Introduction
Renal cancer is a deadly disease with a global
annual incidence of 143,000 cases of cancer-associated mortality,
with the 9th highest incidence rate in men and 14th highest in
women, as reported in 2012 (1).
Renal cell carcinoma (RCC) is the most common form of renal
neoplasm, accounting for >90% of cases in adults regardless of
sex, worldwide (1). The high
metastatic rate of RCC and its resistance to traditional
chemotherapy and radiotherapy results in an unpredictable
presentation as well as adverse clinical outcomes. Although
surgical treatment remains the gold standard for treating primary
RCC, 30–40% patients may undergo progression or recurrence
following the initial radical nephrectomy, and the 5-year survival
rate of metastatic patients was only 12% in 2015 (2). Therefore, there is an urgent need to
investigate novel approaches for treating RCC.
Cancer stem cells (CSCs), a minor initiating cell
subpopulation within solid tumors, are associated with unique
characteristics, including clonogenesis, high self-renewal capacity
and multilineage differentiation properties (3). Emerging evidence has demonstrated that
CSCs are implicated in tumorigenesis, progression, chemoresistance
and relapse of malignancies (3–5). To
date, CSCs have been isolated and identified in various types of
solid malignancy or established cell lines, including glioblastoma,
breast cancer and renal cancer (6–8).
Bussolati et al (9) isolated
renal CSCs from the tumor samples of patients with clear cell RCC
using fluorescence-activated cell sorting in 2008. Subsequently,
Zhong et al (10) enriched
CSCs from the RCC cell line SK-RC-42 using a sphere-formation
assay. In addition, Huang et al (11) identified a cancer stem-like cell side
population in the 769P cell line. Therefore, understanding the
biology of renal CSCs for selective targeting can be an effective
interventional strategy in preventing and treating renal
cancer.
The sonic hedgehog (Shh) pathway has been considered
as an oncogenic pathway, serving a crucial role in the maintenance
of tissue polarity and CSC properties (12). Aberrant activation of the Shh pathway
has been reported to be involved in various types of human
malignancy (13). The binding of
hedgehog ligands, including Shh protein, to the transmembrane
receptor patched 1 (PTCH) results in the activation of the Shh
pathway. PTCH represses the activity of the transmembrane protein
smoothened (Smo) in the absence of Shh protein, which can thereby
activate an endocellular signal transduction cascade via the
glioma-associated oncogene (Gli) transcription factor family,
including Gli1, Gli2 and Gli3 (14).
Gli1 acts as a transcription activator, while Gli2 and Gli3 can
function either as repressors or activators depending on the
context (14,15).
Recently, numerous studies have illustrated that
dietary factors exert chemo-preventative functions and can decrease
the risk of multiple types of cancer (16–18).
Notably, certain botanical compounds can directly regulate CSC
activity; compounds that have now been identified as promising
anticancer and therapeutic agents. Among these compounds, genistein
(4,5,7-trihydroxyisoflavone), a major isoflavone component that can
be isolated from soybeans and soy products, possesses anticancer
potential by targeting the CSCs of different tumors (19,20).
Genistein exerts anticancer effects on various types of malignancy,
including breast, colon and ovarian cancer (19,21,22).
Additionally, it has been reported that genistein inhibits the
pluripotency of prostate and gastric cancer cells by targeting the
hedgehog-Gli1 pathway (20,23).
However, to the best of our knowledge, the
anticancer properties of genistein against renal CSCs remain to be
elucidated. Therefore, the aim of the present study was to evaluate
the anticancer effects of genistein on renal CSCs, and to evaluate
whether the Shh pathway may be involved in the genistein-induced
anticancer activity. The findings of the present study may provide
valuable avenues in the search for a potential interventional
target of genistein in renal CSCs.
Materials and methods
Chemicals and reagents
Genistein and DMSO were purchased from
Sigma-Aldrich; Merck KGaA. Vismodegib and purmorphamine were
purchased from MedChemExpress and dissolved in DMSO at a stock
solution concentration of 10 mM. Epidermal growth factor (EGF),
basic fibroblast growth factor (bFGF) and insulin were purchased
from PeproTech, Inc.. In addition, 2% B27 was acquired from Gibco;
Thermo Fisher Scientific, Inc..
The primary antibodies that were used in the present
study, including Shh (cat. no. 20697-1-AP), Gli1 (cat. no.
66905-1-Ig), Gli2 (cat. no. 18989-1-AP), CD44 (cat. no.
15675-1-AP), CD133 (cat. no. 18470-1-AP), aldehyde dehydrogenase 1
family member A1 (ALDH1A1) (cat. no. 15910-1-AP), Oct4 (cat. no.
11263-1-AP), Nanog (cat. no. 14295-1-AP), cyclin D1 (cat. no.
60186-1-Ig), proliferating cell nuclear antigen (PCNA) (cat. no.
10205-2-AP), Bcl-2 (cat. no. 12789-1-AP), Bax (cat. no.
50599-2-Ig), and GAPDH (cat. no. 10494-1-AP) were purchased from
ProteinTech, Inc.. Cleaved caspase 3 (cat. no. 9661), cleaved
caspase 8 (cat. no. 8592) and cleaved caspase 9 (cat. no. 20750)
were obtained from Cell Signaling Technology, Inc.. Smo (cat. no.
DF5152) was obtained from Affinity Biosciences, Inc. Anti-rabbit
(cat. no. SA00001-2) and anti-mouse (cat. no. SA00001-1) second
antibodies were purchased from ProteinTech, Inc..
Cell culture and sphere formation
assay
The renal cancer 786-O and ACHN cell lines were
purchased from Type Culture Collection of the Chinese Academy of
Sciences and cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 100 IU/ml penicillin and 100 µg/ml streptomycin.
All cells were cultured at 37°C with 5% CO2.
The 786-O and ACHN cells were washed twice, seeded
into a low-attachment plate at a density of 5,000 cells/well, and
cultured at 37°C in a humidified incubator with 5% CO2
in serum-free DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.)
containing 4 mg/ml insulin, 20 ng/ml EGF, 20 ng/ml bFGF, B27 (1:50
dilution) and 0.4% BSA (Beyotime Institute of Biotechnology). Half
fresh medium was renewed and chemicals (genistein, vismodegib or
purmorphamine) were added every other day. Tumor sphere formation
was observed and images were captured using a light microscope
(Nikon Corporation).
To investigate the effect of genistein and
purmorphamine on the tumorsphere formation capacity of renal CSCs,
various concentrations of genistein (0, 30, 60 and 90 µM) or
purmorphamine (1 µM) were added to each well with 0.1% DMSO as the
control. After 5 days treatment at 37°C in a humidified incubator
with 5% CO2, the number of tumor spheres in each group
with a diameter >50 µm was counted.
