Introduction
Gliomas are the most common malignant primary brain
tumors, and are categorized, according to the 2016 World Health
Organization (WHO) classification, as low-grade (grade I–II) and
high-grade (grade III–IV) (1).
Low-grade gliomas (LGGs) are infiltrative neoplasms that most
frequently arise in the cerebral hemispheres of adults, and include
astrocytomas, oligodendrogliomas and oligoastrocytomas (1). LGGs generally occur in young adults
between 35 and 44 years of age (2).
Despite a better prognosis compared with HGG, complete
neurosurgical resection of LGGs is challenging due to their
invasive nature, increased vascularity and lack of a well-defined
tumor capsule. Furthermore, certain cases LGG may rapidly progress
to WHO grade IV glioma, becoming a glioblastoma multiforme (GBM),
whereas others may remain stable for a long time (3). The survival time of patients with LGG
ranges from 1 to 15 years (4).
Although traditional histopathological methods are
regarded as the gold standard for LGG classification, they do not
adequately predict clinical outcome. Consequently, an increasing
number of studies have shown that biomarkers may improve the
clinical decision-making process (5). Hypermethylation of the
O6-methylguanine-DNA methyltransferase promoter can
effectively predict the responsiveness to temozolomide (6). Mutations in isocitrate dehydrogenase
(NADP+) 1 (IDH1) and IDH2 may result in an improved
prognosis for patients with LGG. Mutations in IDH and the
heterozygous deletion at the chromosomal position 1p/19q may result
in improved responses to radiotherapy and chemotherapy, and
patients with these mutations have longer survival times than
patients without these mutations (7–10).
The present study investigated novel prognostic and
predictive biomarkers for LGG using an mRNA PCR array to screen for
genes with altered expression in LGG. The prognostic value of newly
identified biomarkers was subsequently investigated using the
OncoLnc and Gene Expression Profiling Interactive Analysis (GEPIA)
databases, and biomarker mRNA level was analyzed using the Oncomine
database.
Materials and methods
Patient specimens
The study included 53 glioma tissues and
formalin-fixed paraffin-embedded tumor tissues. Glioma tissues were
fixed overnight at 4°C in 10% neutral-buffered formalin, dehydrated
by soaking in 70, 85, 95 and 100% ethanol for 45 min each time at
room temperature, washed in xylene twice for 15 min each at room
temperature, soaked twice in paraffin for 1 h each at 56°C,
embedded in paraffin and subsequently stored at 4°C until use. Six
patients with LGG and six patients with GBM were recruited from the
Neurology Institute of Lanzhou University Second Hospital (Lanzhou,
China) between January 2013 and December 2017. Of the patients
recruited in the present study, 31 were male and 22 were female,
with a mean age of 48 years (range, 20–72 years). A total of five
normal brain tissue samples were obtained from patients without
glioma who underwent surgery for other reasons, including cerebral
trauma. The glioma and normal tissue specimens were snap-frozen in
liquid nitrogen following surgery, and subsequently stored at −80°C
until use. Histological diagnosis was based on the criteria stated
by the WHO. According to the 2016 World Health Organization
Classification of Tumors of the Central Nervous System (1), gliomas were divided into four
categories: i) WHO I (pilocytic astrocytoma); ii) WHO II (diffuse
astrocytoma, oligoastrocytoma and oligodendroglioma); iii) WHO III
(anaplastic astrocytoma, anaplastic oligodendroglioma and
anaplastic oligoastrocytoma); and iv) WHO IV (glioblastoma).
Patients undergoing surgery who had not been previously treated
with radiotherapy or chemotherapy were included in the present
study. Approval for the study was obtained from The Medical Ethics
Committee of The Affiliated Second Hospital of Lanzhou University.
Written informed consent was obtained from all recruited
patients.
Human mRNA PCR array
After dewaxing, total RNA was extracted from the
tumor samples of 12 randomly selected patients using
TRIzol® reagent (Thermo Fisher Scientific, Inc.). First
strand cDNA was synthesized using the mRNA First-Strand cDNA
Synthesis kit (cat. no. AS-MR-004; Arraystar, Inc.) according to
the manufacturer's protocol. The cDNA products were amplified using
TB SYBR green Premix Ex Taq II (Takara Biotechnology Co., Ltd.).
The Human mRNA PCR Array (cat. no. AS-MR-0033, Arraystar, Inc.) was
used with the ABI PRISM7900 system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The thermocycling condition of the qPCR
were: Initial denaturation at 95°C for 1 min, followed by 40 cycles
of denaturation at 95°C for 15 sec, and annealing and extension at
60°C for 30 sec. Each 364-well miRStar Human Cancer Focus miRNA and
Target mRNA PCR Array (Arraystar, Inc.) contains 177 genes
associated with human cancer, six wells for housekeeping genes, a
genomic DNA contamination control, three replicates of reverse
transcription (RT) controls and three replicates of positive PCR
controls. The raw data were processed by performing the following
analyses: Background detection, robust multi-array average global
background correlation, quantile normalization, median adjustment
and log2-transformation with miRNA QC tool version 1.1 (Affymetrix;
Thermo Fisher Scientific, Inc.).
