Introduction
The incidence rate of thyroid carcinoma has
increased markedly by 4.73 times from 2.40/100,000 in 2003 to
13.75/100,000 in 2012, with an average annual increase of 20% in
China (1). Papillary thyroid
carcinoma (PTC) is one type of thyroid carcinoma, which is fast
growing (2). The majority of PTCs
have low invasiveness and a good prognosis, but a number of tumors
exhibit high invasiveness, mainly manifesting as lymph node
metastasis, outer capsule invasion and distant metastasis. The
B-Raf proto-oncogene, serine/threonine kinase (BRAF)V600E mutation
is a common mutation found in papillary carcinomas (3). Multiple studies have revealed that BRAF
mutations are associated with the invasiveness of PTC (4–6).
Conventional ultrasound and contrast-enhanced ultrasound are
important methods for diagnosing thyroid nodules. The present study
aimed to analyze the associations between the ultrasound features
of PTC and the BRAFV600E mutation and to determine whether
ultrasound can facilitate a preliminary prediction of the
invasiveness of PTC, which can assist clinicians by providing
alternative choices (either observation or surgery). If these
features of conventional and contrast-enhanced ultrasound are
associated with BRAF gene mutations, despite a diagnosis of the
thyroid nodule as PTC, BRAF gene analysis should be performed to
stratify the risk of progression and guide clinical treatment
(7).
Materials and methods
Patients
All patients were examined and treated at the Second
Affiliated Hospital of Chongqing Medical University (Chongqing,
China). A total of 201 patients with thyroid nodules were examined
with conventional ultrasound and contrast-enhanced ultrasound
between October 2016 and April 2018. The patients included 62 males
and 139 females, with ages ranging from 23 to 59 years, and a mean
age of 44.58±7.684 years. A total of 6 patients had 2 thyroid
nodules each; therefore, 207 nodules were included in this study.
Following examination, ultrasound-guided fine-needle aspiration
biopsy (FNAB) was performed on all of the nodules and the specimens
were evaluated for the BRAFV600E mutation. Following resection,
nodules were fixed with 10% formaldehyde for 6 h at room
temperature, the 5 µm thick paraffin-embedded sections, were
stained with hematoxylin and eosin for 80 min at room temperature,
and subsequently confirmed as PTC under optical microscopy (×400
magnificaiton).
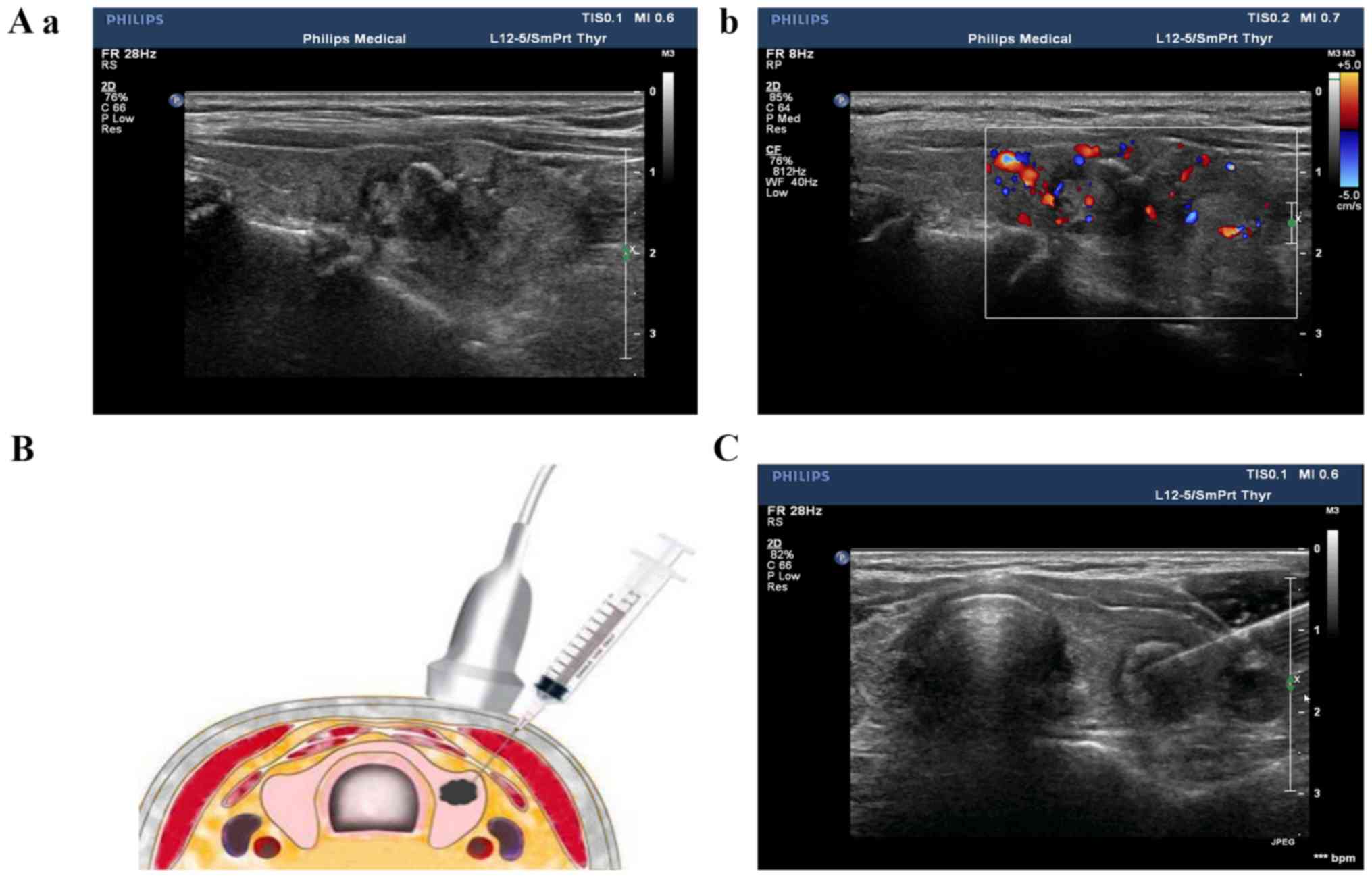
Conventional ultrasound and
contrast-enhanced ultrasound examination of thyroid nodules
The nodules were examined with a color Doppler
ultrasound (Philips IU22, L12-5 line array probe, 5–12 MHz; Philips
Healthcare), which revealed the shape, boundary, internal echo,
aspect ratio, microcalcification of and blood flow to the nodules
(Fig. 1). Contrast-enhanced
ultrasound of the thyroid nodules was used with a color Doppler
ultrasound (Philips IU22, L9-3 line array probe, 3–9 MHz,
mechanical index 0.07; Philips Healthcare). A median vein within
the elbow was selected, and subsequently an ultrasound contrast
