Introduction
The development of immune checkpoint inhibition has
led to important clinical advances in the treatment of advanced
solid tumors (1). However, patients
with recurrent ovarian cancer have responded poorly to single-agent
immune checkpoint blockade to date (2). Challenges to this strategy include the
distinct immune microenvironment that each ovarian cancer patient
may have and the lack of reliable biomarkers (1).
Tumors with DNA repair deficiency, e.g., mismatch
repair defects, are recognized to have high mutational load,
express more neoantigens, and are potentially susceptible to immune
checkpoint inhibitors (3). Tumor
mutational burden and associated neoantigen expression correlate
with the clinical activity of immune checkpoint blockade in lung
cancer and melanoma (4,5). In ovarian cancer, germline BRCA
mutation (gBRCAm)-related high-grade serous ovarian cancer (HGSOC)
has higher mutational load and associated neoantigen expression
compared with BRCA wild-type (BRCAwt) disease (6), which may lead to recruitment of
tumor-infiltrating lymphocytes (TILs) and host immune response
(6). In support of this, increased
intratumoral CD3+ TILs are present in gBRCAm HGSOC but
not in BRCAwt tumors (7).
HGSOC is an immunogenic tumor (8). The presence of T cells in the tumor
microenvironment is associated with improved survival (8). Immunosuppressive pathways, such as
regulatory T cells (Tregs) and myeloid-derived suppressor cells
(MDSCs), are prominent in HGSOC; these can be barriers to antitumor
immunity and adversely affect clinical outcomes (9). Studies also suggest that a high density
of Tregs is associated with a poor prognosis, possibly due to their
suppressive effects on antitumor cytotoxic T cells (10,11).
Additionally, MDSCs play a key immunosuppressive role in various
types of cancer, including ovarian cancer. Wu et al reported
ovarian cancer patients had significantly higher numbers of MDSCs
in both peripheral blood and ascites compared to healthy donors,
and ovarian cancer patients with higher levels of monocytic MDSC
had a shorter relapse-free survival (12). Thus, the characterization of MDSC
phenotypes and their generation in blood and/or ascites from
recurrent ovarian cancer remains to be elucidated.
gBRCAm status is a favorable prognostic factor in
HGSOC due to platinum-based chemosensitivity within the first
decade after diagnosis. However, more recent data suggest that
gBRCAm status may have a negative prognostic impact on
disease-specific and all cause-survival beyond a decade
post-diagnosis (13). DNA repair
deficiency in tumors is found to be predictive of higher 5-year
survival probability, but at 10 years post-diagnosis, the benefit
appears to be lost (6). It is
possible that changes in the immune milieu may partly contribute to
the lack of long-term survival benefit in these patients. We
therefore hypothesize that the gBRCAm HGSOC is associated with a
more robust circulating immune response during their early disease
course, compared to BRCAwt disease.
The immune system responds dynamically to variations
in the tumor microenvironment (14).
Many studies indicate that altered compositions of peripheral
immune cells, e.g., lymphocyte proportion, neutrophil proportion,
and neutrophil-to-lymphocyte ratios in the peripheral blood, are
potential markers for survival in cancer patients (15,16).
Thus, monitoring these varying immune responses over time and
treatment may uncover new vulnerabilities. In this pilot study, our
aim was to quantify the immune subsets and functional markers using
blood samples from HGSOC patients.
Materials and methods
Patients and isolation of PBMCs
This study was approved by the Institutional Review
Boards of the Center for Cancer Research, National Cancer Institute
and Dana-Farber Cancer Institute (DFCI). Peripheral blood
mononuclear cells (PBMC) were collected before treatment from
recurrent HGSOC patients enrolled on one of the two phase I PARP
inhibitor olaparib trials at the Clinical Center of National Cancer
Institute (NCT01445418 and NCT01237067) for Cohort 1 (17,18). All
patients had at least a 4 weeks wash-out period from previous
therapy before enrollment. BRCA mutation status was confirmed by a
commercial BRCA testing (Myriad Genetic Laboratories) prior to
enrollement on study.
To examine the findings from Cohort 1 in a broad
patient population, PBMC samples were also obtained from unselected
advanced stage or recurrent ovarian cancer patients enrolled on the
blood collection protocols at DFCI or National Cancer Institute
(Cohort 2). Blood samples were collected in cell preparation tubes
with sodium citrate (BD Vacutainer CPT Tubes; BD Biosciences).
PBMCs were isolated and viably frozen within 2 h from collection at
National Cancer Institute and within 24 h for the samples collected
at the DFCI; a minimum of 1×105 cells were acquired for
each analysis. All patients reviewed and signed an informed consent
form approved by the National Cancer Institute or DFCI
Institutional Review Board for collection of blood samples.
Flow cytometric analysis
Multiparameter flow cytometric analysis was
performed as described previously (19). Briefly, cells were incubated with
LIVE/DEAD Fixable Aqua Dead Cell Stain (1:100 dilution) (Thermo
Fisher Scientific, Inc.) for 15 min at 4°C, then incubated with Fc
receptor blocking agent (1:10 dilution) (Miltenyi Biotec) and
stained for 20 min at 4°C in a dark room with the monoclonal
antibodies listed in Table SI. For
intracellular staining for Foxp3 expression, cells were fixed and
permeabilized using a Fix/Perm buffer (eBiosciences) according to
the manufacturer's instructions, then stained with anti-Foxp3
antibody. The immunophenotypic markers used to define immune cell
subsets are listed in Table SII.
All antibodies were purchased from BioLegend. Live cells were
discriminated by means of the LIVE/DEAD stain, and dead cells were
excluded from all analyses. All flow cytometric analyses were
performed using a MACSQuant Analyzer (Miltenyi Biotec). As
indicated, flow cytometric data were quantified either as a
percentage of cells or as the median fluorescence intensity (MFI).
Data were analyzed using FlowJo software (FlowJo LLC.).
Statistical analysis
An exact Wilcoxon rank sum test was used to compare
differences in marker values between gBRCAm and BRCAwt patients.
All statistical tests were two-tailed and the reported P-values are
calculated using a formal correction for multiple comparisons
(i.e., the Holm-Bonferroni method). This method sorts P-values in
ascending order and compares them to a corresponding pre-defined
value. Since there were 13 distinct hypotheses to be tested within
each immune subset group, statistically significant P-values in our
case were considered to be Pi<0.05/i, where i ranges
from 13 to 1 in descending order. Thus, in order to declare
statistical significance, the following significance thresholds
were calculated: P1<0.003846,
P2<0.004167, P3<0.004545, …,
P13<0.05, for the first, second, third …, and
thirteenth tested hypothesis, respectively.