Oncomine database analysis
The expression level of CD44 in LGG, GBM and matched
normal tissues were retrieved from the Oncomine database
(http://www.oncomine.org). The expression analysis
of CD44 in the Oncomine database was based on a previous study
(11). A total of 157 brain and
central nervous system tumors and 23 normal brain samples were
analyzed on an Affymetrix U133 Plus 2.0 platform (Affymetrix;
Thermo Fisher Scientific, Inc.). Normal tissue samples were
provided in the Oncomine database. The-fold-change of mRNA
expression in LGG and GBM samples, compared with their matched
normal tissues, was determined using the following parameters: i)
P<1×10−3; and ii) fold-change ≥2-fold.
Kaplan-Meier plotter analysis
The prognostic value of CD44 was analyzed using
OncoLnc (www.oncolnc.org/search_results/?q=CD44) and GEPIA
(gepia.cancer-pku.cn). Patients were divided into high-level and
low-level groups based on the median value of CD44. The overall
survival (OS) rates of the patients in the high-level and low-level
CD44 groups were evaluated using Kaplan-Meier analysis
(gepia.cancer-pku.cn/detail.php?gene=&clicktag=survival).
RT-quantitative PCR (RT-q) PCR
Total RNA was extracted from normal brain and glioma
tissues using Trizol® reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Total RNA (2 µg)
was reverse transcribed using the PrimeScript RT reagent kit with
genomic DNA Eraser (Takara Biotechnology Co., Ltd.). Reverse
transcription was performed as follows: 37°C for 15 min and 85°C
for 5 sec. The cDNA products were amplified using TB SYBR green
Premix Ex Taq II (Takara Biotechnology Co., Ltd.) on a Bio-Rad
CFX96 real-time PCR system (Bio-Rad Laboratories, Inc.). The qPCR
procedure was performed as follows: Pre-denaturation at 95°C for 1
min, followed by 40 cycles of denaturation at 95°C for 15 sec, and
annealing and extension at 60°C for 30 sec. The relative mRNA
expression data were analyzed using the 2−ΔΔCq method
(10), and expression values were
normalized to the internal control GAPDH. The following primers
were used: CD44 forward, 5′-CCAACTCCATCTGTGCAG-3′ and reverse,
5′-AACCTCCTGAAGTGCTGC-3′; GAPDH, forward 5′-GGACCTGACCTGCCGTCTAG-3′
and reverse, 5′-TAGCCCAGGAGGATGCCCTTGAG-3′.
Western blotting
The glioma tissues were homogenized and lysed with
cell lysis buffer (Beijing Solarbio Science & Technology Co.,
Ltd.) containing 1% PMSF. The lysates were centrifuged at 12,000 ×
g for 5 min at 4°C. The protein concentration was determined using
a bicinchoninic acid assay. The proteins (30 µg total protein/lane)
were separated using SDS-PAGE on a 12% gel. Proteins were
transferred to PVDF membranes (EMD Millipore), blocked with 5%
fat-free milk for 25°C for 1 h and incubated with primary
antibodies against CD44 (cat. no. D190741; 1:750; Sangon Biotech
Co., Ltd.) and GAPDH (cat. no. TA-08; 1:1,000; Beijing Zhongshan
Jinqiao Biotechnology Co., Ltd.) at 4°C overnight. The membranes
were washed with PBS and 0.1% Tween, and incubated with goat
anti-mouse secondary antibody (cat. no. abs20001; 1:2,000; Absin
Bioscience Inc.) at 25°C for 1 h. Protein bands were visualized
using the enhanced chemiluminescence (PerkinElmer, Inc.) method and
a Bio-Rad gel imaging system (Bio-Rad Laboratories, Inc.), and
densitometry analysis was performed using ImageJ version 1.8.0
software (National Institutes of Health).
Data analysis
The median mRNA expression levels of CD44 mRNA was
used as the cut-off point, and the patients were divided into a
high-level and a low-level group. The χ2 test was used
to analyze the association between CD44 mRNA expression and
clinicopathological characteristics. The OS rates of LGG in the
high- and low-level groups were evaluated using Kaplan-Meier
analysis. Furthermore, univariate and multivariate Cox proportional
hazard regression analysis was performed to evaluate the prognostic
value of multiple variables, including CD44 mRNA expression, sex,
age and Karnofsky Performance Scale (KPS) (12).
Statistical analysis
All analyses were performed using SPSS software
(version 17; SPSS, Inc.). The data are presented as the mean ± SEM.
Data were analyzed using one-way ANOVA followed by the least
significant difference post hoc test or an unpaired Student's
t-test. Survival curves were estimated using the Kaplan-Meier
method with the log-rank test. Univariate and multivariate Cox
regression analysis was employed to estimate the risk factor of
gliomas. P<0.05 was considered to indicate a statistically
significant difference. The analyses were performed using GraphPad
Prism software (version 5; GraphPad Software, Inc.).
