Targets Ther. 7:925–935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li XL, Chen XQ, Zhang MN, Chen N, Nie L,
Xu M, Gong J, Shen PF, Su ZZ, Weng X, et al: SOX9 was involved in
TKIs resistance in renal cell carcinoma via Raf/MEK/ERK signaling
pathway. Int J Clin Exp Pathol. 8:3871–3881. 2015.PubMed/NCBI
|
|
18
|
Heng DY, Xie W, Regan MM, Harshman LC,
Bjarnason GA, Vaishampayan UN, Mackenzie M, Wood L, Donskov F, Tan
MH, et al: External validation and comparison with other models of
the International metastatic renal-cell carcinoma database
consortium prognostic model: A population-based study. Lancet
Oncol. 14:141–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Huang JH, Qu QH, Xia Q, Wang DS, Jin
L and Sheng C: Evaluating the microRNA-target gene regulatory
network in renal cell carcinomas, identification for potential
biomarkers and critical pathways. Int J Clin Exp Med. 8:7209–7219.
2015.PubMed/NCBI
|
|
20
|
Bustin S and Nolan T: Talking the talk,
but not walking the walk: RT-qPCR as a paradigm for the lack of
reproducibility in molecular research. Eur J Clin Invest.
47:756–774. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Daugas E, Nochy D, Ravagnan L, Loeffler M,
Susin SA, Zamzami N and Kroemer G: Apoptosis-inducing factor (AIF):
A ubiquitous mitochondrial oxidoreductase involved in apoptosis.
FEBS Lett. 476:118–123. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohiro Y, Garkavtsev I, Kobayashi S,
Sreekumar KR, Nantz R, Higashikubo BT, Duffy SL, Higashikubo R,
Usheva A, Gius D, et al: A novel p53 inducible apoptogenic gene,
PRG3, encodes a homologue of the apoptosis-inducing factor (AIF).
FEBS Lett. 524:163–171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seth R, Yang C, Kaushal V, Shah SV and
Kaushal GP: p53-dependent caspase-2 activation in mitochondrial
release of apoptosis-inducing factor and its role in renal tubular
epithelial cell injury. J Biol Chem. 280:31230–31239. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agarwal E, Chaudhuri A, Leiphrakpam PD,
Haferbier KL, Brattain MG and Chowdhury S: Akt inhibitor MK-2206
promotes anti-tumor activity and cell death by modulation of AIF
and Ezrin in colorectal cancer. BMC Cancer. 14:1452014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu JY, Zheng ZH, Son YO, Shi X, Jang YO
and Lee JC: Mycotoxin zearalenone induces AIF- and ROS-mediated
cell death through p53- and MAPK-dependent signaling pathways in
RAW264.7 macrophages. Toxicol In Vitro. 25:1654–1663. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeong EG, Lee JW, Soung YH, Nam SW, Kim
SH, Lee JY, Yoo NJ and Lee SH: Immunohistochemical and mutational
analysis of apoptosis-inducing factor (AIF) in colorectal
carcinomas. APMIS. 114:867–873. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Skyrlas A, Hantschke M, Passa V, Gaitanis
G, Malamou-Mitsi V and Bassukas ID: Expression of
apoptosis-inducing factor (AIF) in keratoacanthomas and squamous
cell carcinomas of the skin. Exp Dermatol. 20:674–676. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun Y, Zhang Y, Wang X, Blomgren K and Zhu
C: Apoptosis-inducing factor downregulation increased neuronal
progenitor, but not stem cell, survival in the neonatal hippocampus
after cerebral hypoxia-ischemia. Mol Neurodegener. 7:172012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiong Z, Guo M, Yu Y, Zhang FF, Ge MK,
Chen GQ and Shen SM: Downregulation of AIF by HIF-1 contributes to
hypoxia-induced epithelial-mesenchymal transition of colon cancer.
Carcinogenesis. 37:1079–1088. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu H, Li J, Zhao Y and Liu D: TNFα-induced
downregulation of microRNA-186 contributes to apoptosis in rat
primary cardiomyocytes. Immunobiology. 222:778–784. 2017.
View Article : Google Scholar : PubMed/NCBI
|