Introduction
Esophageal cancer, one of the top 10 malignant
tumors, has a high morbidity that ranks 8th among all malignant
tumors (1). Generally, surgery and
chemoradiotherapy are the main clinical treatments for esophageal
cancer. At present, surgery is the optimal option for patients
suitable for surgical resection. However, it leads to a low 5-year
survival rate of 15–20% (2) and a
great possibility of pulmonary complications and anastomotic
leakage (3). Considering the great
toxic side effects that chemoradiotherapy brings, the exploration
of a method to improve the postoperative survival and provide
accurate postoperative treatment is in an urgent need.
In recent years, a high expression level of Xenopus
kinesin-like protein 2 (TPX2) in patients with non-small cell lung
cancer (4), cervical (5), and oral cancer (6) compared to healthy individuals has been
reported, suggesting the possible involvement of TPX2 in the
occurrence and development of a variety of tumors. Chang et
al (7) found that the high
expression of TPX2 in cervical cancer cells causes a more active
proliferation of cancer cells and predicts the deterioration of
cervical cancer, leading to speculation that the high expression of
TPX2 may be a tumorigenic mechanism. Lowering the expression of
TPX2 can effectively inhibit the growth and invasion of tumor cells
(8). NIK-IKK-β binding protein
(NIBP), which has gained attention in the medical field, is highly
expressed in various tumor tissues such as colon (9), gastric (10), and esophageal (11) cancer, and has proved to promote the
proliferation and invasion of tumor cells (12). In their study on NIBP expression in
colon cancer, Xu et al (9)
found that NIBP is overexpressed in colon cancer, and patients with
high NIBP expression have much lower overall survival than patients
with lower levels of NIBP. They also showed that the expression
level of NIBP is an important prognostic factor in colon cancer
patients.
There is little research on the expression of TPX2
and NIBP in esophageal cancer and their roles in prognosis. The
expression of TPX2 and NIBP in esophageal cancer was investigated,
and their clinical significance in the occurrence, development, and
prognosis of esophageal cancer was explored in this study.
Patients and methods
General information
Tissue samples from 250 patients who received
radical resection of esophageal cancer in Weihai Central Hospital
(Weihai, China) from March 2011 to February 2014 were collected.
The same size of esophageal cancer tissue and adjacent cancer
tissue were taken from the patients. Histopathological typing
showed 198 cases of squamous cell carcinoma, 29 cases of
adenocarcinoma, 13 cases of undifferentiated carcinoma, and 10
cases of adenosquamous carcinoma.
Inclusion criteria were: patients diagnosed with
esophageal cancer by pathology; patients with no history of
anti-tumor therapy such as radiotherapy and chemotherapy before the
radical surgery for esophageal cancer; patients with complete
clinical data. The TNM staging referred to the Union for
International Cancer Control (UICC) Cancer Staging Manual (13). Exclusion criteria were: patients
combined with severe liver and kidney dysfunction, hypertension,
diabetes, or other malignant tumors; patients with mental disorders
or communication disorders. This study was approved by the Ethics
Committee of Weihai Central Hospital. Informed consent was signed
by the patients or the guardians.
Reagents and equipment
StepOnePlus Real-Time PCR System was purchased from
Thermo Fisher Scientific, Inc. DR5000 UV–V spectrophotometer was
purchased by HACH. SYBR-Green Quantitative RT-qPCR Kit (cat. no.
QR0100) was purchased from Takara. TRIzol extraction kit was
purchased from Wuhan Chundu Biotechnology Co., Ltd. (cat. no.
CDLG-4396). The reverse transcription kit was purchased from
GeneCopoeia, Inc. Rabbit anti-human TPX2 polyclonal antibody (cat.
no. PAB11993) and rabbit anti-human NIBP polyclonal antibody (cat.
no. PAB0321) were purchased from Abnova. HRP-labeled rabbit
secondary antibody (cat. no. 6401-05) was purchased from BioVision.