agent [2.4 ml SonoVue (Bracco Suisse SA), diluted with 0.9% saline
to 5 ml] was rapidly infused into the vein. Subsequently, 5 ml 0.9%
saline was immediately injected. The entry of the SonoVue until its
disappearance was observed.
Ultrasound-guided FNAB of thyroid
nodules
The thyroid nodules were examined with ultrasound to
determine the insertion site, angle and depth of the needle, while
the patients were in a supine position with their back raised and
their head tilted back. Following routine sterilization of the
injection site, 1–2 ml of 2% lidocaine was used for local
anesthesia. The probe was fixed and pressurized, with the puncture
point on one side of the probe, and the puncture route was set at
30–40° to the long axis of the probe surface (Fig. 1). Once the fine needle was positioned
inside the thyroid nodule, the needle was repeatedly inserted and
rotated 10 to 20 times and subsequently quickly withdrawn. The
specimen was fixed onto a slide and visually designated suitable
for further analysis and microscopic observations, prior to
delivery to the Department of Pathology of the Second Affiliated
Hospital of Chongqing Medical University (Chongqing, China) for
cytological examination [the slide was fixed in 95% ethanol for 15
min at room temperature, stained with hematoxylin and eosin for 20
min at room temperature, and observed under light microscope (×400
magnification)], and subsequently forwarded to the Molecular
Diagnostic Laboratory of the Second Affiliated Hospital of
Chongqing Medical University for BRAFV600E mutation analysis.
Materials for BRAFV600E mutation
detection
The following materials were used for BRAFV600E
mutation detection: A human BRAFV600E mutation detection kit (cat.
no. ADx-BR04; Amoy Diagnostics Co., Ltd.), containing Taq enzyme,
reaction mixture (containing the specific primer, the bi-loop
probe, 50 nM dNTPs, 750 nM magnesium chloride, ammonium sulfate and
potassium chloride) and a positive control (a mixture of 300
copies/µl of BRAFV600E mutation plasmid and 10 ng/µl of wild-type
BRAF human genome), a nucleic acid extraction reagent
(formalin-fixed paraffin-embedded DNA; cat. no. ADx-T101; Amoy
Diagnostics Co., Ltd.), purified water (self-prepared), a mini
centrifuge [WTL-6K; Cence, China (http://www.xiangyilxj.com/)] and patient thyroid
nodule cytological samples (FNA sample).
Methods of BRAFV600E mutation
detection
Each patient thyroid nodule cytological sample was
tested and analyzed along with a positive and negative control
(purified water). The reaction mixture was thawed at room
temperature, mixed on a vortex for 15 sec and subsequently
centrifuged at 2,000 × g for 15 sec at room temperature. The
reaction mixture (35 µl) was mixed with 0.4 µl Taq enzyme and
subsequently dispensed into a PCR tube (kept on ice). Following
this, 5 µl DNA sample (2–5 ng), 5 µl positive control and 5 µl
negative control were added separately to each PCR tube. The PCR
tubes were centrifuged at 2,000 × g for 1 min at room temperature
and a thermocycler (CFX96; Bio-Rad Laboratories, Inc.) was used.