Results
Gene expression analysis
The mRNA profile was analyzed for LGG (n=6) and GBM
(n=6) using the miRStar Human Cancer Focus mRNA PCR Array. The
median expression levels of each mRNA were calculated in both
groups, and the differences between them were determined using a
t-test. P<0.05 was considered to indicate a significant
difference and a-fold change >2 was used as the cut-off value.
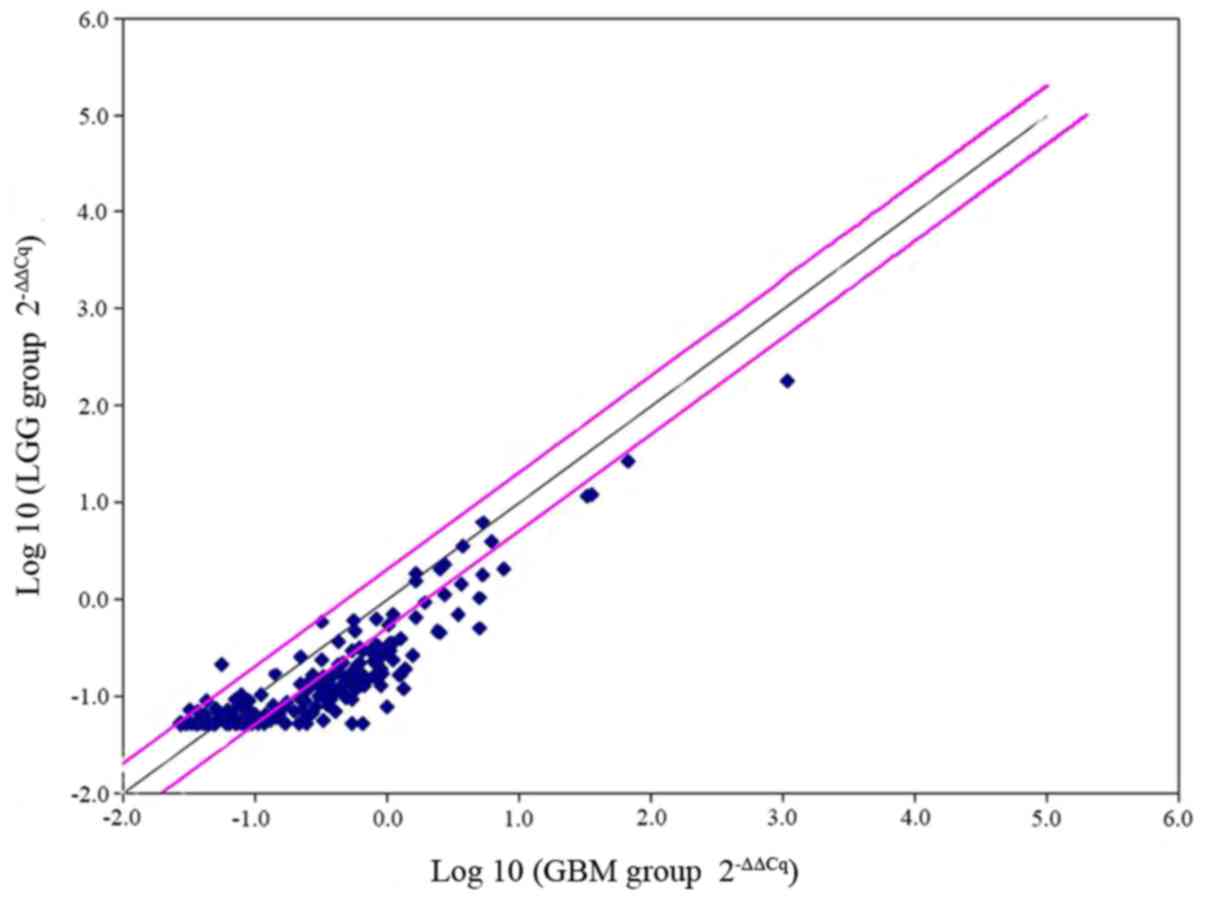
The results indicated that of the 177 genes, 44 difference genes
were upregulated. CD44 was identified to exhibit a 3-fold increase
in GBM (P=0.02; Fig. 1). The results
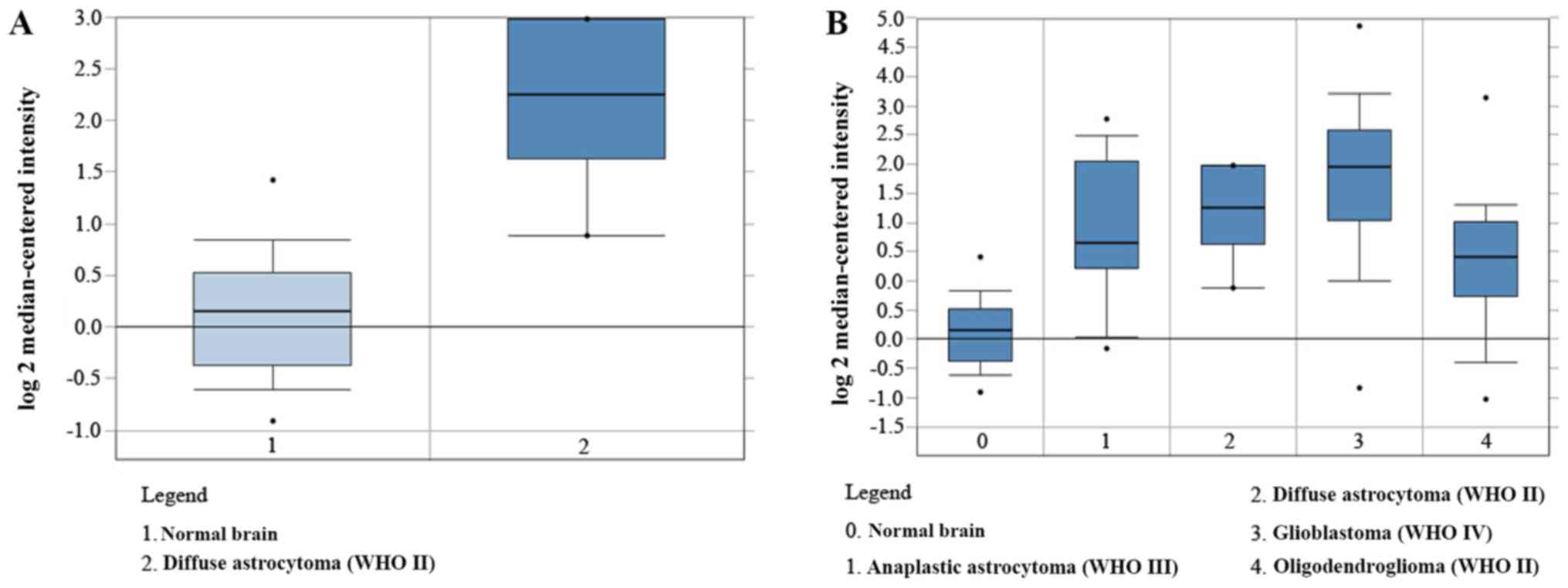
were further validated using Oncomine analysis, which demonstrated
that the CD44 mRNA level was significantly higher in GBM compared
with LGG and normal brain tissue (Fig.
2A and B).
CD44 expression in glioma tissues
To validate the mRNA PCR array and Oncomine database
