Introduction
Melanoma is a common skin cancer that has a high
mortality rate due to its propensity to metastasize (1). According to the Global Burden of
Disease Study of melanoma in 2015, there were known 351,800 cases
of melanoma worldwide in 2015 with an age-standardized rate of five
cases per 100,000 individuals (1).
Melanoma was also responsible for 59,782 global deaths with an
age-standardized rate of one death per 100,000 individuals during
the same year (1). Melanomas which
have been diagnosed at an early stage can usually be removed by
surgical resection with no further complications for the patient.
However, melanomas frequently have a high metastatic potential, and
once metastasis occurs, they become very difficult to successfully
treat (2). Indications for
radiotherapy and chemotherapy as main therapeutic approaches to
treat metastatic melanoma are limited because melanoma is
considered to be radio- and chemotherapy resistant (2). Therefore, the novel therapeutic options
are required for treating patients with advanced melanoma.
Vitamin C is a water-soluble vitamin found in
certain foodstuffs and serves as a dietary supplement (3). There are numerous studies demonstrating
the effects of vitamin C as a preventative treatment for various
diseases. McCormick (4) showed that
vitamin C deficiency served an important role in carcinogenesis.
Numerous studies have investigated the use of vitamin C alone as a
potential treatment to remedy cancer. Yun et al (5) demonstrated that high doses of vitamin C
may selectively kill colorectal cancer cells with mutations in KRAS
or BRAF. Mark Levine's group at The National Cancer Institute
(National Institutes of Health, Bethesda, MD, USA) demonstrated
that vitamin C killed cancer cells whilst doing less damage to
healthy cells (6–8). Furthermore, vitamin C has been shown to
induce cell death in various types of cancer cells, including
mesothelioma, pancreatic cancer, leukemia and renal cell carcinoma
cells (9–11). Taken together, the findings of all
these studies indicate that high vitamin C doses may prevent the
growth of tumor cells.
Vitamin C is taken into cells through active
transport and simple diffusion (5).
Because there are two biological forms of vitamin C, the transport
process is mediated by two families of transport proteins,
including the solute carrier gene family 23 and the SLC2 family of
glucose transporters. The solute carrier gene family 23, consisting
of sodium-dependent vitamin C transporters, are responsible for
transporting ascorbic acid (reduced form of vitamin C), and glucose
transporters transport dehydroascorbic acid (oxidized form of
vitamin C) (5,12). The conversion between the two forms
of vitamin C is regulated by redox reactions (12). Dehydroascorbic acid is reduced back
to vitamin C by glutathione (GSH), and the GSH becomes oxidized
glutathione. Consequently, the cellular redox state is changed.
Vitamin C is sensitive to changes in the redox potential in the
internal cellular environment and also regulates the redox state in
cancer cells (12). Chen et
al (8) demonstrated that high
vitamin C doses increased the levels of reactive oxygen species
(ROS) in cancer cells resulting in the apoptosis of these cells.
However, the molecular mechanisms through which vitamin C affects
melanoma cells are not completely understood.
The present study demonstrated the anti-tumor
effects of vitamin C in the human melanoma A375 cell line for the
first time, to the best of our knowledge. Additionally, a possible
underlying mechanism resulting in the apoptosis of melanoma cells
following vitamin C treatment was investigated.
Materials and methods
Chemicals and reagents
Vitamin C was purchased from Selleck Chemicals
(Houston, TX, USA). MTT, phenylmethylsulfonyl fluoride (PMSF), RIPA
lysis buffer and 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl
enzamidazolocarbocyanin iodide (JC-1) were obtained from
Sigma-Aldrich; Merck KGaA, (Darmstadt, Germany). Fluorescein
isothiocyanate (FITC) Annexin V Apoptosis Detection kit with
propidium iodide (PI) was purchased from BioLegend, Inc. (San
Diego, CA, USA) and bicinchoninic acid (BCA) protein assay kit was
purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China).
Mitochondria Isolation kit for Cultured Cells was purchased from
Beyotime Institute of Biotechnology (Haimen, China). Cytochrome
c (cat. no. 11940S), Bax (cat. no. 5023S), Bcl-2 (cat. no.
4223S), caspase-3 (cat. no. 14220S), caspase-9 (cat. no. 9502S) and
β-actin (cat. no. 4970S) antibodies were purchased from CST
Biological Reagents Co., Ltd. (Shanghai, China) and horseradish
peroxidase-conjugated anti-rabbit Immunoglobulin G (cat. no.
D110065) was purchased from Sangon Biotech Co., Ltd (Shanghai,
China). Other common chemicals were purchased from Shanghai
Titanchem Co., Ltd. (Shanghai, China).
Cell culture
The human melanoma cell line, A375, was purchased
from American Type Culture Collection (Manassas, VA, USA) and
cultured in Dulbecco's modified Eagle's medium (HyClone; GE
Healthcare, Logan, UT, USA) supplemented with 10% heat-inactivated
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), 100 U ml−1 penicillin and 100 mg
ml−1 streptomycin (both from HyClone; GE Healthcare) and
subsequently incubated in a cell culture incubator at 37°C and 5%
CO2, and the media was changed every 2 days.
Cell viability assay
Cells were seeded into 96-well plates at a density
of 5×103 cells/well at 37°C overnight, and treated with
different concentrations (0, 0.6, 1 and 1.4 mM) of vitamin C for 24
h. After treatment with vitamin C, 0.5 mg/ml MTT solution was added
to each well, and cells were further incubated for 2 h at 37°C. The
culture media was removed and 100 µl DMSO was added to each well.
