Introduction
The first-line therapy for carcinoma of the oral
cavity floor and/or oral surface of the tongue (OCC) is surgery,
which is typically combined with adjuvant radiation therapy when
the disease is highly advanced. The basic prognostic factors that
aid in the selection of patients for postoperative treatment are
the status of the surgical margins and disease advancement.
Additional factors that require the initiation of adjuvant therapy
are the unfavorable prognostic factors observed upon
histopathological examination such as close surgical margins,
perineural invasion, lymphovascular invasion and extensive lymph
node invasion (1). On the other
hand, the histological grade of the tumor (G) is controversial in
regard to its prognostic significance in OCC (2,3).
Unfortunately, a large proportion of patients
develop locoregional recurrence (4,5), which
suggests that despite the early stage detection of the disease, a
more aggressive treatment is required in some cases. Therefore, it
is necessary to identify novel prognostic factors that may identify
groups of patients with a high risk of recurrence. The Hippo
pathway effectors YAP1/Yes-associated protein 1 (YAP) and
transcriptional co-activator with PDZ-binding motif, WWTR1 (TAZ),
potentially meet the criteria for oncogenes, and appear to be
promising markers.
YAP and TAZ are close paralogues that are mainly
involved in the transduction of signals in the Hippo signaling
pathway (6). These proteins are
transcriptional modules of the Hippo signaling pathway. They
function as transcriptional coactivators that shift from the
cytoplasm to the cell nucleus where, mainly via interactions with
TEAD transcriptional factors, they induce the expression of genes
responsible for proliferation, cell growth, epithelial-mesenchymal
transition (EMT) and apoptosis inhibition (7).
From the classical point of view, YAP and TAZ meet
the criteria for oncogenes. Amplification in the region of the
11q21-22 chromosome that encodes YAP is observed in many types of
cancers, and in squamous cell carcinoma (SCC) of the oral cavity in
5–15% of cases (8). In addition, it
was previously reported that YAP and TAZ are the nuclear
transducers of mechanical signals produced by the stiffness of the
extracellular matrix (ECM) and cell shape (9). This regulation requires Rho GTPase
activity and actomyosin cytoskeleton tension, and is independent
from the Hippo signaling pathway. YAP and TAZ activity is
positively correlated with ECM stiffness (9,10).
Experimental studies have shown that in dense cell populations,
where the contact between cells is maintained, YAP and TAZ are not
active and they are located in the cytoplasm, whereas in sparse
cell populations YAP and TAZ are located in the cell nucleus and
trigger proliferation (9,11). However, mechanical stretching of the
cells may result in prolonged activation of RhoA and myosin, which
in turn induces YAP/TAZ migration to the cell nucleus, stimulating
proliferation even in contact-inhibited epithelial cells. YAP/TAZ
may have a key role in the contact-induced inhibition of cell
proliferation, as its dysregulation is one of the main markers of
cancerous cell transformation (12).
Nevertheless, it was observed that YAP, depending on the cell
context, functions not only as a protooncogene but also as a
carcinogenesis suppressor (13).
A few reports have been published recently
highlighting the role of tyrosine-protein phosphatase non-receptor
type 14 (PTPN14) as a suppressor of YAP, the key oncoprotein of the
Hippo signaling pathway that controls organ development through the
regulation of proliferation and cell apoptosis (14,15).
PTPN14 protein belongs to the family of protein tyrosine
phosphatases, which is a large family of enzymes that are involved
in the phosphorylation of tyrosine proteins and act contrarily to
protein tyrosine kinases, being the key regulator of many
physiological processes within a cell, such as metabolism, growth
and cell differentiation (16).
PTPN14 is a 130 kDa non-receptor protein encoded by a gene located
on 1q32.2 chromosome (17). In
vitro studies have demonstrated that the dysregulated
expression of this protein results in changes in the adhesion,
growth and structure of the cell cytoskeleton (18). Depending on its localization within
the cell, it regulates the maintenance of cell junctions and
adhesion, as well as proliferation (19). Changes in PTNP14 levels during
embryogenesis have also been revealed to influence transforming
growth factor (TGF)-β induction and EMT, inducing morphological and
functional changes, and the acquisition of migratory features by
the cells (20).
The minichromosome maintenance 7, DNA replication
licensing factor 7 (MCM7) protein is a marker of cell proliferation
considered necessary for the initiation and continuation of DNA
replication process in eukaryotic cells (21,22). It
has also been revealed that YAP/TAZ contributes to the
proliferation of non-small cell lung cancer, and breast and head
and neck cancers via the upregulation of MCM7 (23).
In our previous study conducted on a similar though
slightly larger group of patients (24), it was demonstrated that there was a
significant effect of MCM7 and PTPN14 proteins on the prognosis of
patients with OCC. To the best of our knowledge, this was the first
study that evaluated the expression of PTPN14 in human tumors. It
was demonstrated that the high expression of PTPN14 was an
unfavorable prognostic factor and its expression in >75% of
cancer cells allowed for the identification of the group patients
with a high risk of relapse (24).
These results inspired new experiments and the design of the
present study in order to evaluate the potential relationship of
the previously tested PTPN14 with YAP and TAZ.
The aim of the present study was to analyze the
correlation between the expression of YAP and TAZ, and PTPN14 and
to determine whether the increased expression of the YAP and TAZ
proteins had an effect on the level of tumor cell proliferation as
assessed by MCM7 levels. Their prognostic value in the group of
patients recruited was also determined.
Materials and methods
Patients
Ethical approval from the Bioethical Commission at
the Medical University of Wroclaw was obtained (no. KB299/2013);
due to the retrospective nature of the study the need for written
informed consent from patients was waived. In total, 127 patients
(25 women and 102 men) treated by postoperative radiation at the
Lower Silesian Oncology Center between 2000 and 2011 were enrolled
in the present study. Enrollment criteria included the diagnosis of
SCC of the oral cavity floor (C04) or oral surface of the tongue
(C02) and radical resection of the primary tumor (R0) with
simultaneous surgery of the regional lymphatic system.
Disease advancement stage was defined in line with
the 7th edition of TNM classification of 2010 (25). Table I
presents the studied clinicopathological characteristics of the
patients. All patients were subjected to postoperative radiation
therapy and the irradiated area was the resection site with a
margin and regional draining lymph node basin. The radiation dose
was between 50 and 68 Gy (mean: 60 Gy, median: 60 Gy). The median
time that lapsed between surgery to the start of radiation therapy
was 74 days (mean: 77 days). The treatment that had been applied
was in line with currently accepted standards of care (26).
 | Table I.Clinical characteristics of the
patients recruited to the present study. |
Table I.