findings, the expression level of CD44 was determined in the glioma
tissue samples (n=53) and normal brain tissues (n=5) using RT-qPCR
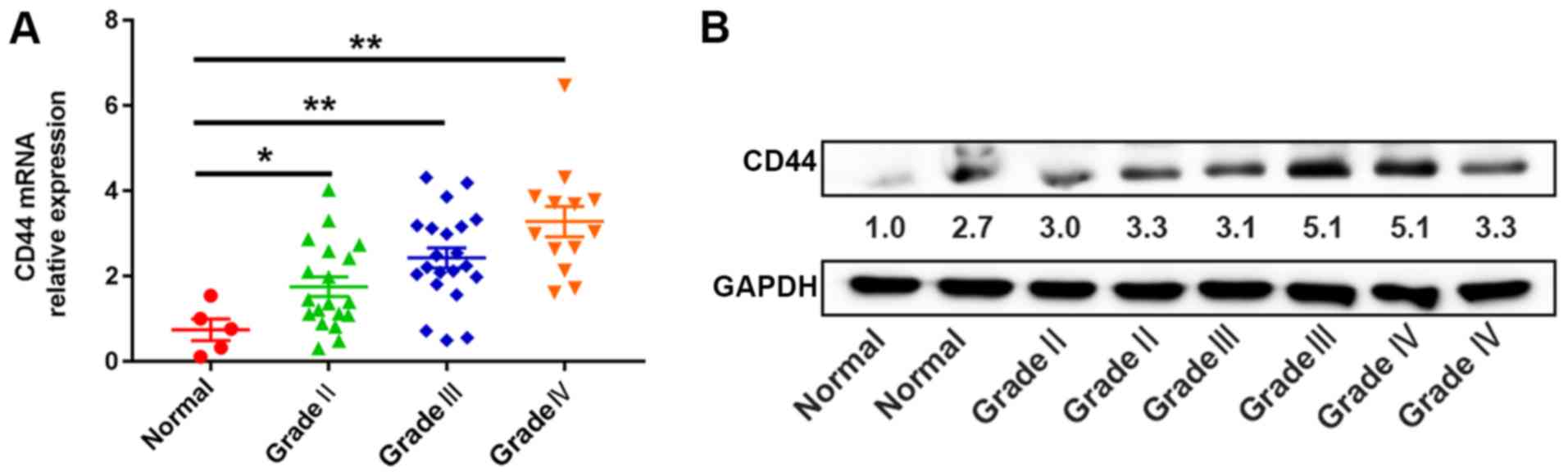
and western blotting. The CD44 expression level in glioma tissues
was significantly increased compared with normal brain tissues
(P<0.05; Fig. 3A and B). The data
were consistent with the results of the mRNA PCR array and Oncomine
database analysis, in which patients were divided into high-level
(n=26) and low-level groups (n=27), with the median expression
level of CD44 mRNA as the cut-off value. The expression level of
CD44 mRNA was associated with KPS (P<0.01) and WHO grade
(P<0.01; Table I). However, it
was not significantly associated with other clinicopathological
parameters, including age and sex.
 | Table I.Association of the expression level of
CD44 mRNA with clinicopathological factors of low-grade glioma and
glioblastoma multiforme. |
Table I.
