Introduction
Thyroid cancer is rapidly increasing in incidence
(1). Follicular thyroid carcinoma
(FTC) and papillary thyroid carcinoma (PTC) account for ~94% of
cases of thyroid cancer, with FTC being more aggressive and having
a poorer prognosis than PTC (1–3). The
clinical therapy of FTC, including surgery, radioactive iodine
ablation and thyroid-stimulating hormone suppression, leads to
complications, such as developing resistance to hormones, which can
be disabling and may be life-threatening (4,5).
Therefore, there is an urgent need to examine the mechanism and
identify novel targets involved in FTC to improve early diagnosis,
develop better treatments and prolong survival time.
The traditional Chinese medicine matrine can induce
apoptosis in V600EBRAF-harboring melanoma, lung cancer,
prostate cancer, PTC, breast cancer and T cell lymphoblastic
leukemia cells (6–13). The PTEN/Akt pathway serves an
important role in the inhibitory effect of matrine on cancer cells
(6–8,11,12).
However, to the best of our knowledge, the pro-apoptotic effect and
underlying mechanism of matrine in FTC have not been investigated
previously.
MicroRNA-21 (miR-21) is one of the most frequently
upregulated microRNAs in malignant tumors (14,15).
Previous studies have shown that miR-21 is a potential regulator of
PTEN in various types of cancer, including thyroid cancer (11), breast cancer (12,16),
non-small cell lung cancer (14) and
human hepatocytes (17). PTEN is a
tumor suppressor gene with phospholipase activity, which suppresses
the phosphorylation of Akt and is involved in the occurrence and
development of cancer (12). In the
present study, the action and underlying mechanism of miR-21 on
matrine-induced inhibition of the FTC-133 human FTC cell line were
investigated.
Materials and methods
Matrine and cell line
Matrine (C15H24N2O;
MW 248.36; CAS: 519-02-8, purity ≥98%) was purchased from Shanghai
Aladdin Biochemical Technology Co., Ltd. and was dissolved in 0.9%
NaCl solution for use. The FTC-133 human FTC cells were donated by
Changchun Institute of Applied Chemistry, Chinese Academy of
Sciences (Beijing, China). The cells were cultured in RPMI-1640
medium (Hyclone; GE Healthcare Life Sciences) supplemented with 10%
fetal bovine serum (Hyclone; GE Healthcare Life Sciences) and
incubated at 37°C at a humidity of 95 and 5% CO2.
MTT assay
The FTC-133 cells were seeded in a 96-well plate
(200 µl/well) at a density of 1×104 cells per well.
After 12 h, the cells were treated with matrine at concentrations
of 0, 40, 80, 200 and 400 µM at 37°C. Following incubation at 37°C
for 24, 48 and 72 h, 20 µl of 5 mg/ml MTT reagent (Beijing Solarbio
Science & Technology Co., Ltd.) was added to each well for 4 h.
The medium was then removed and 150 µl DMSO was added to dissolve
the formazan crystals. Finally, the absorbance was measured at a
wavelength of 570 nm using a SpectraMax Plus 384 microplate reader
(Molecular Devices, LLC).
To investigate the effect of matrine on FTC-133
cells with a high expression of miR-21, following treatment of the
FTC-133 cells with matrine and miR-21 mimics, cell viability was
measured again using an MTT assay. Each MTT assay was repeated
three times. The results are expressed as the percentage growth
inhibition with respect to the normal control cells.
Flow cytometry
The FTC-133 cells were treated with matrine at
concentrations of 0, 80, 200 and 400 µM in a 6-well plate (Corning,
Inc.) for 24 h. Cell apoptosis was detected using the Annexin
V-FITC Detection kit according to the manufacturer's protocol
(Bioworld Technology, Inc.). Following staining, the samples were
analyzed using a Guava easyCyte6-2L flow cytometer (Merck
KGaA).
Transfection of miR-21 mimics and
miR-21 inhibitor
The FTC-133 cells were seeded at a density of
1×105 cells/ml for 24 h. When the cells reached 80%
confluence, they were transfected either with miR-21 mimics (sense,
5′-UAGCUUAUCAGACUGAUGUUGA-3′; antisense,
5′-UCAACAUCAGUCUGAUAAGCUA-3′) or miR-21 inhibitor
(5′-UCAACAUCAGUCUGAUAAGCUA-3′; Biomics Biotechnologies Co., Ltd.)
at final concentrations of 0 (negative control; NC), 30 and 50 nM
using X-tremeGENE siRNA transfection reagent following the
manufacturer's protocol (Roche Diagnostics). Based on our
preliminary experiments, transfection with a low concentration had
no significant effects, whereas a high concentration of
transfection produced cytotoxicity. Therefore, the transfection
concentrations of 30 and 50 nM were selected. To examine the
biological effects of transfection on FTC-133 cells, the expression
of miR-21 was determined by reverse transcription-quantitative PCR
(RT-qPCR) analysis 48 h after transfection, and the mRNA and
protein levels of PTEN, Akt and phosphorylated (p)-Akt were
detected.
RT-qPCR analysis of miR-21, PTEN and
Akt
Total RNA was extracted from FTC-233 cells with
TRIzol® reagent following the manufacturer's
instructions (Ambion; Thermo Fisher Scientific, Inc.). The
concentration of RNA was determined using the Nanodrop 2000
instrument (Thermo Fisher Scientific, Inc.). The optical density
260/280 rate was between 1.8 and 2.0. Nucleic acid electrophoresis
was performed for quality analysis of RNA in Tris-Borate-EDTA
solution.
