Introduction
Acute myeloid leukemia (AML) is a heterogeneous
disease resulting from multiple genetic and epigenetic
abnormalities that affect differentiation, proliferation and
apoptosis of myeloid cells (1). The
traditional treatment for leukemia is chemotherapy; however,
chemotherapy has serious side effects and may result in treatment
failure due to treatment-associated mortality or the emergence of
drug resistance (2). Despite recent
advances in treatment strategies, AML remains an incurable disease
(3). In recent years, traditional
Chinese medicine has been extensively used and has demonstrated
success in the treatment of leukemia. For example, arsenic trioxide
can achieve a high complete remission rate in the treatment of
acute promyelocytic leukemia (APL) and is particularly effective
for patients with recurrent APL (4–6).
Matrine, an alkaloid extracted from a Chinese herb,
has been widely used to treat viral hepatitis, cardiac arrhythmia
and skin inflammation, and exhibits chemotherapeutic potential
through its ability to trigger caspase-independent programmed cell
death (7). Matrine has also been
demonstrated to exert potential activities against different types
of leukemia by inhibiting cancer cell proliferation, accelerating
apoptosis, inducing cell cycle arrest and suppressing metastasis
(8,9). A number of studies have reported that
matrine inhibits AML cell proliferation via a variety of
mechanisms, including cancer cell differentiation and apoptosis,
altering the tumor cell cycle and inhibiting telomerase activity
(7,10,11).
The phosphoinositide 3-kinase (PI3K)/protein kinase
B (Akt)/mechanistic target of rapamycin kinase (mTOR) signaling
pathway is involved in cell growth, proliferation and
differentiation, and serves important roles in tumor occurrence,
development, treatment and outcome (9). A number of studies have reported that
there is continuous activation of the PI3K/Akt/mTOR signaling
pathway in leukemia cells (12,13).
However, to the best of our knowledge, a systematic scientific
evaluation of matrine has not been performed for AML and its
anticancer mechanisms on AML cell lines remain unclear. The present
study investigated the anticancer activity of matrine in THP-1
cells and revealed an induction of apoptosis and inhibition of the
PI3K/Akt/mTOR signaling pathway following treatment with
matrine.
Materials and methods
Chemicals and reagents
Human AML THP-1 cells were purchased from the Cell
Bank of Chinese Academy of Sciences. Matrine was purchased from
Tianyuan Biological Agent Plant. The Cell Counting Kit-8 (CCK-8)
was purchased from Dojindo Molecular Technologies, Inc. RPMI-1640
medium, fetal bovine serum (FBS), dimethyl sulfoxide dissolving
(DMSO), penicillin and streptomycin were purchased from Invitrogen;
Thermo Fisher Scientific, Inc. The ELISA reader ELx800 was obtained
from Bio-Tek Instruments, Inc. Inverted phase contrast microscope
was obtained from Olympus Corporation. LY294002 was purchased from
Cell Signaling Technology, Inc. and was dissolved in DMSO according
to the manufacturer's instructions. Antibodies, including
anti-PI3K, anti-phosphorylated (p)-PI3K, anti-mTOR, anti-p-mTOR,
anti-Akt, anti-p-Akt and anti-β-actin were purchased from ABclonal
Biotech Co., Ltd. The current study was approved by the Ethics
Committee of Bengbu Medical College (Bengbu, China).
Cell culture
THP-1 cell lines were maintained in RPMI-1640 medium
supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C with 5% CO2. A total of
5×103 cells in 100 µl RPMI-1640 medium were plated in
96-well plates. When the cell growth reached the log growth phase,
the cells were treated with trypsin to obtain a single cell
suspension, and were then seeded into different culture plates or
dishes for subsequent experiments, according to the experimental
requirements.
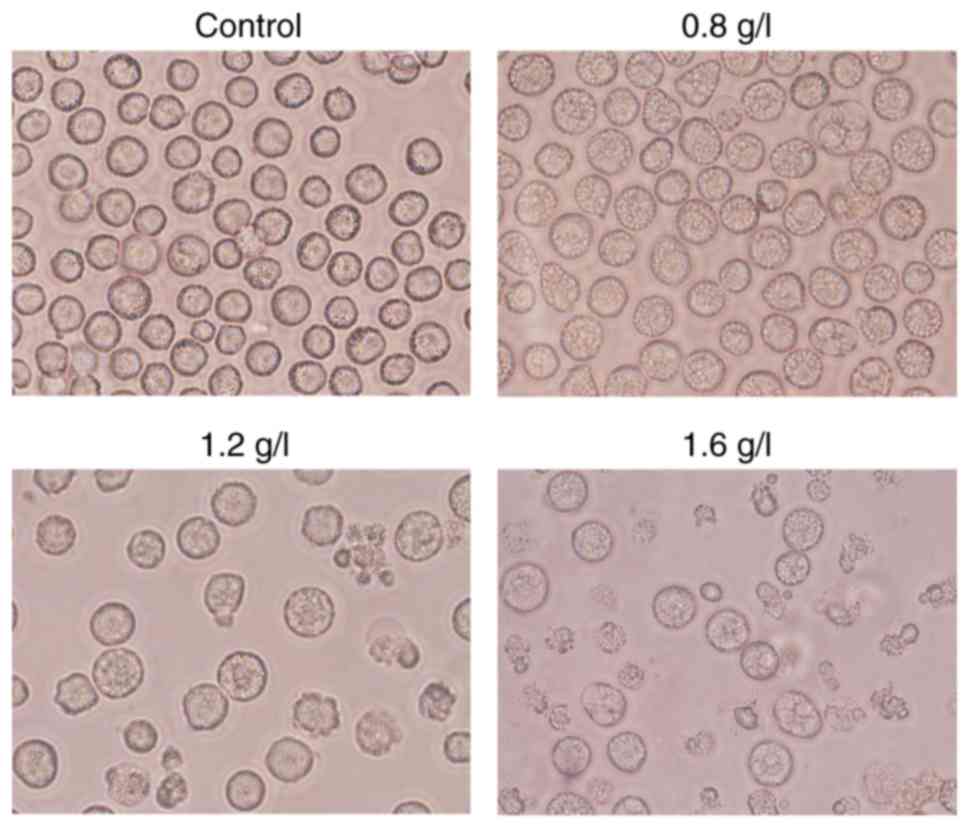
Morphology change
THP-1 cells, at a density of 2.0×105
cells/well, were equally seeded in 24-well flat bottom microtiter
plates at room temperature, and then treated with matrine at the
concentration of 0, 0.8, 1.2 and 1.6 g/l. After 24 h of treatment,
the morphology of THP-1 cells was observed under an inverted phase
contrast microscope (magnification, ×100).