Clinical characteristics of the
patients recruited to the present study.
|
Characteristics | Number of
patients | Percentage (%) |
|---|
| Sex |
|
|
|
Male | 102 | 80 |
|
Female | 25 | 20 |
| Primary site of the
tumor in the oral cavity |
|
|
| Oral
surface of the tongue | 42 | 33 |
| Oral
cavity floor | 69 | 54 |
| Both
the floor and the tongue are affected-primary location cannot be
established | 16 | 13 |
| Histological grade
of the tumor |
|
|
| G1 | 38 | 30 |
| G2 | 57 | 45 |
| G3 | 32 | 25 |
| Keratosis features
present |
|
|
|
Keratodes | 109 | 86 |
|
Akeratodes | 18 | 14 |
| T value |
|
|
| T1 | 3 | 2 |
| T2 | 57 | 45 |
| T3 | 37 | 29 |
| T4 | 27 | 21 |
| N value |
|
|
| N0 | 73 | 57 |
| N1 | 20 | 16 |
| N2 | 33 | 26 |
| N3 | 1 | 1 |
| TNM stage |
|
|
| II | 39 | 31 |
|
III | 36 | 28 |
| IV | 52 | 41 |
| Scope of
surgery |
|
|
| Partial
resection of the tongue, oral cavity floor and mandible | 85 | 67 |
| Partial
resection of the tongue and oral cavity floor | 17 | 13 |
| Partial
resection of the tongue or oral cavity floor | 25 | 20 |
| Bilateral vs
unilateral lymphadenectomy |
|
|
|
Bilateral | 100 | 79 |
|
Unilateral | 27 | 21 |
| Type of
lymphadenectomy on the tumor side |
|
|
|
Radical, using Crile's
method | 33 | 26 |
|
Functional | 14 | 11 |
|
Selective | 80 | 63 |
| Type of
lymphadenectomy on the opposite side of the tumor |
|
|
|
None | 27 | 21 |
|
Functional | 15 | 12 |
|
Selective | 85 | 67 |
| Radiation therapy
2D vs. 3D |
|
|
| 2D | 79 | 62 |
| 3D | 48 | 38 |
During a 5-year follow-up, 37 patients (29%)
developed recurrence, of whom 8 patients (6%) developed metastases
without locoregional recurrence. The average time between surgery
and the detection of recurrence was 12.8 months (4.6–39.4 months).
The average time between surgery and death due to cancer
progression was 19.3 months (8–60 months).
Immunohistochemical examination
Studies were performed on the tissue materials
acquired during surgical treatment fixed in 10% buffered formalin,
and then embedded in paraffin blocks. Immunohistochemical
determination of YAP1 (clone H9; cat. no. sc-271134; Santa Cruz
Biotechnology, Inc.; dilution: 1:100), TAZ (clone 1H9; cat. no.
LS-C173295; LifeSpan Biosciences Seattle, WA, USA; dilution 1:150),
PTPN14 (clone 448701; cat. no. MAB 4458; R&D Systems, Inc.;
dilution 1:200), MCM7: clone immunoglobulin (Ig)-G1 DCS-141.1 (cat.
no. NCL-MCM7; Novocastra; dilution 1:30), α-smooth muscle actin
(SMA; clone 1A4; cat. no. IR611; Dako; Agilent Technologies, Inc.;
ready-to-use), Podoplanin (clone D2-40; cat. no. IR072; Dako;
Agilent Technologies, Inc.; ready-to-use) and Vimentin (clone V9;
cat. no. IR630; Dako; Agilent Technologies, Inc.; ready-to-use) was
performed on 4 µm-thick paraffin sections mounted on salinized
slides (S3003; cat. no. Dako; Agilent Technologies, Inc.), which
were then subjected to deparaffinization, rehydration and heat
induced epitope retrieval performed using PT Link, with EnVision
FLEX Target Retrieval Solution High pH used for 20 min incubation
at 97°C. An Autostainer Link48 was used to perform immunological