To perform RT-qPCR of miR-21, 25 ng RNA was reverse
transcribed to cDNA using the mirVana RT-qPCR miRNA Detection kit
(Ambion; Thermo Fisher Scientific, Inc.). The reaction conditions
were as follows: 37°C for 30 min and 95°C for 10 min. The levels of
miR-21 were determined by RT-qPCR using the mirVana RT-qPCR miRNA
Detection kit following the manufacturer's instructions (Ambion;
Thermo Fisher Scientific, Inc.) and normalized to U6 as the
internal reference. The reactions were performed on the Aligent
Mx3000P PCR system (Agilent Technologies, Inc.). The primer
sequences used for RT-qPCR were as follows: miR-21,
5′-GCGCGCGTAGCTTATCAGACTGA-3′ (forward) and
5′-ATCCAGTGCAGGGTCCGAGG-3′ (reverse); U6, 5′-CTCGCTTCGGCAGCACA-3′
(forward) and 5′-AACGCTTCACGAATTTGCGT-3′ (reverse). The
amplification program was as follows: 95°C for 3 min, 40 cycles of
95°C for 15 sec and 60°C for 30 sec.
To investigate the mRNA expression levels of PTEN
and Akt, 50 ng RNA was reverse transcribed to cDNA using the
FastQuant RT kit (Beijing Tiangen Biotech Co. Ltd.). The reaction
conditions were as follows: 42°C for 15 min and 95°C for 3 min.
RT-qPCR was performed with SYBR Premix Ex Taq (Takara Bio, Inc.)
and normalized to GAPDH as the internal reference. The primer
sequences used were as follows: PTEN,
5′-GCAATATGTTCATAACGATGGCTGTGG-3′ (forward),
5′-GAACTGGCAGGTAGAAGGCAACTC-3′ (reverse); Akt,
5′-GCAGGATGTGGACCAACGTGAG-3′ (forward), 5′-GCAGGCAGCGGATGATGAAGG-3′
(reverse). The thermocycling conditions for PTEN and Akt was as
follows: 95°C for 30 sec, 40 cycles of 95°C for 5 sec and 60°C for
30 sec. The mRNA expression level was calculated using the
2−ΔΔCq method (18),
normalizing to the Cq value of the reference gene GAPDH.
Western blotting
The cells were collected and washed with cold PBS
and then lysed in lysis buffer (Beijing Solarbio Science &
Technology Co., Ltd.) supplemented with protease inhibitor,
phosphatase inhibitor and PMSF (lysis buffer: Protease inhibitor:
Phosphatase inhibitor: PMSF=100:1:1:1). The concentrations of
proteins were determined using a Bicinchoninic Acid Protein Assay
kit (Beijing Solarbio Science & Technology Co., Ltd.). In
total, 30 µg protein was separated by SDS-PAGE on 10% gels and
electroblotted onto PVDF membranes (Beijing Solarbio Science &
Technology Co., Ltd.). The membranes were blocked with 5% non-fat
milk in TBS with Tween-20 at 37°C for 1 h and incubated with
primary antibodies of PTEN (26H9) mouse mAb (cat. no. 9556,
1:1,000; Cell Signaling Technology, Inc.), p-Akt (Ser-473) mouse
mAb (cat. no. 12694, 1:1,000; Cell Signaling Technology, Inc.) and
Akt1 (2H10) mouse mAb (cat. no. 2967, 1:1,000; Cell Signaling
Technology, Inc.) overnight at 4°C. Following washing with TBST,
the membranes were incubated with horseradish peroxidase-labeled
horse anti-mouse secondary antibodies (cat. no. 7076, 1:1,000; Cell
Signaling Technology, Inc.) at 37°C for 1 h. The protein signal was
visualized using 3,3′diaminobenzine reagent (Beijing Solarbio
Science & Technology Co., Ltd.). GAPDH (TransGen Biotech Co.,
Ltd.) was used as the internal reference.
Statistical analysis
All data are presented as the mean ± SD. Analyses
were performed with GraphPad Prism 5.0 (GraphPad Software, Inc.).
Statistical significance of the results were analyzed by Student's
t-test for two group comparison and one-way ANOVA followed by
Dunnett's test for multiple comparisons. P<0.05 was considered
to indicate a statistically significant difference.