Cell cycle analysis
Following culture in serum-supplemented medium (SSM)
or serum-free medium (SFM) for 5 days, 786-O and ACHN cells were
collected at a density of 1×106/ml, and then fixed with
75% ice-cold ethanol at −20°C overnight. Subsequently, the cells
were washed twice with PBS and treated with 20 mg/ml propidium
iodide (PI; Invitrogen; Thermo Fisher Scientific, Inc.), 0.1%
Triton X-100 (Invitrogen; Thermo Fisher Scientific, Inc.) and 0.2
mg/ml RNase (PureLink; Thermo Fisher Scientific, Inc.) at room
temperature in the dark for 15 min. Subsequently, flow cytometry
(FCM) using a FACSCalibur™ flow cytometer (BD Biosciences) was used
to analyze the cell cycle phase distribution and the percentage of
cells in different phases. The data were analyzed using FlowJo
software (v10.0.7; FlowJo LLC).
Western blotting
786-O and ACHN tumor spheres were harvested and
washed with ice-cold PBS. The cell precipitates were homogenized in
RIPA buffer (Beyotime Institute of Biotechnology) containing
complete protease inhibitors, followed by centrifugation at 15,000
× g for 30 min at 4°C. The supernatants were collected and
denatured. BCA protein assay (Pierce; Thermo Fisher Scientific,
Inc.) was utilized to determine the concentration of each protein.
The proteins (50 µg per lane) were separated via 10% SDS-PAGE and
then transferred onto PVDF membranes (EMD Millipore). Subsequently,
the PVDF membranes were treated with 5% skimmed milk at room
temperature for 2 h and incubated with the primary antibodies
against CD133 (1:500 dilution), Gli1 (1:500 dilution), Gli2 (1:500
dilution), CD44 (1:1,000 dilution), ALDH1A1 (1:1,000 dilution),
CD44 (1:1,000 dilution), Oct4 (1:1,000 dilution), Nanog (1:1,000
dilution), Bcl2 (1:1,000 dilution), Bax (1:1,000 dilution), cleaved
caspase 3 (1:1,000 dilution), cleaved caspase 9 (1:1,000 dilution),
cleaved caspase 8 (1:1,000 dilution), cyclin D1 (1:1,000 dilution),
PCNA (1:1,000 dilution), Shh (1:1,000 dilution), Smo (1:1,000
dilution), and GAPDH (1:1,000 dilution) at 4°C overnight. Membranes
were rinsed and incubated with the corresponding
peroxidase-conjugated secondary antibodies (1:5,000 dilution) for 1
h at 25°C. Chemiluminescent detection was performed using an ECL
kit (Beyotime Institute of Biotechnology). GAPDH was used as an
internal control in order to measure the relative expression of
each protein. ImageJ software (v1.46; National Institutes of
Health) was used to quantify the protein bands of interest.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total cellular RNA was extracted from renal cancer
adherent or tumor sphere cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), and subsequently
reverse-transcribed into cDNA using 5X All-In-One RT master mix
(Applied Biological Materials, Inc.), according to the
manufacturer's protocol, as follows: 25°C for 10 min, 42°C for 15
min for cDNA synthesis, then 85°C for 5 min followed by chilling on
ice. qPCR was performed using the Power SYBR Green Master mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and a LC96
real-time PCR system. The amplification reactions were as follows:
An initial hold step at 95°C for 5 min and 45 cycles of a two-step
PCR (95°C for 15 sec, 54°C for 30 sec and 72°C for 30 sec). GAPDH
levels were used as normalization controls. Fold changes in gene
expression were calculated using a comparative threshold cycle (Cq)
method using the formula 2−ΔΔCT (24). The forward and reverse primers used
for qPCR are presented in Table
I.
 | Table I.Oligonucleotide sequences of primers
for reverse transcription-quantitative PCR. |
Table I.
Oligonucleotide sequences of primers
for reverse transcription-quantitative PCR.
| Genes | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| CD133 |
TACAACGCCAAACCACGACTGT |
TCTGAACCAATGGAATTCAAGACCCTTT |
| CD44 |
AGCAACCAAGAGGCAAGAAA |
GTGTGGTTGAAATGGTGCTG |
| ALDH1A1 |
GCACGCCAGACTTACCTGTC |
CCTCCTCAGTTGCAGGATTAAAG |
| Oct4 |
GACAACAATGAAAATCTTCAGGAGA |
TTCTGGCGCCGGTTACAGAACCA |
| Nanog |
TTTGTGGGCCTGAAGAAAACT |
AGGGCTGTCCTGAATAAGCAG |
| GAPDH |
GACTGTGGATGGCCCCTCCGG |
AGGTGGAGGAGTGGGTGTCGC |
Detection of apoptotic cells by
FCM
The apoptosis assay was performed using a
fluorescein isothiocyanate (FITC)-Annexin-V apoptosis detection kit
purchased from BD Biosciences. Following treatment with genistein
at different concentrations (0, 30, 60 and 90 µM) for 5 days at
37°C with 5% CO2, 786-O tumor spheres were harvested and
washed twice with PBS. Subsequently, cells were resuspended with
100 µl binding buffer (BD Biosciences) at a density of
1×106/ml, incubated with Annexin V-fluorescein
isothiocyanate (5 µl) and PI (5 µl) at room temperature in the dark
for 15 min, and then detected using FACSCalibur™ (BD Biosciences)
flow cytometer within 1 h. The data were analyzed using FlowJo
software (v10.0.7; FlowJo LLC).
Immunofluorescence staining
Following genistein treatment for 5 days at 37°C
with 5% CO2, the tumor spheres were washed three times
and fixed with 4% paraformaldehyde for 15 min at 25°C. The tumor
spheres were subsequently washed three times with PBS-Tween 20
(PBST) then blocked with 5% BSA (Beyotime Institute of
Biotechnology) for 1 h at 25°C, following which the tumor spheres
were incubated with a rabbit CD44 or Smo (1:100 dilution) antibody
at 4°C overnight, followed by washing with PBST three times.
Subsequently, tumor spheres were stained with Cy3-conjugated goat
anti-rabbit secondary antibody (1:1,000 dilution; cat. no. P0173;
Beyotime Institute of Biotechnology) for 2 h at 25°C, followed by
counterstaining with DAPI for 10 min at 25°C. Images were obtained
using a fluorescence microscope (Olympus Corporation) at a
magnification of ×100.
Statistical analysis
Each experiment was repeated three times
independently. A two-tailed Student's t-test was used to analyze
the statistical differences between two groups. One-way analysis of
variance with Tukey post hoc test was utilized for comparisons
among multiple groups. The results are presented as the mean ±
standard deviation. All analyses were performed using SPSS version
11.0 software (SPSS, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Tumor sphere formation in renal cancer
cells through culture in SFM
A number of studies have reported that CSCs have the
ability to grow in suspension and to form spheres through culturing
under SFM conditions (10,25,26).