Association of the expression level of
CD44 mRNA with clinicopathological factors of low-grade glioma and
glioblastoma multiforme.
|
|
| CD44 mRNA
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | Patients, n | Low, n | High, n | P-value |
|---|
| Age |
|
|
| 0.126 |
|
>50 | 24 | 15 | 9 |
|
| ≤50 | 29 | 12 | 17 |
|
| Sex |
|
|
| 0.659 |
| Male | 31 | 15 | 16 |
|
|
Female | 22 | 12 | 10 |
|
| World health
organization grade |
|
|
| <0.01 |
| II | 19 | 14 | 5 |
|
|
III–IV | 34 | 13 | 21 |
|
| Karnofsky performance
status |
|
|
| <0.01 |
|
>80 | 19 | 15 | 4 |
|
| ≤80 | 34 | 12 | 22 |
|
Survival analysis
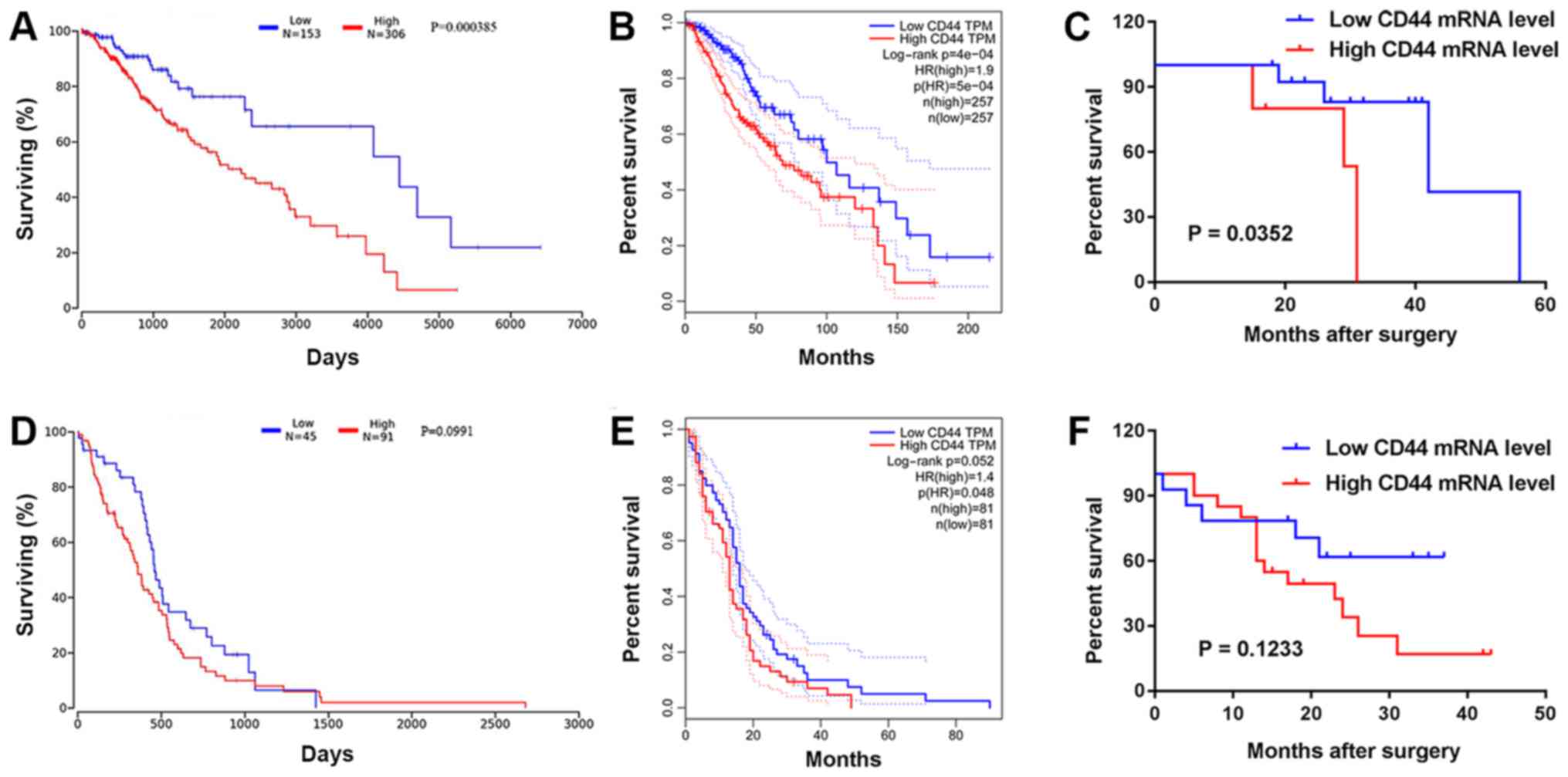
Prognostic value analysis of CD44 mRNA was performed
in patients with LGG and GBM using data from the OncoLnc and GEPIA
databases. Kaplan-Meier analysis revealed that a high expression
level of CD44 in LGG was a predictor of short OS and poor patient
outcome, as assessed by OncoLnc (P<0.01) and GEPIA (P<0.01)
(Fig. 4A and B). The level of CD44
mRNA did not significantly influence patient outcome, as assessed
by OncoLnc (P=0.099) and GEPIA (P=0.052) in GBM (Fig. 4D and E). In addition, Kaplan-Meier
analysis and the log-rank test were used to evaluate the prognostic
value of CD44 mRNA expression in patients with LGG and GBM
according to clinical follow-up data. The present results suggested
that patients with LGG with a high expression level of CD44 had a
significantly shorter OS than the low expression group (P=0.035;
Fig. 4C). There was no significant
difference in patients with GBM (P=0.123; Fig. 4F). Furthermore, Cox regression
analysis revealed that the expression level of CD44 was
significantly associated with the OS of LGG patients (hazard ratio,
3.7012; 95% CI, 0.927–11.215; P=0.032; Table II).