Results
Matrine inhibits the growth of FTC-133
cells
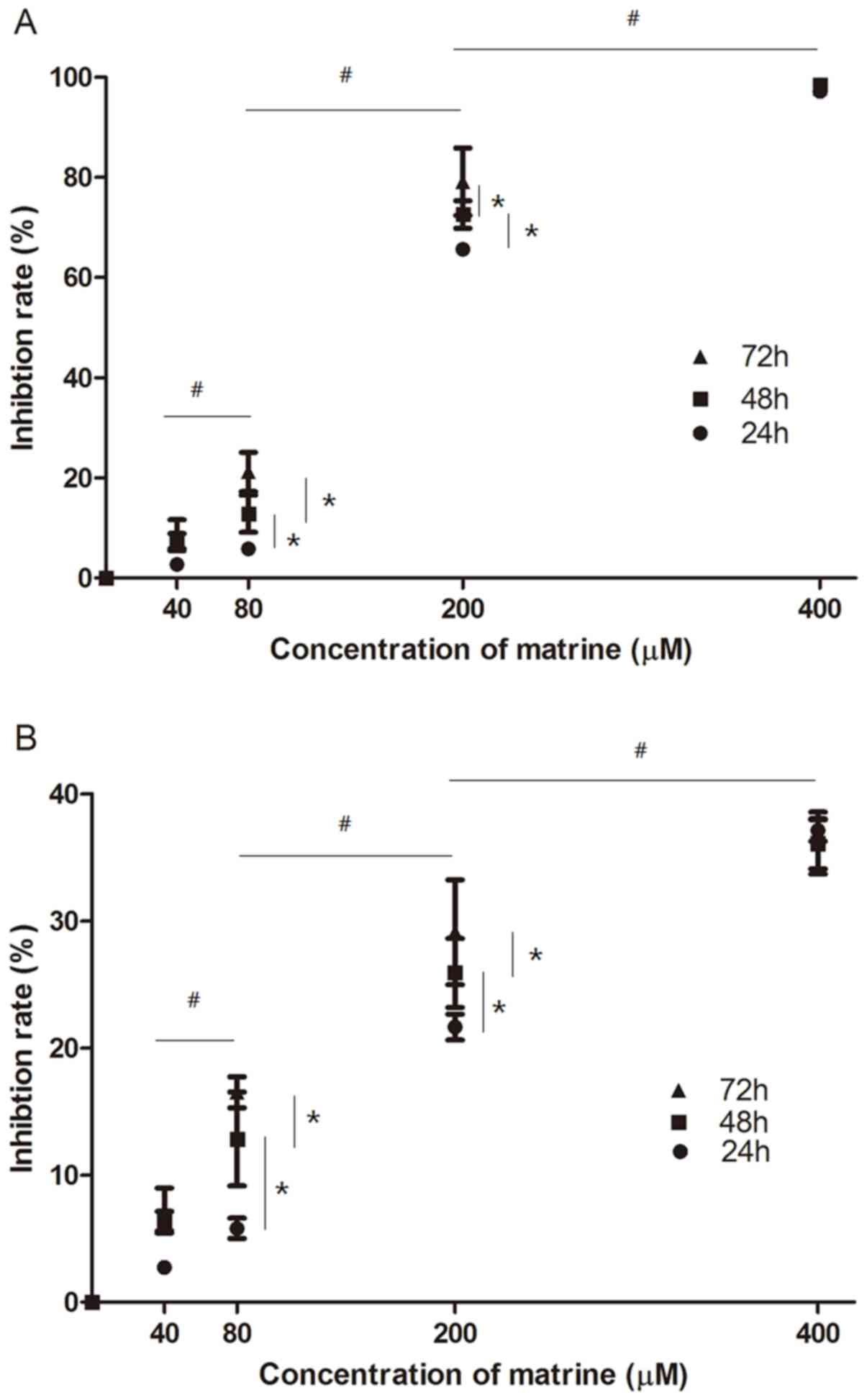
To detect the biological effect of matrine on
FTC-133 cells, an MTT assay was performed. The FTC-133 cells were
treated with matrine at concentrations of 0, 40, 80, 200 and 400 µM
for 24, 48 and 72 h. The results showed that matrine had an
inhibitory effect on FTC-133 cells in a dose- and time-dependent
manner with an IC50 of 154.8 µM at 48 h (Fig. 1A). Compared with the control, the
treatment group with a low concentration of 40 µM had no
significant inhibitory effect (P>0.05). The inhibition rate was
~80% for 200 µM at 72 h. When the concentration of matrine reached
400 µM, growth of the FTC-133 cells was almost completely
inhibited.
Following transfection of the FTC-133 cells with
miR-21 mimics at a final concentration of 50 nM, the cells were
treated with matrine at concentrations of 0, 40, 80, 200 and 400 µM
for 24, 48 and 72 h. Cell viability was measured again using an MTT
assay. Compared with the control group, the marked inhibitory
effect of matrine on the transfected FTC-133 cells was still
observed. However, compared with the matrine treatment group, the
inhibition rate was decreased significantly (Fig. 1B). This suggested that high
expression of miR-21 reduced the inhibitory effect of matrine on
FTC-133 cells.
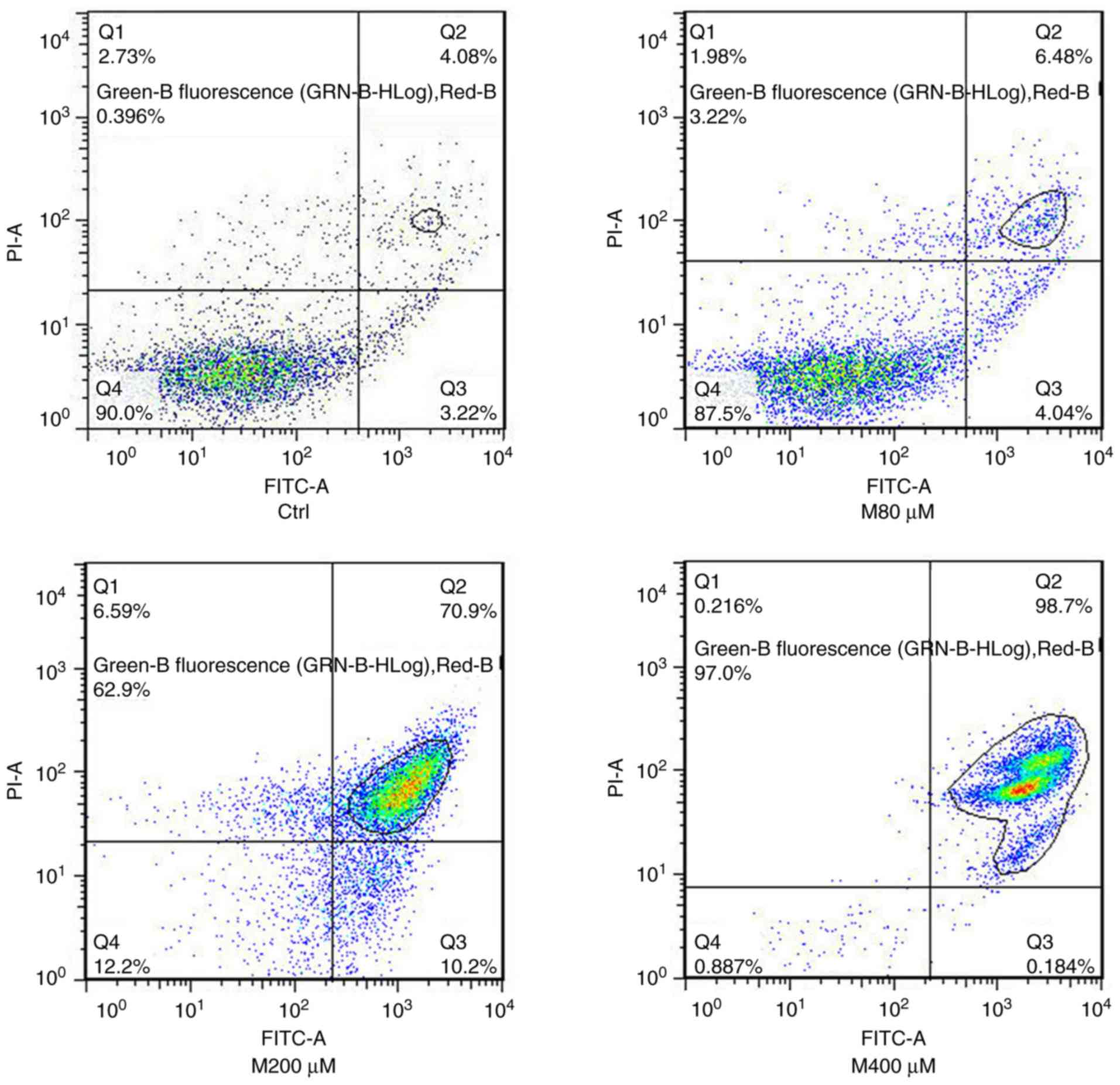
Matrine induces cell apoptosis
To investigate the pro-apoptotic effect of matrine
on FTC-133 cells, the cells were exposed to matrine at
concentrations of 0, 80, 200 and 400 µM. Cell apoptosis was
detected by flow cytometry and the results are presented in
Fig. 2. When the cells were treated
with a high concentration of 200 µM, the microscopic morphology
revealed no necrosis (Fig. S1).
Therefore, the impact of matrine on cell survival was not
associated with necrosis but apoptosis. The apoptosis fractions for