Cell viability assay
THP-1 cells at a density of 2.0×105
cells/well were plated in 96-well microtiter plates and treated
with various doses of matrine (0, 0.4, 0.8, 1.2, 1.6 and 2.0 g/l),
dissolved in RPMI-1640 medium, for 12, 24 and 48 h at 37°C with 5%
CO2. Cell viability was subsequently measured using the
CCK-8 assay according to the manufacturer's protocol. Briefly, 20
µl CCK-8 solution was added to the culture medium 24 h following
matrine treatment. The cells were incubated for 4 h at 37°C. The
absorbance was read at a wavelength of 450 nm using the ELx800
ELISA reader. All experiments were repeated a minimum of four
times. The absorbance value was measured and the proliferation
inhibition rate (IR) was calculated as: IR=(control group
absorbance-experimental group absorbance)/control group absorbance
×100%. The cell viability rate (%)=A450 (matrine)/A450 (control)
×100%.
Apoptosis assay
The ability of matrine to induce apoptosis in AML
cells was assessed by Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) double staining. AML cells were plated
at a density of 2.0×105 cells/well in 6-well plates. The
cells were then treated with 0, 0.8, 1.2 and 1.6 g/l of matrine or
20 µmol/l LY294002, dissolved in DMSO, for 24 h or 1.2 g/l matrine
at 37°C for three different time points (12, 24 and 48 h). The
cells in 6-well plates were stained with DAPI (3 µM). The cells
were then washed with PBS and fixed with 10% formaldehyde at room
temperature for 20 min. The DAPI-stained cells were observed with a
fluorescence microscope (magnification, ×100) for estimation of the
percentage of cells not undergoing apoptosis. After treatment, the
cells were collected and stained at 25°C with Annexin V-FITC and PI
using an Annexin V-FITC Apoptosis Detection kit (BD Biosciences).
Subsequently, apoptosis analysis was performed using FITC signal
detector (FL1) and PI signal detector (FL2) using a flow cytometer
(BD Accuri C6) and analyzed using the FACSCalibur system (BD
Biosciences). The apoptosis ratio of THP-1 cells contains the
percentage of early-phase apoptotic cells and early-phase apoptotic
cells. The experiment was repeated three times independently with
similar results.
Western blot analysis
To further investigate whether the PI3K/Akt/mTOR
signaling pathway is associated with the inhibitory effects of
matrine in THP-1 cells, the PI3K-specific inhibitor LY294002 was
used to treat THP-1 cells prior to western blot analysis. Following
treatment with matrine or 20 µmol/l LY294002, changes in protein
expression levels were detected using western blot analysis. THP-1
cells were collected from each group and total protein was
extracted using radioimmunoprecipitation assay buffer (cat. no.
P0013B; Beyotime Institute of Biotechnology; Haimen, China).
Protein concentration was determined by the bicinchoninic acid
assay. A total of 20 µg protein was separated by SDS-PAGE on a 10%
gel and then transferred to polyvinylidene fluoride (PVDF)
membrane. The PVDF membrane was then incubated with anti-p-PI3K
(dilution 1:1,000; cat. no. Ap0427), anti-PI3K (dilution 1:2,000;
cat. no. A11177), anti-p-Akt (dilution 1:2,000, cat. no. AP0140),
anti-Akt (dilution 1:2,000; cat. no. A11016), anti-p-mTOR (dilution
1:2,000; cat. no. AP0094), anti-mTOR (dilution 1:2,000; cat. no.
A2445) or anti-β-actin (dilution 1:200,000; cat. no. AC026) (all
from ABclonal Biotech Co., Ltd.) at 4°C overnight. Subsequently,
the membrane was washed with washing buffer and incubated with
secondary antibody goat anti-rabbit IgG (H+L) (1:3,000; cat. no.
s0001; Beyotime Institute of Biotechnology) for 1 h at 25°C. The
protein bands were visualized by an ECL Advanced Western Blot
Detection kit (EMD Millipore).