testing using detection reagents EnVision FLEX (cat. no. K8002;
Dako; Agilent Technologies, Inc.).
For each of the antibodies used, positive and
negative controls were employed. The assessment of all of the
specimens was conducted by two of the authors who used a light
microscope (OLYMPUS BX41). For each protein, all visual fields were
analyzed; the entire tissue specimen irrespective of its size was
assessed, taking into account the percentage of positively stained
cells and the intensity of staining.
A semi-quantitative method was used to evaluate
MCM7, PTPN14, YAP and TAZ expression in SCC. The two
immunohistochemical reaction parameters used were the percentage of
cells with a positive cytoplasmic or nuclear reaction (the
percentage of reactive tissue) and the intensity of reaction. The
semi-quantitative ImmunoReactive Score (IRS) scale of Remmele and
Stenger (27) was used to calculate
the final immunohistochemical reaction results. In this approach,
points were given depending on the percentage of reactive cells
(0–4 points: 0, 0%; 1, 1–25%; 2, 26–50%; 3, 51–75%; and 4, >75%)
and the intensity of reaction (0–3 points). The scores for these
two parameters were multiplied to give the final result, which was
referred to as the IRS factor or score (0–12 points).
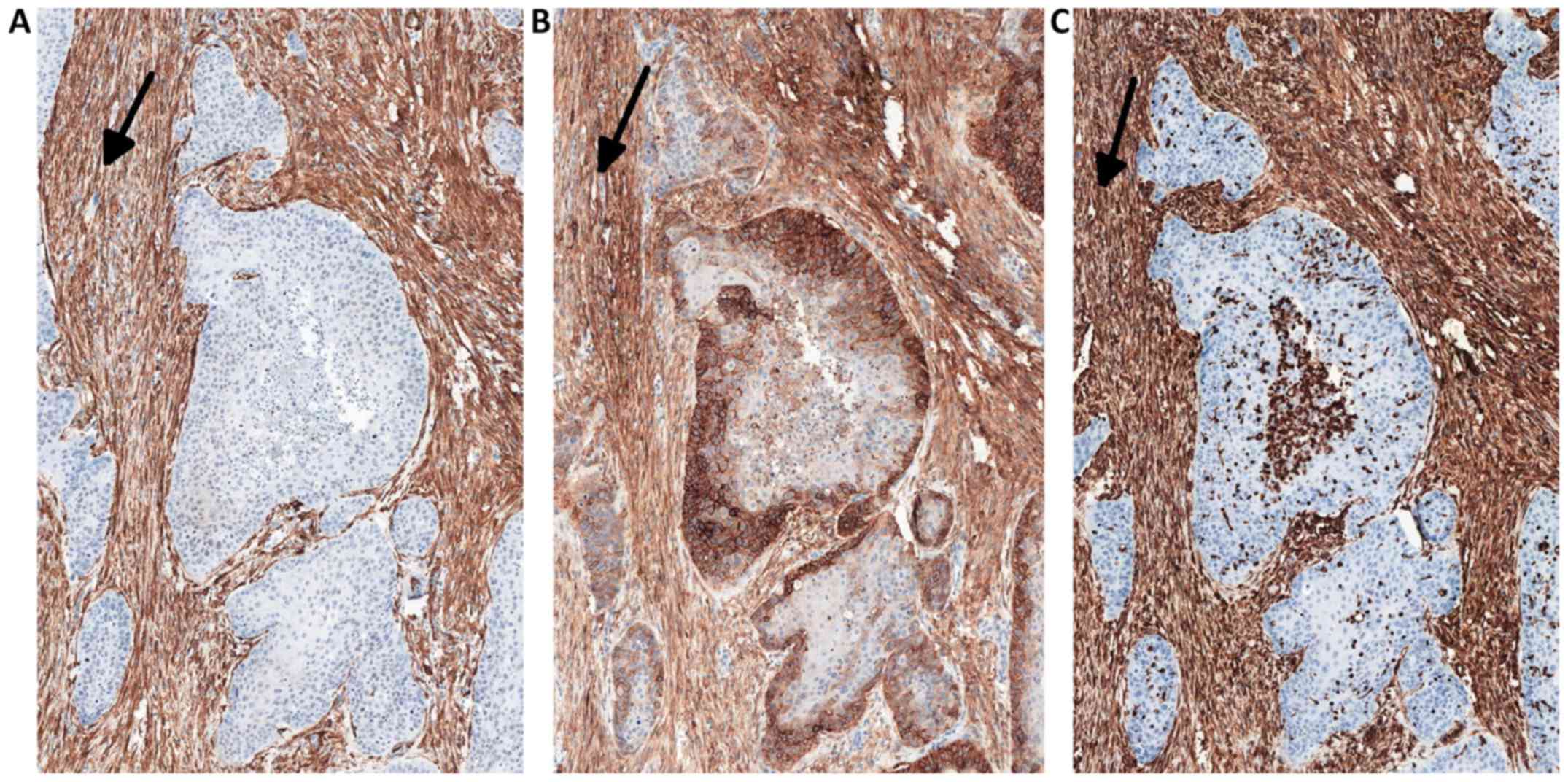
The expression of YAP protein in the stromal
compartment (cancer-associated fibroblasts; CAFs) of the tumor was
calculated semi-quantitatively as negative (0: no staining), weak
(1: either diffuse weak staining or strong staining in <10% of
the analyzed cells), intermediate (2: either diffuse intermediate
staining or strong staining in ≥10–30% of the analyzed cells) and
strong (3: defined as strong staining in >30% of the analyzed
cells). CAFs were confirmed by the positive immunohistochemical
reactions for α-SMA, vimentin and podoplanin (D2-40), which are all
characteristic markers of CAFs (Fig.
1).
Statistical analysis
All statistical analyses were performed using the
Statistica 10 (StatSoft, Inc.) package, employing the Kaplan-Meier
method, Cox's proportional hazard model and Spearman's rank
correlation coefficient. Evaluation of the prognostic value of the
variables in the present study was performed using overall survival
(OS), defined as the survival duration from the day of surgery to
death, disease specific survival (DSS), defined as the survival
duration from the day of surgery to death due to cancer
progression, disease free survival (DFS), defined as the survival
from the day of surgery to the recurrence of cancer (metastases
and/or local locoregional relapse), and locoregional
recurrence-free survival (LRFS) defined as the survival from the
day of surgery until the onset of locoregional relapse.
Results
Expression patterns of the analyzed
proteins
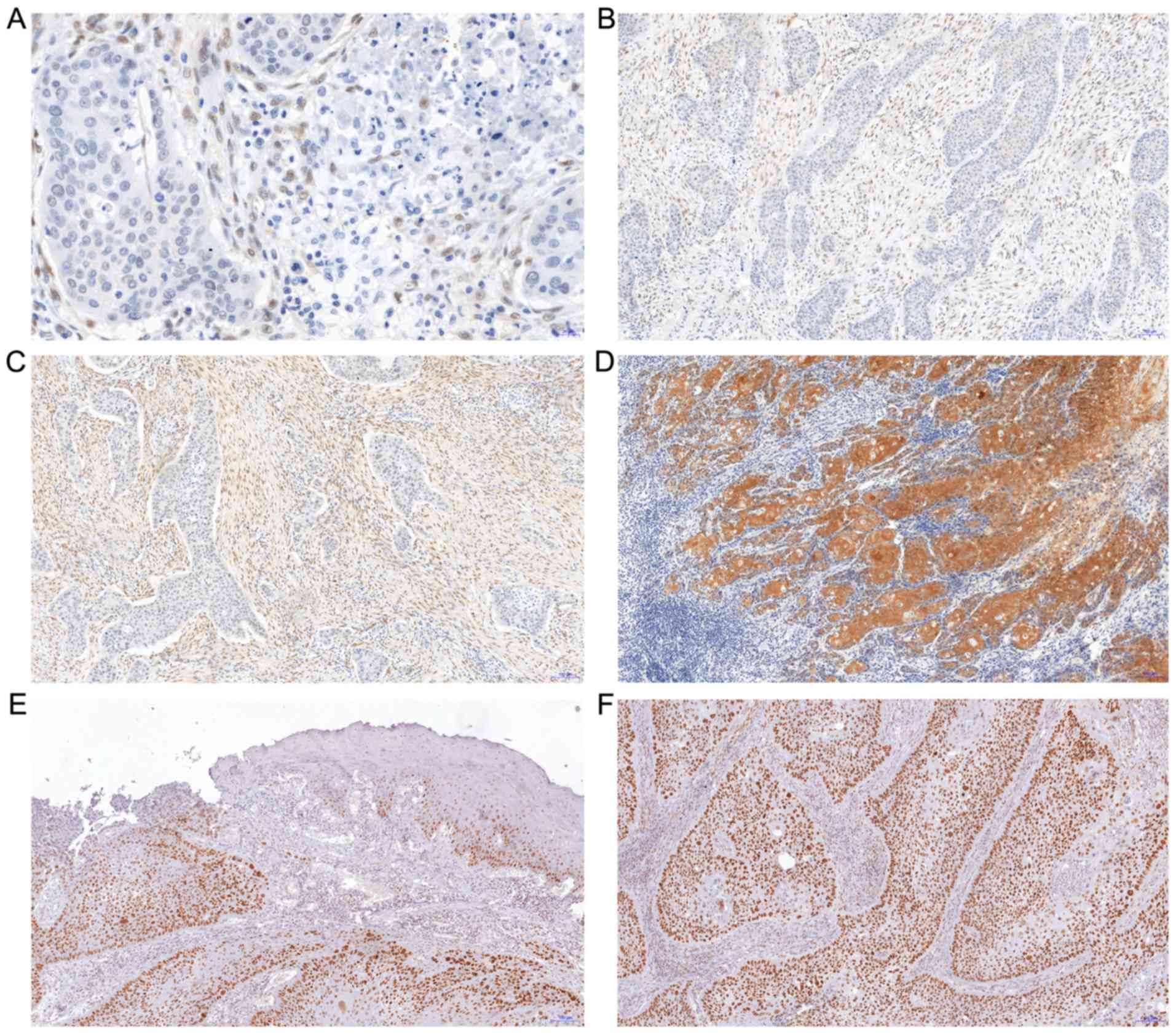
The expression of YAP was observed in two cell
populations: Cancer cells (only cytoplasmic localization) and in
the stromal compartment of the tumor (CAFs with nuclear-cytoplasmic
distribution). The median IRS for YAP expression in cancer cells
was 6 (range: 0–12, mean: 5.25±3.7; Fig.