0, 80, 200 and 400 µM were 0.396, 3.22, 62.9 and 97.0%,
respectively. The pro-apoptotic effect was in a matrine
concentration-dependent manner. Therefore, in the following
experiments, matrine was used at the concentrations of 80 and 200
µM.
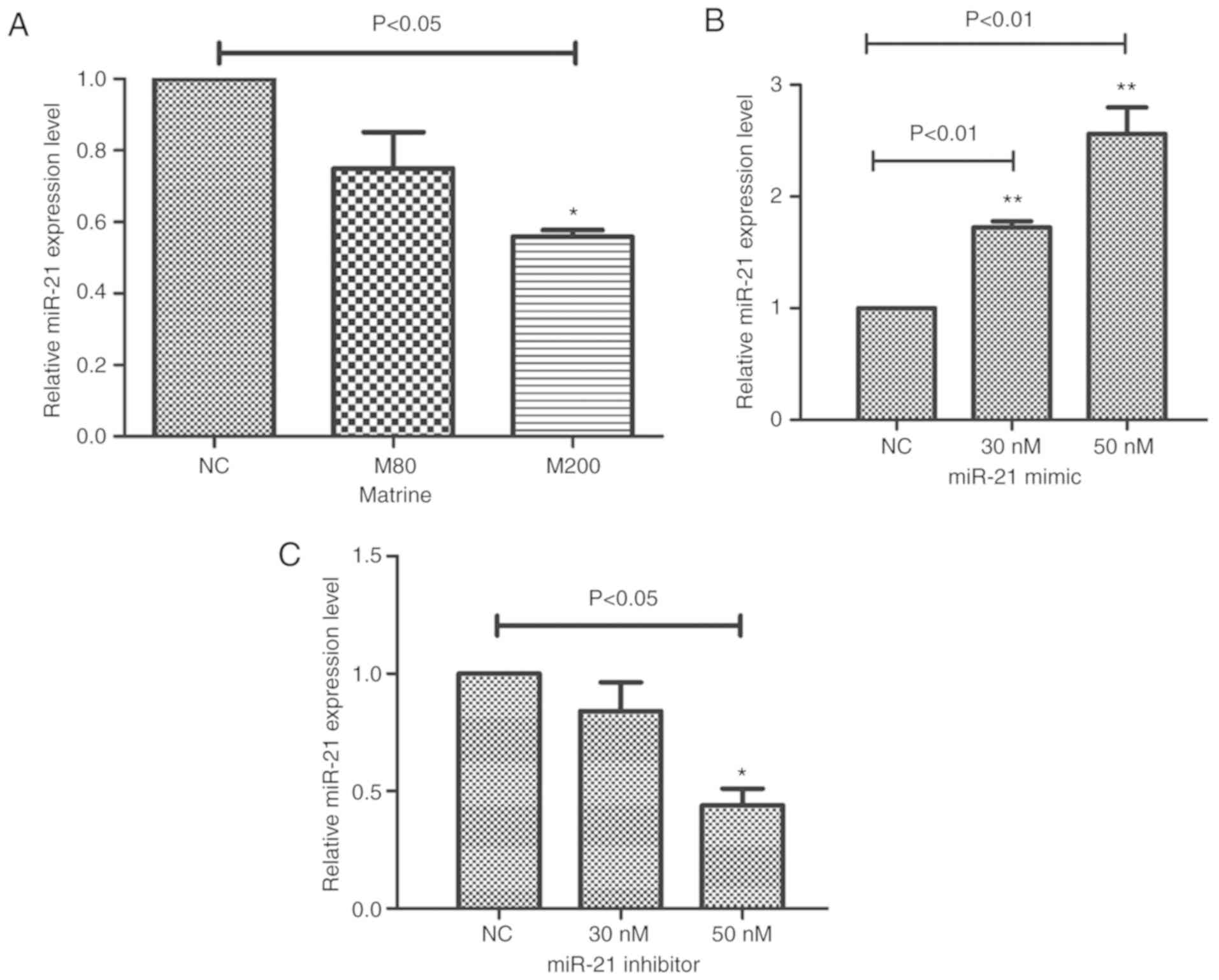
Effect of matrine, miR-21 mimics and
miR-21 inhibitor on the expression of miR-21 in FTC-133 cells
The FTC-133 cells were exposed to matrine at
concentrations of 0, 80 and 200 µM for 48 h. The expression level
of miR-21 was examined by RT-qPCR analysis. As shown in Fig. 3A, the expression level of miR-21 was
significantly downregulated when the cells were treated with 200 µM
matrine for 48 h (M200/NC: 0.56±0.026-fold). The expression of
miR-21 was examined following transfection of the cells with 0
(NC), 30 and 50 nM of either miR-21 mimics or miR-21 inhibitor. As
shown in Fig. 3B and C, as expected,
the overexpression and inhibition of miR-21 were achieved by
transfection with miR-21 mimics or miR-21 inhibitor, respectively.
miR-21 mimics increased the level of miR-21 (30 nM/NC:
1.72±0.08-fold; 50 nM/NC: 2.71±0.13-fold). The miR-21 inhibitor
decreased the level of miR-21 (50 nM/NC: 0.44±0.10-fold). These
results suggested that the inhibitory effect of matrine on FTC-133
cells was associated with the downregulation of miR-21.
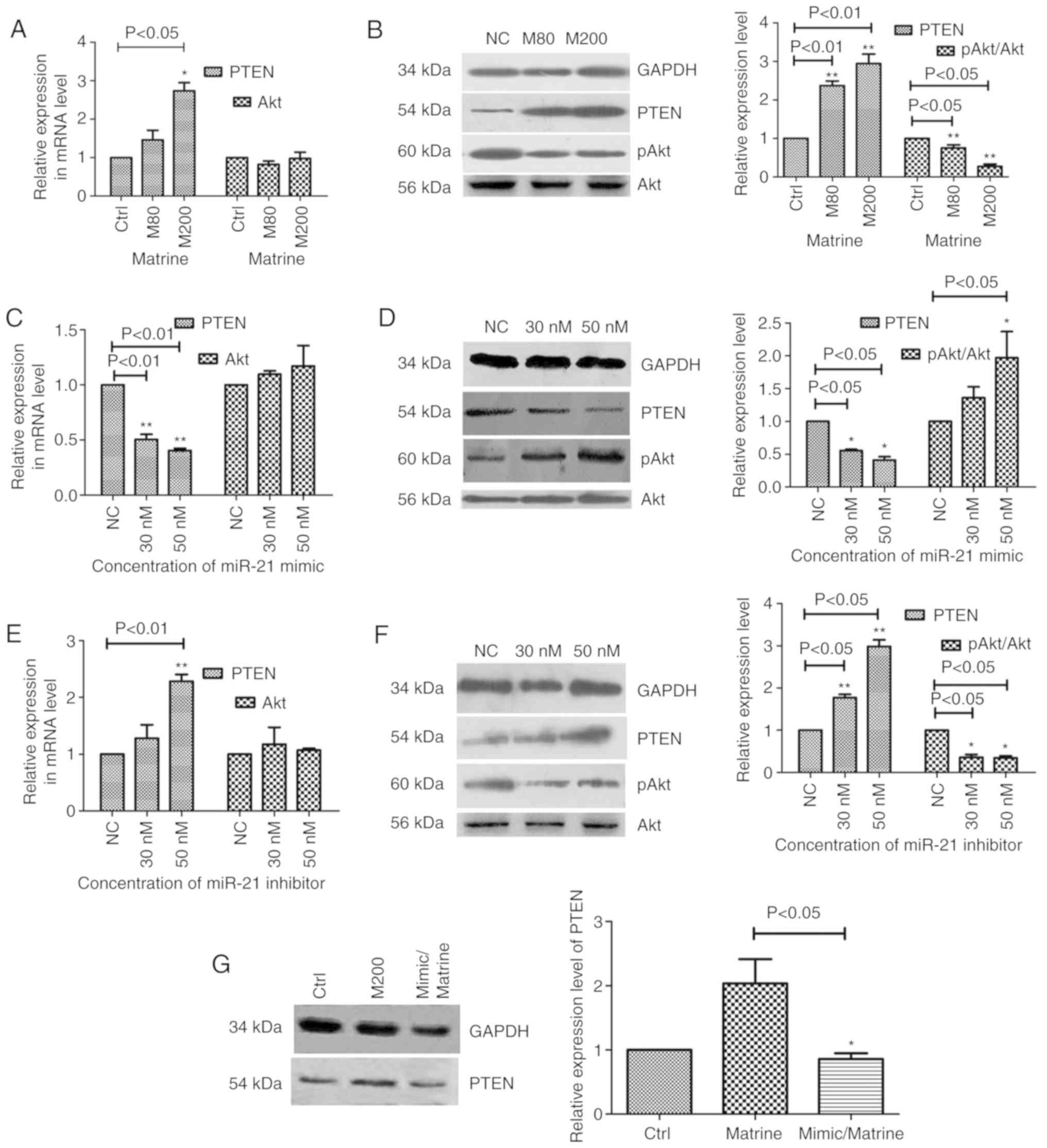
Effects of matrine, miR-21 mimics and
miR-21 inhibitor on the expression of PTEN and Akt in FTC-133
cells
Following treatment of the FTC-133 cells with
matrine at 0, 80 and 200 µM for 48 h, the mRNA and protein
expression levels of PTEN and Akt (or p-Akt) were examined by
RT-qPCR analysis and western blotting (Fig. 4A and 4B), respectively. As shown in Fig. 4A, the mRNA expression levels of PTEN
were upregulated 1.46±0.35-fold for M80/NC and 2.74±0.30-fold for