Rabbit anti-human GAPDH polyclonal antibody (cat. no. GV357911) was
purchased from Shanghai Yiji Industial Co., Ltd. The design and
synthesis of TPX2, NIBP, and GAPDH internal reference primers were
from Takara Biotechnology Co., Ltd. The primer sequences are shown
in Table I.
 | Table I.Primer sequences of TPX2, NIBP, and
GAPDH. |
Table I.
Primer sequences of TPX2, NIBP, and
GAPDH.
| Genes | Forward primers | Reverse primers |
|---|
| TPX2 |
5′-ACCTTGCCCTACTAAGATT-3′ |
5′-AATGTGGCACAGGTTGAGC-3′ |
| NIBP |
5′-GAACTGCCTTAGCCCTGAAGACAT-3′ |
5′-AGCCTTGATGCACGCTTCC-3′ |
| GAPDH |
5′-GCACCGTCAAGGTGAGAAC-3′ |
5′-TGGTGAAGACGCCAGTGGA-3′ |
Experimental methods
RT-qPCR
Cancer tissues and adjacent normal tissues (4 cm
away or farther from the edge of cancer tissue) were collected from
each patient during the surgical resection, and then cut into cubes
(0.5 cm × 0.5 cm × 0.5 cm) before storing at −80°C in
liquid-nitrogen. 3 mm3 esophageal cancer tissues and 3
mm3 adjacent normal tissues were ground in
liquid-nitrogen, and the tissue suspension was taken to extract
total RNA according to the TRIzol extraction kit. The density and
purity of total RNA were detected by DR5000 UV-visible
spectrophotometer. Then reverse transcription was performed based
on the instructions of the kit. Conditions for reverse
transcription reaction were: 1 h at 37°C, 5 min at 85°C for
inactivation, and the reaction was terminated at 4°C. The
synthesized cDNA samples were stored in a refrigerator at −20°C.
PCR reaction system (a total volume of 25 µl): 5 µl CTV cDNA, 1 µl
upstream primer, 1.25 µl downstream primer, 1 µl 25 mM
MgCl2, 2.5 pµl 10X PCR buffer, 0.3 µl Taq enzyme and
13.95 µl water. PCR Premix, 50 ng double-distilled water, 50 ng ROX
Dye, and 200 nm primers. GAPDH was used as the internal reference
gene, and the reaction conditions were: 35 cycles in 1 min of 95°C
for 4 min, 95°C for 30 sec, 58°C for 30 sec and 72°C, and extension
at 72°C for 10 min. The experiment was repeated 3 times. The data
were analyzed with 2−ΔCq method (14).
Western blot analysis
The total protein of the tissue was frozen with
liquid nitrogen, and the protein concentration was determined by
the BCA method. TPX2 protein concentration detection:
electrophoresis was performed by 6% SDS-PAGE electrophoresis.
Denatured protein sample (30 µl) was separated and transferred to a
PVDF membrane. The PVDF membrane was removed and washed three times
with TBST (T1082) for 15 min each time. Then, 5% skim milk powder
was used for blocking for 1 h. TPX2 primary antibody (1:1,500) and
GAPDH primary antibody (1:3,000) were added and incubated overnight
at 4°C in a refrigerator. The next day, they were washed with PBST
3 times, and then incubated with HRP rabbit secondary antibody
(1:5,000) at 20°C for 1 h. After washing 3 times, ECL
chemiluminescence was used to visualize, and the images were
preserved. The operation of NIBP protein concentration detection
was similar to that of the TPX2 protein concentration detection,
and the NIBP primary antibody dilution was (1:100).
Follow-up
The follow-up was performed every 3 months via
telephone or visit to 250 patients, lasting for 5 years until
January 2019. The overall survival time referred to the time from
the first day after the surgery to the last day of follow-up or the
day of non-survival.