The following thermocycling conditions were used for the
quantitative PCR: Initial denaturation at 95°C for 5 min; 15 cycles
of 95°C for 25 sec, 64°C for 20 sec and 72°C for 20 sec; followed
by an additional 31 cycles of 93°C for 25 sec, 60°C for 35 sec and
72°C for 20 sec. Carboxyfluorescein (FAM) and
hexachloro-flororescein (HEX) signals were detected at 60°C during
the final set of cycling conditions. The 2−ΔΔCq method
was used to quantify the relative amount DNA (8). If the Cq value of the FAM signal of the
sample was ≥28, the sample was considered negative for the
BRAFV600E mutation; if the Cq value of the FAM signal of the sample
was <28, the sample was considered positive for the BRAFV600E
mutation, according to manufacturer's protocol.
Statistical analysis
Statistical analysis was performed using SPSS
(v23.0; IBM Corp.). Univariate logistic regression analysis was
performed to determine the ultrasound factors associated with the
BRAF gene mutation, and the variables with statistical significance
on the univariate analysis were analyzed by multivariate logistic
regression analysis. A two-sided P-value of <0.05 was considered
to indicate a statistically significant difference.
Results
Ultrasonographic features and
univariate analysis of the BRAF gene mutation
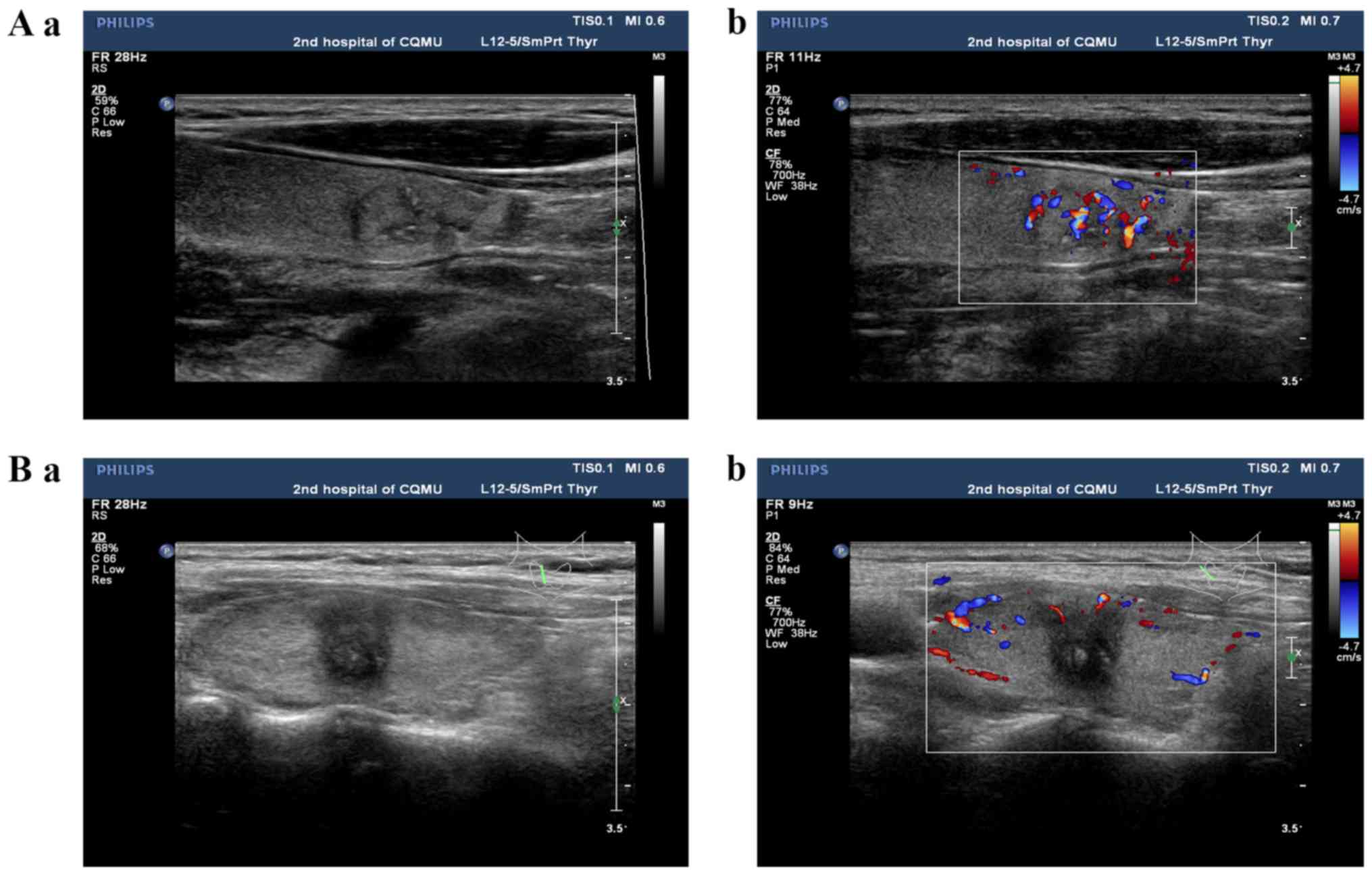
The conventional ultrasonographic features of PTC
include either isoechoic or hypoechoic nodules, regular or
irregular morphology, a clear or unclear boundary, an aspect ratio
<1 or ≥1, the absence or presence of microcalcification, and a
rich or poor blood flow signal (Fig.