The Kaplan-Meier method was used to obtain estimates
of progression-free survival (PFS). PFS curves were compared with a
two-tailed log-rank test (α=0.05). We separated marker values at
their corresponding observed baseline median values. An
unstratified Cox regression model was used to estimate the hazard
ratio (HR) for the high marker group relative to the low marker
group. All analyses were performed using GraphPad Prism software
version 6.0 (GraphPad Software Inc.).
Results
Patients
Patient characteristics are detailed in Tables I and II. Cohort 1 contains pretreatment samples
from 41 heavily pretreated recurrent HGSOC patients (16 gBRCAm
[39%]; 25 BRCAwt [61%]). The median time from initial diagnosis was
4.27 years (<5 years [54% (22/41)] vs. >5 years
post-diagnosis [46% (19/41)]) upon enrollment. Of the 19 patients
who had more than 5 years from diagnosis, 17 patients relapsed
within 5 years. Cohort 2 represents samples from unselected either
primary or recurrent ovarian cancer patients in whom approximately
25% were gBRCAm carriers. The median time from initial diagnosis
for all patients was 3.2 years (Table
II). For Cohort 2, most patients (75% [55/73]) were <5 years
post-diagnosis. Of the 18 patients who had more than 5 years from
diagnosis, 17 patients relapsed within 5 years. We therefore chose
a 5-year cut-off as a surrogate of the first decade after diagnosis
given all except one patient had less than 10 years of
follow-up.
 | Table I.Cohort 1 patient characteristics. |
Table I.
Cohort 1 patient characteristics.
|
Characteristics | gBRCAm (n=16) | BRCAwt (n=25) | All (n=41) |
|---|
| Age, median
(range), years | 52 (28–71) | 65 (49–73) | 65 (28–73) |
| Tumor status |
|
|
|
| Primary
stage III/IV | 0 | 0 | 0 |
|
Recurrent | 16 (100%) | 25 (100%) | 41 (100%) |
| Histology |
|
|
|
|
HGSOC | 16 (100%) | 25 (100%) | 41 (100%) |
| Clear
cell | 0 | 0 | 0 |
| Years from initial
diagnosis |
|
|
|
| <5
years | 10 (63%) | 12 (48%) | 22 (54%) |
| ≥5
years | 6
(37%) | 13 (52%) | 19 (46%) |
| Number of previous
lines of therapy, median (range) | 5 (2–8) | 7
(3–14) | 6
(2–14) |
| Platinum
sensitivity |
|
|
|
|
Platinum-sensitive recurrent
disease | 5
(31%) | 8
(32%) | 13 (32%) |
|
Platinum-resistant recurrent
disease | 11 (69%) | 17 (68%) | 28 (68%) |
| Prior
bevacizumab |
|
|
|
|
Yes | 5
(31%) | 19 (76%) | 24 (58%) |
| No | 11 (69%) | 6
(24%) | 17 (42%) |
| Prior immune
checkpoint inhibitors | 0 | 0 | 0 |