The absorbance was measured at 490 nm using an Infinite M200 PRO
microplate reader (Tecan Group, Ltd., Mannedorf, Switzerland). All
values were normalized to the control treatment.
Apoptosis assay
Apoptosis was examined using a FITC Annexin V
Apoptosis Detection kit with PI, followed by flow cytometry
according to the manufacturer's protocol. Cells were treated with
vitamin C for 24 h, then harvested by trypsinization, and suspended
in binding buffer (BioLegend, Inc.) at a density of
1×106 cells/ml. The cell suspension was stained with
Annexin V-FITC and PI in the dark for 10–20 min at room temperature
(20–25°C and analyzed using a FACS Calibur flow cytometer (BD
Biosciences, San Jose, CA, USA). Data were analyzed using Flow Jo
Version 7.2 (Tree Star, Inc., Ashland, OR, USA).
Mitochondrial membrane potential
analysis
The effects of vitamin C on the mitochondrial
membrane potential were determined using JC-1. A375 cells were
seeded into 6-well plates at a density of 1×106
cells/well. Cells were incubated for 24 h prior to treating with
various concentrations (0, 0.6, 1 and 1.4 mM) of vitamin C for 6 h,
after which the cells were washed three times with PBS and
incubated with JC-1 (10 µg/ml) at 37°C for 30 min. The fluorescence
intensity of vitamin C-treated cells was examined by flow
cytometry.
Western blotting
After incubation with vitamin C for 6 h, cells were
washed three times with PBS. Cells were harvested and homogenized
in ice-cold RIPA lysis buffer supplemented with 1 mM PMSF for 30
min, followed by centrifugation at 12,000 × g for 30 min at 4°C.
Cell lysates were collected, and protein concentrations were
determined using the BCA Protein assay kit (Nanjing KeyGen Biotech
Co., Ltd.). Proteins were mixed with 4X loading buffer (Sangon
Biotech Co., Ltd.) and boiled for 15 min to denature the proteins.
Equivalent quantities of protein were loaded on a 10%
polyacrylamide gel for electrophoresis and subsequently transferred
onto a 0.22 µm polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were blocked with 5% non-fat
dried milk dissolved in Tris-buffered saline containing 0.05%
Tween-20 (TBST) for 1 h at room temperature. The blot was then
probed with the following primary antibodies: Bax (1:1,000), Bcl-2
(1:1,000), caspase-3 (1:1,000), caspase-9 (1:1,000) and β-actin
(1:1,000) antibodies at 37°C for 4 h. After washing three times
with TBST, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibody (1:1,000) for 2 h at room
temperature, and the bound antibody was visualized using an
enhanced chemiluminescence solution (Pierce; Thermo Fisher
Scientific, Inc.). The densitometry analysis of the integrated
density values of the bands was performed using Image J (National
Institutes of Health), and the calculated values were normalized to
those of β-actin.
Western blot analysis for cytochrome
c
Untreated and drug-treated cells were harvested by
centrifugation at 1,000 g for 10 min at 4°C. The cell pellets were
washed once with ice-cold PBS and resuspended with ice-cold PBS for
cell counting. Mitochondria Isolation kit for cultured cells
(Beyotime Institute of Biotechnology) was used to isolate the
mitochondria according to the manufacturer's protocol. The protein
was extracted from mitochondria using the included mitochondria
lysis buffer. Subsequently, mitochondrial lysates were harvested,
and western blotting was performed as described above. The blot was
probed with a cytochrome c antibody (1:800) at 37°C for 4 h.
After washing three times with TBST, membranes were incubated with
1:1,000 dilution of horseradish peroxidase-conjugated secondary
antibody for 2 h at room temperature. Signals were visualized and
quantified as described above.
Statistical analysis
GraphPad PRISM version 4.0 (GraphPad Software, Inc.,
La Jolla, CA, USA) was used for all statistical analysis and graph
plotting. All values are represented as the mean ± standard
deviation (SD) from at least three independent experiments. The
data were analyzed using one-way analysis of variance followed by
Fisher's multiple comparisons test or an unpaired Student's t-test,
as appropriate. P<0.05 was considered to indicate a
statistically significant difference.