Therefore, a tumor sphere formation assay via culturing in SFM is
an efficient way to isolate and enrich CSCs. In the present study,
786-O and ACHN cell lines were able to form stable cell spheres
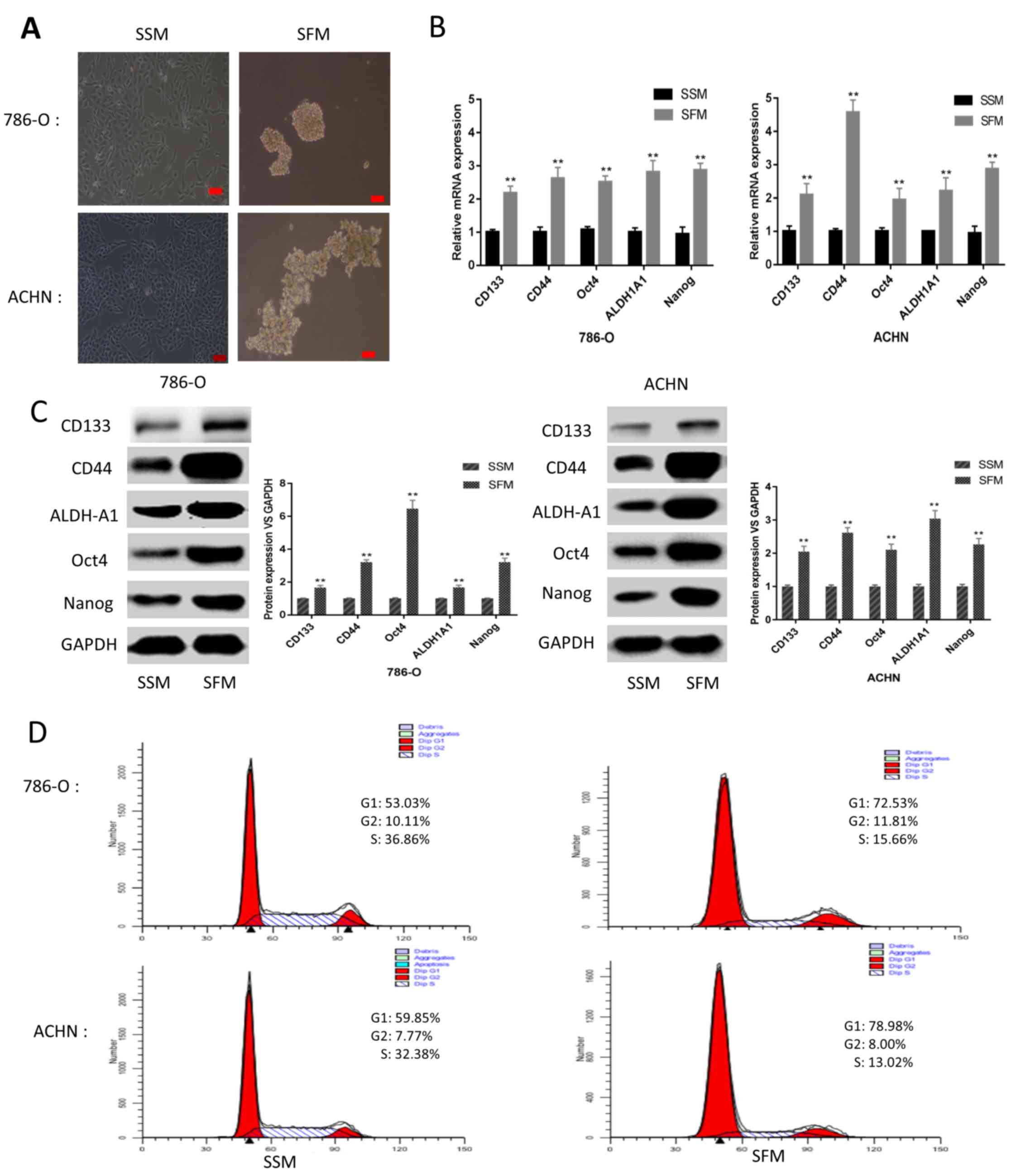
following culturing in SFM for 5 days (Fig. 1A). To further elucidate the CSC
characteristics of the sphere-forming cells, mRNA and protein
expression levels of renal CSC markers, including Nanog, Oct4,
ALDH1A1, CD44 and CD133, were detected. Following culturing under
SFM conditions for 5 days, the mRNA and protein expression levels
of CSC markers were markedly upregulated compared with adherent
cells cultured under SSM conditions (Fig. 1B and C). Furthermore, FCM analysis of
the cell cycle distribution suggested that the sphere-forming cells
were primarily arrested at G0/G1 phase
instead of S1 phase, in contrast to the adherent cells
(Fig. 1D). These results illustrated
the CSC characteristics of the cells cultured under SFM
conditions.
Genistein diminishes the
characteristics of renal CSCs
Genistein has been demonstrated to target CSCs in
several types of malignancy (20,23,27). In
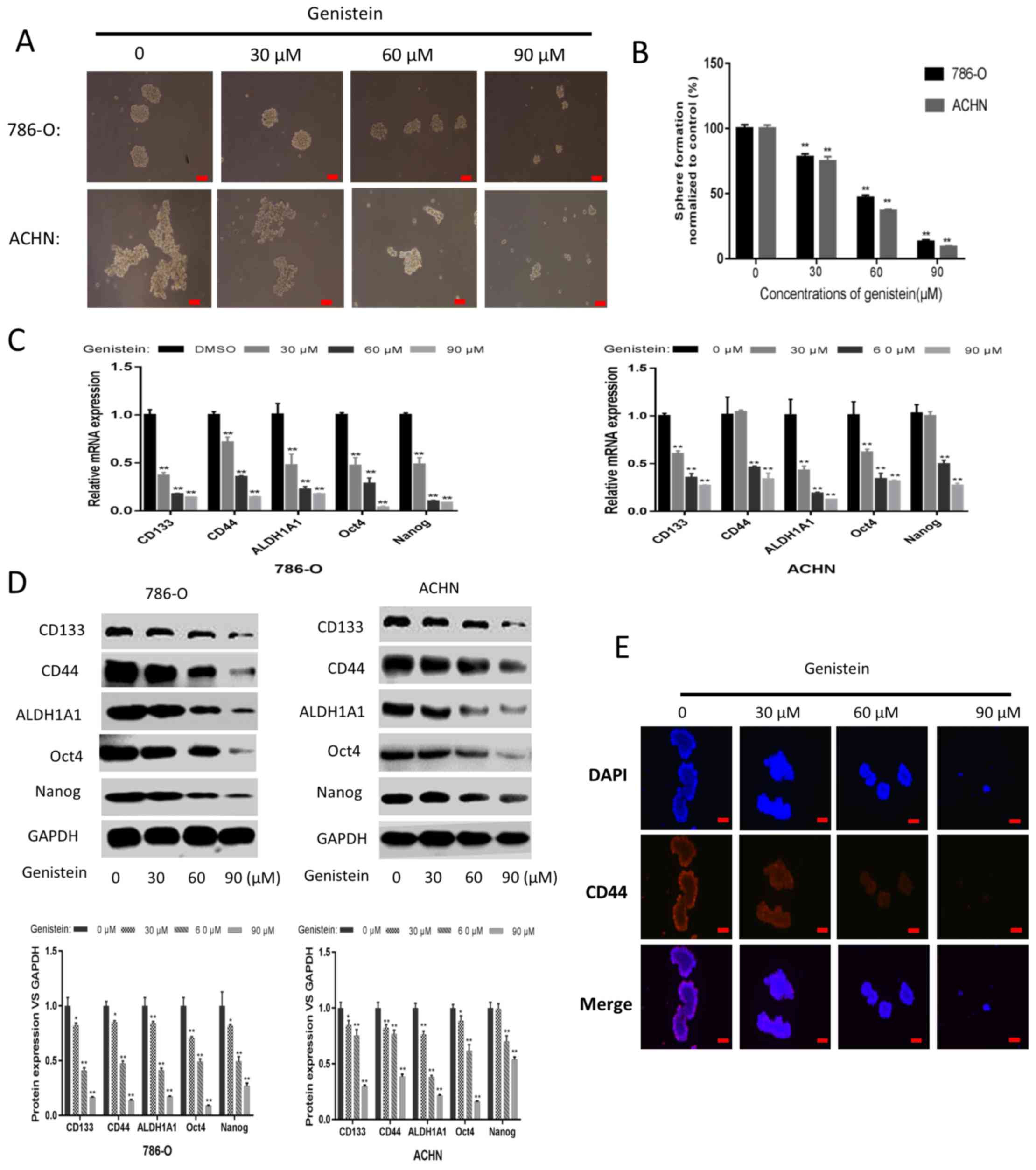
the present study, 786-O and ACHN sphere-forming cells were treated
with genistein at different concentrations (0, 30, 60 and 90 µM)
for 5 days, in order to investigate whether genistein could exhibit
its suppressive effect on renal CSCs. As presented in Fig. 2A and B, the tumor sphere formation
assay revealed that genistein-treatment could, in a dose-dependent