 | Table II.Cohort 2 patient characteristics. |
Table II.
Cohort 2 patient characteristics.
|
Characteristics | gBRCAm (n=18) | BRCAwt (n=55) | All (n=73) |
|---|
| Age, median
(range), years | 57.5 (36–78) | 65 (30–84) | 64 (30–84) |
| Tumor status |
| Primary
stage III/IV | 1 (6%) | 8
(15%) | 9
(12%) |
|
Recurrent | 17 (94%) | 47 (85%) | 64 (88%) |
| Histology |
|
|
|
|
HGSOC | 18 (100%) | 52 (95%) | 70 (96%) |
| Clear
cell | 0 | 3 (5%) | 3 (4%) |
| Years from initial
diagnosis |
|
|
|
| <5
years | 13 (72%) | 42 (76%) | 55 (75%) |
| ≥5
years | 5
(28%) | 13 (24%) | 18 (25%) |
| Number of previous
lines of therapy, median (range) | 3
(1–11) | 5
(1–14) | 4
(1–14) |
| Platinum
sensitivity |
|
|
|
| On
active treatment with first line carboplatin/taxol vs. | 0 (0%) | 8
(15%) | 8
(10%) |
|
Platinum-sensitive recurrent
disease vs. | 9
(50%) | 7
(13%) | 16 (22%) |
|
Platinum-resistant recurrent
disease | 9
(50%) | 40 (72%) | 49 (68%) |
| Prior
bevacizumab |
|
|
|
|
Yes | 7
(64%) | 28 (51%) | 35 (48%) |
| No | 11 (36%) | 27 (49%) | 38 (52%) |
| Prior CTLA-4
inhibitor, prior vaccine and/or PD-1/PDL-1 blockade | 1
(5.6%) | 2
(3.6%) | 3
(4.1%) |
Peripheral immune characteristics of
recurrent HGSOC patients (Cohort 1)
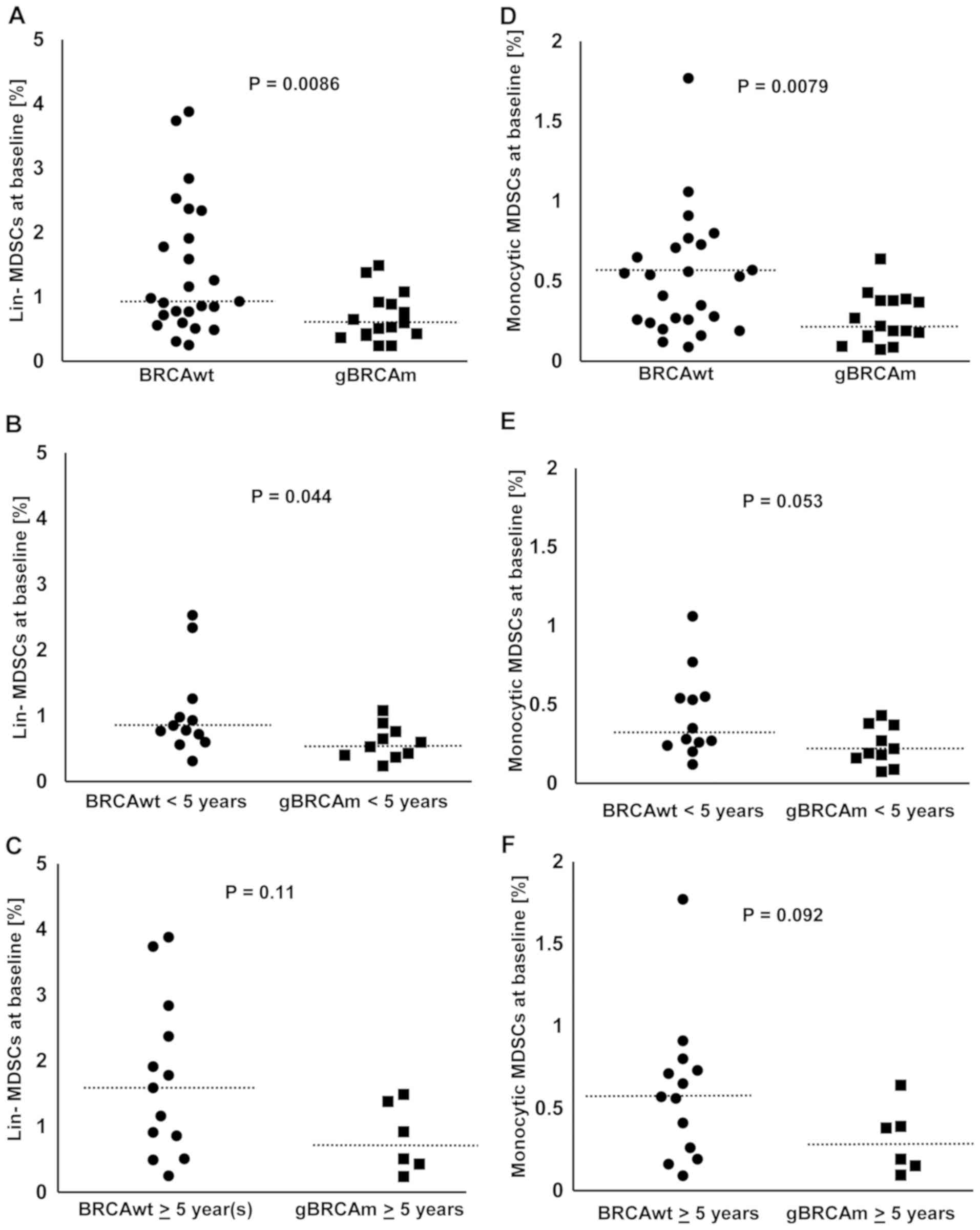
The percentage of MDSCs among viable
CD45+ PBMCs was lower in gBRCAm HGSOC compared to BRCAwt
disease (Fig. 1A-C). The percentage
of lineage (lin)-MDSCs was overall lower in gBRCAm carriers (BRCAwt
vs. gBRCAm, median 0.93 vs. 0.565%, p=0.0086; Fig. 1A). This difference was observed in
gBRCAm patients <5 years post-diagnosis (BRCAwt vs. gBRCAm,
median 0.815 vs. 0.565%, p=0.044; Fig.
1B) but not at >5 years post-diagnosis (Fig. 1C). Similarly, the percentage of
monocytic MDSCs was overall lower in gBRCAm carriers (BRCAwt vs.
gBRCAm, median 0.53 vs. 0.205%, p=0.0079; Fig. 1D). There was a trend of difference
observed in gBRCAm patients <5 years post-diagnosis (Fig. 1E) but not at >5 years
post-diagnosis (Fig. 1F).
Additionally, gBRCAm HGSOC patients had fewer circulating lin-MDSCs
and monocytic MDSCs independent of platinum-sensitivity and prior
exposure to bevacizumab (Fig.
S1A-D).
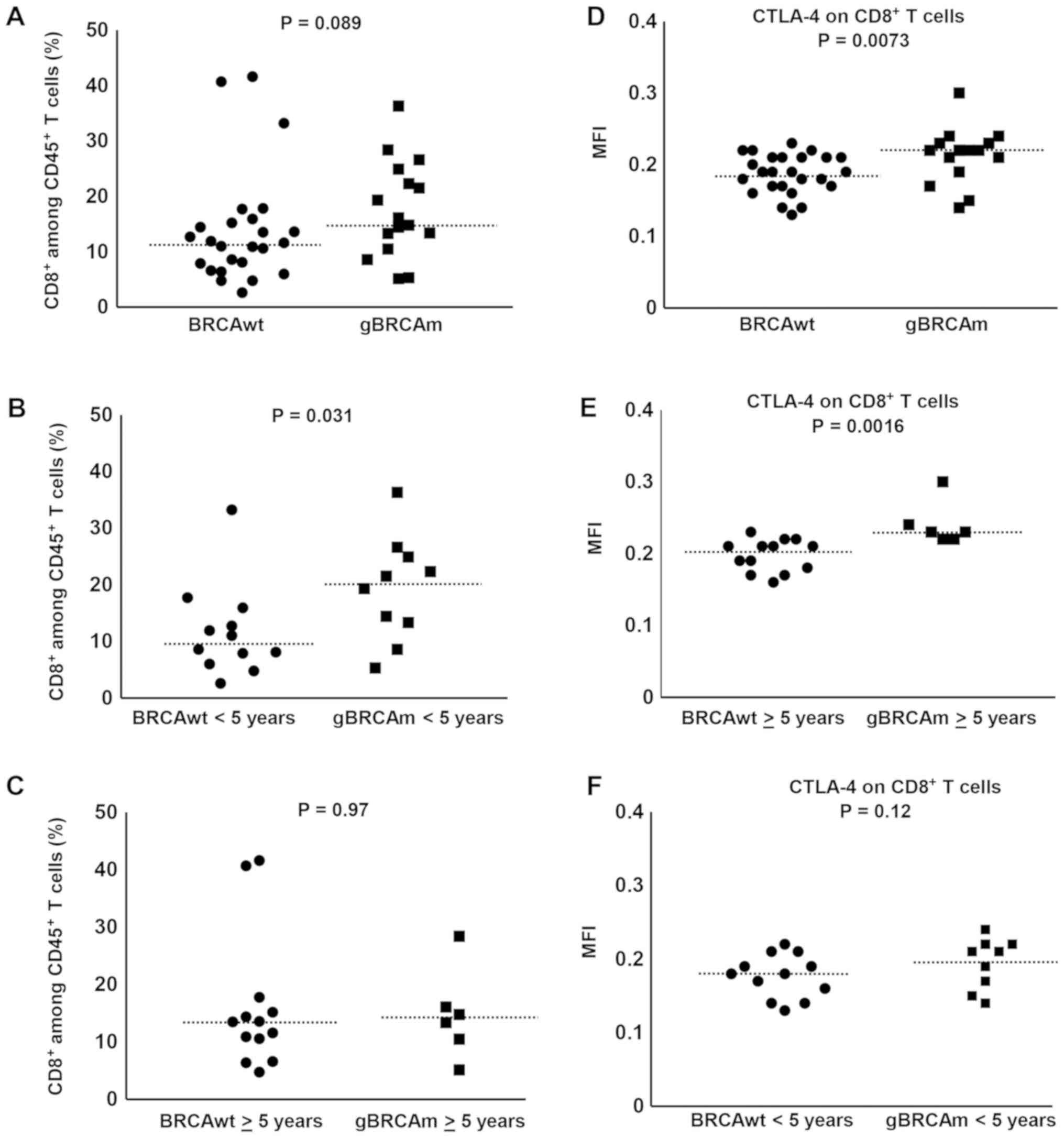
Overall, there was a trend of difference observed in
gBRCAm patients in the percentage of circulating CD8+ T
cells among viable CD45+ T cells (Fig. 2A) independent of platinum-sensitivity
and prior exposure to bevacizumab (Fig.