Results
Vitamin C inhibits proliferation of
A375 cells
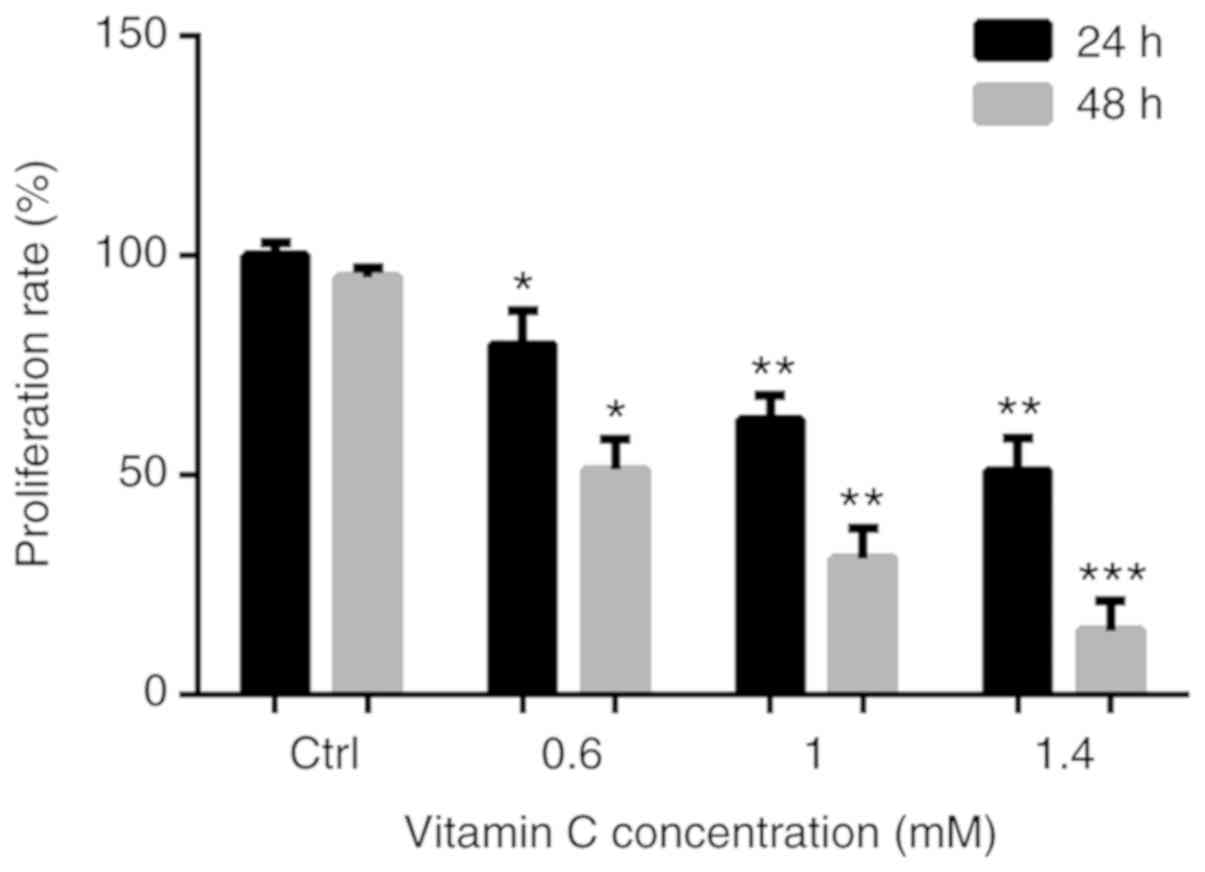
To determine the anti-proliferative activity of
vitamin C on human melanoma A375 cells, cells were treated with 0,
0.6, 1 and 1.4-mM vitamin C for 24 h, and the viability of the
cells was measured using an MTT assay. After treatment with vitamin
C, the proliferation of A375 cells was significantly inhibited in a
dose dependent manner. The proliferation rates were 79.64±7.75,
62.53±5.52 and 48.22±8.40% after treatment with 0.6, 1 and 1.4 mM
of vitamin C for 24 h, respectively. Proliferation rates were
51.23±6.81, 31.02±6.79 and 14.53±6.77% after treatment with 0.6, 1
and 1.4 mM of vitamin C for 48 h, respectively (Fig. 1). As treatment with vitamin C for 48
h resulted in too many dead cells, it was decided that for all
subsequent experiments, cells would be treated with vitamin C at
the varying concentrations for 24 h only.
Vitamin C induces apoptosis in A375
cells
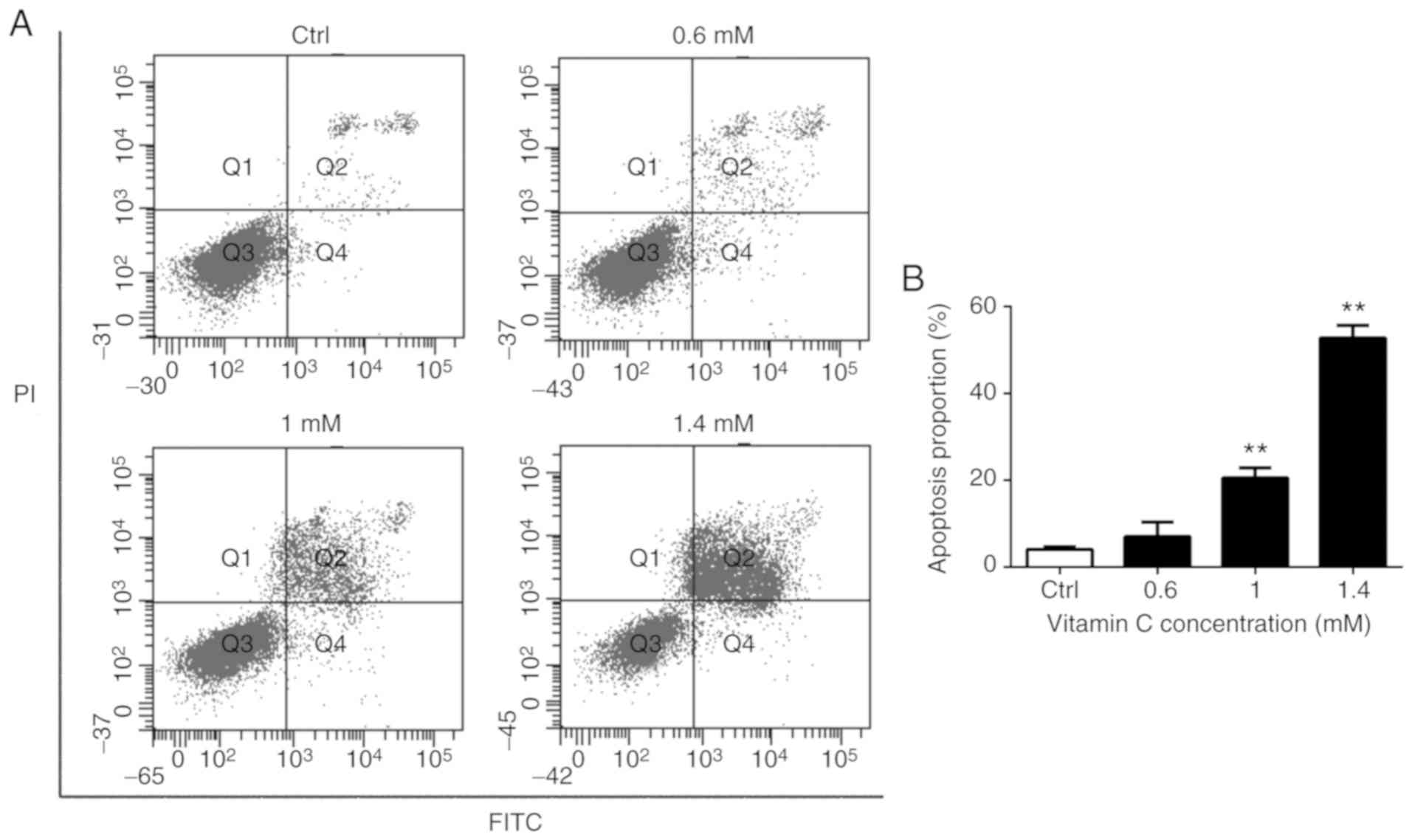
To verify the effects of vitamin C on apoptosis of
A375 cells, Annexin V-FITC/PI double staining and flow cytometry
was used to detect the induction of apoptosis in A375 cells treated
with various concentrations of vitamin C. As presented in Fig. 2, there was a dose-dependent increase
in the number of both early apoptotic cells (Q4 quadrant) and late
apoptotic cells (Q2 quadrant) following treatment with vitamin C.