2).
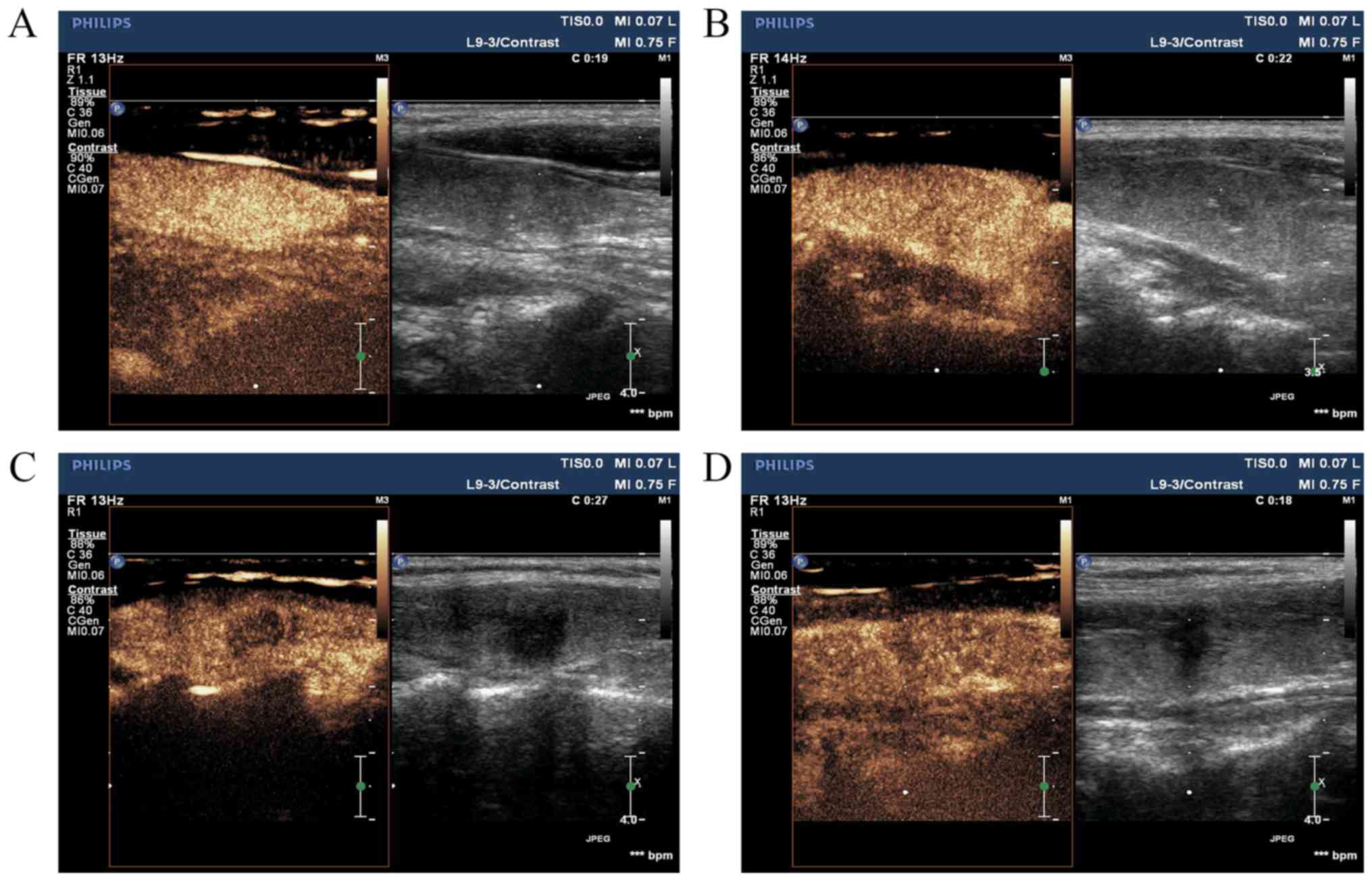
Contrast-enhanced ultrasonographic features of PTC
are characterized by the following: Earlier/synchronous/later
enhancement of the nodule compared with that of the surrounding
parenchyma, divergent/centripetal enhancement mode,
homogeneous/heterogeneous high/equal/low enhancement at the peak
(nodule contrast enhancement reached the peak), no change/increase
in nodule size following angiography and earlier/synchronous/later
clearance time (when SonoVue was completely cleared) to the
surroundings (Fig. 3).