S1E-H). The percentage of circulating CD8+ T cells
among viable CD45+ T cells was higher in gBRCAm carriers
<5 years post-diagnosis over BRCAwt (BRCAwt vs. gBRCAm, median
9.78 vs. 20.4%, p=0.031; Fig. 2B).
This difference was lost in survivors >5 years post-diagnosis
(Fig. 2C). Further, there was
significantly higher expression of CTLA-4 on CD8+ T cells in gBRCAm
carriers (Fig. 2D) and this
difference remained in gBRCAm carriers >5 years post-diagnosis
compared to BRCAwt patients (BRCAwt vs. gBRCAm, median fluorescence
intensity (MFI) 0.21 vs. 0.23, p=0.0016; Fig. 2E). This difference was lost in
survivors <5 years post-diagnosis (Fig. 2F). Additionally, gBRCAm HGSOC
patients had higher CTLA-4+ CD8+ T cells independent of
platinum-resistant disease and prior exposure to bevacizumab
(Fig. S1I-L).
We next evaluated the expression of other
immunosuppressive markers, e.g., TIM-3 and PD-1 on CD8+ T cells. No
significant differences were observed between gBRCAm and BRCAwt
patients in expression of TIM-3 and PD-1 on CD8+ T cells
(Fig. S2A-F).
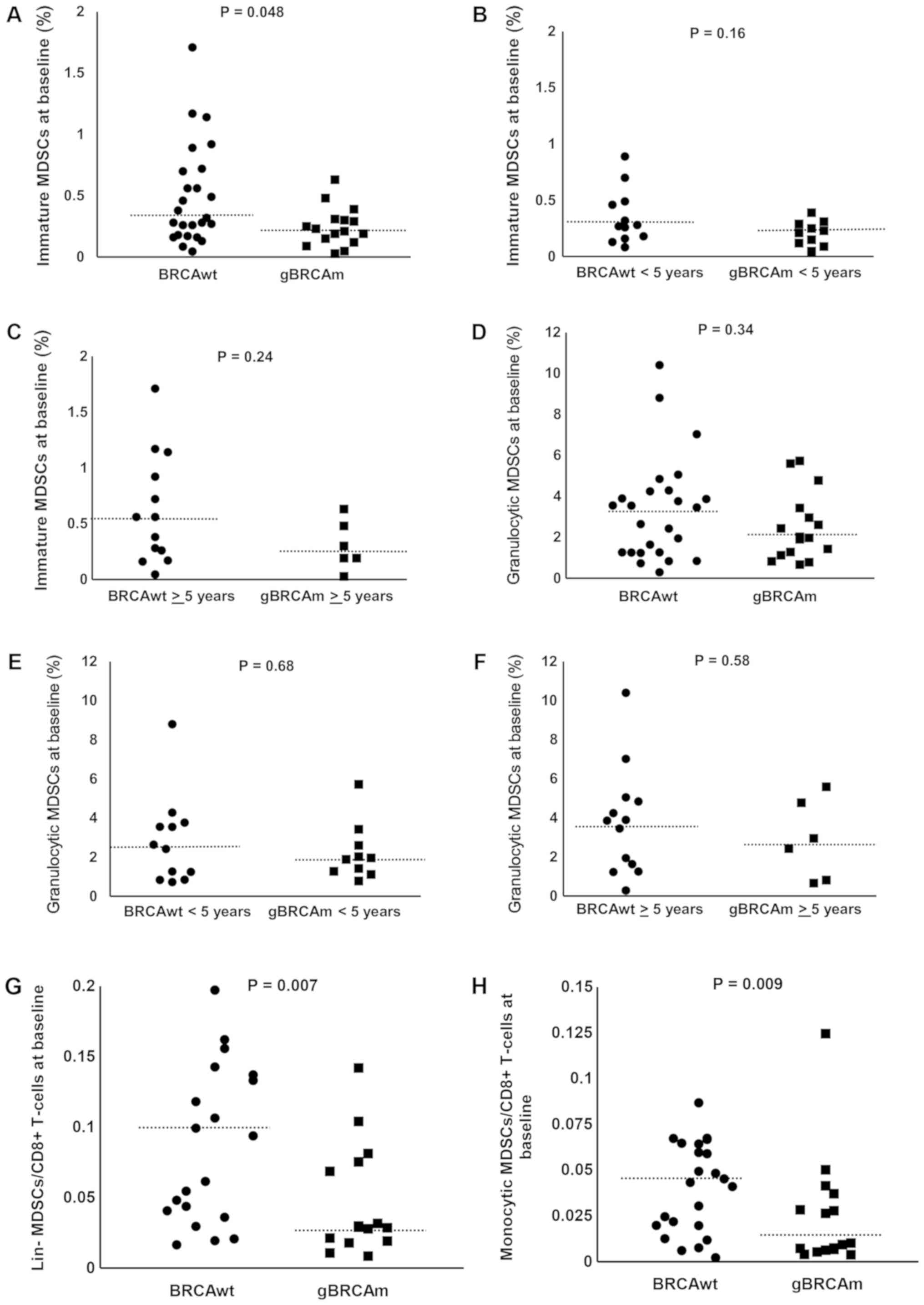
No significant differences were observed between
gBRCAm and BRCAwt patients in the percentage of immature MDSCs
(Fig. 3A-C). No significant
differences were observed between gBRCAm and BRCAwt patients in the
percentage of granulocytic MDSCs (Fig.
3D-F). We also evaluated the ratio of each subtype of MDSCs to
circulating CD8+ T cells. There was a trend of
difference observed in gBRCAm patients in the ratio of lin- and
monocytic MDSCs to CD8+ T cells (Fig. 3G and H) but no other differences in
proportion of MDSCs were found (data not shown). No difference in
the percent/proportion of Tregs was found between gBRCAm and BRCAwt
patients (data not shown).