The proportion of apoptotic cells is represented by the sum of
proportion of early apoptotic cells and late apoptotic cells.
Treatment with 1 and 1.4 mM vitamin C significantly increased the
proportion of apoptotic cells compared with the control (P<0.01)
as shown in Fig. 2B.
Vitamin C decreases the mitochondrial
membrane potential in A375 cells
The loss of mitochondrial membrane potential is a
common early event in apoptosis induced by a variety of stimuli
(13,14). The integrity of the mitochondrial
membrane potential was monitored during vitamin C-induced apoptosis
using the mitochondrial membrane potential-sensitive dye JC-1 and
flow cytometry (Fig. 3A). The
vitamin C-treated cells were stained with JC-1, which accumulates
in healthy mitochondria and fluoresced red, while cells with a
depolarized mitochondrial membrane potential showed green
fluorescence. The ratio of red to green fluorescence intensities
was calculated. There was a decrease in the red/green ratio in
vitamin C-treated A375 cells, indicating the loss of mitochondrial
membrane potential following treatment with vitamin C (Fig. 3B). Treatment with 1.4 mM vitamin C
significantly reduced the red/green ratio compared with the control
(P<0.01; Fig. 3B).
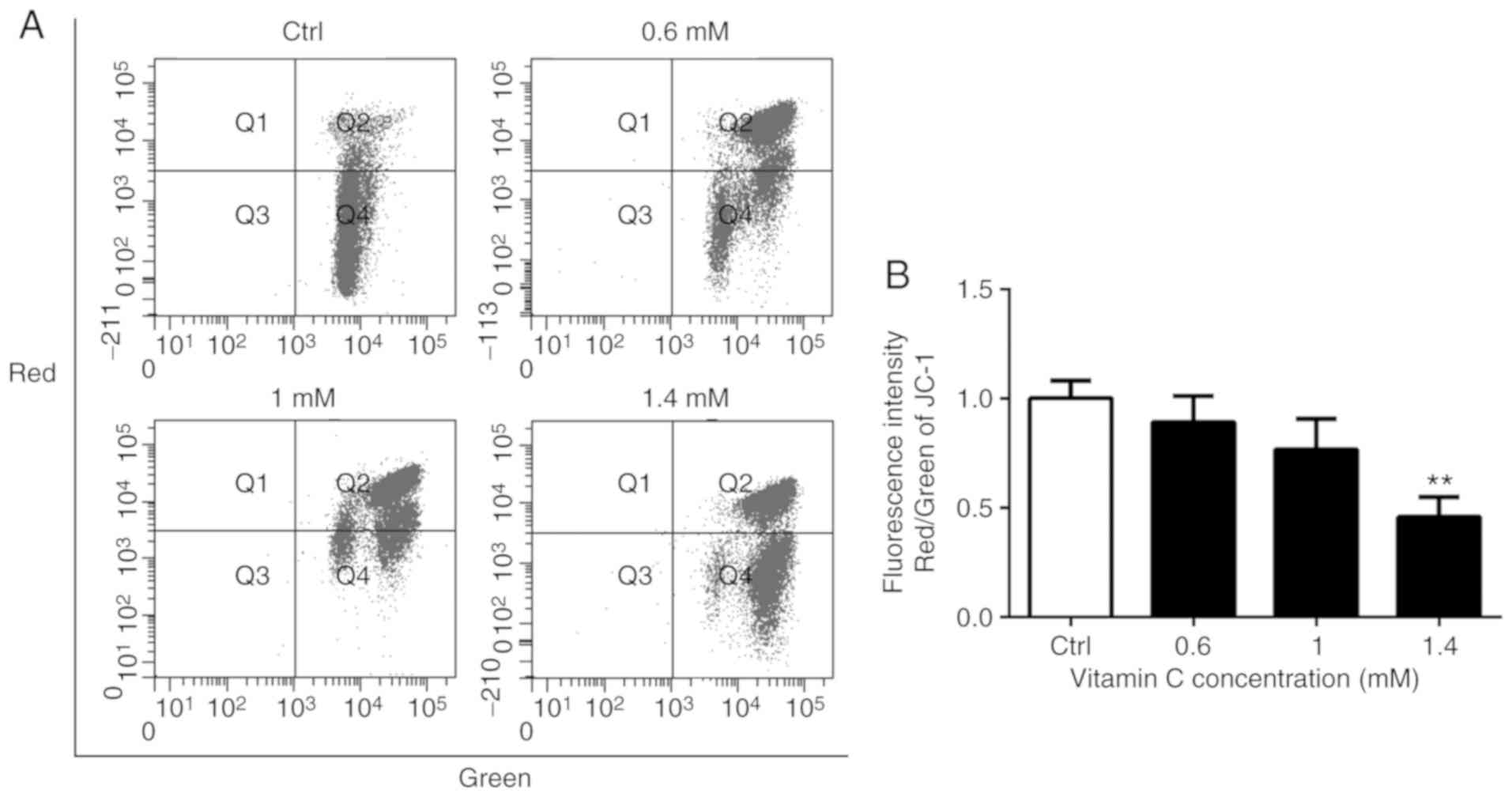 | Figure 3.Vitamin C decreases mitochondrial