Statistics analysis
Statistical analysis was performed using SPSS
software (version 17; SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation and are representative
of at least three independent experiments. One-way analysis of
variance followed by the Bonferroni correction was used to assess
the differences among the groups treated with different doses of
matrine. P<0.05 was considered to indicate a statistically
significant difference.
Results
Matrine inhibits the proliferation of
AML cells
The effects of matrine or LY294002 on the
proliferation of the AML cell line THP-1 cells were examined.
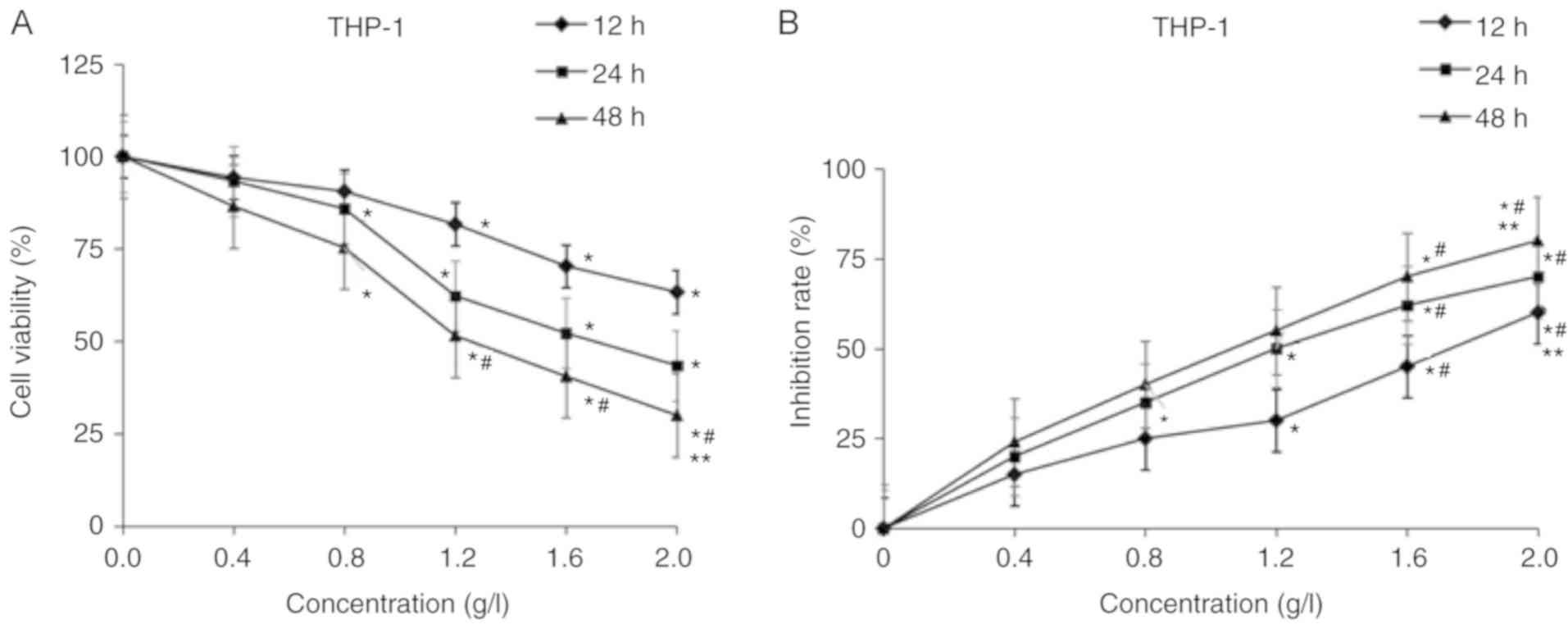
Matrine inhibited the proliferation of THP-1 cells in a dose- and
time-dependent manner, with a half-maximal inhibitory concentration
(IC50) of 1.2 g/l at 24 h (Fig. 1A). Following treatment with different
doses of matrine, it was identified that matrine decreased the cell
viability in a dose-dependent manner. The cell proliferation rates
of the groups treated with 0.8, 1.2, 1.6 and 2.0 g/l matrine were
significantly lower compared with the group treated with 0 g/l
matrine (treated with DMSO only) for 24 and 48 h (P<0.05). The
cell proliferation rates of the 1.6 and 2.0 g/l matrine-treated
groups were significantly lower compared with that of the 0.4 g/l
matrine-treated group for 48 h (P<0.05). The cell proliferation
rate of the 2.0 g/l matrine-treated group was significantly lower
compared with the 1.2 g/l matrine-treated group for 48 h
(P<0.05). Pair-wise comparisons of the cell proliferation
activity were statistically significant among the groups treated
with different doses of matrine (P<0.05; Fig. 1A). Compared with the 12 h group, the
inhibitory rate of THP-1 cell proliferation of 48 h increased from
22 to 56% following exposure to 1.2 g/l matrine (P<0.05;
Fig. 1B). Matrine decreased the
proliferation of AML cells. In addition, during the prolonged
treatments (24 and 48 h), 1.6 g/l matrine demonstrated a
significantly greater inhibitory effect compared with that at 12 h
(P<0.05). Furthermore, matrine induced changes in the morphology
of the AML THP-1 cells, such as the presence of autophagy vesicles
(Fig. 2). The IC50
concentration of AML cells treated with matrine for 24 h was 1.2
g/l. These results indicated that matrine may exert its anticancer
effects by decreasing the proliferation of AML cells and affecting
their morphology.