 | Table II.Univariate and multivariate analyses
of the prognostic parameters of patients with low-grade glioma. |
Table II.
Univariate and multivariate analyses
of the prognostic parameters of patients with low-grade glioma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (>50 vs.
≤50) | 2.932 | 0.679–5.214 | 0.127 | 1.515 | 0.212–2.534 | 0.099 |
| Sex (male vs.
female) | 0.501 | 0.038–3.011 | 0.450 | 3.715 | 0.611–8.425 | 0.679 |
| Karnofsky
performance status (>80 vs. ≤80) | 0.246 | 0.001–1.091 | 0.014 | 0.417 | 0.046–1.914 | 0.034 |
| CD44 mRNA
expression (low vs. high) | 3.7012 | 0.927–11.215 | 0.032 | 2.862 | 0.436–5.534 | 0.042 |
Discussion
Despite recent progresses in neurosurgery, the
survival rate of patients with LGG varies widely. To improve
prediction accuracy for LGG, molecular markers such as ATRX
chromatin remodeler, p53 and telomerase reverse transcriptase have
been established (13,14). Furthermore, IDH mutation and 1p/19q
codeletion were used in the 2016 WHO classification of central
nervous system tumors (1).
IDH-wild-type and IDH-mutants are observed in 90 and 10%,
respectively, of all GBM cases (15). Present studies for the classification
of glioma are focused on high-grade glioma; therefore, biomarkers
relevant for the prognostic stratification of patients with LGG
remain limited.
In recent years, there have been rapid developments
in molecular biology techniques, including chromatin
immunoprecipitation and high-throughput sequencing, as well as in
the application of bioinformatics methods. Advances in these
techniques have allowed the investigation of the occurrence and
development of glioma at the molecular level (16–18). The
present study used a PCR array, and identified CD44 expression
level to be associated with the histopathological grade of gliomas.
Western blotting suggested that CD44 expression was higher in
patients with high-grade glioma compared with patients with LGG and
controls. Similar results were obtained using an mRNA PCR array and
Oncomine analysis. However, there was no significant difference
between grade III and IV glioma.
CD44 primarily regulates cell-to-cell and
cell-to-extracellular matrix adhesion to preserve the
organizational structure of tissues and organ. Additionally, CD44
is involved in cell migration, signaling, proliferation,
angiogenesis, lymphocyte function and metastasis (19). In glioma, CD44 serves as an oncogene,
and can promote GBM cell migration and invasion by influencing the
mitogen-activated protein kinase/EPH receptor B2, epidermal growth
factor and protein kinase B signaling pathways (20–22). Xu
et al (23) demonstrated that
CD44 is upregulated in GBM and promoted GBM cell growth and
increased resistance to reactive oxygen species- and cytotoxic
agent-induced stress by inhibiting the Hippo signaling pathway.
Although CD44 expression was positively correlated with malignancy
in GBM, higher levels of CD44 were additionally correlated with
decreased survival rates in these patients (24). However, the present study showed no
statistically significant association between CD44 expression and
OS in patients with GBM. The reason may be that the tumorigenicity
of primary GBM differs between
CD44low/CD133high and
CD44high/CD133low for gene expression
profiles (25). Furthermore,
molecular heterogeneity among tumors affects the prognostic value
of CD44 in GBM (26).
CD44 is highly upregulated in prostate, lung and
pancreatic cancer, and is associated with poor prognosis (27–29).
Nevertheless, the expression pattern and functional role of CD44 in
LGG has not been fully elucidated. The present study identified
that a high expression level of CD44 in patients with LGG were
significantly associated with poor OS, and Kaplan-Meier analysis
demonstrated that the expression level of CD44 may be used as a
prognostic marker for patients. A recent study revealed that cells
with low expression level of CD44 exhibited more glioma stem cell
(GSC) traits, suggesting that CD44 is not an appropriate marker for
GSCs, but CD44 can be used for predicting glioma-associated
invasion and migration (30).