membrane potential in A375 cells. (A) Cells were cultured and
treated for 6 h in a medium containing 0, 0.6, 1 or 1.4-mM vitamin
C. Cells were loaded with JC-1 and then analyzed by flow cytometry.
Fluorescence intensities were measured with FACSCalibur flow
cytometer. (B) Histograms indicate the ration of red to green
fluorescence intensities in A375 cells after vitamin C treatment.
**P<0.01 vs. Ctrl. JC-1,
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl enzamidazolocarbocyanin
iodide; Ctrl, control. |
Effects of vitamin C on the Bax- and
Bcl-2-mediated mitochondrial pathway
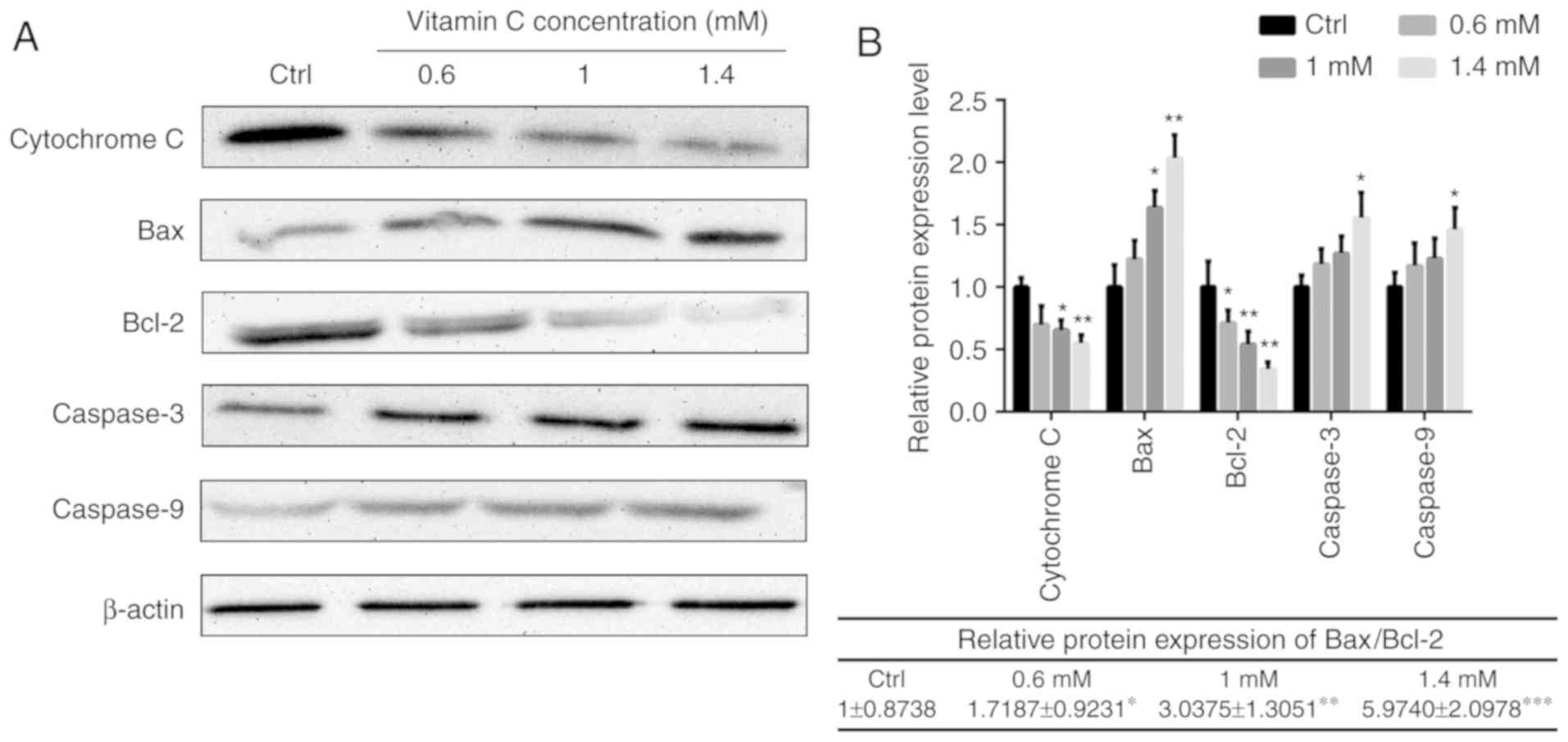
To illustrate the intracellular signaling pathway
involved in the regulation of apoptosis by vitamin C, changes in
the Bax- and Bcl-2-mediated mitochondrial pathway were investigated
in human melanoma A375 cells. As presented in Fig. 4, the levels of cytochrome c in
the mitochondrial fractions were decreased in cells treated with
vitamin C compared with the control in a dose-dependent manner. The
relative protein expression of Bax/Bcl-2 was 1±0.8738 in control
group. The ratio was 1.7187±0.9231, 3.0375±1.3051 and 5.9740±2.0978
when treated with different concentration of vitamin C. So
following vitamin C treatment, the ratio of Bax/Bcl-2 was
significantly increased compared with control group in a
dose-dependent manner. In addition, the protein expression levels
of caspase-3 and caspase-9 were also increased after vitamin C
treatment (Fig. 4).