Statistical analysis
Statistical analysis was performed using SPSS 21.0
(IBM Corp.). Chi-square test was used to compare the enumeration
data between groups. The independent samples t-test was used to
compare the measurement data between the two groups. The paired
t-test was used for comparison before and after operation. The
Kaplan-Meier method was used to separately establish survival
curves for TPX2, NIBP high and low expression populations. The
log-rank test was used to measure the difference in the survival
curves between the two groups. The Cox regression equation was used
to detect independent prognostic factors for esophageal cancer. The
correlation between NIBP and TPX2 was analyzed by Pearsons
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
General information
A total of 250 samples of adjacent normal tissues
were obtained from 250 esophageal cancer patients with complete
clinical data (including 168 male patients and 82 females, aged
30–83 years). Of the 250 subjects whose tumor diameter ranged from
3.2 to 6.3 cm, 183 cases had a tumor diameter <5.0 cm, and 67
cases had a tumor diameter ≥5.0 cm (15); 52 cases were <58 years of age, 198
cases were ≥58 years; 68 cases had their tumors located in the
upper thoracic portion, 128 cases in the middle thoracic portion,
and 54 cases in the posterior thoracic portion; 110 cases were TNM
stage I, 88 cases were stage II, 32 cases were stage III, and 20
cases had reached stage IV. There were 198 cases of squamous cell
carcinoma, 29 cases of adenocarcinoma, 13 cases of undifferentiated
carcinoma, and 10 cases of adenosquamous carcinoma (Table II).
 | Table II.General clinical and pathological data
of patients with esophageal cancer [n (%)]. |
Table II.
General clinical and pathological data
of patients with esophageal cancer [n (%)].
| Factors | Case no. (%) |
|---|
| Sex |
| Male | 168 (67.20) |
|
Female | 82 (32.80) |
| Age (years) |
|
<58 | 52 (20.80) |
| ≥58 | 198 (79.20) |
| Tumor diameter
(cm) |
|