2). In 23 patients, no YAP expression was observed in tumor
cells. YAP expression in CAFs was identified in 82 patients; in 30
patients it was scored 1, in 28 patients scored 2 and in 24
patients scored 3 points on a 4-point scale (0–3; Fig. 2).
TAZ protein exhibited exclusively nuclear expression
in cancer cells, without any immunoreactivity in the stroma
(Fig. 2). The median IRS for TAZ
expression was 6 (range: 0–12; mean: 5±3.9). In 33 patients no
expression of this marker was observed in tumor cells. MCM7
expression tended to be exclusively nuclear in nature; it was
observed in all 127 patients. The IRS median for MCM7 expression
was 4 (range: 1–12; mean: 5±3.4).
PTPN14 expression exhibited cytoplasmic
localization. In the majority of cases it was weakly expressed. The
median IRS for PTPN14 expression was 1 (range: 0–8, mean: 2±1.99).
In 31 patients, no PTPN14 expression was observed in tumor
cells.
Correlations between analyzed proteins
and clinicopathological parameters
In the studied group of patients, a weak positive
correlation between the percentage of cells showing MCM7 expression
and the presence of YAP protein in the tumor stroma were observed
(rs=0.207; P=0.019). However, no significant correlation
with TAZ or YAP expression was identified in tumor cells. In
addition, a weak correlation between MCM7 and the percentage of
cells containing PTPN14 protein was identified
(rs=0.202; P=0.022). A weak negative correlation between
YAP and TAZ expression in tumor cells was also noted
(rs=−0.220, P=0.012).
A weak positive correlation was revealed between the
histological grade of the tumor G and stromal expression of YAP
(rs=0.275; P=0.001) and the percentage of cells
highlighted the expression of MCM7 as a proliferation marker
(rs=0.175; P=0.048).
Furthermore, a weak negative correlation between the
size of the primary tumor (pT) and the percentage of cells
exhibiting YAP expression (rs=−0.196; P=0.028), and a
weak positive correlation with the intensity of the color reaction
PTPN14 (rs=0.184; P=0.039), were observed. The present
study also demonstrated a weak negative correlation between pTNM
and the percentage of cells exhibiting YAP expression
(rs=−0.226; P=0.010).
The results did not present a correlation between
the level of PTPN14 expression, and YAP and TAZ proteins.
Impact of clinical features on
patients' survival
Among the clinical and pathological factors under
assessment only the disease advancement stage significantly
affected the prognosis of patients following univariate analysis.
Statistically significant differences in the 5-year survival rates
of patients were revealed dependent on pT, pN and pTNM. The best
prognosis was noted for patients with TNM stage II for whom the
5-year LRFS, DFS, DSS and OS were 92, 89, 89 and 66%, respectively,
whilst in patients with TNM stage III, the survival decreased to
78, 73, 73 and 54%, respectively, and to 60, 50, 52 and 42%,
respectively for stage IV.
Impact of the analyzed protein
expression on patients' survival
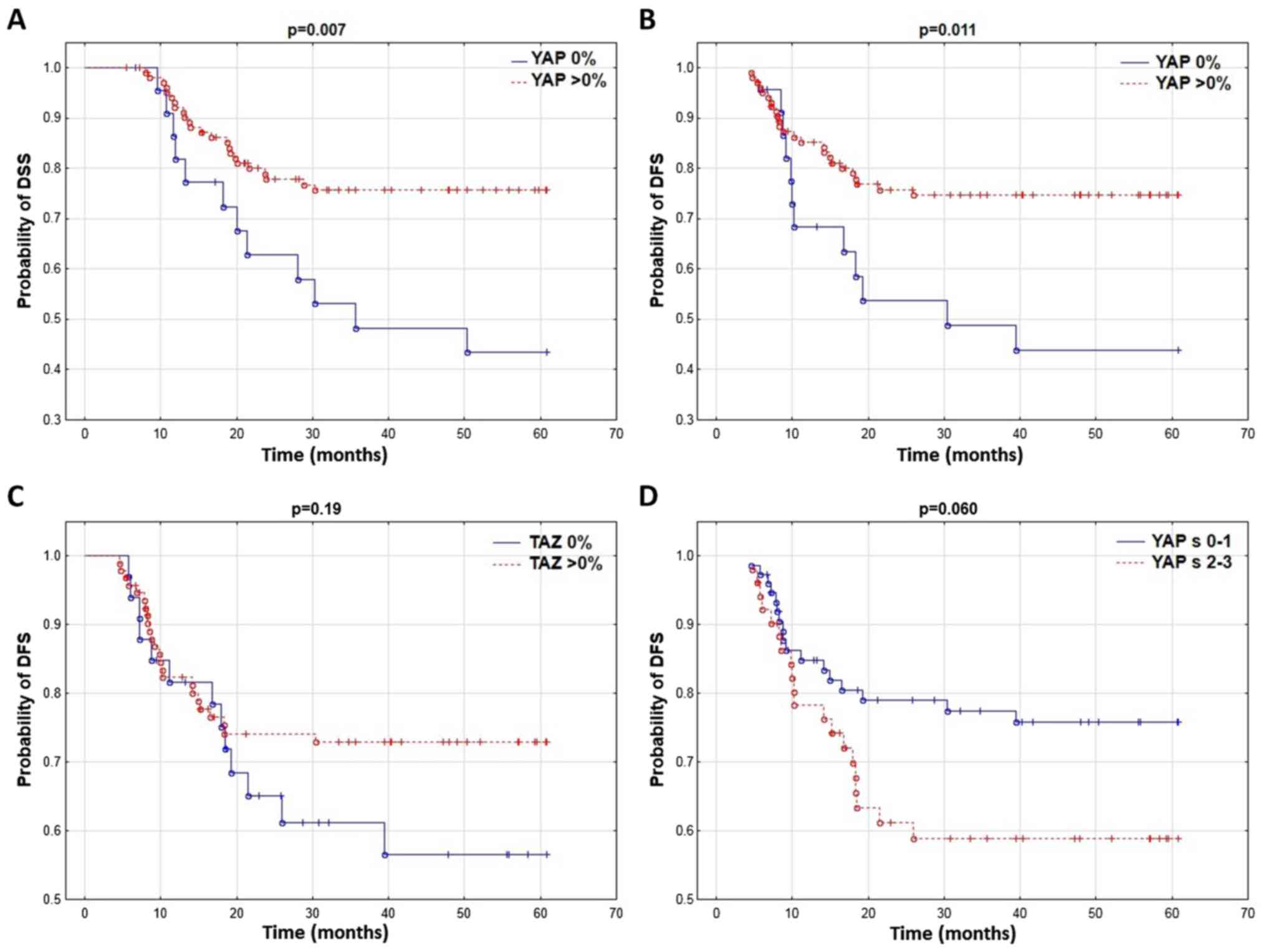
It was revealed that patients without YAP expression
in the tumor cell cytoplasm had a significantly shorter DFS and DSS
(Table II; Fig. 3A and B). No correlation between YAP
protein and LRFS and OS was identified. YAP expression in the tumor
stroma markedly influenced DFS and OS (Table II; Fig.