Matrine induces apoptosis of AML
cells
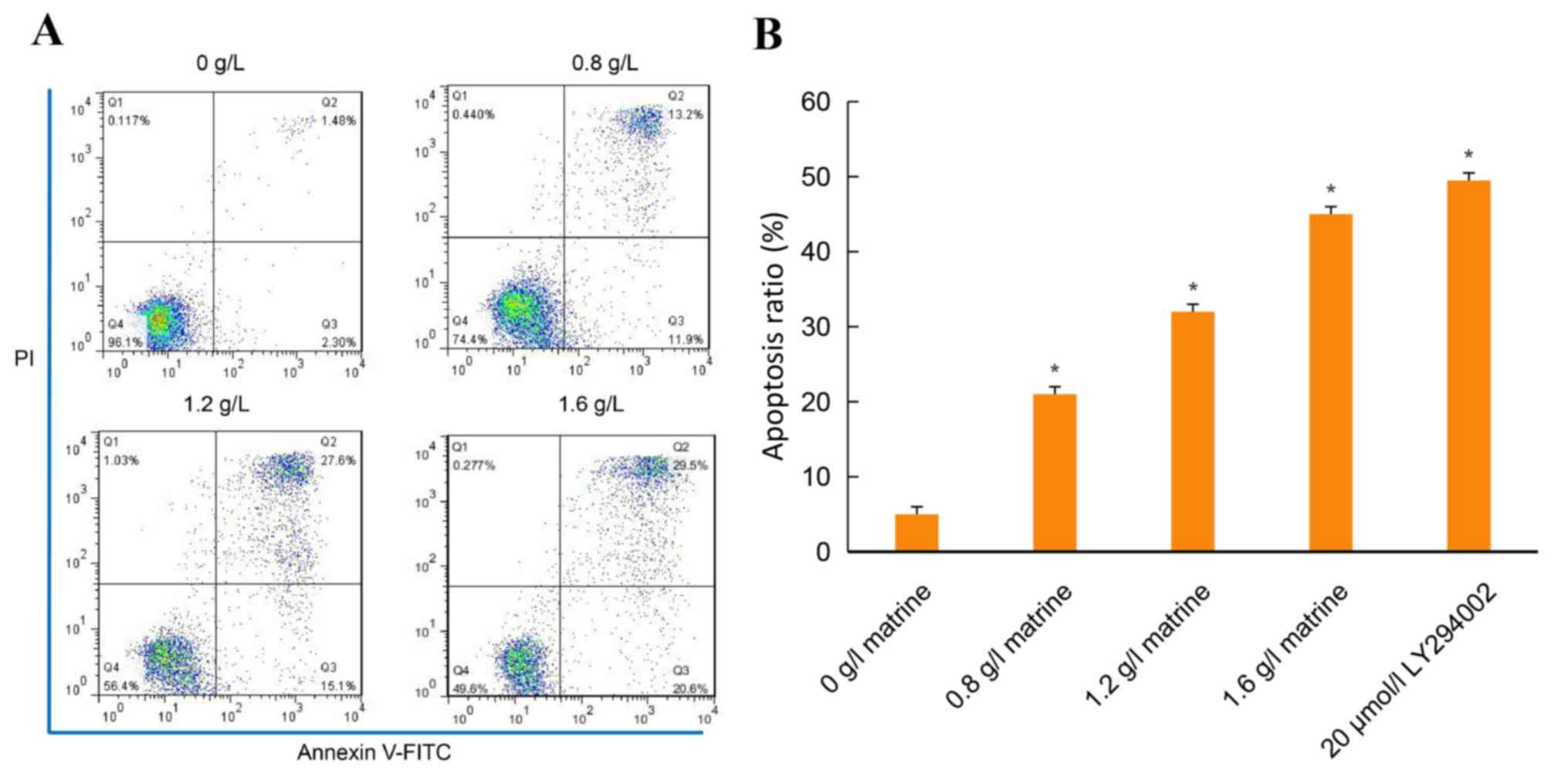
Compared with the blank control group, treatment
with various concentrations of matrine (0.8, 1.2 and 1.6 g/l) for
24 h, significantly induced apoptosis of THP-1 cells (Fig. 3A). The apoptosis ratio for 24 h in
the control group was 0.037±0.0012, whereas the apoptosis ratios in
the 0.8, 1.2 and 1.6 g/l-treated groups were 0.234±0.0011,
0.412±0.0013 and 0.485±0.0010, respectively (P<0.05; Fig. 3A and B). Compared with the control
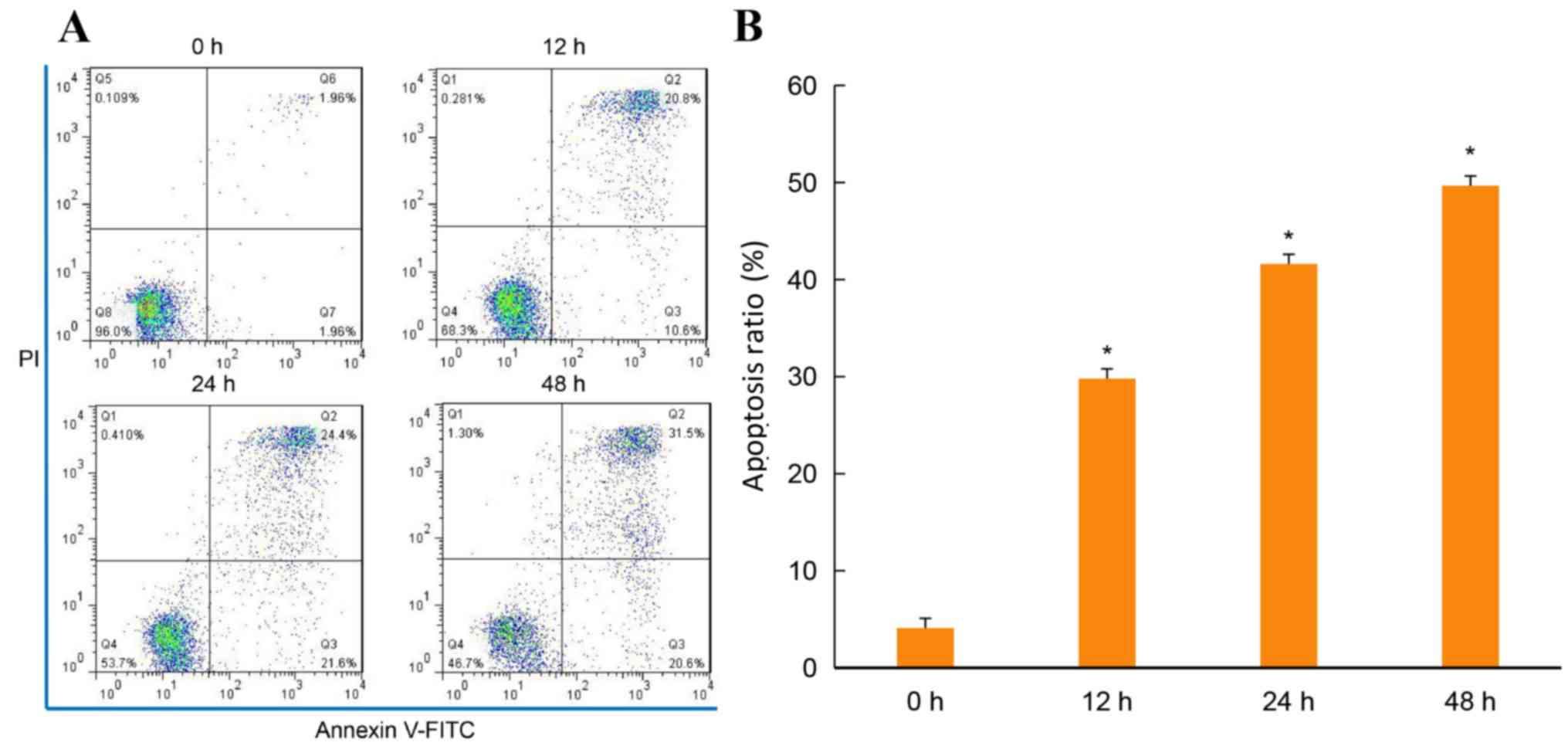
group, the apoptosis of THP-1 cells treated with 1.2 g/l matrine
for 12, 24 and 48 h was significantly increased. The apoptosis
ratio in the control group was 0.039±0.0013, whereas the apoptosis
ratios in the groups treated for 12, 24 and 48 h were 0.305±0.0010,
0.435±0.0015 and 0.513±0.0012, respectively (P<0.05; Fig. 4A and B). The apoptosis ratio of THP-1
cells treated with 20 µmol/l LY294002 was similar to that of 1.2
g/l matrine (Fig. 3B). The annexin V
FITC/PI staining revealed that the apoptosis ratio increased in a
dose- and time-dependent manner in the matrine-treated THP-1
cells.