Discussion
Vitamin C has been reported to serve an important
role in the treatment and the prevention of various types of cancer
(15,16). Although there has been a considerable
amount of effort devoted to discovering its mode of action, the
mechanisms underlying these effects remained obscure. In the
present study, an attempt at clarifying the specific mechanism of
cell death induced by vitamin C was made. Vitamin C inhibited cell
growth and induced cell apoptosis in the human melanoma A375 cell
line, and that this occurred through a decrease in mitochondrial
membrane potential and the subsequent release of cytochrome
c. Furthermore, the expression of the downstream apoptotic
pathway proteins caspase-9 and caspase-3 significantly increased
following treatment with vitamin C. These results indicate that
vitamin C induced apoptosis via the mitochondria-dependent pathway.
Additionally, the expression of the apoptotic protein Bax
increased, while the expression of the anti-apoptotic protein Bcl-2
decreased following treatment with vitamin C.
The mammalian Bcl-2 protein family is divided into
two categories: i) Proapoptotic proteins including Bcl-2 antagonist
killer 1, Bax and possibly Bcl-2-related ovarian killer; ii)
anti-apoptotic proteins consisting of Bcl-2, Bcl-xL, Bclw, myeloid
cell leukemia 1 and Bcl-2-related gene A1. Their functions are
regulated by a third class of Bcl-2 family, the BH3-only proteins
(17,18). The total intracellular ratio of Bax
and Bcl-2 impacts cell fate, either apoptosis or survival (19). Bax redistributes cytochrome c
to the cytosol, while Bcl-2 prevents the collapse of the
mitochondrial membrane potential and prevents the release of
cytochrome c (20). In the
present study, vitamin C increased the ratio of Bax/Bcl-2
expression levels and this may result in an increase of cytochrome
c release into the cytoplasm and subsequent activation of
caspases. The activated caspases may subsequently degrade
structural and nuclear proteins, resulting in the apoptosis of
cells. The apoptosis effector caspase-3 and the apoptosis initiator
caspase-9 were activated following treatment with vitamin C,
suggesting that each of these caspases contributed to vitamin
C-induced apoptosis in A375 cells.
Vitamin C is an important antioxidant and an
essential nutrient (21), and the
results of the present study suggest that it exhibits an anti-tumor
activity through promoting apoptosis. Mata et al (22) conducted a series of non-clinical
studies using different tumor cell lineages to examine the effect
of different concentrations of vitamin C for the treatment and/or
prevention of these types of cancer. They demonstrated that vitamin
C at a concentration range of 0.1–4 mM significantly inhibited the
proliferation of these cancer cell lines (22). Chen et al (6–8)
additionally demonstrated that the growth and weight of ovarian,
pancreatic and glioblastoma tumor xenografts in athymic nude mice
decreased when intraperitoneally injected with vitamin C, and they
demonstrated that mM concentrations of vitamin C killed cancer
cells, but had less of an effect on normal cells (23). Clinical studies regarding the
application of vitamin C have also been conducted. A previous study
reported three cases of patients with cancer who received
intravenous vitamin C as their primary therapy assisting additional
treatments such as other minerals, botanicals and other vitamins
(24). Although the
histopathological examination of these cases suggested poor
prognoses, patients had a longer overall survival duration
following treatment compared with the control group (24). In contrast, mixed results have been
reported in other studies where vitamin C was combined with other
treatments. A small study consisting of 14 patients with advanced
pancreatic cancer, intraperitoneal injection of vitamin C was
administered alongside chemotherapy. The patients experienced very
few side effects of the vitamin C treatment (25); however, other studies reported
adverse side effects when vitamin C was used in combination with
other drugs in patients with acute myeloid leukemia, metastatic
melanoma and refractory metastatic colorectal cancer (26–28),
indicating that vitamin C treatment may be associated with serious
side effects and disease progression.
According to in vitro studies, vitamin C less
than the concentration of 4 mM is an antioxidant that inactivates
ROS. However, at higher concentrations of up to 20 mM, vitamin C
acts as a pro-oxidant and generates oxidative species (22,29).
Therefore, the effects of vitamin C can vary depending on its
concentration. One study demonstrated that high concentrations of
vitamin C induced cytotoxicity in malignant melanoma but promoted
tumor growth at lower concentrations (30). In another example, at low mM
concentrations, vitamin C could kill some cell lines in
vitro by producing superoxide radicals and hydrogen peroxide,
which are responsible for its cytotoxic activity in vivo;
however, 20 mM vitamin C did not pose any risk to nonmalignant cell
lines (31). Other studies have
confirmed that high vitamin C doses are effective in inducing
apoptosis in cancer cell in vitro as well as in vivo
(32–37). However, the mechanism through which
vitamin C induces cancer cell apoptosis in vitro has not
been completely established. Previously, vitamin C was thought to
induce apoptosis in vitro through endoplasmic reticulum
stress-associated pathways and the mitochondrial pathway (38–40). In
the present study, the apoptosis-inducing activity of high
concentrations of vitamin C was investigated and a potential
mechanism for induction of apoptosis was identified. The present
study has certain limitations. The effects of lower concentrations
of vitamin C on the human melanoma A375 cells were not tested.