M200/NC. There was no significant change in the mRNA level of Akt
(P>0.05). The western blotting results are presented in Fig. 4B; the protein expression levels of
PTEN were markedly upregulated in the matrine treatment groups
(M80/NC: 2.37±0.12-fold; M200/NC: 2.94±0.24-fold). The protein
expression levels of Akt did not change between groups. p-Akt
(Ser-473 phosphorylation) was detected and the p-Akt/Akt ratio
levels were downregulated compared with those in the control
(M80/NC: 0.76±0.06-fold; M200/NC: 0.27±0.06-fold; Fig. 4B). Matrine upregulated the expression
of PTEN at the transcriptional and translation levels, suggesting
that PTEN was an important member of the cellular signaling pathway
in which matrine affects FTC-133 cells. The results showed that the
PTEN/Akt signaling pathway may serve an important role in matrine
inhibiting cell growth and inducing cell apoptosis.
To further determine the association between miR-21
and the PTEN/Akt signaling pathway in FTC-133 cells, the mRNA and
protein expression levels of PTEN and Akt (or p-Akt) following
transfection with either miR-21 mimics or miR-21 inhibitor were
examined by RT-qPCR analysis and western blotting (Fig. 4C-F). The mRNA levels of PTEN were
downregulated by the miR-21 mimics (30 nM/NC: 0.50±0.06-fold; 50
nM/NC: 0.40±0.03-fold; Fig. 4C) and
upregulated by the miR-21 inhibitor (50 nM/NC: 2.28±0.17-fold;
Fig. 4E). The transfection had no
effect on the mRNA levels of Akt (P>0.05; Fig. 4C and E).
At the protein expression level (Fig. 4D and F), miR-21 mimics suppressed the
expression of PTEN (30 nM/NC: 0.55±0.02-fold; 50 nM/NC:
0.41±0.05-fold), increased the p-Akt/Akt ratio (50 nM/NC:
1.97±0.39-folds) but had no effect on the expression levels of Akt.
The miR-21 inhibitor increased the expression levels of PTEN (30
nM/NC: 1.87±0.05-fold; 50 nM/NC: 3.08±0.13-fold) and suppressed the