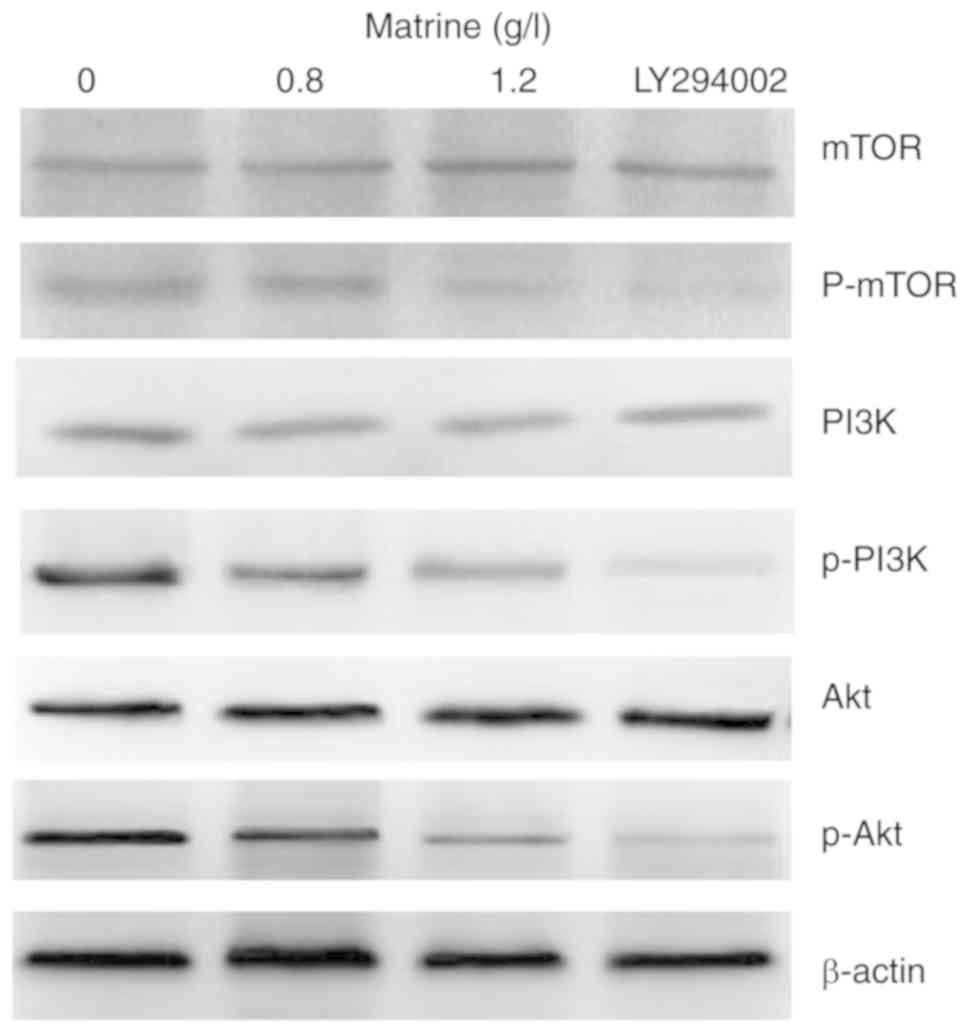
Matrine inhibits the PI3K/Akt/mTOR
signaling pathway
In the present study, the effect of matrine on the
PI3K/Akt/mTOR signaling pathway was investigated. Matrine decreased
the expression of p-PI3K, p-Akt and p-mTOR in a
concentration-dependent manner. The expression of p-PI3K, p-Akt and
p-mTOR was higher in the 0 g/l matrine-treated group compared with
the groups treated with 20 µmol/l LY294002 or 1.2 g/l matrine.
However, no notable differences in the expression levels of PI3K,
Akt and mTOR following treatment with matrine or 20 µmol/l LY294002
were revealed (Fig. 5).
Discussion
AML is a type of tumor of the blood characterized by
an abnormal increase of immature white blood cells (14). Due to the high toxicity and
associated side effects of traditional chemotherapy agents commonly
used in clinical practice, as well as the high recurrence rates,
there is a requirement for the development of novel drugs (15). For decades, matrine, a traditional
Chinese herbal medicine, has been demonstrated to possess
cytoprotective effects and biological safety, and has been used in
the treatment of a number of diseases, including hepatic fibrosis,
atherosclerosis, arrhythmias and infectious diseases (16,17). It
has also been reported that matrine inhibits hepatocellular
carcinoma and leukemia cell proliferation via various mechanisms,
including inducing cancer cell differentiation and apoptosis,
altering the tumor cell cycle and inhibiting telomerase activity
(7,10,11).
Uncontrolled proliferation is a key aspect of
tumorigenesis and inhibiting cell proliferation arrests the growth
of tumor cells (9,16). To investigate the role of matrine in
THP-1 cells, the present study first performed a cell viability
assay, which revealed that matrine significantly inhibited THP-1
cell viability in a dose- and time-dependent manner. To observe the
cytotoxicity of matrine, concentrations of 0, 0.4, 0.8, 1.2, 1.6
and 2.0 g/l matrine were selected for subsequent experiments. In
the current study, the anticancer effects were identified to be
dose-dependent and the IC50 value was determined as 1.2
g/l in THP-1 cells. The cell viability results indicated that
compared with the control, various concentrations of matrine
significantly reduced the viability of THP-1 cells.
To further investigate the mechanisms underlying the
anticancer effects of matrine, the present study performed DAPI
staining and observed that matrine, may exert its anticancer
effects via induction of apoptosis (data not show). It was
demonstrated that matrine significantly increased the apoptosis of
AML cells in vitro; the rate of apoptosis was 42.70% for AML
cells treated with the IC50 concentration of matrine of
1.2 g/l for 24 h. Furthermore, the apoptotic effects of matrine
were concentration-dependent and the apoptotic cell populations
increased with an increase in the concentration of matrine, as
demonstrated by annexin V/PI staining. It has previously been
reported that matrine exerts its antitumor effects by inhibiting
the proliferation and inducing the apoptosis of AML cells (11,18,19).
Consistent with these previous studies, the present study
demonstrated that matrine induced AML cell apoptosis in a
dose-dependent manner in the range of 0.4–2.0 g/l. Apoptosis is
considered one of the important mechanisms that prevents the
development of disease, including cancer, as abnormal cells are
eliminated via this process (18).