Additionally, only one human melanoma cell line was tested, and a
control healthy skin cell line was not used. Therefore, further
research is required to elucidate the effect of lower
concentrations of vitamin C on a range of melanoma cells. The
effect of vitamin C at different concentrations is being studied by
our group on a range of different melanoma cell lines.
Nevertheless, the present study provides an insight into the
underlying mechanisms of vitamin C induced-apoptosis and provides
additional evidence for the potential use of vitamin C as either a
mainline or adjuvant therapy in the treatment of cancer.
Acknowledgements
Not applicable.
Funding
The present study was funded by The National Natural
Science Foundation of China (grant nos. 81872162, 81602556 and
31870338), The Natural Science Foundation of Shandong Province
(grant no. ZR2017JL030), Taishan Scholars Construction Engineering
of Shandong Province, Yantai High-End Talent Introduction Plan
‘Double Hundred’, The Scientific Research Foundation of Binzhou
Medical University (grant nos. BY2016KYQD01 and BY2018KYQD07) and
The Dominant Disciplines' Talent Team Development Scheme of Higher
Education of Shandong Province.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the author on reasonable request.
Authors' contributions
XYC, YC, DFL and QZ conceived and designed the
study. XYC, YC, YQ and WJL performed the experiments. Statistical
analysis was performed by ZHP, CJQ and XZ under the supervision of
DFL and QZ. ZHP prepared the figures. All authors contributed to
the analysis and interpretation of the data. XYC wrote the
manuscript with contributions from all other authors. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Bcl-2
|
BCL2 apoptosis regulator
|
|
Bax
|
BCL2 associated X
|
|
ROS
|
reactive oxygen species
|
|
JC-1
|
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl enzamidazolocarbocyanin
iodide
|
References
|
1
|
Agarwala SS: Current systemic therapy for
metastatic melanoma. Expert Rev Anticancer Ther. 9:587–595. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller AJ and Mihm MC Jr: Melanoma. N Engl
J Med. 355:51–65. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blake CJ: Analytical procedures for
water-soluble vitamins in foods and dietary supplements: A review.
Anal Bioanal Chem. 389:63–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCormick WJ: Cancer: A collagen disease,
secondary to a nutritional deficiency. Arch Pediatr. 76:166–171.
1959.PubMed/NCBI
|
|
5
|
Yun J, Mullarky E, Lu C, Bosch KN,
Kavalier A, Rivera K, Roper J, Chio II, Giannopoulou EG, Rago C, et
al: Vitamin C selectively kills KRAS and BRAF mutant colorectal
cancer cells by targeting GAPDH. Science. 350:1391–1396. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Q, Espey MG, Krishna MC, Mitchell JB,
Corpe CP, Buettner GR, Shacter E and Levine M: Pharmacologic
ascorbic acid concentrations selectively kill cancer cells: Action
as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl
Acad Sci USA. 102:13604–13609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Q, Espey MG, Sun AY, Lee JH, Krishna
MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR and Levine
M: Ascorbate in pharmacologic concentrations selectively generates
ascorbate radical and hydrogen peroxide in extracellular fluid in
vivo. Proc Natl Acad Sci USA. 104:8749–8754. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Q, Espey MG, Sun AY, Pooput C, Kirk
KL, Krishna MC, Khosh DB, Drisko J and Levine M: Pharmacologic
doses of ascorbate act as a prooxidant and decrease growth of
aggressive tumor xenografts in mice. Proc Natl Acad Sci USA.
105:11105–11109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takemura Y, Satoh M, Satoh K, Hamada H,
Sekido Y and Kubota S: High dose of ascorbic acid induces cell
death in mesothelioma cells. Biochem Biophys Res Commun.
394:249–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du J, Martin SM, Levine M, Wagner BA,
Buettner GR, Wang SH, Taghiyev AF, Du C, Knudson CM and Cullen JJ:
Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer.
Clin Cancer Res. 16:509–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia L, Jia Q, Shang Y, Dong X and Li L:
Vitamin C intake and risk of renal cell carcinoma: A meta-analysis.
Sci Rep. 5:179212015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian W, Wang Y, Xu Y, Guo X, Wang B, Sun
L, Liu L, Cui F, Zhuang Q, Bao X, et al: The hypoxia-inducible
factor renders cancer cells more sensitive to vitamin C-induced
toxicity. J Biol Chem. 289:3339–3351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burke PJ: Mitochondria, bioenergetics and
apoptosis in cancer. Trends Cancer. 3:857–870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee KE, Hahm E, Bae S, Kang JS and Lee WJ:
The enhanced tumor inhibitory effects of gefitinib and L-ascorbic
acid combination therapy in non-small cell lung cancer cells. Oncol
Lett. 14:276–282. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Francesco EM, Bonuccelli G, Maggiolini
M, Sotgia F and Lisanti MP: Vitamin C and Doxycycline: A synthetic
lethal combination therapy targeting metabolic flexibility in
cancer stem cells (CSCs). Oncotarget. 8:67269–67286. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Frommel TO and Zarling EJ: Chronic
inflammation and cancer: Potential role of Bcl-2 gene family
members as regulators of cellular antioxidant status. Med
Hypotheses. 52:27–30. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Volkmann N, Marassi FM, Newmeyer DD and
Hanein D: The rheostat in the membrane: BCL-2 family proteins and
apoptosis. Cell Death Differ. 21:206–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Raisova M, Hossini AM, Eberle J, Riebeling
C, Wieder T, Sturm I, Daniel PT, Orfanos CE and Geilen CC: The
Bax/Bcl-2 ratio determines the susceptibility of human melanoma
cells to CD95/Fas-mediated apoptosis. J Invest Dermatol.