3D) while the TAZ expression level did not affect patient
survival (Table II; Fig. 3C).
 | Table II.Percentage of 5-year LRFS, DFS, DSS
and OS in the patients depending on the expression of MCM7, PTPN14,
YAP, stromal YAP and TAZ in squamous carcinoma cells. |
Table II.
Percentage of 5-year LRFS, DFS, DSS
and OS in the patients depending on the expression of MCM7, PTPN14,
YAP, stromal YAP and TAZ in squamous carcinoma cells.
|
| 5-year LRFS
(%) | 5-year DFS (%) | 5-year DSS (%) | 5-year OS (%) |
|---|
| MCM7% |
|
|
|
|
|
≤25% | 88 | 85 | 85 | 64 |
|
>25% | 71 | 64 | 65 | 49 |
|
P-value | 0.029 | 0.014 | 0.018 | 0.072 |
| MCM7 INT |
|
|
|
|
| 1 | 86 | 81 | 83 | 61 |
| 2 | 77 | 69 | 69 | 57 |
| 3 | 59 | 54 | 54 | 37 |
| P
value | 0.005 | 0.005 | 0.006 | 0.041 |
| MCM7 IRS |
|
|
|
|
|
<4 | 86 | 81 | 83 | 62 |
| ≥4 | 69 | 62 | 62 | 47 |
|
P-value | 0.018 | 0.009 | 0.005 | 0.054 |
| YAP% |
|
|
|
|
|
>0% | 79 | 75 | 76 | 39 |
| 0% | 58 | 44 | 43 | 56 |
|
P-value | 0.12 | 0.011 | 0.007 | 0.11 |
| YAPs |
|
|
|
|
|
0–1 | 79 | 76 | 76 | 61 |
|
2–3 | 69 | 59 | 61 | 41 |
|
P-value | 0.17 | 0.060 | 0.11 | 0.064 |
| TAZ% |
|
|
|
|
|
>0% | 79 | 73 | 73 | 58 |
| 0% | 62 | 57 | 59 | 39 |
|
P-value | 0.13 | 0.19 | 0.30 | 0.12 |
| PTPN14 % |
|
|
|
|
|
≤75% | 78 | 71 | 71 | 54 |
|
>75% | 37 | 37 | 50 | 37 |
|
P-value | 0.007 | 0.033 | 0.13 | 0.24 |
Furthermore, similar to our previous report
(24), patients with high expression
levels of MCM7 in tumor cells had a significantly shorter LRFS,
DFS, DSS and OS. In a group of patients with >75% tumor cells
exhibiting PTPN14 expression, the 5-year LRFS and DFS were
significantly worse.
Multivariate analysis
The present multivariate analysis included the
parameters of YAP and TAZ expression that had a statistically
significant effect on survival or produced non-significant but
marked prognostic trends in univariate analysis. In the
multivariate analysis, patients' age had a significantly
unfavorable influence on OS, and its effect on DFS and DSS was
marked but not significantly different. High YAP expression in
stromal cells at the level of 2 or 3 had an unfavorable effect on
DFS and OS. No YAP expression in the cytoplasm of cancer cells was
an independent unfavorable prognostic factor for DFS and DSS
(Table III), and was markedly
different in the case of LRFS and OS. Multivariate analysis
confirmed the findings of our previous paper (24): A high percentage (>75%) of cells
exhibiting cytoplasmic PTPN14 expression was an unfavorable
prognostic factor for LRFS, DFS and DSS, and several times
increased the risk of disease progression or death. In addition,
pT, pN, and color reaction intensity with MCM7 protein were
revealed to be independent prognostic factors for LRFS, DFS, DSS
and OS (Table III).
 | Table III.Final multivariate analysis for LRFS,
DFS, OS and DSS. |
Table III.
Final multivariate analysis for LRFS,
DFS, OS and DSS.
| Prognostic
factors | P-value | Hazard ratio
(HR) | 95% PU HR
lower | 95% PU HR
upper |
|---|
| LRFS |
|
|
|
|
| YAP
% | 0.068 | 0.432 | 0.176 | 1.063 |
| pT | 0.001 | 2.703 | 1.650 | 4.428 |
| pN | 0.012 | 1.717 | 1.127 | 2.615 |
| MCM7
INT | 0.001 | 3.091 | 1.729 | 5.527 |
| PTPN14
% | 0.001 | 6.077 | 2.069 | 17.854 |
| DFS |
|
|
|
|
| YAP
% | 0.009 | 0.360 | 0.166 | 0.779 |
|
YAPs | 0.042 | 2.094 | 1.027 | 4.270 |
| pT | 0.001 | 2.316 | 1.477 | 3.633 |
| pN | 0.008 | 1.674 | 1.141 | 2.455 |
| MCM7
INT | 0.001 | 2.700 | 1.608 | 4.535 |
| PTPN14
% | 0.006 | 4.262 | 1.507 | 12.054 |
|
Age | 0.061 | 1.041 | 0.998 | 1.087 |
| DSS |
|
|
|
|
| YAP
% | 0.004 | 0.327 | 0.153 | 0.703 |
| pT | 0.001 | 2.355 | 1.522 | 3.645 |
| pN | 0.001 | 1.932 | 1.314 | 2.840 |
| MCM 7
INT | 0.001 | 2.436 | 1.478 | 4.015 |
| PTPN14
% | 0.016 | 3.891 | 1.283 | 11.805 |
|
Age | 0.061 | 1.044 | 0.998 | 1.093 |
| OS |
|
|
|
|
| YAP
% | 0.084 | 0.569 | 0.301 | 1.078 |
| pT | 0.001 | 1.819 | 1.295 | 2.555 |
| pN | 0.020 | 1.426 | 1.058 | 1.920 |
| MCM 7
INT | 0.008 | 1.619 | 1.136 | 2.307 |
|
Age | 0.014 | 1.045 | 1.009 | 1.082 |
|
YAPs | 0.041 | 1.770 | 1.025 | 3.058 |
Discussion
In the studied group, pT and pN were independent
prognostic factors that significantly influenced patient survival.
The results were in agreement with the previously established fact
that the primary, important prognostic factors in patients with OCC
are the disease advancement stage TNM, pT and pN (25,28). In
addition, the previously tested markers MCM7 and PTPN14 had a
significant impact on the prognosis of patients. Of the newly
tested proteins, YAP showed an effect on patient survival, while
TAZ did not meet the criterion of a prognostic factor.