In conclusion, CD44 expression levels were
associated with the histopathological grade of gliomas. Higher
expression levels of CD44 mRNA were found in LGG and GBM tissues
compared with normal brain tissues. A high expression level of CD44
mRNA was identified to be associated with a poor survival rate in
LGG. The present results suggested that CD44 may be a novel
prognostic biomarker that may improve OS prediction in LGG and
serve as a potential therapeutic target for glioma. However, a
larger sample size is required to further investigate the present
results. Further experimental studies are required to elucidate the
functions of CD44 in the progression of glioma.
Acknowledgements
Not applicable.
Funding
This project was supported by the Natural Science
Foundation of Gansu (grant nos. 18JR3RA365 and 18JR3RA309), the
Research Fund from The Project of the Health and Family Planning
Commission of Gansu (grant no. GSWSKY-2014-31/2015-58), the Lanzhou
Science and Technology Bureau Project (grant no. 2018-1-109), and
the Doctoral Research Fund and Cuiying Science and Technology fund
of Lanzhou University Second Hospital (grant nos.
YNBSKYJJ2015-1-02/2015-2-11/2015-2-5 and
CY2017-MS12/-MS15/CYXZ-01).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request. The datasets generated and/or analyzed during the current
study are available in the OncoLnc (http://www.oncolnc.org/search_results/?q=CD44) and
GEPIA (http://gepia.cancer-pku.cn/detail.php?gene=&clicktag=survival)
databases.
Authors' contributions
YP, GY and QD conceived the project. QD, QL, MW and
JH performed the experiments. QD, QL and GY analyzed the data. QL,
JH, LN and JD interpreted the data and revised the manuscript. YP,
GY, QL and QD wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by The Ethical Committee of
The Second Hospital of The Lanzhou University (Gansu, China).
Written informed consent was provided by each patient or their
guardians prior to their participation in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarellabranger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ostrom QT, Gittleman H, Xu J, Kromer C,
Wolinsky Y, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United States in 2009–2013. Neuro Oncol. 18
(Suppl_5):v1–v75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van den Bent MJ: Practice changing mature
results of RTOG study 9802: Another positive PCV trial makes
adjuvant chemotherapy part of standard of care in low-grade glioma.
Neuro Oncol. 16:1570–1574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Behin A, Hoangxuan K, Carpentier AF and
Delattre JY: Primary brain tumours in adults. Lancet.
379:1984–1996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roszkowski K, Furtak J, Zurawski B,
Szylberg T and Lewandowska M: Potential role of methylation marker
in glioma supporting clinical decisions. Int J Mol Sci.
17:18762016. View Article : Google Scholar
|
|
6
|
Yuan G, Niu L, Zhang Y, Wang X, Ma K, Yin
H, Dai J, Zhou W and Pan Y: Defining optimal cutoff value of MGMT
promoter methylation by ROC analysis for clinical setting in
glioblastoma patients. J Neurooncol. 133:193–201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Houillier C, Wang X, Kaloshi G, Mokhtari
K, Guillevin R, Laffaire J, Paris S, Boisselier B, Idbaih A,
Laigle-Donadey F, et al: IDH1 or IDH2 mutations predict longer
survival and response to temozolomide in low-grade gliomas.
Neurology. 75:1560–1566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Felsberg J, Wolter M, Seul H, Friedensdorf
B, Göppert M, Sabel MC and Reifenberger G: Rapid and sensitive
assessment of the IDH1 and IDH2 mutation status in cerebral gliomas
based on DNA pyrosequencing. Acta Neuropathol. 119:501–507. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Delgado-López PD and Corrales-García EM:
Survival in glioblastoma: A review on the impact of treatment
modalities. Clin Transl Oncol. 18:1062–1071. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Phan K, Ng W, Lu VM, McDonald KL, Fairhall
J, Reddy R and Wilson P: Association between IDH1 and IDH2
mutations and preoperative seizures in patients with low-grade
versus high-grade glioma: A systematic review and meta-analysis.
World Neurosurg. 111:e539–e545. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun L, Hui AM, Su Q, Vortmeyer A,
Kotliarov Y, Pastorino S, Passaniti A, Menon J, Walling J, Bailey
R, et al: Neuronal and glioma-derived stem cell factor induces
angiogenesis within the brain. Cancer Cell. 9:287–300. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chambless LB, Kistka HM, Parker SL,
Hassam-Malani L, Mcgirt MJ and Thompson RC: The relative value of
postoperative versus preoperative Karnofsky Performance Scale
scores as a predictor of survival after surgical resection of
glioblastoma multiforme. J Neurooncol. 121:359–364. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li G, Shen J, Cao J, Zhou G, Lei T, Sun Y,
Gao H, Ding Y, Xu W, Zhan Z, et al: Alternative splicing of human
telomerase reverse transcriptase in gliomas and its modulation
mediated by CX-5461. J Exp Clin Cancer Res. 37:782018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takano S, Ishikawa E, Sakamoto N, Matsuda
M, Akutsu H, Noguchi M, Kato Y, Yamamoto T and Matsumura A:
Immunohistochemistry on IDH 1/2, ATRX, p53 and Ki-67 substitute
molecular genetic testing and predict patient prognosis in grade
III adult diffuse gliomas. Brain Tumor Pathol. 33:107–116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gulluoglu S, Tuysuz EC and Sahin M:
Simultaneous miRNA and mRNA transcriptome profiling of glioblastoma
samples reveals a novel set of OncomiR candidates and their target
genes. Brain Res 1700. 199–210. 2018.