117:333–340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Antonsson B, Montessuit S, Sanchez B and
Martinou JC: Bax is present as a high molecular weight
oligomer/complex in the mitochondrial membrane of apoptotic cells.
J Biol Chem. 276:11615–11623. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coulter ID, Hardy ML, Morton SC, Hilton
LG, Tu W, Valentine D and Shekelle PG: Antioxidants vitamin C and
vitamin e for the prevention and treatment of cancer. J Gen Intern
Med. 21:735–744. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mata AM, Carvalho RM, Alencar MV,
Cavalcante AA and Silva BB: Ascorbic acid in the prevention and
treatment of cancer. Rev Assoc Med Bras (1992). 62:680–686. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Frei B and Lawson S: Vitamin C and cancer
revisited. Proc Natl Acad Sci USA. 105:11037–11038. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Padayatty SJ, Sun H, Wang Y, Riordan HD,
Hewitt SM, Katz A, Wesley RA and Levine M: Vitamin C
pharmacokinetics: Implications for oral and intravenous use. Ann
Intern Med. 140:533–537. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Monti DA, Mitchell E, Bazzan AJ, Littman
S, Zabrecky G, Yeo CJ, Pillai MV, Newberg AB, Deshmukh S and Levine
M: Phase I evaluation of intravenous ascorbic acid in combination
with gemcitabine and erlotinib in patients with metastatic
pancreatic cancer. PLoS One. 7:e297942012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Subbarayan PR, Lima M and Ardalan B:
Arsenic trioxide/ascorbic acid therapy in patients with refractory
metastatic colorectal carcinoma: A clinical experience. Acta Oncol.
46:557–561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bael TE, Peterson BL and Gollob JA: Phase
II trial of arsenic trioxide and ascorbic acid with temozolomide in
patients with metastatic melanoma with or without central nervous
system metastases. Melanoma Res. 18:147–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Welch JS, Klco JM, Gao F, Procknow E, Uy
GL, Stockerl-Goldstein KE, Abboud CN, Westervelt P, DiPersio JF,
Hassan A, et al: Combination decitabine, arsenic trioxide, and
ascorbic acid for the treatment of myelodysplastic syndrome and
acute myeloid leukemia: A phase I study. Am J Hematol. 86:796–800.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carr A and Frei B: Does vitamin C act as a
pro-oxidant under physiological conditions? FASEB J. 13:1007–1024.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang G, Yan Y, Ma Y and Yang Y: Vitamin C
at high concentrations induces cytotoxicity in malignant melanoma
but promotes tumor growth at low concentrations. Mol Carcinog.
56:1965–1976. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
McCarty MF and Contreras F: Increasing
superoxide production and the labile iron pool in tumor cells may
sensitize them to extracellular ascorbate. Front Oncol. 4:2492014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ohwada R, Ozeki Y and Saitoh Y: High-dose
ascorbic acid induces carcinostatic effects through hydrogen
peroxide and superoxide anion radical generation-induced cell death
and growth arrest in human tongue carcinoma cells. Free Radic Res.
51:684–692. 2017.PubMed/NCBI
|
|
33
|
Perez-Cruz I, Cárcamo JM and Golde DW:
Caspase-8 dependent TRAIL-induced apoptosis in cancer cell lines is
inhibited by vitamin C and catalase. Apoptosis. 12:225–234. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hong SW, Jin DH, Hahm ES, Yim SH, Lim JS,
Kim KI, Yang Y, Lee SS, Kang JS, Lee WJ, et al: Ascorbate (vitamin
C) induces cell death through the apoptosis-inducing factor in
human breast cancer cells. Oncol Rep. 18:811–815. 2007.PubMed/NCBI
|
|
35
|
Zou W, Yue P, Lin N, He M, Zhou Z, Lonial
S, Khuri FR, Wang B and Sun SY: Vitamin C inactivates the
proteasome inhibitor PS-341 in human cancer cells. Clin Cancer Res.
12:273–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Reddy VG, Khanna N and Singh N: Vitamin C
augments chemotherapeutic response of cervical carcinoma HeLa cells
by stabilizing P53. Biochem Biophys Res Commun. 282:409–415. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
An SH, Kang JH, Kim DH and Lee MS: Vitamin
C increases the apoptosis via up-regulation p53 during cisplatin
treatment in human colon cancer cells. BMB Rep. 44:211–216. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nagappan A, Park KI, Park HS, Kim JA, Hong
GE, Kang SR, Lee DH, Kim EH, Lee WS, Won CK and Kim GS: Vitamin C
induces apoptosis in AGS cells by down-regulation of 14-3-3σ via a
mitochondrial dependent pathway. Food Chem. 135:1920–1928. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Aumailley L, Warren A, Garand C, Dubois
MJ, Paquet ER, Le Couteur DG, Marette A, Cogger VC and Lebel M:
Vitamin C modulates the metabolic and cytokine profiles, alleviates
hepatic endoplasmic reticulum stress, and increases the life span
of Gulo-/-mice. Aging (Albany NY). 8:458–483. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lim JY, Kim D, Kim BR, Jun JS, Yeom JS,
Park JS, Seo JH, Park CH, Woo HO, Youn HS, et al: Vitamin C induces
apoptosis in AGS cells via production of ROS of mitochondria. Oncol
Lett. 12:4270–4276. 2016. View Article : Google Scholar : PubMed/NCBI
|