manner, significantly reduce the number and size of sphere-forming
cells in the two cell lines. Following treatment with genistein for
5 days, the mRNA and protein expression levels of renal CSC markers
were markedly downregulated in the sphere-forming cells of the two
cell lines (Fig. 2C and D). In
addition, immunofluorescent staining indicated that the number of
CD44-positive 786-O sphere-forming cells was decreased following
genistein treatment in a dose-dependent manner (Fig. 2E). Overall, these data revealed that
genistein could effectively diminish the characteristics of renal
CSCs.
Genistein inhibits the proliferation
and induces the apoptosis of renal CSCs
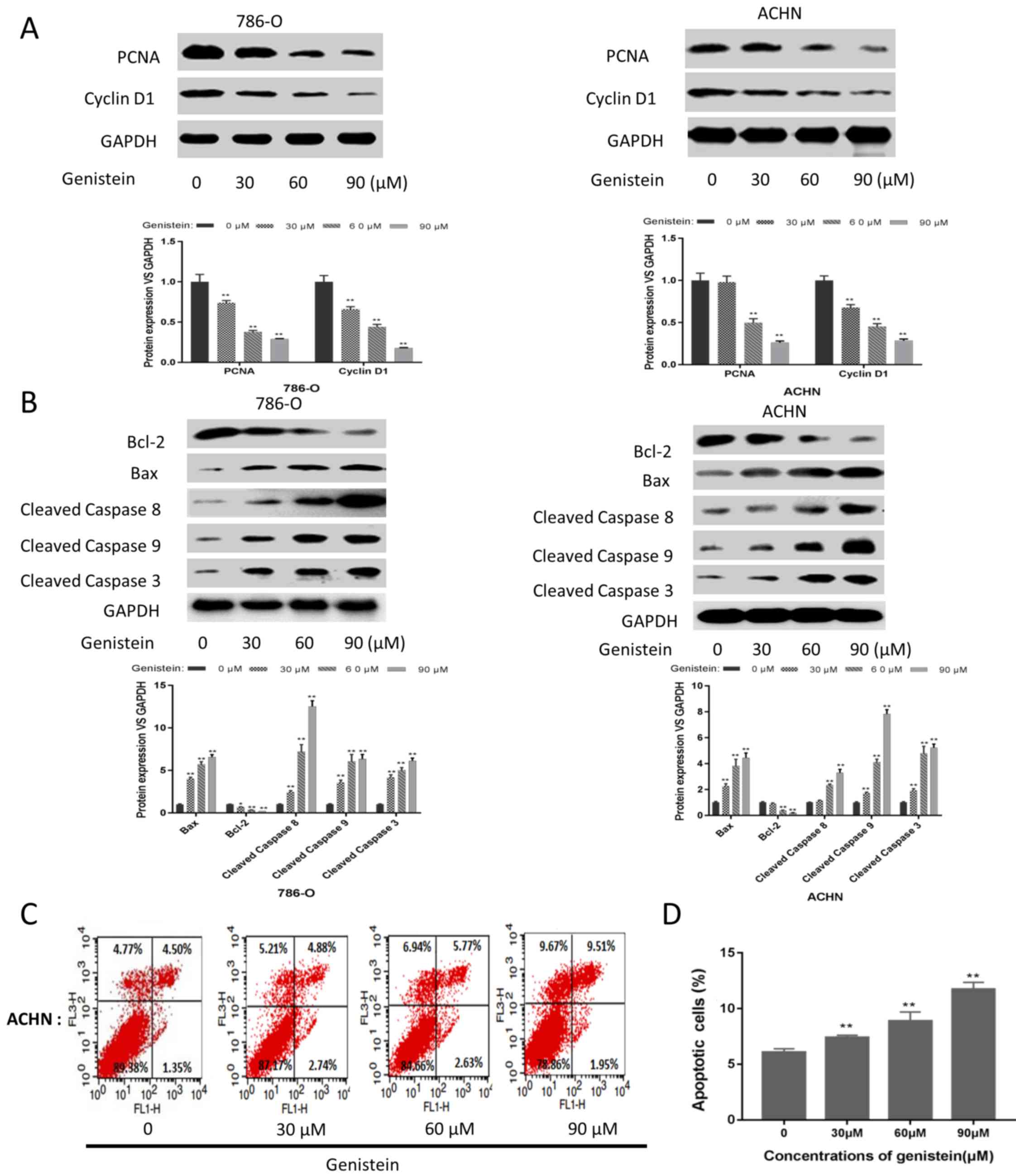
Furthermore, the effects of genistein on cell
proliferation and apoptosis were investigated in the present study.
As presented in Fig. 3A, the
expression levels of proliferation-associated proteins, including
PCNA and cyclin D1, were markedly downregulated in the 786-O and
ACHN sphere-forming cells following genistein treatment.
Additionally, the expression level of the anti-apoptotic protein
Bcl-2 was decreased, while the expression levels of the
pro-apoptotic proteins Bax, cleaved caspase 8, cleaved caspase 9
and cleaved caspase 3 were markedly upregulated following
genistein-treatment (Fig. 3B). In
addition, FCM indicated that genistein could significantly induce
apoptosis in ACHN sphere-forming cells (Fig. 3C and D). These results revealed that
genistein could inhibit the proliferation and induce the apoptosis
of renal CSCs.