|
|
16
|
Valder CR, Liu JJ, Song YH and Luo ZD:
Coupling gene chip analyses and rat genetic variances in
identifying potential target genes that may contribute to
neuropathic allodynia development. J Neurochem. 87:560–573. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parkinson H, Sarkans U, Kolesnikov N,
Abeygunawardena N, Burdett T, Dylag M, Emam I, Farne A, Hastings E,
Holloway E, et al: ArrayExpress update-an archive of microarray and
high-throughput sequencing-based functional genomics experiments.
Nucleic Acids Res. 39(Database Issue): D1002–D1004. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sedlazeck FJ, Lee H, Darby CA and Schatz
MC: Piercing the dark matter: Bioinformatics of long-range
sequencing and mapping. Nat Rev Genet. 19:329–346. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dalal S, Zha Q, Daniels CR, Steagall RJ,
Joyner WL, Gadeau AP, Singh M and Singh K: Osteopontin stimulates
apoptosis in adult cardiac myocytes via the involvement of CD44
receptors, mitochondrial death pathway, and endoplasmic reticulum
stress. Am J Physiol Heart Circ Physiol. 306:H1182–H1191. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng C, Zhang Y, Yin J, Li J, Abounader R
and Zuo Z: Regulatory factor X1 is a new tumor suppressive
transcription factor that acts via direct downregulation of CD44 in
glioblastoma. Neuro Oncol. 16:1078–1085. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Monaghan M, Mulligan KA, Gillespie H,
Trimble A, Winter P, Johnston PG and McCormick D: Epidermal growth
factor up-regulates CD44-dependent astrocytoma invasion in vitro. J
Pathol. 192:519–525. 2015. View Article : Google Scholar
|
|
22
|
Zhao LH, Lin QL, Wei J, Huai YL, Wang KJ
and Yan HY: CD44v6 expression in patients with stage II or stage
III sporadic colorectal cancer is superior to CD44 expression for
predicting progression. Int J Clin Exp Pathol. 8:692–701.
2015.PubMed/NCBI
|
|
23
|
Xu Y, Stamenkovic I and Yu Q: CD44
attenuates activation of the Hippo signaling pathway and is a prime
therapeutic target for glioblastoma. Cancer Res. 70:2455–2464.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei KC, Huang CY, Chen PY, Feng LY, Wu TW,
Chen SM, Tsai HC, Lu YJ, Tsang NM, Tseng CK, et al: Evaluation of
the prognostic value of CD44 in glioblastoma multiforme. Anticancer
Res. 30:253–259. 2010.PubMed/NCBI
|
|
25
|
Fu J, Yang QY, Sai K, Chen FR, Pang JC, Ng
HK, Kwan AL and Chen ZP: TGM2 inhibition attenuates ID1 expression
in CD44-high glioma-initiating cells. Neuro Oncol. 15:1353–1365.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishikawa M, Inoue A, Ohnishi T, Kohno S,
Ohue S, Matsumoto S, Suehiro S, Yamashita D, Ozaki S, Watanabe H,
et al: Significance of Glioma Stem-Like cells in the tumor
periphery that express high levels of CD44 in tumor invasion, early
progression, and poor prognosis in glioblastoma. Stem Cells Int
2018. 53870412018.
|
|
27
|
Zeng Y, Wodzenski D, Gao D, Shiraishi T,
Terada N, Li Y, Vander Griend DJ, Luo J, Kong C, Getzenberg RH and
Kulkarni P: Stress-response protein RBM3 attenuates the stem-like
properties of prostate cancer cells by interfering with CD44
variant splicing. Cancer Res. 73:4123–4133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo JY, Hsu HS, Tyan SW, Li FY, Shew JY,
Lee WH and Chen JY: Serglycin in tumor microenvironment promotes
non-small cell lung cancer aggressiveness in a CD44-dependent
manner. Oncogene. 36:2457–2471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao S, Chen C, Chang K, Karnad A,
Jagirdar J, Kumar AP and Freeman JW: CD44 expression level and
isoform contributes to pancreatic cancer cell plasticity,
invasiveness and response to therapy. Clin Cancer Res.
22:5592–5604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang HH, Liao CC, Chow NH, Huang LL,
Chuang JI, Wei KC and Shin JW: Whether CD44 is an applicable marker
for glioma stem cells. Am J Transl Res. 9:4785–4806.
2017.PubMed/NCBI
|