Overall, 74.9% (155/207) of the PTC nodules had the
BRAFV600E mutation, while 25.1% (52/207) had the wild-type BRAF
allele. Conventional and contrast-enhanced ultrasonographic
features of the nodules were univariately analyzed between the
mutant and wild-type groups for the BRAFV600E mutation. There were
a significantly higher number of nodules in the two categories with
regard to the aspect ratio, microcalcification, nodule size
following enhancement, enhancement mode and enhancement time in the
mutant BRAF group compared with that in the wild-type BRAF group
(P<0.05) (Table I).
 | Table I.Association between ultrasonographic
features and B-Raf proto-oncogene serine/threonine kinase gene
mutation. |
Table I.
Association between ultrasonographic
features and B-Raf proto-oncogene serine/threonine kinase gene
mutation.
| Factors | Mutant (n=155) | Wild-type (n=52) | χ2 | P-value |
|---|
| Morphology |
|
| 0.584 | 0.445 |
|
Irregular | 84 | 25 |
|
|
|
Regular | 71 | 27 |
|
|
| Boundary |
|
| 0.849 | 0.357 |
|
Unclear | 80 | 23 |
|
|
|
Clear | 75 | 29 |
|
|
| Hypoechoic
nodule |
|
| 0.853 | 0.356 |
| No | 72 | 28 |
|
|
| Yes | 83 | 24 |
|
|
| Aspect ratio |
|
| 5.216 | 0.022 |
|
<1 | 70 | 33 |
|
|
| ≥1 | 85 | 19 |
|
|
|
Microcalcification |
|
| 18.565 | <0.001 |
| No | 57 | 37 |
|
|
| Yes | 98 | 15 |
|
|
| Blood flow
signal |
|
| 0.129 | 0.719 |
| Poor | 79 | 28 |
|
|
| Rich | 76 | 24 |
|
|
| Nodule size following
enhancement |
|
| 11.660 | 0.001 |
| No
change | 62 | 35 |
|
|
|
Increase | 93 | 17 |
|
|
| Enhancement
uniformity |
|
| 1.004 | 0.316 |
|
Heterogeneous | 81 | 23 |
|
|
|
Homogeneous | 74 | 29 |
|
|
| Enhancement
degree |
|
| 1.169 | 0.280 |
| Equal
or high | 76 | 30 |
|
|
|
Low | 79 | 22 |
|
|
| Enhancement
mode |
|
| 11.614 | 0.001 |
|
Divergent | 51 | 31 |
|
|
|
Centripetal | 104 | 21 |
|
|
| Enhancement
time |
|
| 9.743 | 0.002 |
|
Synchronous or fast in | 57 | 32 |
|
|
| Slow
in | 98 | 20 |
|
|
| Clearance time |
|
| 0.477 | 0.490 |
|
Synchronous or slow out | 89 | 27 |
|
|
| Fast
out | 66 | 25 |
|
|
Multivariate logistic regression
analysis of ultrasonographic features associated with BRAF gene
mutations
The variables with statistical significance in the
univariate analysis were analyzed using multivariate logistic
regression analysis and the BRAFV600E mutation was associated with
microcalcification [odds ratio (OR), 2.256; 95% confidence interval
(CI), 1.160–5.500; P=0.020] and nodule size following enhancement
(OR, 2.119; 95% CI, 1.039–4.321; P=0.039) (Table II).
 | Table II.Multivariate logistic regression
analysis of ultrasonographic features associated with B-Raf
proto-oncogene serine/threonine kinase gene mutation. |
Table II.
Multivariate logistic regression
analysis of ultrasonographic features associated with B-Raf
proto-oncogene serine/threonine kinase gene mutation.
| Factors | OR | 95% CI | P-value |
|---|
| Aspect ratio | 0.612 | 0.179–2.094 | 0.434 |
|
Microcalcification | 2.256 | 1.160–5.500 | 0.020 |
| Nodule size
following enhancement | 2.119 | 1.039–4.321 | 0.039 |
| Enhancement
mode | 1.913 | 0.717–5.099 | 0.195 |
| Enhancement
time | 1.752 | 0.592–5.184 | 0.311 |
Discussion
With the continuous advancement of thyroid
ultrasound diagnostic technologies and increasing attention to
thyroid diseases, the incidence rate of thyroid cancer,
particularly PTC, has been increasing over the last four decades
(2). The majority of cases of PTC
have good prognosis and the progression are slow therefore the
patients could be monitored. The frequency of evaluation depends
upon the sonographic features of the nodules, 6 to 12 months for
subcentimeter nodules with suspicious characteristics, 12 to 24
months for nodules with low to intermediate suspicion on
ultrasound, 2 to 3 years for very-low-risk nodules (9). The majority of thyroid nodules are
stable during observation; however, a small number develop invasive
manifestations, including tumor enlargement, lymph node or distant
metastasis. The BRAF gene is a risk factor and may be associated
with lymph node and distant metastasis (10). Preliminarily evaluation of the
thyroid nodule to determine whether it is benign or malignant
through non-invasive ultrasound examination would assist thyroid
surgeons to provide patients with different treatment options.