Genistein diminishes renal CSC
characteristics by suppressing the Shh pathway
The Shh pathway is understood to be an important
regulator of renal CSCs (28–30). In
the present study, it was demonstrated that sphere-forming cells
treated with 15 µM vismodegib for 5 days, a Smo inhibitor that
suppressed Shh activation, exhibited downregulated expression
levels of renal CSC markers (Fig.
4A). Additionally, the present study investigated whether
genistein could inhibit the Shh pathway in renal CSCs. As presented
in Fig. 4B, genistein-treatment
resulted in markedly decreased expression levels of the Shh
pathway-associated proteins in 786-O and ACHN sphere-forming cells.
Furthermore, immunofluorescent staining indicated that the number
of Smo-positive 786-O sphere-forming cells decreased following
genistein-treatment in a dose-dependent manner (Fig. 4C). These data indicate that genistein
may inhibit the Shh pathway in renal CSCs.
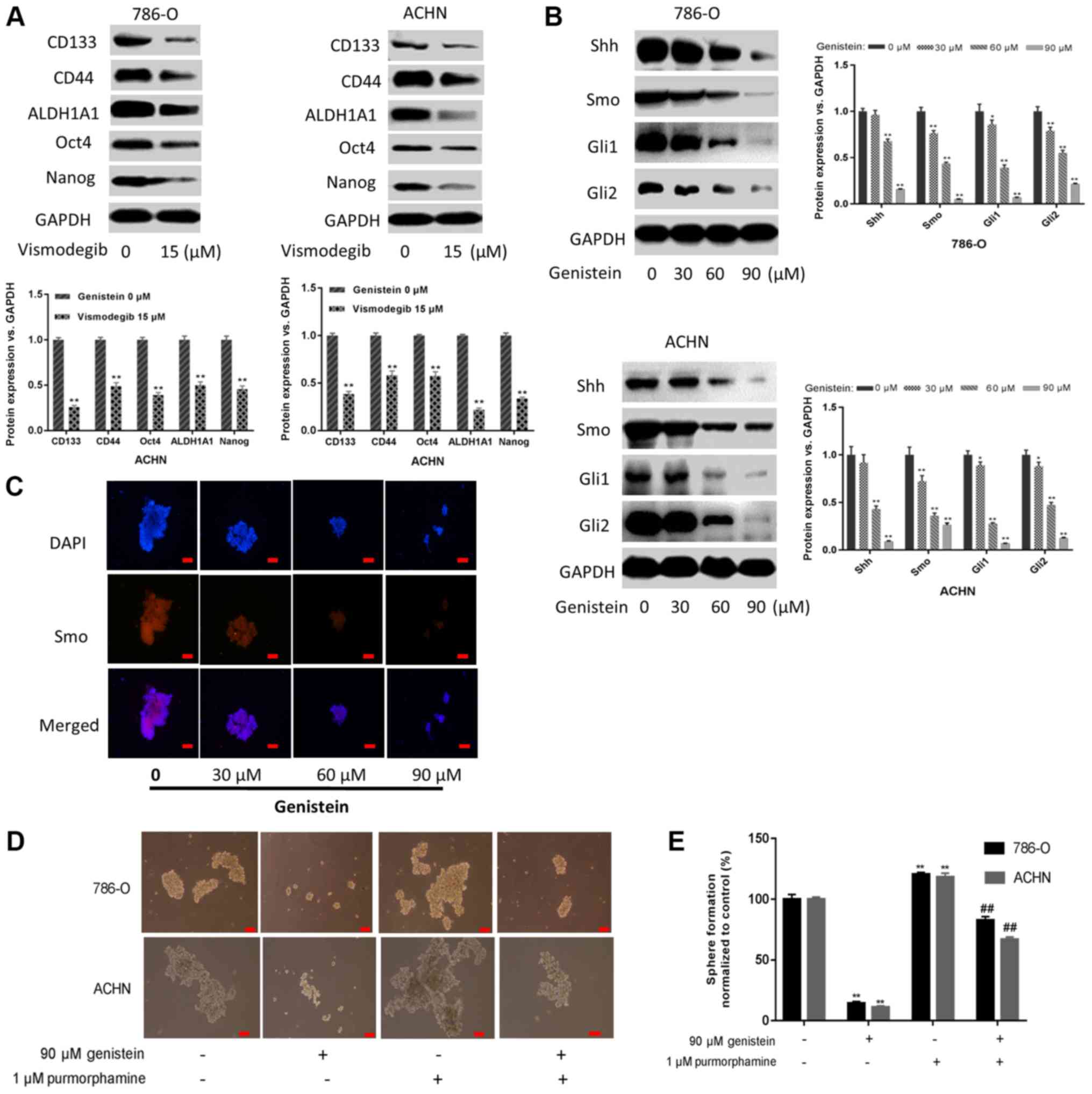 | Figure 4.Genistein blocks the activity of the
Shh pathway. (A) 786-O and ACHN sphere-forming cells were treated
with the Smo inhibitor vismodegib (15 µM) for 5 days, and the
expression levels of renal CSC markers were assessed by western
blotting. (B) 786-O and ACHN sphere-forming cells were treated with
different concentrations of genistein (0, 30, 60 and 90 µM) for 5
days. The expression levels of critical components of the Shh
pathway were measured by western blotting. (C) Immunofluorescence
staining images of 786-O tumor spheres were captured to determine
Smo expression. Scale bar, 100 µm. (D) 786-O and ACHN
sphere-forming cells were treated with 90 µM genistein in the
presence or absence of 1 µM purmorphamine for 5 days.
Representative images of sphere-forming cells. Scale bar, 100 µm.
(E) Numbers of sphere-forming cells were counted and normalized to
the control group (genistein 0 µM and purmorphamine 0 µM).
*P<0.05, **P<0.01 vs. genistein 0 µM. ##P<0.01
vs. genistein 90 µM. ALDH1A1, aldehyde dehydrogenase 1 family
member A1; CSC, cancer stem cell; Gli, glioma-associated oncogene;
Shh, sonic hedgehog; Smo, smoothened. |
In addition, purmorphamine, an activator of Smo that
activates the Shh pathway, was used to further determine the role
of the Shh pathway in the suppressive effect exerted by genistein
on renal CSCs. The results of the present study revealed that
purmorphamine could reverse the suppressive effects of genistein on
sphere formation (Fig. 4D and E).
Additionally, purmorphamine-treatment increased the expression
levels of renal CSC markers in 786-O and ACHN sphere-forming cells
and reversed the inhibitory effect of genistein on the Shh pathway
(Fig. 5A and B). Furthermore, the
inhibitory effects of genistein on cell proliferation and
apoptosis-associated proteins were reversed following
purmorphamine-treatment (Fig. 5C and
D). In conclusion, these results indicate a suppressive effect
of genistein on renal CSCs via the inhibition of the Shh
pathway.