The hypothesis that MCM proteins may be good
proliferation markers has been tested in many previous studies
(29–32). One of the first reports on the
prognostic significance of MCM2 as a cell proliferation marker in
OCC was the work by our team (33)
performed on a different, smaller group of patients. In this study
the expression of MCM2 was compared with the recognized
proliferation marker Ki-67 and the results revealed a strong
correlation between the expression levels of these two markers in
oral SCC and additionally it was demonstrated to be a better
prognostic factor than Ki-67. In the case of the MCM7 protein, only
one study has been reported that assessed the effect of its
expression on prognosis in OCC (30), in which shorter survival was typical
for patients with MCM7 expression in >49% of cancer cells.
However, this effect was limited to a subgroup of TNM stage III and
IV patients, and was not noticed in the group of stage I and II
patients. On the other hand, in our present and previous studies,
MCM7 expression had an effect on patient prognosis at all stages of
advancement, and in the multivariate analysis, along with pT and pN
grade, it was the only independent prognostic factor for each of
the assessed survival parameters.
The relationship between proliferation and PTPN14
was described by Wadham et al (19), who noticed a correlation between
PTPN14 translocation to the nucleus and proliferation initiation,
which indicates its important role in the process. In the present
study, PTPN14 was located in the cytoplasm and upon
immunohistochemical examination, its nuclear expression was not
observed. However, a high percentage of cells (>75%) containing
this protein were correlated with the studied proliferation marker
MCM7 and was in turn related with poor prognosis.
Furthermore, in the present study high expression of
PTPN14 had the highest effect on locoregional recurrence with a
decreasing effect on DFS and DSS. Studies on Zebrafish (Danio
reiro) embryos reported that PTPN14 overexpression induced TGFβ
secretion and via the canonical Smad-related pathway induced
changes in the expression of genes associated with EMT including
Snail, Slug, ZEB1 and ZEB2, and the loss of E-cadherin expression,
which resulted in morphological and functional changes and the
acquisition of migratory features by the cells (20). It is possible that the high
expression of PTPN14 results in the increased migration of cancer
cells and, despite negative margins of resection, cancer cell
deposits may be near the postoperative field, which is associated
with an almost 6-fold higher risk of local recurrence.
In 2012 and 2013, three papers were published
reporting that PTPN14 inhibits the activity of YAP by promoting its
cytoplasmic location (14,34,35). In
these studies, it was noticed that the combination of PTPN14
protein with YAP inhibited its translocation to the nucleus and
transcription of target genes for YAP. On the other hand, lack of
PTPN14 resulted in YAP overexpression and increased cell migration
(14,34). In 2013, another article was published
indicating that PTPN14 was a negative regulator of YAP
transcriptional activity. However, the inhibition of YAP
translocation to the cell nucleus induced by PTPN14 was not
described (15). In 2014, Wilson
et al (36) demonstrated that
PTPN14 not only directly, but also indirectly through activation of
large tumor suppressor drosophila homolog 1, negatively regulated
YAP and TAZ. However, the present study did not observe a
correlation between PTPN14, and YAP and TAZ expression.
In the present study, TAZ was located only in the
cancer cell nuclei and despite an evident tendency for a poorer
prognosis in the group of patients whose cancer cells did not
exhibit TAZ expression, no significant effect on survival was
observed. In the case of YAP in cancer cells, only a cytoplasmic
reaction was identified, while in CAFs a nuclear-cytoplasmic
reaction was observed. In the present study, no cytoplasmic YAP
expression in cancer cells was related with poor prognosis, and was
an independent prognostic factor for DFS and DSS. Similar results
highlighted YAP as a suppressor in SCC of the head and neck area as
presented by Ehsanian et al (37), in 2010. This study demonstrated that
cells with YAP knockdown exhibited increased proliferation,
survival, migration abilities and resistance to cisplatin. On the
other hand, YAP expression increased cell apoptosis and
chemosensitivity; however, YAP's function as a suppressor may be
dysregulated by AKT or ΔNp63 (37).
Nevertheless, the majority of papers indicate that there is a
correlation between YAP and TAZ overexpression in cancer cells and
the presence of aggressive clinicopathological features and
unfavorable prognosis of cancer patients and chemoresistance
(38–41).
At the same time some findings have suggested that
YAP may function not only as a protooncogene but also as a
suppressor of tumors depending on the cell context (13,42).
Recently, papers have been published that report that YAP and TAZ
may suppress WNT signal transduction amongst other signals by
sequestering β-catenin in the cytoplasm or intensifying its
degradation (43,44).
It was previously revealed that YAP is silenced in
highly aggressive human colorectal cancers and its re-expression
may limit cancer growth, which indicates its potential role as a
suppressor (13,45). It seems that YAP and TAZ expression
in the cytoplasm function as β-catenin inhibitors, whereas with the
nuclear localization they are positive mediators of WNT-associated
transformation (44).
Furthermore, it was noted that in normal hematologic
cells oncogene-mediated DNA damage induces ataxia telangiectasia
mutated and then JUN N-terminal kinase activation, which
phosphorylates 14-3-3 adaptor protein causing Abelson
tyrosine-protein kinase 1 (ABL1) to shift from the cytoplasm to the
cell nucleus (46,42). Upon entry into the cell nucleus, ABL1
phosphorylates the YAP tyrosine residue. Phosphorylated YAP forms a
complex with the TP73 tumor suppressor and maintains the
transcription of pro-apoptotic genes such as BAX and
PUMA (42). MST1-induced
activation of the Hippo signaling pathway, via phosphorylation of
serine residues YAP, inhibits its pro-apoptotic activity (42). Low expression of YAP prevents the
apoptosis induced by ABL1 in the case of DNA damage in hematologic
cancers. On the other hand, the re-occurrence of YAP expression in
tumor cells promotes apoptosis and growth arrest (42).
A possible explanation for this contradictory
information concerning YAP function in cancer is that it may form
complexes with various transcriptional factors that have different
functions depending on the cancer type. TAZ and YAP may have
varying effects on carcinogenesis by associating with different
transcriptional factors depending on the cell context.
The present study observed a weak positive
correlation between YAP expression in CAFs and the percentage of
tumor cells presenting MCM7 expression. Furthermore, the results
demonstrated an unfavorable effect of high YAP expression in CAFs
on DFS and OS. This finding suggests a direct relationship between
the presence of YAP in stromal cells and the increased
proliferation of cancer cells and poorer patient prognoses. This
phenomenon may be explained by earlier studies that suggested that
YAP activation in CAFs promoted stiffening of the matrix by
increased deposition of collagen (47). Furthermore, stiffening of the matrix
causes tension within the CAFs, which results in Src kinase
activation and YAP translocation to the nucleus and as a result,
YAP and TAZ promote the expression of regulators of the
cytoskeleton such as anillin actin binding protein and diaphanous
related formin 3, and stabilizing actomyosin proteins. This further
stiffens the matrix, increases proliferation and results in the
formation of the self-enforcing loop during carcinogenesis
(47).