The BRAF gene is a member of the RAF gene family and
is located on chromosome 7q34. The BRAFV600E mutation is a
substitution mutation within exon 15 at amino acid 600, which
changes to glutamic acid from valine and is one of the most common
gene mutations in PTC (9). The
prevalence of this mutation has been reported to be 40–70%
(11–14). The BRAFV600E mutation promotes
tumorigenesis and progression by increasing kinase activity and
overactivating the mitogen-activated protein kinase pathway
(15). The BRAFV600E mutation is
associated with the invasiveness of PTC. Clinicopathological
analyses have reported that PTC that has the BRAFV600E mutation is
highly invasive and prone to capsular invasion, lymph node
metastasis and a poor prognosis (5,16). In
the present study, the BRAF gene mutation rate was 74.8%, which is
consistent with previous reports (11–14).
PTC presents as unclear hypoechoic nodules on
conventional ultrasound and is often accompanied by
microcalcification, irregular morphology and an aspect ratio ≥1.
The diagnostic accuracy of conventional ultrasound for PTC, the
main examination method for the clinical diagnosis of this disease,
is 74–82% (17). Among the various
ultrasonographic features, hypoechogenicity and microcalcification
have high specificity for the diagnosis of PTC. Contrast-enhanced
ultrasound involves the introduction of an ultrasound contrast
agent into the tumor microcirculation to observe tumor vascular
distribution and blood perfusion. The main features of using this
ultrasound include the centripetal entry of contrast agents, with a
slow in and fast out enhancement, heterogeneous low enhancement and
larger nodular size compared with that in 2-dimensional grayscale
ultrasound. The aforementioned features of contrast-enhanced
ultrasound were utilized in the present study. The distribution of
blood and perfusion in PTC nodules are associated with tumor size.
The smaller nodules (<1 cm) may have poor blood flow signals,
while larger nodules (>2 cm) may display the opposite pattern
(18–20). Therefore, the present study included
tumors ≤2 cm in size. Furthermore, the guidelines for the clinical
application of contrast-enhanced ultrasound developed by the
European Federation of Societies for Ultrasound in Medicine and
Biology (21) still recommend that
determination of the results of the contrast-enhanced ultrasound
must be performed on 2-dimensional grayscale and color Doppler
ultrasound. Therefore, the present study combined the features of
2-dimensional grayscale, color Doppler ultrasound and
contrast-enhanced ultrasound.
A number of reports indicate a correlation between
BRAF gene mutations and ultrasound features, particularly for
contrast-enhanced ultrasound, but the results are controversial.
Cong et al (22) reported
that the BRAFV600E mutation was associated with the blood flow
signal in ultrasound, while Kabaker et al (23) identified an association between the
BRAFV600E mutation and the boundary, aspect ratio and
microcalcification of thyroid nodules upon ultrasound.
Sastre-Marcos et al (24)
reported an association between the BRAFV600E mutation with at
least one of the ultrasound features, and microcalcification and
hypoechogenicity were two independently associated factors. Luo
et al (25) reported that the
BRAFV600E mutation was associated with the features of the nodules
on elastography, but not with the features on 2-dimensional
ultrasound. Li et al (26)
also reported that the BRAFV600E mutation was not associated with
the features of 2-dimensional ultrasound (including tumor size,
tumor border, or calcification). In the present study, a
statistical significant difference was identified between five
features of conventional and contrast-enhanced ultrasound, namely,
microcalcification, aspect ratio, nodule size following
enhancement, enhancement mode and enhancement time, and the
BRAFV600E gene mutation status, using univariate logistic
regression analysis. Further analysis using multivariate logistic
regression revealed that only microcalcification and nodule size
following enhancement were significantly associated with BRAF gene
mutations (P<0.05). Previous studies have revealed that hard
thyroid nodules are more prone to extravasation and may have a
higher degree of relative malignancy (27,28). The
hardness of PTC nodules may be derived from their
microcalcification, and microcalcification may be associated with
BRAF gene mutations. The boundary of the benign nodule is often
clear, the nodule size following enhancement is not increased, and
its boundary of contrast-enhanced ultrasound is the same as that of
2-dimensional ultrasound. However, the malignant nodule has the
nature of invasive growth, and its boundary is often unclear in
2-dimensional ultrasound, therefore the measured size of the nodule
using 2-dimensional ultrasound could be smaller than its true size.
After contrast-enhanced ultrasound, the boundary can be clearly
displayed, which is larger compared with that of 2-dimensional
ultrasound (21). Therefore, the
increase in nodule size following angiography could be due to the
invasive growth of thyroid cancer, and the invasive nature of the
tumor may also be an important outcome of BRAF gene mutations.