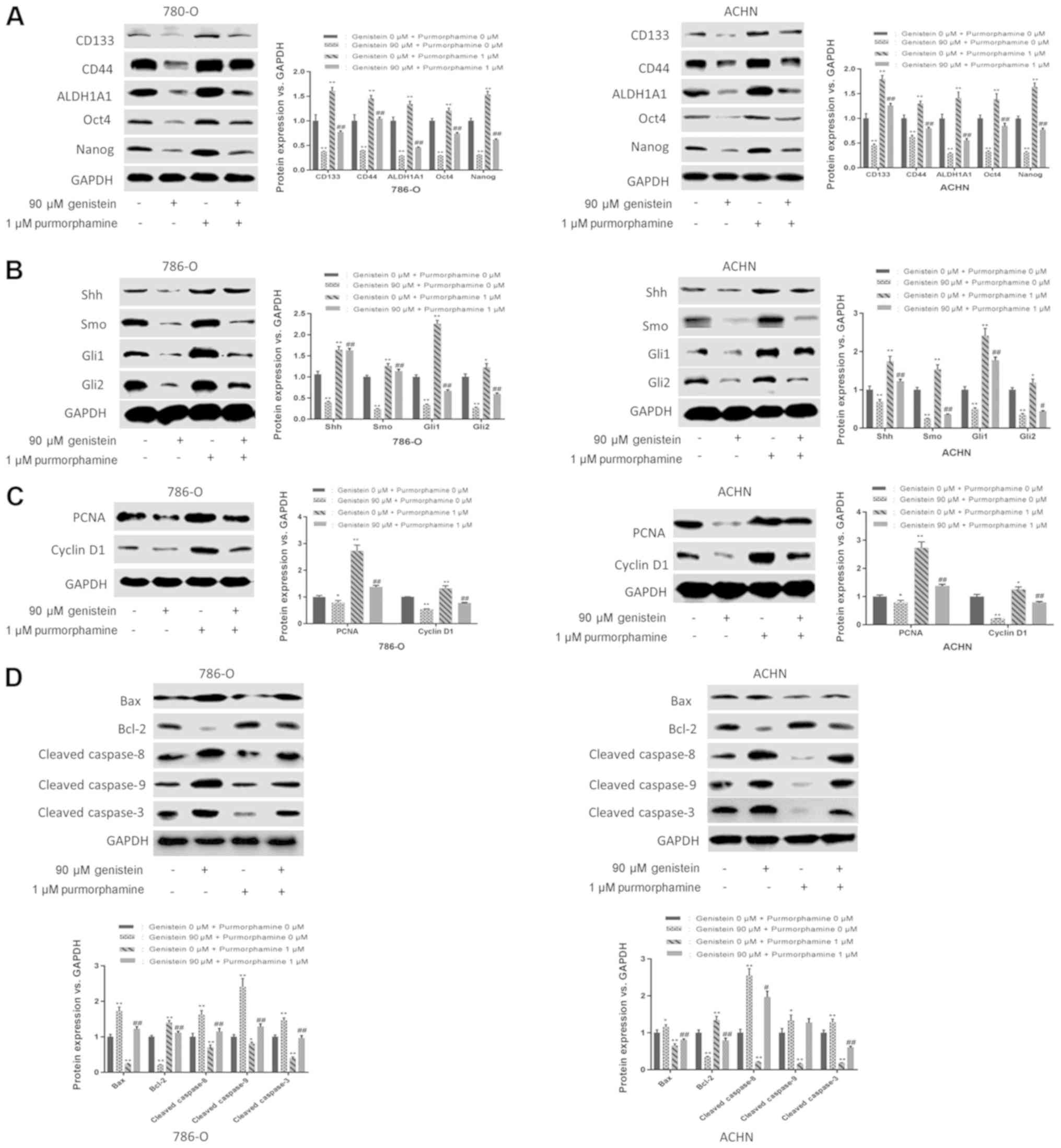 | Figure 5.Genistein diminishes renal CSC
characteristics by suppressing the Shh pathway. 786-O and ACHN
sphere-forming cells were treated with 90 µM genistein in the
presence or absence of 1 µM purmorphamine for 5 days. (A) Western
blot analysis of renal CSC markers. (B) Western blot analysis of
the critical components of the Shh pathway. Western blotting was
used to analyze the expression levels of (C)
proliferation-associated proteins (PCNA and cyclin D1) and (D)
apoptosis-associated proteins (Bcl-2, Bax, cleaved caspase 8,
cleaved caspase 9 and cleaved caspase 3). *P<0.05, **P<0.01
vs. genistein 0 µM. #P<0.05, ##P<0.01
vs. genistein 90 µM. ALDH1A1, aldehyde dehydrogenase 1 family
member A1; CSC, cancer stem cell; Gli, glioma-associated oncogene;
PCNA, proliferating cell nuclear antigen; Shh, sonic hedgehog; Smo,
smoothened. |
Discussion
It has been demonstrated that CSCs serve a vital
role in cancer development, progression and drug resistance. The
Shh signaling pathway has been implicated in maintaining the
pluripotency of CSCs. Genistein, a major isoflavone component that
is isolated from soybeans and soy products, has been reported to
possess anticancer activity (31).
The results of the present study suggested that genistein may
inhibit the activities of renal CSCs by suppressing the Shh
signaling pathway.
The formation of 3D spheres under non-adherent and
serum-free conditions is considered to be one of the major
characteristics of CSCs (32), and
this trait provides a convenient and effective way to isolate and
enrich putative CSCs. To date, several markers have been used to
distinguish renal CSCs, including CD133, CD44, ALDH1A1, Nanog and
Oct4 (6,33). CD133-positive progenitors contribute
to tumor angiogenesis, which can notably enhance tumor development
and growth when co-transplanted with renal carcinoma cells
(9,34). CD44-positive populations derived from
786-O, Caki-1, ACHN and HEK293T renal cancer cells have
self-renewal properties and sphere formation capabilities (35,36). In
addition, clinical data have demonstrated that CD44 is associated
with poor prognosis, cancer cell invasion and metastasis, as well
as resistance to sunitinib-treatment (37,38).
ALDH1-positive cells are associated with higher sphere-forming
ability, self-renewal ability and tumorigenicity compared with
their ALDH1-negative counterparts (39). Additionally, aberrant expression of
Oct4 and Nanog is considered to be characteristic of the
pluripotent stem cells of an embryo and embryonic stem cells, which
are involved in maintaining the self-renewal ability and
pluripotency of CSCs (40). In the
present study, renal CSCs were enriched from the adherent renal
cancer cell lines 786-O and ACHN, which exhibited sphere formation
capabilities when cultured under SFM conditions. Additionally, the
results of the present study indicated that sphere-forming cells
cultured under SFM conditions exhibited markedly elevated
expression levels of renal CSC markers, including CD133, CD44,
ALDH1A1, Oct4 and Nanog. An increasing amount of evidence has
suggested that the quiescent stem-like populations may contribute
to the formation of CSCs (41,42). In
line with the aforementioned findings, the present study also
revealed that cells cultured under SFM conditions were more
frequently arrested at the G0/G1 phase
compared with the adherent cells cultured under SSM conditions.
These results illustrated the CSC characteristics of these cells
when cultured in SFM.