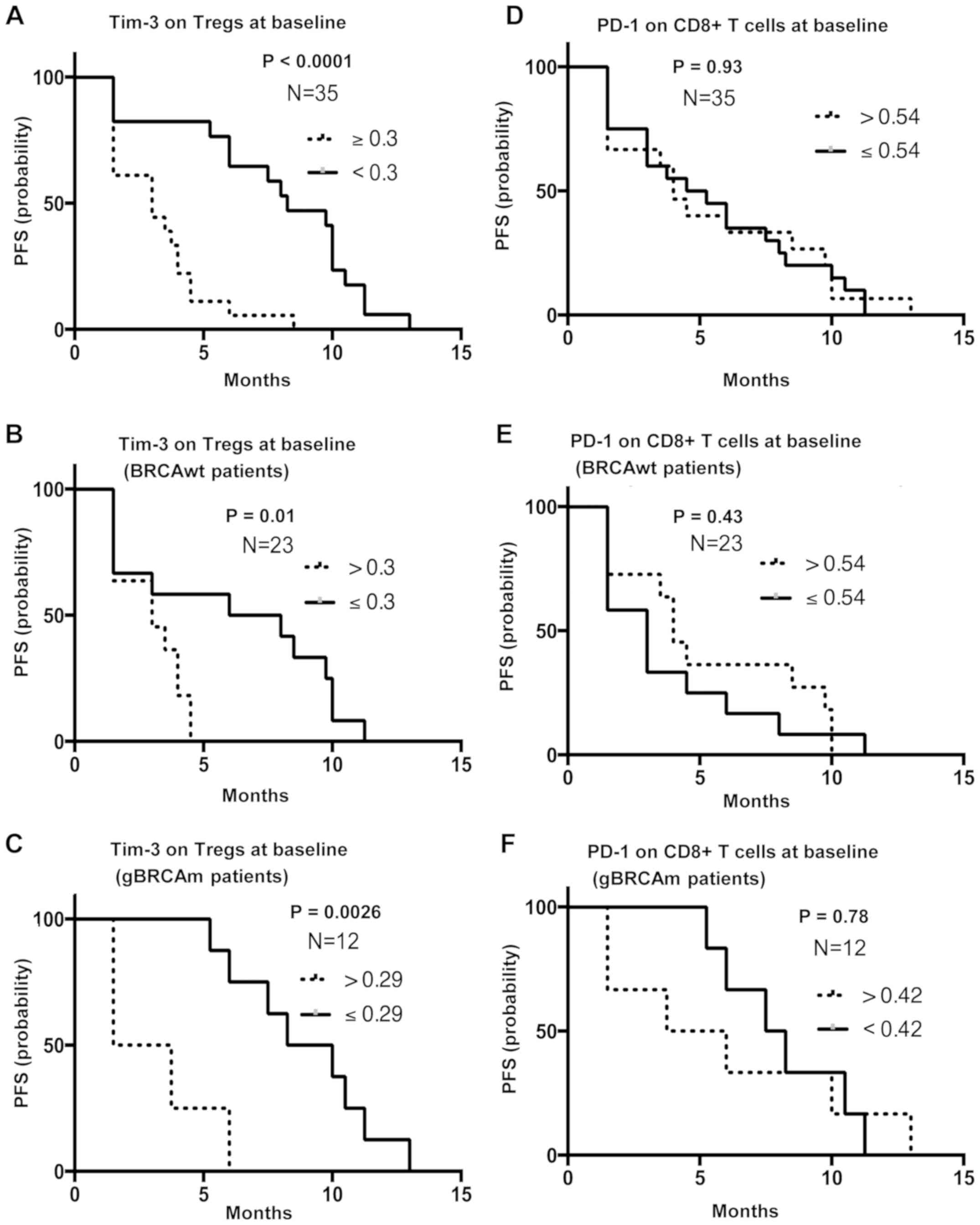
High baseline TIM-3 expression on
Tregs is associated with poor PFS, independent of gBRCAm
status
PFS analysis was performed using survival and
progression data from Cohort 1. Patients with greater than and
equal to the median MFI of TIM-3 on Tregs (n=18) were associated
with poor survival compared to those with lower than the median MFI
[n=17; median PFS 3 mo (1.5–8.5 mo) vs. 8.25 mo (1.5–13 mo);
P<0.0001; HR, 0.2; 95% CI, 0.09–0.46; P<0.001, Fig. 4A]. This difference was observed in
both BRCAwt and gBRCAm patients (HR, 0.3; 95% CI, 0.1–0.88;
P=0.021; Fig. 4B, and HR, 0.09; 95%
CI, 0.015–0.55; P=0.007; Fig. 4C,
respectively). Other immune subsets did not differ between the two
groups. Specifically, no significant differences were observed in
PD-1 on CD8+ T cells expression, CTLA-4 on Tregs
expression or percentage of granulocytic MDSCs between gBRCAm and
BRCAwt patients (Fig. 4D-F and
Fig. S3A-F).
Peripheral immune characteristics of
Cohort 2 unselected primary or recurrent HGSOC patients
We next examined our findings from Cohort 1 in the
unselected Cohort 2 ovarian cancer patient population who enrolled
on the blood collection protocols (Table II). Approximately 88% (64 of 73) had
recurrent HGSOC and 25% (18 of 73) were gBRCAm carriers. The
percentage of MDSCs among single CD45+ viable cells was
not significantly different (data not shown). CTLA-4, PD-1 or TIM-3
expression on CD8+ T cells did not show any differences
in gBRCAm HGSOC patients compared to BRCAwt HGSOC regardless of
platinum-sensitivity, prior exposure to bevacizumab or years from
initial diagnosis (data not shown). Survival and progression data
were not available for PFS analysis.
Discussion
Patients with gBRCAm ovarian cancer have increased
therapeutic susceptibility to platinum agents and have a longer
median survival time compared to those without gBRCAm. This earlier
chemosensitivity does not appear to persist into long-term survival
beyond a decade from diagnosis (20). Our findings suggest that gBRCAm
recurrent HGSOC patients may have fewer circulating
immunosuppressive MDSCs and more CD8+ T cells early in
their disease course. This is the description of a potentially
active immune microenvironment that could enhance response to
therapy. The loss of this benefit and/or equivocation over time
could presage immune exhaustion and reduced treatment
susceptibility. It is possible that the loss of this differential
coincides with the progressive loss of platinum sensitivity and
multiplicity of treatment regimens, and may be partly associated
with immune tolerance and loss of long-term survival advantage.