p-Akt/Akt ratio (30 nM/NC: 0.38±0.02-fold; 50 nM/NC:
0.33±0.13-fold). However, the miR-21 inhibitor did not affect the
levels of Akt. Combining the results of the transcriptional and
translational levels of PTEN and Akt in FTC-133 cells, it was
identified that miR-21 directly affected the expression of PTEN,
and PTEN suppressed the phosphorylation of Akt at the Ser-473 site.
Additionally, matrine significantly reduced the expression of
miR-21 and increased the expression of PTEN in FTC-133 cells. These
results suggested that matrine inhibited the growth of FTC-133
cells and induced cell apoptosis through the miR-21/PTEN/Akt
signaling pathway. To further validate this result, the expression
of PTEN was detected in FTC-133 cells treated with miR-21
mimics/matrine. The western blotting results showed that there was
no significant change in the expression of PTEN in the miR-21
mimics/matrine group compared with that in the control group
(Fig. 4G). Simultaneously, compared
with the matrine treatment group, the expression of PTEN in the
miR-21 mimics/matrine group was reduced. This suggested that PTEN,
which was reduced by miR-21 mimics, was replenished by matrine. It
was demonstrated that matrine exerted inhibitory effects on the
cells through the miR-21 and PTEN pathway.
Discussion
The morbidity and mortality rates of thyroid cancer
are increasing year by year worldwide. Surgical resection is the
main treatment for thyroid cancer. The prevention, diagnosis and
treatment of thyroid cancer, as with other cancerous diseases, have
become an increasingly concerning problem. At present, research on
the antitumor effects of the active ingredients of traditional
Chinese medicine has attracted increasing attention. Additionally,
the antitumor effect of traditional Chinese medicine has been
confirmed in clinical practice.