FNA is suitable for detecting the BRAFV600E mutation
(7), but it is an invasive
examination. If the nodules are not suitable to determine a
diagnosis, further FNA procedures would be required, resulting in
increased invasiveness and additional complications, including
bleeding. As a non-invasive method, ultrasound is the first choice
for thyroid screening (7). The aim
of the present study was to determine which characteristic
ultrasound feature could reliably be used to screen suspicious BRAF
mutations and avoid non-essential FNA. BRAFV600E mutation detection
is recommended if the microcalcification and nodule size following
enhancement ultrasound features are present, otherwise detection is
not recommended. BRAF detection has two advantages: i) Diagnosis:
Simple cytological testing has a low positive detection rate for
thyroid cancer and is contingent on subjective factors. However,
BRAF detection is objective and if a mutation is present, the
nodule has an estimated malignancy risk of >95% (7); ii) treatment: The 2015 recommendations
of the American Thyroid Association indicate that BRAF mutations
increase the risk of thyroid cancer recurrence and are associated
with other risk factors, such as lymph node metastasis. Aggressive
treatment, such as surgery, is recommended for thyroid nodules with
BRAF mutations, and patients should be closely monitored if surgery
is refused. Observation is recommended if a wild-type BRAF gene is
detected (7). Therefore, cytological
examination consisting of FNA and BRAF mutation detection will
increase the diagnostic rate (2).
The present study identified associations between
microcalcification and nodule size following enhancement and the
BRAFV600E mutation. If these two features are present, BRAF
mutation detection is recommended. Otherwise, BRAF mutation
detection should be limited. These criteria provide clinicians with
a framework for diagnosis and treatment, and a basis for clinicians
and patients to undergo observation. Therefore, ultrasound
assessment is a non-invasive method for early PTC screening that
may detect BRAF mutations. Following screening, closer follow-up or
more aggressive intervention may be required. Even in the case of
substantial tissue invasion, following early intervention, the
prognosis will be improved.
A limitation to the present study is the small
number of patients with PTC included and the fact that the majority
of the analysis was qualitative with subjective indexes. Although
the conventional and contrast-enhanced ultrasound procedures were
performed by experienced sonographers, subjective factors may have
affected the data. A number of risk factors can affect the
prognosis of PTC, including the BRAF mutation and ultrasound
features. Further investigation is required to analyze the clinical
course and clinicopathological risk factors of BRAF mutation and
ultrasound features. The lymph node dissection was not performed in
all of the patients in the present study and the presence of
pathological lymph node metastasis in the other patients was
unknown. Thus, lymph node conditions should be included to analyze
the prognosis of patients in future studies. In addition, patients
should be followed over a longer postoperative time period to
evaluate distant metastasis and/or aggressive tumors that may
develop with a positive BRAFV600E mutation status.
In conclusion, the BRAFV600E mutation was associated
with microcalcification and nodule size following enhancement. If
ultrasound reveals microcalcification or an increase in nodule size
following angiography, surgical treatment and BRAF gene detection
should be performed.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81701709), Chongqing
Municipal Health and Family Planning Commission (grant no.
2016MSXM029) and Chongqing Science and Technology Commission (grant
no. cstc2016jcyjA0295).
Availability of data and materials
All data generated or analyzed during this study are
included in this article.
Authors' contributions
YL, LH, JC, and LY designed the study and wrote the
main manuscript text. YL, LH and GY analyzed all data. GY and LC
prepared all figures. LC and BZ performed the FNAB. GY and LY
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This retrospective review study was approved by the
Ethics Committee of the Second Affiliated Hospital of Chongqing
Medical University. The requirement for consent was waived due to
the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Du L, Li R, Ge M, Wang Y, Li H, Chen W and
He J: Incidence and mortality of thyroid cancer in China,
2008–2012. Chin J Cancer Res. 31:144–151. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jung CK, Little MP, Lubin JH, Brenner AV,
Wells SA Jr, Sigurdson AJ and Nikiforov YE: The increase in thyroid
cancer incidence during the last four decades is accompanied by a
high frequency of BRAF mutations and a sharp increase in RAS
mutations. J Clin Endocrinol Metab. 99:E276–E285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Czarniecka A, Oczko-Wojciechowska M and
Barczyński M: BRAF V600E mutation in prognostication of papillary
thyroid cancer (PTC) recurrence. Gland Surg. 5:495–505. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li F, Chen G, Sheng C, Gusdon AM, Huang Y,
Lv Z, Xu H, Xing M and Qu S: BRAFV600E mutation in papillary
thyroid microcarcinoma: A meta-analysis. Endocr Relat Cancer.
22:159–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Czarniecka A, Kowal M, Rusinek D,
Krajewska J, Jarzab M, Stobiecka E, Chmielik E, Zembala-Nozynska E,
Poltorak S, Sacher A, et al: The risk of relapse in papillary
thyroid cancer (PTC) in the context of BRAFV600E mutation status
and other prognostic factors. PLoS One. 10:e01328212015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Y, Sadow PM, Suh H, Lee KE, Choi JY,
Suh YJ, Wang TS and Lubitz CC: BRAF(V600E) is correlated with
recurrence of papillary thyroid microcarcinoma: A systematic
review, Multi-institutional primary data analysis, and
meta-analysis. Thyroid. 26:248–255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brito JP, Ito Y, Miyauchi A and Tuttle RM:
A clinical framework to facilitate risk stratification when
considering an active surveillance alternative to immediate biopsy
and surgery in papillary microcarcinoma. Thyroid. 26:144–149. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xing M, Alzahrani AS, Carson KA, Viola D,
Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, et al:
Association between BRAF V600E mutation and mortality in patients
with papillary thyroid cancer. JAMA. 309:1493–1501. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prescott JD: BRAF V600E status adds
incremental value to current risk classification systems in
predicting papillary thyroid carcinoma recurrence. Surgery.