Several signaling pathways such as PI3K/AKT or Notch
regulate cell proliferation and apoptosis. Molecular biology
studies have been instrumental in deciphering the pathogenesis of
AML (13). The inhibition of
signaling pathways, such as PI3K/AKT, are considered to be the most
important factors in determining the response to chemotherapy and
the outcome of AML (20,21). The PI3K/Akt/mTOR signaling pathway is
an important pathway that has been reported to be activated in
several types of cancer (22–24).
PI3K, a signaling protein with catalytic activity within cells, is
activated by the action of extracellular cytokines, drugs, stress
and other factors. Once activated, PI3K phosphorylates
phosphatidylinositol (4,5)-bisphosphate to phosphatidylinositol
(3,4,5)-trisphosphate, and promotes Akt
activation to regulate the expression of a variety of cell
proliferation-associated genes and other genes (12). It has been reported that patients
with AML and hyperactivation of Akt signaling in AML cells exhibit
a worse prognosis and shorter survival time compared with patients
with normal levels of Akt activation (25,26).
Therefore, drugs targeting this pathway may prove useful in the
treatment of different types of malignancy.
The present study investigated the effect of matrine
on the expression of p-PI3K, PI3K, p-Akt, Akt, p-mTOR and mTOR. It
was identified that matrine decreased the expression of p-PI3K,
p-Akt and p-mTOR in a concentration-dependent manner, indicating
that the anticancer effects of matrine may in part be due to
inhibition of the PI3K/Akt/mTOR signaling pathway. In the current
study, treatment with matrine inhibited the activity of PI3K and
Akt in THP-1 cells, as demonstrated by a decrease in their
phosphorylated levels, which lowers their downstream kinase
activity and inhibits the pathway (19). To further clarify whether the
PI3K/Akt signaling pathway is targeted and regulates the inhibitory
effects of matrine in THP-1 cells, the PI3K-specific inhibitor
LY294002 was used to treat THP-1 cells. The expression of p-PI3K,
p-Akt and p-mTOR was markedly lower in the group treated with 20
µmol/l LY294002 compared with the control group. This further
suggested that matrine-induced apoptosis may be attributed, at
least partially, to PI3K/Akt inactivation. Similarly, previous
studies have demonstrated that matrine suppresses the
phosphorylation of Akt in AML THP-1 cells (26,27). The
identification of matrine as a novel Akt inhibitor may have
implications for cancer biology and treatment.
In conclusion, the present study demonstrated that
matrine exhibited anticancer effects in THP-1 cells in
vitro. The anticancer activity of matrine may be attributed to
its inhibition of proliferation, promotion of apoptosis of THP-1
cells by inhibition of the PI3K/Akt/mTOR signaling pathway. Given
that matrine has been demonstrated to inhibit proliferation and
induce apoptosis of THP-1 cells, matrine may be a useful candidate
as a chemotherapeutic agent in the treatment of AML.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Nature
Science Key Program of College and University of Anhui Province
(grant no. KJ2018A0216).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding authors on reasonable
request.
Authors' contributions
YH, NW and DL conceived and designed the study. NZ,
HY and YZ were involved in data acquisition. HX, CZ and YZ
performed the data analysis. NZ, YH, YZ, NW and DL wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Bengbu Medical College
(Bengbu, China) approved the study protocol.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Döhner H, Weisdorf DJ and Bloomfield CD:
Acute myeloid leukemia. N Engl J Med. 373:1136–1152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Estey EH: Therapeutic options for acute
myelogenous leukemia. Cancer. 92:1059–1073. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jabo B, Morgan JW, Martinez ME, Ghamsary M
and Wieduwilt MJ: Sociodemographic disparities in chemotherapy and
hematopoietic cell transplantation utilization among adult acute
lymphoblastic and acute myeloid leukemia patients. PLoS One.
12:e01747602017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwong YL, Au WY, Chim CS, Pang A, Suen C
and Liang R: Arsenic trioxide- and idarubicin-induced remissions in
relapsed acute promyelocytic leukemia: Clinicopathological and
molecular features of a pilot study. Am J Hematol. 66:274–279.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
George B, Mathews V, Vishwabandhya A,
Srivastava A and Chandy ML: Arsenic Trioxide (As2O3) in the
treatment of patients with newly diagnosed acute promyelocytic
leukemia (APML)-Toxicity and outcome. Blood. 104:8892004.PubMed/NCBI
|
|
6
|
Lazo G, Kantarjian H, Estey E, Thomas D,
O'Brien S and Cortes J: Use of arsenic trioxide (As2O3) in the
treatment of patients with acute promyelocytic leukemia: The M. D.