In conclusion, the present demonstrated the
significant effect of increased YAP expression in cancer-associated
fibroblasts on the unfavorable prognosis of patients with squamous
OCC. Additionally, a weak positive correlation was confirmed
between proliferation in cancer cells and YAP expression in the
stroma. This finding highlights the key role of the ECM in cancer
progression. The results also demonstrated that the lack of YAP
expression in the cytoplasm of tumor cells may be a poor prognostic
factor for DFS and DSS, suggesting its suppressive effect on cancer
in this region. No statistically significant correlations were
identified between the level of YAP and TAZ expression and PTPN14,
nor was a correlation between the level of cell proliferation and
the presence of YAP and TAZ protein in tumor cells observed.
However, due to the fact that the findings reported by other
authors are inconclusive, the present observations require further
validation in a future study on another group of patients.
Acknowledgements
Not applicable.
Funding
The presented results of studies performed as part
of the subject as per records in the Simple system number
ST.C280.17.010 and SUB.C280.19.050 were financed through a
statutory subsidy by the Minister of Science and Higher
Education.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to our institutional
policy but are available from the corresponding author on
reasonable request.
Authors' contributions
JS undertook study design, data collection, analysis
of data, manuscript preparation and the Literature search. PD
performed data collection, analysis of data, manuscript preparation
and microphotographs. KRW analyzed the data, prepared the
manuscript and took microphotographs. AH analyzed the data and
reviewed the manuscript. DZ and KL were involved in drafting the
manuscript, revising it critically for important intellectual
content and given final approval of the version to be published, in
addition they took part in th eacquisition of data and analysis and
interpretation of data. AM analyzed the data and reviewed the
manuscript. ELW undertook the literature search, data collection
and manuscript preparation. AP analyzed the data, prepared the
manuscript and took microphotographs. RM was responsible for study
design, analyzing the data and reviewing the manuscript.
Ethics approval and consent to
participate
The consent of the bioethical commission was
obtained (consent no. KB299/2013).
Patient consent for publication
Due to the retrospective nature of the work and the
lack of influence of the conducted research on the treatment
method, no written consent for conducting the research was obtained
from patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Protocol for the Examination of specimens
from Patients with Carcinomas of the Lip and Oral Cavity.
https://cap.objects.frb.io/protocols/cp-headandneck-lip-oralcavity-17protocol-4001.pdfMay
1–2017
|
|
2
|
Lindenblatt Rde C, Martinez GL, Silva LE,
Faria PS, Camisasca DR and Lourenço Sde Q: Oral squamous cell
carcinoma grading systems-analysis of the best survival predictor.
J Oral Pathol Med. 41:34–39. 2012. View Article : Google Scholar
|
|
3
|
Bhargava A, Saigal S and Chalishazar M:
Histopathological grading systems in oral squamous cell carcinoma:
A review. J Int Oral Health. 2:1–9. 2010.
|
|
4
|
Brandwein-Gensler M and Smith RV:
Prognostic indicators in head and neck oncology including the new
7th edition of the AJCC staging system. Head Neck Pathol. 4:53–61.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zwetyenga N, Majoufre-Lefebvre C,
Siberchicot F, Demeaux H and Pinsolle J: Squamous-cell carcinoma of
the tongue: Treatment results and prognosis. Rev Stomatol Chir
Maxillofac. 104:10–17. 2003.(In French). PubMed/NCBI
|
|
6
|
Pan D: The hippo signaling pathway in
development and cancer. Dev Cell. 19:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnson R and Halder G: The two faces of
Hippo: Targeting the Hippo pathway for regenerative medicine and
cancer treatment. Nat Rev Drug Discov. 13:63–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baldwin C, Garnis C, Zhang L, Rosin MP and
Lam WL: Multiple microalterations detected at high frequency in
oral cancer. Cancer Res. 65:7561–7567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dupont S, Morsut L, Aragona M, Enzo E,
Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M,
Bicciato S, et al: Role of YAP/TAZ in mechanotransduction. Nature.
474:179–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sanz-Moreno V, Gaggioli C, Yeo M,
Albrengues J, Wallberg F, Viros A, Hooper S, Mitter R, Féral CC,
Cook M, et al: ROCK and JAK1 signaling cooperate to control
actomyosin contractility in tumor cells and stroma. Cancer Cell.
16:229–245. 2011. View Article : Google Scholar
|
|
11
|
Aragona M, Panciera T, Manfrin A, Giulitti
S, Michielin F, Elvassore N, Dupont S and Piccolo S: A mechanical
checkpoint controls multicellular growth through YAP/TAZ regulation
by actin-processing factors. Cell. 154:1047–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao B, Wei X, Li W, Udan R, Yang Q, Kim
J, Xie J, Ikenoue T, Yu J, Li L, et al: Inactivation of YAP
oncoprotein by the Hippo pathway is involved in cell contact
inhibition and tissue growth control. Genes Dev. 21:2747–2761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barry ER, Morikawa T, Butler BL, Shrestha
K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, et
al: Restriction of intestinal stem cell expansion and the
regenerative response by YAP. Nature. 493:106–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Yang N, Figel SA, Wilson KE,
Morrison CD, Gelman IH and Zhang J: PTPN14 interacts with and
negatively regulates the oncogenic function of YAP. Oncogene.
32:1266–1273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang JM, Nagatomo I, Suzuki E, Mizuno T,
Kumagai T, Berezov A, Zhang H, Karlan B, Greene MI and Wang Q: YAP
modifies cancer cell sensitivity to EGFR and survivin inhibitors
and is negatively regulated by the non-receptor type protein
tyrosine phosphatase 14. Oncogene. 32:2220–2229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neel BG and Tonks NK: Protein tyrosine
phosphatases in signal transduction. Curr Opin Cell Biol.
9:193–204. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gyapay G, Morissette J, Vignal A, Dib C,
Fizames C, Millasseau P, Marc S, Bernardi G, Lathrop M and
Weissenbach J: The 1993–94 Généthon human genetic linkage map. Nat
Genet. 7:246–339. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ogata M, Takada T, Mori Y, Uchida Y, Miki
T, Okuyama A, Kosugi A, Sawada M, Oh-hora M and Hamaoka T:
Regulation of phosphorylation level and distribution of PTP36, a
putative protein tyrosine phosphatase, by cell-substrate adhesion.
J Biol Chem. 274:20717–20724. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wadham C, Gamble JR, Vadas MA and
Khew-Goodall Y: Translocation of protein tyrosine phosphatase
Pez/PTPD2/PTP36 to the nucleus is associated with induction of cell
proliferation. J Cell Sci. 113:3117–3123. 2000.PubMed/NCBI
|
|
20
|
Wyatt L, Wadham C, Crocker LA, Lardelli M
and Khew-Goodall Y: The protein tyrosine phosphatase Pez regulates
TGFbeta, epithelial-mesenchymal transition, and organ development.