Matrine is a major alkaloid extracted from the
leguminous plant Sophora flavescens, it has shown antitumor
activity against a variety of cancer cells, including gastric,
breast, lung cancer, pancreatic and colon cancer. However, to the
best of our knowledge, the effect of matrine on FTC has not been
reported previously. In the present study, it was found that
matrine exhibited an anticancer effect and pro-apoptotic activity
on FTC-133 cells. The results of MTT assays showed that matrine
inhibited the growth of FTC-133 cells in a dose- and time-dependent
manner; this result is consistent with those of previous studies
(6–13). Furthermore, previous studies showed
that the sensitivity of different cancer cells to matrine is
varied. In the present study, FTC-133 cells had a moderate
IC50 of 154.8 µM. Several previous studies have shown
that the pro-apoptotic activity of matrine contributes to cancer
inhibition (6,7,11,12). The
pro-apoptotic activity of matrine on FTC-133 cells was also
determined in the present study by flow cytometry; the result
showed that the pro-apoptotic effect also occurred in a matrine
concentration-dependent manner. Therefore, the inhibitory effect of
matrine on FTC-133 cells may be achieved mainly by promoting
apoptosis.
miR-21 has been reported to be related to the
formation of cancer (19). In
several types of cancer, the abnormal expression level of miR-21
increases markedly. Furthermore, the overexpression of miR-21 can
initiate the apoptosis of breast cancer cells (16). At present, miR-21 has become one of
the important markers of several types of cancer (20). PTEN is a target gene of miR-21
(17). As a classical anti-oncogene,
similar to p53, PTEN has been investigated for many years. The
inhibition of PTEN is key in the process of cell apoptosis, mainly
relying on the phosphorylation and dephosphorylation of Akt. As an
important protein kinase in several signaling pathways, activated
Akt has anti-apoptotic activity and promotes proliferation,
invasion and angiogenesis. PI3K can activate Akt through a series
of cascade reactions. PI3K can also be dephosphorylated by PTEN,
thereby, avoiding the activation of Akt, which likely activates Bad
to trigger intrinsic apoptotic cascades (21–23). The
mechanism underlying the effect of matrine on other cancer cells
predominantly involves the miR-21/PTEN/Akt signaling pathway
(11,12). Therefore, it was hypothesized that
the miR-21/PTEN/Akt signaling pathway may be involved in the
regulatory mechanism of matrine inhibiting FTC cells. In the
present study, when FTC-133 cells were treated with matrine, the
expression of miR-21 was significantly downregulated and the
expression of PTEN was upregulated. The expression of p-Akt was
also downregulated. The regulatory effect of miR-21 on PTEN/Akt in
FTC-133 cells was investigated, and the FTC-133 cells were
transfected with miR-21 mimics and inhibitor. The results showed
that the miR-21 mimics downregulated the expression of PTEN and
upregulated the level of p-Akt, whereas the miR-21 inhibitor,
similar to matrine, upregulated PTEN and downregulated p-Akt. The
phosphorylation level of Akt at Ser-473 increased but the level of
Akt did not alter significantly, suggesting that PTEN regulated the
activity of Akt in the present study. The results showed that the
miR-21/PTEN/Akt signaling pathway may be important in the effect of
matrine inhibiting cell growth and inducing cell apoptosis. To
further validate the results, the expression of PTEN was detected
in FTC-133 cells treated with miR-21 mimics/matrine and it was
determined that PTEN, which was reduced by miR-21 mimics, was
increased by matrine. These results demonstrated that matrine
inhibited the growth of FTC-133 cells and induced cell apoptosis
through the miR-21/PTEN/Akt signaling pathway. The results of the
present study were consistent with the previously reported effect
of matrine in PTC and breast cancer cells (11,12).
In the process of the transcription of miR-21, STAT3
regulates the upstream enhancer encoding miR-21 (24). In addition, previous studies have
demonstrated that the expression of p-STAT3 was significantly
downregulated by matrine in human cholangiocarcinoma cells and
H1975 cells (25,26). According to these results, it was
hypothesized that matrine may inhibit the expression of miR-21 at
the transcriptional level by decreasing the phosphorylation of
STAT3.
In conclusion, the present study showed that matrine
inhibited the growth of FTC-133 cells and induced cell apoptosis
via the miR-21/PTEN/Akt signaling pathway. Matrine may be a
potential drug for the treatment of FTC and miR-21 may be a
potential target. The present study provided a theoretical and
experimental basis for drug development and the treatment of
FTC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The current study was supported by the Special
Foundation for New Pharmaceutical Health Development of Jilin
Province of China (grant no. YYZX201725), Development and Reform
Commission Project of Jilin Province of China (grant nos.
3J115AK93429 and 2014G072), Jilin Agricultural University Doctorate
Fund of China (grant no. 2015016), Science and Technology Project
of Jilin Province of China (grant nos. 20180101260JC and
20160312008ZG) and the National Undergraduate Innovation and
Entrepreneurship Project of China (grant no. 201810193033).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TL and QL designed the experiment. QL performed the
MTT assay, flow cytometry, RT-qPCR analysis and western blotting
and composed the manuscript. SZ and MW performed the MTT assay,
cell transfection and RT-qPCR analysis. YW and SD collected data
and performed statistical analysis. TL and YF critically reviewed
and revised the manuscript. YF also conceived and designed the
experiment and performed the flow cytometery. XW, SL and GC were
responsible for flow cytometry and image editing. All authors
agreed to the final version.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Carling T and Udelsman R: Thyroid cancer.