Anderson experience. Cancer. 97:2218–2224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou H, Xu M, Gao Y, Deng Z, Cao H, Zhang
W, Wang Q, Zhang B, Song G, Zhan Y and Hu T: Matrine induces
caspase-independent program cell death in hepatocellular carcinoma
through bid-mediated nuclear translocation of apoptosis inducing
factor. Mol Cancer. 13:592014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Zhang H, Yu P, Liu Q, Liu K, Duan
H, Luan G, Yagasaki K and Zhang G: Effects of matrine against the
growth of human lung cancer and hepatoma cells as well as lung
cancer cell migration. Cytotechnology. 59:191–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang S, Zhang Y, Zhuang Y, Wang J, Ye J,
Zhang S, Wu J, Yu K and Han Y: Matrine induces apoptosis in human
acute myeloid leukemia cells via the mitochondrial pathway and Akt
inactivation. PLoS One. 7:e468532012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang LP, Jiang JK, Tam JW, Zhang Y, Liu
XS, Xu XR, Liu BZ and He YJ: Effects of matrine on proliferation
and differentiation in K-562 cells. Leuk Res. 25:793–800. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yong-Qing Z, Gao-Sheng H and Hong-Jun L:
Cells differential orientation in K562 cell line with matrine
induction. J Oncol. 10:25–27. 2004.
|
|
12
|
Xu Q, Simpson SE, Scialla TJ, Bagg A and
Carroll M: Survival of acute myeloid leukemia cells requires PI3
kinase activation. Blood. 102:972–980. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kubota Y, Ohnishi H, Kitanaka A, Ishida T
and Tanaka T: Constitutive activation of PI3K is involved in the
spontaneous proliferation of primary acute myeloid leukemia cells:
Direct evidence of PI3K activation. Leukemia. 18:1438–1440. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dores GM, Devesa SS, Curtis RE, Linet MS
and Morton LM: Acute leukemia incidence and patient survival among
children and adults in the United States, 2001–2007. Blood.
119:342012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krug U, Röllig C, Koschmieder A, Heinecke
A, Sauerland MC, Schaich M, Thiede C, Kramer M, Braess J,
Spiekermann K, et al: Complete remission and early death after
intensive chemotherapy in patients aged 60 years or older with
acute myeloid leukaemia: A web-based application for prediction of
outcomes. Lancet. 376:2000–2008. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao HY, Li GY, Lou MM, Li XY, Wei XY and
Wang JH: Hepatoprotective effect of Matrine salvianolic acid B salt
on carbon tetrachloride-induced hepatic fibrosis. J Inflamm (Lond).
9:162012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen JX, Shen HH, Niu M, Guo YM, Liu XQ,
Han YZ, Zhang YM, Zhao YL, Bai BK, Zhou WJ and Xiao XH:
Anti-hepatitis B virus effect of matrine-type alkaloid and
involvement of p38 mitogen-activated protein kinase and tumor
necrosis factor receptor-associated factor 6. Virus Res.
215:104–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma L, Zhu Z, Jiang L, Sun X, Lu X, Zhou M,
Qian S and Li J: Matrine suppresses cell growth of human chronic
myeloid leukemia cells via its inhibition of the
interleukin-6/Janus activated kinase/signal transducer and
activator of transcription 3 signaling cohort. Leuk Lymphoma.
56:2923–2930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Guo JX, Shao ZQ and Gao JP:
Matrine inhibits bladder cancer cell growth and invasion in vitro
through PI3K/AKT signaling pathway: An experimental study. Asian
Pac J Trop Med. 10:515–519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishikawa Y: Genetic abnormalities in core
binding factor acute myeloid leukemia. Rinsho Ketsuek (Japanese).
58:991–998. 2017.
|
|
21
|
Illendula A, Pulikkan JA, Zong H,
Grembecka J, Xue L, Sen S, Zhou Y, Boulton A, Kuntimaddi A, Gao Y,
et al: Chemical biology. A small-molecule inhibitor of the aberrant
transcription factor CBFβ-SMMHC delays leukemia in mice. Science.
347:779–784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morgensztern D and Mcleod HL:
PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer
Drugs. 16:797–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin R, Jin YY, Tang YL, Yang HJ, Zhou XQ
and Lei Z: GPNMB silencing suppresses the proliferation and
metastasis of osteosarcoma cells by blocking the PI3K/Akt/mTOR
signaling pathway. Oncol Rep. 39:3034–3040. 2018.PubMed/NCBI
|
|
24
|
Qi L, Sun K, Zhuang Y, Yang J and Chen J:
Study on the association between PI3K/AKT/mTOR signaling pathway
gene polymorphism and susceptibility to gastric cancer. J BUON.
22:1488–1493. 2017.PubMed/NCBI
|
|
25
|
Tamburini J, Elie C, Bardet V, Chapuis N,
Park S, Broët P, Cornillet-Lefebvre P, Lioure B, Ugo V, Blanchet O,
et al: Constitutive phosphoinositide 3-kinase/Akt activation
represents a favorable prognostic factor in de novo acute
myelogenous leukemia patients. Blood. 110:1025–1028. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park S, Chapuis N, Tamburini J, Bardet V,
Cornilletlefebvre P, Willems L, Green A, Mayeux P, Lacombe C and
Bouscary D: Role of the PI3K/AKT and mTOR signaling pathways in
acute myeloid leukemia. Haematologica. 95:819–828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu J, Hu G, Dong Y, Ma R, Yu Z, Jiang S,
Han Y, Yu K and Zhang S: Matrine induces Akt/mTOR signalling
inhibition-mediated autophagy and apoptosis in acute myeloid
leukaemia cells. J Cell Mol Med. 21:1171–1181. 2017. View Article : Google Scholar : PubMed/NCBI
|