J Cell Biol. 178:1223–1235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishimi Y: A DNA helicase activity is
associated with an MCM4, −6, and −7 protein complex. J Biol Chem.
272:24508–24513. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Labib K, Tercero JA and Diffley JF:
Uninterrupted MCM2-7 function required for DNA replication fork
progression. Science. 288:1643–1647. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lo Sardo F, Forcato M, Sacconi A, Capaci
V, Zanconato F, Di Agostino S, Del Sal G, Pandolfi PP, Strano S,
Bicciato S and Blandino G: MCM7 and its hosted miR-25, 93 and 106b
cluster elicit YAP/TAZ oncogenic activity in lung cancer.
Carcinogenesis. 38:64–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szelachowska J: Różnice w długości
przeżycia chorych na płaskonabłonkowego raka dna jamy ustnej lub
trzonu języka w zależności od poziomu ekspresji białka MCM7, RB,
E-kadheryny, p16, TGFb i PTPN14 w komórkach guza. (Wrocław).
Uniwersytet Medyczny im. Piastów Śląskich. 2015.(In Polish).
|
|
25
|
Edge S, Byrd D, Compton C, Fritz A, Greene
F and Trotti A: AJCC Cancer Staging Manual. (7th). Springer. (New
York, NY). 2010.
|
|
26
|
National Comprehensive Cancer Network.
Bone Cancer (Version 1.2019). https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf2019
05 07
|
|
27
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.PubMed/NCBI
|
|
28
|
Leemans CR, Tiwari R, Nauta JJ, van der
Waal I and Snow GB: Recurrence at the primary site in head and neck
cancer and the significance of neck lymph node metastases as a
prognostic factor. Cancer. 73:187–190. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gueiros LA, Coletta RD, Kowalski LP and
Lopes MA: Clinicopathological features and proliferation markers in
tongue squamous cell carcinomas. Int J Oral Maxillofac Surg.
40:510–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tamura T, Shomori K, Haruki T, Nosaka K,
Hamamoto Y, Shiomi T, Ryoke K and Ito H: Minichromosome
maintenance-7 and geminin are reliable prognostic markers in
patients with oral squamous cell carcinoma: Immunohistochemical
study. J Oral Pathol Med. 39:328–334. 2010.PubMed/NCBI
|
|
31
|
Feng CJ, Li HJ, Li JN, Lu YJ and Liao GQ:
Expression of Mcm7 and Cdc6 in oral squamous cell carcinoma and
precancerous lesions. Anticancer Res. 28:3763–3769. 2008.PubMed/NCBI
|
|
32
|
Torres-Rendon A, Roy S, Craig GT and
Speight PM: Expression of Mcm2, geminin and Ki67 in normal oral
mucosa, oral epithelial dysplasias and their corresponding
squamous-cell carcinomas. Br J Cancer. 100:1128–1134. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Szelachowska J, Dziegiel P,
Jelen-Krzeszewska J, Jelen M, Matkowski R, Pomiecko A, Spytkowska
B, Jagas M, Gisterek I and Kornafel J: Mcm-2 protein expression
predicts prognosis better than Ki-67 antigen in oral cavity
squamocellular carcinoma. Anticancer Res. 26:2473–2478.
2006.PubMed/NCBI
|
|
34
|
Michaloglou C, Lehmann W, Martin T,
Delaunay C, Hueber A, Barys L, Niu H, Billy E, Wartmann M, Ito M,
et al: The tyrosine phosphatase PTPN14 is a negative regulator of
YAP activity. PLoS One. 8:e619162013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang W, Huang J, Wang X, Yuan J, Li X,
Feng L, Park JI and Chen J: PTPN14 is required for the
density-dependent control of YAP1. Genes Dev. 26:1959–1971. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wilson KE, Li YW, Yang N, Shen H, Orillion
AR and Zhang J: PTPN14 forms a complex with Kibra and LATS1
proteins and negatively regulates the YAP oncogenic function. J
Biol Chem. 289:23693–23700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ehsanian R, Brown M, Lu H, Yang XP,
Pattatheyil A, Yan B, Duggal P, Chuang R, Doondeea J, Feller S, et
al: YAP dysregulation by phosphorylation or ΔNp63-mediated gene
repression promotes proliferation, survival and migration in head
and neck cancer subsets. Oncogene. 29:6160–6171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ge L, Smail M, Meng W, Shyr Y, Ye F, Fan
KH, Li X, Zhou HM and Bhowmick NA: Yes-associated protein
expression in head and neck squamous cell carcinoma nodal
metastasis. PLoS One. 6:275292011. View Article : Google Scholar
|
|
39
|
Jerhammar F, Johansson AC, Ceder R,
Welander J, Jansson A, Grafström RC, Söderkvist P and Roberg K:
YAP1 is a potential biomarker for cetuximab resistance in head and
neck cancer. Oral Oncol. 50:832–839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Z, Wang Y, Zhu Y, Yuan C, Wang D, Zhang
W, Qi B, Qiu J, Song X, Ye J, et al: The Hippo transducer TAZ
promotes epithelial to mesenchymal transition and cancer stem cell
maintenance in oral cancer. Mol Oncol. 9:1091–1105. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zheng L, Xiang C, Li X, Guo Q, Gao L, Ni
H, Xia Y and Xi T: STARD13-correlated ceRNA network-directed
inhibition on YAP/TAZ activity suppresses stemness of breast cancer
via co-regulating Hippo and Rho-GTPase/F-actin signaling. J Hematol
Oncol. 30:722018. View Article : Google Scholar
|
|
42
|
Cottini F, Hideshima T, Xu C, Sattler M,
Dori M, Agnelli L, ten Hacken E, Bertilaccio MT, Antonini E, Neri
A, et al: Rescue of Hippo coactivator YAP1 triggers DNA
damage-induced apoptosis in hematological cancers. Nat Med.
20:599–606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Attisano L and Wrana JL: Signal
integration in TGF-β, WNT, and Hippo pathways. F1000Prime Rep.
5:172013. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Azzolin L, Panciera T, Soligo S, Enzo E,
Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V,
et al: YAP/TAZ incorporation in the β-catenin destruction complex
orchestrates the Wnt response. Cell. 158:157–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zanconato F, Cordenonsi M and Piccolo S:
YAP/TAZ at the Roots of Cancer. Cancer Cell. 13:783–803. 2016.
View Article : Google Scholar
|
|
46
|
Levy D, Adamovich Y, Reuven N and Shaul Y:
Yap1 phosphorylation by c-Abl is a critical step in selective
activation of proapoptotic genes in response to DNA damage. Mol
Cell. 29:350–361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Calvo F, Ege N, Grande-Garcia A, Hooper S,
Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary
E, Charras G and Sahai E: Mechanotransduction and YAP-dependent
matrix remodelling is required for the generation and maintenance
of cancer-associated fibroblasts. Nat Cell Biol. 15:637–646. 2013.
View Article : Google Scholar : PubMed/NCBI
|