There is correlative evidence that native host
anti-tumor immune mechanisms play a role in clinical outcome of
epithelial ovarian cancer; the presence of greater intra-tumoral
CD3+ T cell infiltrates is shown to prognosticate
improved outcome in advanced ovarian cancer (8). Ovarian tumors with dense
CD3+ CD8+ T cell infiltrates are strongly
associated with favorable clinical outcomes; the five-year overall
survival rate was 38% among patients whose tumors contained
CD3+ T cells and 4.5% among patients whose tumors
contained no T cells (P<0.001) (21). Also, gBRCAm HGSOC is shown to have a
better prognosis when there is increased immune cell infiltrate in
tumors (7,22). Hwang et al reported that a
lack of intraepithelial TILs was associated with a worse survival
outcome among ovarian cancer patients (pooled HR: 2.24, 95% CI;
1.71–2.91) (23). In support,
increased Treg infiltration is related to poor prognosis in ovarian
cancer (24). These data suggest
that host immunity may play a role in delaying or preventing tumor
recurrence after standard treatment and that immunosuppressive
cells suppress the host anti-tumor immunity, leading to poor
outcomes.
In our pilot study, gBRCAm HGSOC patients had fewer
circulating MDSCs and a concomitant increase in circulating CD8+ T
cells during their early disease course. The low numbers of MDSCs
in gBRCAm patients may indicate a lack of widespread
immunosuppression. MDSCs are immunosuppressive cells, known to
down-regulate anti-tumor immunity (25). MDSCs represent a heterogeneous family
of myeloid cells that suppress T cell immunity in tumor-bearing
hosts and promote cancer cell proliferation, epithelial-mesenchymal
transition, and tumor dissemination (26), and that promote immune suppression in
epithelial ovarian cancer mouse models (27). Furthermore, MDSCs suppress the
antigen-specific T cell response induced by both CD4+
and CD8+ T cells, and elevated concentrations of MDSCs
are detected in the peripheral blood of cancer patients when
compared with normal controls (28,29).
Also, it has been proposed that MDSCs may enhance the ovarian
cancer stem cell pool and thus increase the risk of relapse
(30).
Additionally, our findings showed a higher
expression of CTLA-4 on CD8+ T cells in gBRCAm carriers.
Higuchi et al reported a CTLA-4 antibody, but not PD-1/PD-L1
blockade, synergized therapeutically with a PARP inhibitor,
resulting in immune-mediated tumor clearance and improved survival
in immunocompetent BRCA1-deficient murine ovarian cancer models
(31). In this report, authors
demonstrated the survival benefit of this combination was likely
T-cell mediated and dependent on increases in local interferon
(IFN)-γ production in the peritoneal tumor microenvironment. BRCA1
regulates IFN-γ signaling, upregulating the signal transducers and
activators of transcription (STAT)1 and STAT2, involved in type I
IFN signaling and activation of innate immune responses (32). Xu and colleagues also reported loss
of BRCA2 upregulates a subset of IFN-related genes, e.g., APOBEC3F
and APOBEC3G in BRCA2 knockout HCT116 colorectal carcinoma cells
(33). The role of BRCA1 and BRCA2
in regulating IFN-γ signaling and immunosuppressive cells, along
with associated clinical outcomes remains to be further
elucidated.
There are preclinical data suggesting that DNA
damages induced by platinum agents or PARP inhibition activate
cGAS/STING pathway, resulting in activation of type I IFN and
immune responses (34,35). Ding et al (34) reported PARP inhibition induces both
adaptive and innate immune responses through a STING-dependent
antitumor immune response in BRCA1-deficient ovarian mouse models.
gBRCAm HGSOC is shown to have a higher tumor mutational burden and
neoantigen expression compared with BRCAwt disease (6), which may further induce T cell
activation and anti-tumor immunity. These findings suggest the
PARPi and/or carboplatin in combination with immune checkpoint
blockade may be a therapeutic opportunity for subsets of ovarian
cancer patients. Further preclinical and clinical studies are
warranted to explore this possibility.
Shifting the immune balance by the interruption of
pro-tumor immunosuppression can enhance anti-tumor immunity, as
shown in melanoma (36). The
PD-1/PD-L1 interaction promotes this imbalance favoring
tumor-mediated immunosuppression (37). Although we did not find any
differences in peripheral PD-L1 expression on immune cells in our
study, Strickland et al reported a significantly higher
expression of PD-1 and PD-L1 on intraepithelial and peritumoral
immune cells in gBRCAm ovarian cancer compared with HR-proficient
tumors (7). PD-1/PD-L1 axis
upregulation causes ‘exhaustion’ of T cells allowing cancer growth
and disruption of CTL-mediated tumor killing, which may partly
contribute to a poor prognosis in advanced ovarian cancers
(38–43). However, our post-hoc correlative
findings should be interpreted with caution and viewed as
hypothesis generating because of the small number of samples we
assessed.
Recent studies demonstrated an important role of
TIM-3 T cell exhaustion in cancer (44–46). Wu
and colleagues investigated the expression of TIM-3 on peripheral
CD4+ T and CD8+ T cells in ovarian cancer and
showed elevated expression of TIM-3 in T cells were associated with
advanced stage and a higher tumor grade (poorly differentiated)
(44). Kuchroo et al reported
TIM-3 and PD-1 were coexpressed on CD8+ TILs in mice
bearing transplanted tumors as well as on NY-ESO-1-specific
CD8+ T cells in patients with advanced melanoma
(46). Consistent with these
reports, our current study showed higher TIM-3 was associated with
poor prognosis in recurrent ovarian cancer patients, suggesting
TIM-3 negative regulation on various T cell subsets. Blockade of
TIM-3 pathways therefore may be an effective strategy in
controlling tumor growth.
Our study has some limitations. First, our small
sample size and less than a decade follow-up in most cases may
introduce biases in estimating clinical benefit and our correlative
endpoints were exploratory. Also, we were not able to complete the
subgroup analysis e.g., gBRCA1m vs. gBRCA2m due to the small sample
size at this time; future studies on this topic are warranted given
possible survival differences between gBRCA1m and gBRCA2m carriers
(47). We note that the small sample
sizes in the present study prevent the statistical analysis from
being extrapolated to the overall gBRCAm and BRCAwt patient
populations. Therefore, it is possible that this limitation may
affect the clinical and statistical significance of our findings.