Genistein, a predominant isoflavone found in
soybeans, has multiple anticancer effects in various different
types of cancer, including lung, colon, head and neck squamous cell
carcinoma, and hepatocellular carcinoma (21,43–45). A
previous epidemiological study based on observations made in Asian
countries, including China, demonstrated that the incidence rates
of breast and prostate cancer are lower than those in the USA and
Europe, where diets are lower in soy products (46). Accumulating evidence suggests that
genistein exhibits anticarcinogenic effects on CSCs in vivo
and in vitro, including in prostate (27), gastric (20) and breast cancer (19). In line with the aforementioned
studies, the results of the present study suggested that genistein
could efficiently diminish the activity of renal CSCs by repressing
tumor sphere formation, downregulating the expression levels of
renal CSC markers, and inhibiting the proliferation and inducing
the apoptosis of renal CSCs.
The Shh signaling pathway is a major regulator of
cell differentiation, cell proliferation and tissue polarity
(47). To date, a large amount of
evidence has been accumulated that supports the view that the
aberrant activation of Shh signaling is involved in tumorigenesis,
tumor progression and therapeutic response in several types of
cancer, including medulloblastoma, malignant glioma and leukemia,
as well as lung, pancreatic, breast and renal cancer (48,49).
Behnsawy et al (30) reported
that treatment with Shh recombinant, the Shh signaling stimulator,
could enhance RCC cell proliferation, whereas treatment with
cyclopamine, a Smo inhibitor, could inhibit tumor growth. Qian
et al (29) demonstrated that
Shh signaling was involved in the stimulation of renal CSC
pluripotency, induced by cigarette smoking. Therefore, targeting of
the Shh pathway with phytochemicals may provide a potential
strategy for intervention in renal cancer. In the present study,
genistein could inhibit the activation of the Shh pathways in renal
CSCs by downregulating the levels of Shh, Smo, Gli1 and Gli2. By
contrast, the purmorphamine-triggered activation of the Shh pathway
may abolish the inhibitory effects of genistein on tumor sphere
formation, CSC marker expression, and the proliferation and
apoptosis of renal CSCs. Collectively, these results demonstrated
the interventional effect of genistein on renal CSCs via Shh
pathway inhibition. However, the results of the present study are
based on in vitro experiments, therefore further in
vivo studies are required to validate the role of the Shh
signaling pathway in renal CSCs inhibition by genistein. Moreover,
additional in vivo studies are also warranted to explore
whether genistein inhibits other renal CSC models, such as side
population and chemotherapy enrichment.
In conclusion, the present study revealed that
genistein exerts an interventional inhibitory effect on renal CSCs
by suppressing the Shh pathway, which may provide novel insights
into the molecular mechanism of renal CSCs, as well as valuable
avenues for investigating potential interventional targets of
genistein on renal CSCs.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science at
Higher Institutions of Anhui Province (grant no. kj2018A0209) and
Natural Science Foundation of Anhui Province (grant no.
1608085QH173).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
DY and CZ designed the study. EL, TZ, XS, YL and HG
performed the experiments. EL analyzed the data and wrote the
manuscript. All authors read and approved the final manuscript for
publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Znaor A, Lortet-Tieulent J, Laversanne M,
Jemal A and Bray F: International variations and trends in renal
cell carcinoma incidence and mortality. Eur Urol. 67:519–530. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ciardiello C, Leone A and Budillon A: The
crosstalk between cancer stem cells and microenvironment is
critical for solid tumor progression: The significant contribution
of extracellular vesicles. Stem Cells Int. 2018:63921982018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prager BC, Xie Q, Bao S and Rich JN:
Cancer stem cells: The architects of the tumor ecosystem. Cell Stem
Cell. 24:41–53. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lucarelli G, Galleggiante V, Rutigliano M,
Vavallo A, Ditonno P and Battaglia M: Isolation and
characterization of cancer stem cells in renal cell carcinoma.
Urologia. 82:46–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiao X, Rizvanov AA, Cristofanilli M,
Miftakhova RR and Pestell RG: Breast cancer stem cell isolation.
Methods Mol Biol. 1406:121–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jensen SS, Meyer M, Petterson SA, Halle B,
Rosager AM, Aaberg-Jessen C, Thomassen M, Burton M, Kruse TA and
Kristensen BW: Establishment and characterization of a tumor stem
cell-based glioblastoma invasion model. PLoS One. 11:e01597462016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bussolati B, Bruno S, Grange C, Ferrando U
and Camussi G: Identification of a tumor-initiating stem cell
population in human renal carcinomas. FASEB J. 22:3696–3705. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhong Y, Guan K, Guo S, Zhou C, Wang D, Ma
W, Zhang Y, Li C and Zhang S: Spheres derived from the human
SK-RC-42 renal cell carcinoma cell line are enriched in cancer stem
cells. Cancer Lett. 299:150–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang B, Huang YJ, Yao ZJ, Chen X, Guo SJ,
Mao XP, Wang DH, Chen JX and Qiu SP: Cancer stem cell-like side
population cells in clear cell renal cell carcinoma cell line 769P.
PLoS One. 8:e682932013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang L, Xie G, Fan Q and Xie J: Activation
of the hedgehog-signaling pathway in human cancer and the clinical
implications. Oncogene. 29:469–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruch JM and Kim EJ: Hedgehog signaling
pathway and cancer therapeutics: Progress to date. Drugs.
73:613–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gorojankina T: Hedgehog signaling pathway:
A novel model and molecular mechanisms of signal transduction. Cell
Mol Life Sci. 73:1317–1332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruiz i Altaba A, Mas C and Stecca B: The
Gli code: An information nexus regulating cell fate, stemness and
cancer. Trends Cell Biol. 17:438–447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Wang XQ, Zhang Q, Zhu JY, Li Y,
Xie CF, Li XT, Wu JS, Geng SS, Zhong CY and Han HY:
(−)-Epigallocatechin-3-gallate inhibits colorectal cancer stem
cells by suppressing Wnt/β-catenin pathway. Nutrients. 9:E5722017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu J, Wang S, Chen Y, Li X, Jiang Y, Yang
X, Li Y, Wang X, Meng Y, Zhu M, et al: miR-19 targeting of GSK3beta
mediates sulforaphane suppression of lung cancer stem cells. J Nutr
Biochem. 44:80–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Wang X, Xie C, Zhu J, Meng Y, Chen
Y, Li Y, Jiang Y, Yang X, Wang S, et al: Sonic hedgehog and
Wnt/beta-catenin pathways mediate curcumin inhibition of breast
cancer stem cells. Anticancer Drugs. 29:208–215. 2018.PubMed/NCBI
|
|
19
|
Fan PH, Fan SJ, Wang H, Mao J, Shi Y,
Ibrahim MM, Ma W, Yu X, Hou Z, Wang B and Li L: Genistein decreases
the breast cancer stem-like cell population through Hedgehog
pathway. Stem Cell Res Ther. 4:1462013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu D, Shin HS, Lee YS, Lee D, Kim S and
Lee YC: Genistein attenuates cancer stem cell characteristics in
gastric cancer through the downregulation of Gli1. Oncol Rep.