Annu Rev Med. 65:125–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li X, Abdel-Mageed AB, Mondal D and Kandil
E: MicroRNA expression profiles in differentiated thyroid cancer, a
review. Int J Clin Exp Med. 6:74–80. 2013.PubMed/NCBI
|
|
3
|
Jang EK, Song DE, Sim SY, Kwon H, Choi YM,
Jeon MJ, Han JM, Kim WG, Kim TY, Shong YK and Kim WB: NRAS codon 61
mutation is associated with distant metastasis in patients with
follicular thyroid carcinoma. Thyroid. 24:1275–1281. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schlumberger MJ: Papillary and follicular
thyroid carcinoma. N Engl J Med. 338:297–306. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American Thyroid Association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American Thyroid Association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin H, Sun Y, Wang SY and Cheng XD:
Matrine activates PTEN to induce growth inhibition and apoptosis in
V600EBRAF harboring melanoma cells. Int J Mol Sci. 14:16040–16057.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei YP, Wang XH, Liu G, Zhang JF, Yang YX,
Zhang J, Song XL, Li ZD and Zhao LD: Matrine exerts inhibitory
effects in melanoma through the regulation of miR-19b-3p/PTEN. Int
J Oncol. 53:791–800. 2018.PubMed/NCBI
|
|
8
|
Niu HY, Zhang YF, Wu BG, Zhang Y, Jiang HF
and He P: Matrine induces the apoptosis of lung cancer cells
through downregulation of inhibitor of apoptosis proteins and the
Akt signaling pathway. Oncol Rep. 32:1087–1093. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
An Q, Han C, Zhou Y, Li F, Li D, Zhang X,
Yu Z, Duan Z and Kan Q: Matrine induces cell cycle arrest and
apoptosis with recovery of the expression of miR-126 in the A549
non-small cell lung cancer cell line. Mol Med Rep. 14:4042–4048.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang H, Du T, Xu G, Lai Y, Fan XX, Chen
X, Li WJ, Yue F, Li Q, Liu L and Li K: Matrine suppresses invasion
of castration-resistant prostate cancer cells by downregulating
MMP-2/9 via NF-κB signaling pathway. Int J Radiat Oncol.
50:640–648. 2017.
|
|
11
|
Zhao L, Zhang X and Cui S: Matrine
inhibits TPC-1 human thyroid cancer cells via the miR-21/PTEN/Akt
pathway. Oncol Lett. 16:2965–2970. 2018.PubMed/NCBI
|
|
12
|
Li LQ, Li XL, Wang L, Du WJ, Guo R, Liang
HH, Liu X, Liang DS, Lu YJ, Shan HL and Jiang HC: Matrine inhibits
breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells.
Cell Physiol Biochem. 30:631–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tetik Vardarlı A, Düzgün Z, Erdem C,
Kaymaz BT, Eroglu Z and Çetintas VB: Matrine induced G0/G1 arrest
and apoptosis in human acute T-cell lymphoblastic leukemia (T-ALL)
cells. Bosn J Basic Med Sci. 18:141–149. 2018.PubMed/NCBI
|
|
14
|
Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K
and Yang GH: MicroRNA-21 (miR-21) represses tumor suppressor PTEN
and promotes growth and invasion in non-small cell lung cancer
(NSCLC). Clin Chim Acta. 411:846–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moriyama T, Ohuchida K, Mizumoto K, Yu J,
Sato N, Nabae T, Takahata S, Toma H, Nagai E and Tanaka M:
MicroRNA-21 modulates biological functions of pancreatic cancer
cells including their proliferation, invasion, and chemoresistance.
Mol Cancer Ther. 8:1067–1074. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fragni M, Bonini SA, Bettinsoli P, Bodei
S, Generali D, Bottini A, Spano PF, Memo M and Sigala S: The
miR-21/PTEN/Akt signaling pathway is involved in the anti-tumoral
effects of zoledronic acid in human breast cancer cell lines.
Naunyn Schmiedebergs Arch Pharmacol. 389:529–538. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Wang J, Guo C and Fan X:
microRNA-21 mediates epithelial-mesenchymal transition of human
hepatocytes via PTEN/Akt pathway. Biomed Pharmacother. 69:24–28.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pfeffer SR, Yang CH and Pfeffer LM: The
Role of miR-21 in cancer. Drug Dev Res. 76:270–277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Y, Peng W, Tang T, Xia L, Wang XD,
Duan BF and Shu Y: MicroRNAs as promising biomarkers for
tumor-staging: Evaluation of MiR21 MiR155 MiR29a and MiR92a in
predicting tumor stage of rectal cancer. Asian Pac J Cancer Prev.
15:5175–5180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bleau AM, Hambardzumyan D, Ozawa T,
Fomchenko EI, Huse JT, Brennan CW and Holland EC: PTEN/PI3K/Akt
pathway regulates the side population phenotype and ABCG2 activity
in glioma tumor stem-like cells. Cell Stem Cell. 4:226–235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Lea JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Löffler D, Brocke-Heidrich K, Pfeifer G,
Stocsits C, Hackermüller J, Kretzschmar AK, Burger R, Gramatzki M,
Blumert C, Bauer K, et al: Interleukin-6 dependent survival of
multiple myeloma cells involves the Stat3-mediated induction of
microRNA-21 through a highly conserved enhancer. Blood.
110:1330–1333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang N, Han F, Cui H, Huang J, Wang T,
Zhou Y and Zhou J: Matrine suppresses proliferation and induces
apoptosis in human cholangiocarcinoma cells through suppression of
JAK2/STAT3 signaling. Pharmacol Rep. 67:388–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen SF, Zhang ZY and Zhang JL: Matrine
increases the inhibitory effects of afatinib on H1975 cells via the
IL-6/JAK1/STAT3 signaling pathway. Mol Med Rep. 16:2733–2739. 2017.
View Article : Google Scholar : PubMed/NCBI
|