Secondly, we did not assess tissue immune subsets due to limited or
unavailable tissue samples, and thus we cannot address how many of
our patients may have both tumoral and peripheral immune exhaustion
characteristics. Further, we did not perform transcriptome on
clinical samples to evaluate gene signatures for T cell activation
or exhaustion status (48). Lastly,
we did not observe similar findings across immune subsets in Cohort
2, an unselected ovarian cancer sample set, most likely reflecting
the more substantial heterogeneity of that population as well as
the dynamic changes of peripheral immune microenvironment between
newly diagnosed and progressively treated patients. Also, Cohort 2
patients did not have a 4-week washout from previous or active
treatment prior to blood sample collection that may have made
findings difficult to interpret. Examination of these parameters
has been prospectively planned into our ongoing phase 2 clinical
trial of the PD-L1 inhibitor, durvalumab, with the PARP inhibitor,
olaparib, and/or a VEGFR inhibitor, cediranib (NCT02484404), in
which we collect baseline and on-treatment tissues and blood
samples from recurrent ovarian cancer patients.
Overall, disease outcome is influenced by both
patient host and tumor characteristics (20). Among many characteristics, our pilot
data suggest that fewer circulating MDSCs and higher CD8+ T cells
among total PBMCs may be associated with favorable early clinical
outcome of gBRCAm HGSOC patients. It is possible that changes in
the immune milieu over the course of the disease contribute to the
lack of long-term survival benefit. Further studies focusing on
HGSOC are needed to further elucidate the long-term impact of
immune factors on survival of gBRCAm HGSOC patients.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Seth Steinberg
(Biostatistics and Data Management Section, Office of the Clinical
Director, Center for Cancer Research) for his statistical
input.
Funding
The present study was funded by the Intramural
Program of the Center for Cancer Research, NCI, NIH (JML; grant no.
ZIA BC011525) and the Division of Gynecologic Oncology at the
Dana-Farber Cancer Institute (UM, LAM and KMM).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JML, DAB, YT, AY, MJL, ECK, CMA, UM, LAM, JRN, KMM
and JBT contributed to the conception and design of the present
study, and to the collection and assembly of data. JML, DAB, YT,
AY, MJL, ECK, CMA, UM, LAM, JRN, KMM and JBT performed the data
analysis and interpretation, and contributed to writing the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Boards of the Center for Cancer Research, National Cancer Institute
and Dana-Farber Cancer Institute (DFCI). All patients reviewed and
signed an informed consent form approved by the National Cancer
Institute or DFCI Institutional Review Board for collection of
blood samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee JM, Ivy SP and Kohn EC: Challenges and
opportunities for immunotherapies in gynecologic cancers. Oncology
(Williston Park). 30:67–69. 2016.PubMed/NCBI
|
|
2
|
Bourla AB and Zamarin D: Immunotherapy:
New strategies for the treatment of gynecologic malignancies.
Oncology (Williston Park). 30:59–66, 69. 2016.PubMed/NCBI
|
|
3
|
Llosa NJ, Cruise M, Tam A, Wicks EC,
Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS,
et al: The vigorous immune microenvironment of microsatellite
instable colon cancer is balanced by multiple counter-inhibitory
checkpoints. Cancer Discov. 5:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schumacher TN and Schreiber RD:
Neoantigens in cancer immunotherapy. Science. 348:69–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patch AM, Christie EL, Etemadmoghadam D,
Garsed DW, George J, Fereday S, Nones K, Cowin P, Alsop K, Bailey
PJ, et al: Whole-genome characterization of chemoresistant ovarian
cancer. Nature. 521:489–494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Strickland KC, Howitt BE, Shukla SA, Rodig
S, Ritterhouse LL, Liu JF, Garber JE, Chowdhury D, Wu CJ, D'Andrea
AD, et al: Association and prognostic significance of
BRCA1/2-mutation status with neoantigen load, number of
tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high
grade serous ovarian cancer. Oncotarget. 7:13587–13598. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Conejo-Garcia JR, Katsaros D,
Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H,
Schlienger K, Liebman MN, et al: Intratumoral T cells, recurrence,
and survival in epithelial ovarian cancer. N Engl J Med.
348:203–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sucheston-Campbell LE, Cannioto R, Clay
AI, Etter JL, Eng KH, Liu S, Battaglia S, Hu Q, Szender JB,
Minlikeeva A, et al: No evidence that genetic variation in the
myeloid-derived suppressor cell pathway influences ovarian cancer
survival. Cancer Epidemiol Biomarkers Prev. 26:420–424. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, et al: Specific recruitment of regulatory T cells in
ovarian carcinoma fosters immune privilege and predicts reduced
survival. Nat Med. 10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barnett BG, Rüter J, Kryczek I, Brumlik
MJ, Cheng PJ, Daniel BJ, Coukos G, Zou W and Curiel TJ: Regulatory
T cells: A new frontier in cancer immunotherapy. Adv Exp Med Biol.
622:255–260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu L, Deng Z, Peng Y, Han L, Liu J, Wang
L, Li B, Zhao J, Jiao S and Wei H: Ascites-derived IL-6 and IL-10
synergistically expand CD14+HLA-DR-/low myeloid-derived suppressor
cells in ovarian cancer patients. Oncotarget. 8:76843–76856.
2017.PubMed/NCBI
|
|
13
|
Candido-dos-Reis FJ, Song H, Goode EL,
Cunningham JM, Fridley BL, Larson MC, Alsop K, Dicks E, Harrington
P, Ramus SJ, et al: Germline mutation in BRCA1 or BRCA2 and
ten-year survival for women diagnosed with epithelial ovarian
cancer. Clin Cancer Res. 21:652–657. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qi L, Li B, Dong Y, Xu H, Chen L, Wang H,
Li P, Zhao W, Gu Y, Wang C and Guo Z: Deconvolution of the gene
expression profiles of valuable banked blood specimens for studying
the prognostic values of altered peripheral immune cell proportions
in cancer patients. PLoS One. 9:e1009342014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Showe MK, Kossenkov AV and Showe LC: The
peripheral immune response and lung cancer prognosis.
OncoImmunology. 1:1414–1416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sarraf KM, Belcher E, Raevsky E, Nicholson
AG, Goldstraw P and Lim E: Neutrophil/lymphocyte ratio and its
association with survival after complete resection in non-small
cell lung cancer. J Thorac Cardiovasc Surg. 137:425–428. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JM, Hays JL, Annunziata CM, Noonan AM,
Minasian L, Zujewski JA, Yu M, Gordon N, Ji J, Sissung TM, et al:
Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2
mutation-associated breast or ovarian cancer with biomarker
analyses. J Natl Cancer Inst. 106:dju0892014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JM, Peer CJ, Yu M, Amable L, Gordon N,
Annunziata CM, Houston N, Goey AK, Sissung TM, Parker B, et al:
Sequence-specific pharmacokinetic and pharmacodynamic phase I/Ib
study of olaparib tablets and carboplatin in women's cancer. Clin
Cancer Res. 23:1397–1406. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomas A, Rajan A, Szabo E, Tomita Y,
Carter CA, Scepura B, Lopez-Chavez A, Lee MJ, Redon CE, Frosch A,
et al: A phase I/II trial of belinostat in combination with
cisplatin, doxorubicin, and cyclophosphamide in thymic epithelial
tumors: A clinical and translational study. Clin Cancer Res.