152:984–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng X, Wei S, Han Y, Li Y, Yu Y, Yun X,
Ren X and Gao M: Papillary microcarcinoma of the thyroid: Clinical
characteristics and BRAF(V600E) mutational status of 977 cases. Ann
Surg Oncol. 20:2266–2273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Walczyk A, Kowalska A, Kowalik A, Sygut J,
Wypiórkiewicz E, Chodurska R, Pięciak L and Góźdź S: The
BRAF(V600E) mutation in papillary thyroid microcarcinoma: Does the
mutation have an impact on clinical outcome. Clin Endocrinol (Oxf).
80:899–904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moon HJ, Kim EK, Chung WY, Shin DY and
Kwak JY: BRAF mutation in fine needle aspiration specimens as a
potential predictor for persistence/recurrence in patients with
classical papillary thyroid carcinoma larger than 10 mm at a BRAF
mutation prevalent area. Head Neck. 37:1432–1438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peyssonnaux C and Eychène A: The
Raf/MEK/ERK pathway: New concepts of activation. Biol Cell.
93:53–62. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu C, Chen T and Liu Z: Associations
between BRAF(V600E) and prognostic factors and poor outcomes in
papillary thyroid carcinoma: A meta-analysis. World J Surg Oncol.
14:2412016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang B, Jiang YX, Dai Q, et al:
Prospective observation of contrast-enhanced patterns of thyroid
nodules with SonoVue. Chin J Med Imaging Technol. 26:844–847.
2010.
|
|
18
|
Yang YJ, Jian-Min L and Wang BC: Study of
relationship between VEGF expression and tumor progress of primary
hepatocellular carcinoma. China J Modern Med. 15:408–412. 2005.
|
|
19
|
Bartolotta TV, Midiri M, Galia M, Runza G,
Attard M, Savoia G, Lagalla R and Cardinale AE: Qualitative and
quantitative evaluation of solitary thyroid nodules with
contrast-enhanced ultrasound: Initial results. Eur Radiol.
16:2234–2241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Q, Jiang J, Du XP, et al: Value of
contrast-enhanced ultrasound in diagnosis of thyroid papillary
carcinoma. Chin J Ultrasound Med. 27:595–597. 2011.
|
|
21
|
Claudon M, Cosgrove D, Albrecht T, Bolondi
L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D'Onofrio
M, et al: Guidelines and good clinical practice recommendations for
contrast enhanced ultrasound (CEUS)-update 2008. Ultraschall Med.
29:28–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cong X, Zhao S, Wang Y, et al: Ultrasound
features of braf t1799a gene mutation papillary thyroid carcinoma.
Med J Qilu. 29:201–212. 2014.
|
|
23
|
Kabaker AS, Tublin ME, Nikiforov YE,
Armstrong MJ, Hodak SP, Stang MT, McCoy KL, Carty SE and Yip L:
Suspicious ultrasound characteristics predict BRAF V600E-positive
papillary thyroid carcinoma. Thyroid. 22:585–589. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sastre-Marcos J, Val-Zaballos FD,
Cortes-Muñoz C, Campos-Martin Y, Aso-Manso S, Jaen-Diaz I,
Vicente-Delgado A and Lopez-Lopez J: BRAF V600E positive papillary
thyroid carcinoma is associated with suspicious ultrasound feature.
Endocrine. 37:EP8502015.
|
|
25
|
Luo ZY, Hong YR, Wen Q, et al:
Associations of the BRAF V600E mutation with sonographic features
in patients with papillary thyroid carcinoma. Chin J Ultrasonogr.
25:785–789. 2016.
|
|
26
|
Li Q, Yuan J, Wang Y and Zhai Y:
Association between the BRAF V600E mutation and ultrasound features
of the thyroid in thyroid papillary carcinoma. Oncol Lett.
14:1439–1444. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moon HJ, Kim EK, Yoon JH and Kwak JY:
Clinical implication of elastography as a prognostic factor of
papillary thyroid microcarcinoma. Ann Surg Oncol. 19:2279–2287.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin ZQ, Lin MY, Hu WH, Li WY and Bai SJ:
Gray-Scale ultrasonography combined with elastography imaging for
the evaluation of papillary thyroid microcarcinoma: As a prognostic
clinicopathology factor. Ultrasound Med Biol. 40:1769–1777. 2014.
View Article : Google Scholar : PubMed/NCBI
|