31:673–678. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y and Chen H: Genistein attenuates
WNT signaling by up-regulating sFRP2 in a human colon cancer cell
line. Exp Biol Med (Maywood). 236:714–722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Antosiak A, Milowska K, Maczynska K,
Rozalska S and Gabryelak T: Cytotoxic activity of
genistein-8-C-glucoside form Lupinus luteus L. and genistein
against human SK-OV-3 ovarian carcinoma cell line. Med Chem Res.
26:64–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang W, Wan C and Luo Q, Huang Z and Luo
Q: Genistein-inhibited cancer stem cell-like properties and reduced
chemoresistance of gastric cancer. Int J Mol Sci. 15:3432–3443.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
House CD, Hernandez L and Annunziata CM:
In vitro enrichment of ovarian cancer tumor-initiating cells. J Vis
Exp. Feb 18–2015.doi: 10.3791/52446. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pozzi V, Sartini D, Rocchetti R,
Santarelli A, Rubini C, Morganti S, Giuliante R, Calabrese S, Di
Ruscio G, Orlando F, et al: Identification and characterization of
cancer stem cells from head and neck squamous cell carcinoma cell
lines. Cell Physiol Biochem. 36:784–798. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Li L, Jiao M, Wu D, Wu K, Li X,
Zhu G, Yang L, Wang X, Hsieh JT and He D: Genistein inhibits the
stemness properties of prostate cancer cells through targeting
Hedgehog-Gli1 pathway. Cancer Lett. 323:48–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dormoy V, Danilin S, Lindner V, Thomas L,
Rothhut S, Coquard C, Helwig JJ, Jacqmin D, Lang H and Massfelder
T: The sonic hedgehog signaling pathway is reactivated in human
renal cell carcinoma and plays orchestral role in tumor growth. Mol
Cancer. 8:1232009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qian W, Kong X, Zhang T, Wang D, Song J,
Li Y, Li X, Geng H, Min J, Kong Q, et al: Cigarette smoke
stimulates the stemness of renal cancer stem cells via Sonic
Hedgehog pathway. Oncogenesis. 7:242018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Behnsawy HM, Shigemura K, Meligy FY,
Yamamichi F, Yamashita M, Haung WC, Li X, Miyake H, Tanaka K,
Kawabata M, et al: Possible role of sonic hedgehog and
epithelial-mesenchymal transition in renal cell cancer progression.
Korean J Urol. 54:547–554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mukund V, Mukund D, Sharma V, Mannarapu M
and Alam A: Genistein: Its role in metabolic diseases and cancer.
Crit Rev Oncol Hematol. 119:13–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tirino V, Desiderio V, Paino F, De Rosa A,
Papaccio F, La Noce M, Laino L, De Francesco F and Papaccio G:
Cancer stem cells in solid tumors: An overview and new approaches
for their isolation and characterization. FASEB J. 27:13–24. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Corro C and Moch H: Biomarker discovery
for renal cancer stem cells. J Pathol Clin Res. 4:3–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park EK, Lee JC, Park JW, Bang SY, Yi SA,
Kim BK, Park JH, Kwon SH, You JS, Nam SW, et al: Transcriptional
repression of cancer stem cell marker CD133 by tumor suppressor
p53. Cell Death Dis. 6:e19642015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu J, Cui Y, Zhu J, He J, Zhou G and Yue
Z: Biological characteristics of Rh123high stem-like
cells in a side population of 786-O renal carcinoma cells. Oncol
Lett. 5:1903–1908. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Debeb BG, Zhang X, Krishnamurthy S, Gao H,
Cohen E, Li L, Rodriguez AA, Landis MD, Lucci A, Ueno NT, et al:
Characterizing cancer cells with cancer stem cell-like features in
293T human embryonic kidney cells. Mol Cancer. 9:1802010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lichner Z, Saleh C, Subramaniam V,
Seivwright A, Prud'homme GJ and Yousef GM: miR-17 inhibition
enhances the formation of kidney cancer spheres with stem cell/
tumor initiating cell properties. Oncotarget. 6:5567–5581. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mikami S, Mizuno R, Kosaka T, Saya H, Oya
M and Okada Y: Expression of TNF-α and CD44 is implicated in poor
prognosis, cancer cell invasion, metastasis and resistance to the
sunitinib treatment in clear cell renal cell carcinomas. Int J
Cancer. 136:1504–1514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ueda K, Ogasawara S, Akiba J, Nakayama M,
Todoroki K, Ueda K, Sanada S, Suekane S, Noguchi M, Matsuoka K and
Yano H: Aldehyde dehydrogenase 1 identifies cells with cancer stem
cell-like properties in a human renal cell carcinoma cell line.
PLoS One. 8:e754632013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Khan MI, Czarnecka AM, Helbrecht I,
Bartnik E, Lian F and Szczylik C: Current approaches in
identification and isolation of human renal cell carcinoma cancer
stem cells. Stem Cell Res Ther. 6:1782015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu FH, Mu L, Li XL, Hu YB, Liu H, Han LT
and Gong JP: Characterization and functional analysis of a
slow-cycling subpopulation in colorectal cancer enriched by cell
cycle inducer combined chemotherapy. Oncotarget. 8:78466–78479.
2017.PubMed/NCBI
|
|
42
|
Giancotti FG: Mechanisms governing
metastatic dormancy and reactivation. Cell. 155:750–764. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tian T, Li J, Li B, Wang Y, Li M, Ma D and
Wang X: Genistein exhibits anti-cancer effects via down-regulating
FoxM1 in H446 small-cell lung cancer cells. Tumour Biol.
35:4137–4145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li S, Li J, Dai W, Zhang Q, Feng J, Wu L,
Liu T, Yu Q, Xu S, Wang W, et al: Genistein suppresses aerobic
glycolysis and induces hepatocellular carcinoma cell death. Br J
Cancer. 117:1518–1528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ardito F, Di Gioia G, Pellegrino MR and
Muzio LL: Genistein as a potential anticancer agent against head
and neck squamous cell carcinoma. Curr Top Med Chem. 18:174–181.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Spagnuolo C, Russo GL, Orhan IE,
Habtemariam S, Daglia M, Sureda A, Nabavi SF, Devi KP, Loizzo MR,
Tundis R and Nabavi SM: Genistein and cancer: Current status,
challenges, and future directions. Adv Nutr. 6:408–419. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ruiz i Altaba A: Hedgehog signaling and
the Gli code in stem cells, cancer, and metastases. Sci Signal.
4:pt92011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rimkus TK, Carpenter RL, Qasem S, Chan M
and Lo HW: Targeting the sonic Hedgehog signaling pathway: Review
of smoothened and GLI inhibitors. Cancers (Basel). 8:E222016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
D'Amato C, Rosa R, Marciano R, D'Amato V,
Formisano L, Nappi L, Raimondo L, Di Mauro C, Servetto A, Fulciniti
F, et al: Inhibition of Hedgehog signalling by NVP-LDE225
(Erismodegib) interferes with growth and invasion of human renal
cell carcinoma cells. Brit J Cancer. 111:1168–1179. 2014.
View Article : Google Scholar : PubMed/NCBI
|