20:5392–5402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoppenot C, Eckert MA, Tienda SM and
Lengyel E: Who are the long-term survivors of high grade serous
ovarian cancer? Gynecol Oncol. 148:204–212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nelson BH: The impact of T-cell immunity
on ovarian cancer outcomes. Immunol Rev. 222:101–116. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McAlpine JN, Porter H, Kobel M, Nelson BH,
Prentice LM, Kalloger SE, Senz J, Milne K, Ding J, Shah SP, et al:
BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and
presence of immune cell infiltrates in ovarian high-grade serous
carcinoma. Mod Pathol. 25:740–750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hwang WT, Adams SF, Tahirovic E, Hagemann
IS and Coukos G: Prognostic significance of tumor-infiltrating T
cells in ovarian cancer: A meta-analysis. Gynecol Oncol.
124:192–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yigit R, Massuger LF, Figdor CG and
Torensma R: Ovarian cancer creates a suppressive microenvironment
to escape immune elimination. Gynecol Oncol. 117:366–372. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Facciabene A, Motz GT and Coukos G:
T-regulatory cells: Key players in tumor immune escape and
angiogenesis. Cancer Res. 72:2162–2171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Movahedi K, Guilliams M, Van den Bossche
J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P and Van
Ginderachter JA: Identification of discrete tumor-induced
myeloid-derived suppressor cell subpopulations with distinct T
cell-suppressive activity. Blood. 111:4233–4244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang R, Cai Z, Zhang Y, Yutzy WH IV, Roby
KF and Roden RB: CD80 in immune suppression by mouse ovarian
carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res.
66:6807–6815. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Diaz-Montero CM, Salem ML, Nishimura MI,
Garrett-Mayer E, Cole DJ and Montero AJ: Increased circulating
myeloid-derived suppressor cells correlate with clinical cancer
stage, metastatic tumor burden, and doxorubicin-cyclophosphamide
chemotherapy. Cancer Immunol Immunother. 58:49–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen J, Ye Y, Liu P, Yu W, Wei F, Li H and
Yu J: Suppression of T cells by myeloid-derived suppressor cells in
cancer. Hum Immunol. 78:113–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cui TX, Kryczek I, Zhao L, Zhao E, Kuick
R, Roh MH, Vatan L, Szeliga W, Mao Y, Thomas DG, et al:
Myeloid-derived suppressor cells enhance stemness of cancer cells
by inducing microRNA101 and suppressing the corepressor CtBP2.
Immunity. 39:611–621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Higuchi T, Flies DB, Marjon NA,
Mantia-Smaldone G, Ronner L, Gimotty PA and Adams SF: CTLA-4
blockade synergizes therapeutically with PARP inhibition in
BRCA1-deficient ovarian cancer. Cancer Immunol Res. 3:1257–1268.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Buckley NE, Hosey AM, Gorski JJ, Purcell
JW, Mulligan JM, Harkin DP and Mullan PB: BRCA1 regulates IFN-gamma
signaling through a mechanism involving the type I IFNs. Mol Cancer
Res. 5:261–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu H, Xian J, Vire E, McKinney S, Wei V,
Wong J, Tong R, Kouzarides T, Caldas C and Aparicio S:
Up-regulation of the interferon-related genes in BRCA2 knockout
epithelial cells. J Pathol. 234:386–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ding L, Kim HJ, Wang Q, Kearns M, Jiang T,
Ohlson CE, Li BB, Xie S, Liu JF, Stover EH, et al: PARP inhibition
elicits STING-dependent antitumor immunity in brca1-deficient
ovarian cancer. Cell Rep. 25:2972–2980.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ghaffari A, Peterson N, Khalaj K, Vitkin
N, Robinson A, Francis JA and Koti M: STING agonist therapy in
combination with PD-1 immune checkpoint blockade enhances response
to carboplatin chemotherapy in high-grade serous ovarian cancer. Br
J Cancer. 119:440–449. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wolchok JD, Kluger H, Callahan MK, Postow
MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K,
et al: Nivolumab plus ipilimumab in advanced melanoma. N Engl J
Med. 369:122–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nguyen LT and Ohashi PS: Clinical blockade
of PD1 and LAG3-potential mechanisms of action. Nat Rev Immunol.
15:45–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mellman I, Coukos G and Dranoff G: Cancer
immunotherapy comes of age. Nature. 480:480–489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Keir ME, Francisco LM and Sharpe AH: PD-1
and its ligands in T-cell immunity. Curr Opin Immunol. 19:309–314.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Curiel TJ, Wei S, Dong H, Alvarez X, Cheng
P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et
al: Blockade of B7-H1 improves myeloid dendritic cell-mediated
antitumor immunity. Nat Med. 9:562–567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hamanishi J, Mandai M, Iwasaki M, Okazaki
T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N,
et al: Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+
T lymphocytes are prognostic factors of human ovarian cancer. Proc
Natl Acad Sci USA. 104:3360–3365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu J, Liu C, Qian S and Hou H: The
expression of Tim-3 in peripheral blood of ovarian cancer. DNA Cell
Biol. 32:648–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li L, Ma Y, Xu Y and Maerkeya K: TIM-3
expression identifies a distinctive PD-1+follicular helper T cell
subset, with reduced interleukin 21 production and B cell help
function in ovarian cancer patients. Int Immunopharmacol.
57:139–146. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kuchroo VK, Umetsu DT, DeKruyff RH and
Freeman GJ: The TIM gene family: Emerging roles in immunity and
disease. Nat Rev Immunol. 3:454–462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ovarian tumor tissue analysis (OTTA)
consortium, ; Goode EL, Block MS, Kalli KR, Vierkant RA, Chen W,
Fogarty ZC, Gentry-Maharaj A, Tołoczko A, Hein A, et al:
Dose-response association of CD8+ tumor-infiltrating lymphocytes
and survival time in high-grade serous ovarian cancer. JAMA Oncol.
3:e1732902017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kalachand RD, Ruscito I, Dimitrova D,
Panici PB, Sehouli J, Olek S, Braicu EI, Lu L, Katsaros D, Yu H, et
al: Clinical characteristics and survival outcomes in
BRCA1-methylated epithelial ovarian cancer (Bmeth-OC): A pooled
analysis of data for 1,278 patients across five studies. J Clin
Oncol 33:. (Suppl 15):S55262018.
|