<5.0 | 183 (73.20) |
| ≥5.0 | 67 (26.80) |
| Tumor location |
| Upper
thoracic portion | 68 (27.20) |
| Middle
thoracic portion | 128 (51.20) |
| Posterior
thoracic portion | 54 (21.60) |
| TNM stage |
| I | 110 (44.00) |
| II | 88 (35.20) |
| III | 32 (12.80) |
| IV | 20 (8.00) |
| Lymph node
metastasis |
| Yes | 72 (28.80) |
| No | 178 (71.20) |
| Histopathological
typing |
| Squamous
cell carcinoma | 198 (79.20) |
|
Adenocarcinoma | 29 (11.60) |
|
Undifferentiated
carcinoma | 13 (5.20) |
|
Adenosquamous carcinoma | 10 (4.00) |
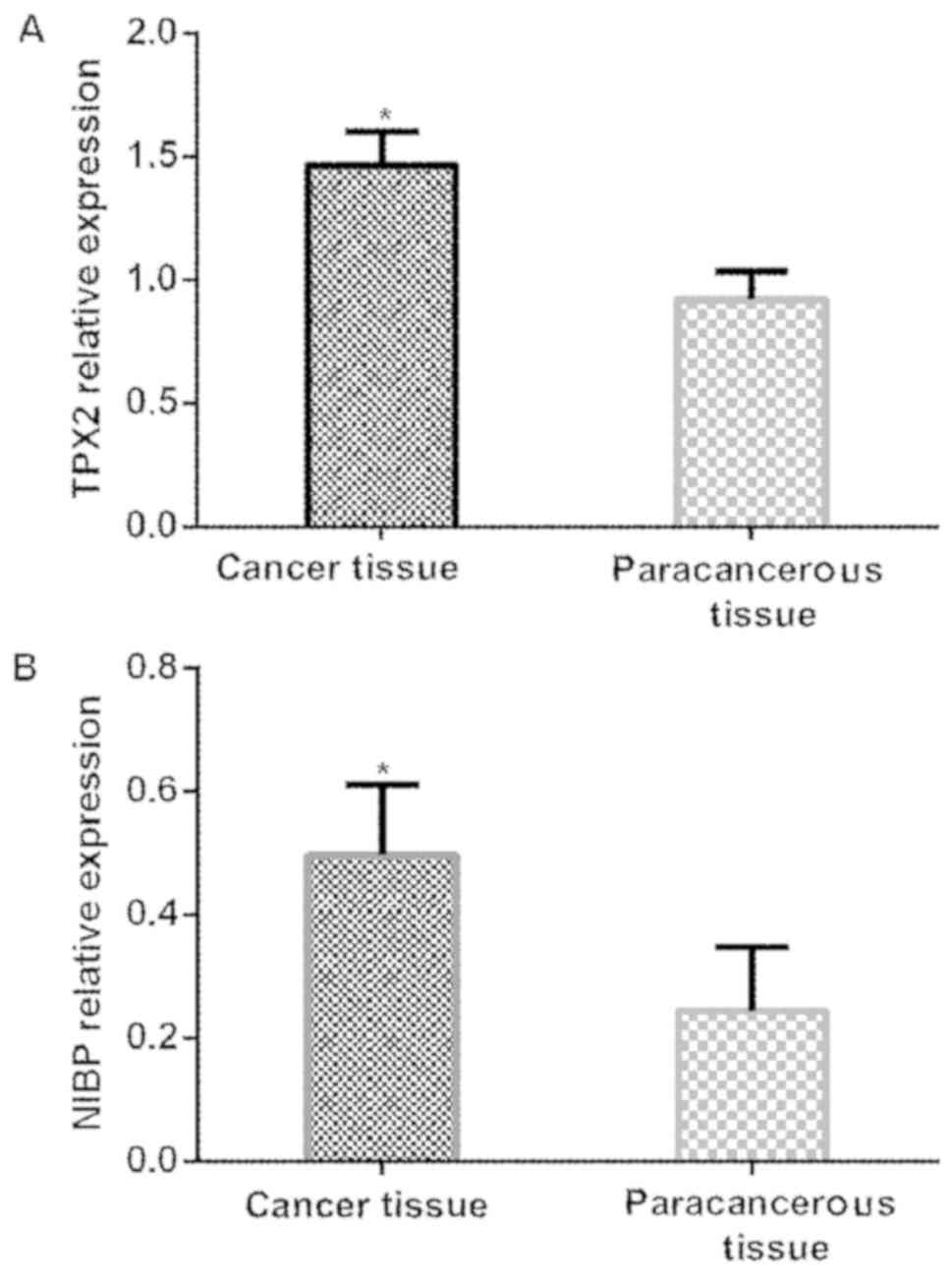
Expression of TPX2 and NIBP genes in
esophageal cancer
The relative expression of TPX2 gene in esophageal
cancer tissues was significantly higher than that in adjacent
tissues (P<0.001). The relative expression of NIBP gene in
cancer tissues was significantly higher than that in adjacent
tissues (P<0.001) (Table III
and Fig. 1).
 | Table III.Expression of TPX2 and NIBP genes in
esophageal cancer (mean ± SD). |
Table III.
Expression of TPX2 and NIBP genes in
esophageal cancer (mean ± SD).
| Groups | No. | TPX2 | NIBP |
|---|
| Cancer tissues | 250 | 1.465±0.136 | 0.498±0.112 |
| Adjacent normal
tissues | 250 | 0.923±0.114 | 0.244±0.104 |
| t-value | – | 48.291 | 26.276 |
| P-value | – | <0.001 | <0.001 |
Expression of TPX2 and NIBP protein in
esophageal cancer
The relative expression of TPX2 protein in
esophageal cancer tissues was significantly higher than that in
adjacent tissues, with statistically significant difference
(P<0.05). The relative expression of NIBP protein in esophageal
cancer tissues was significantly higher than that in adjacent
tissues, with statistically significant difference (P<0.001)
(Table IV).
 | Table IV.Expression of TPX2 and NIBP protein in
esophageal cancer (mean±SD). |
Table IV.
Expression of TPX2 and NIBP protein in
esophageal cancer (mean±SD).
| Groups | No. | TPX2 | NIBP |
|---|
| Cancer tissues | 250 | 0.673±0.078 | 0.248±0.044 |
| Adjacent normal
tissues | 250 | 0.223±0.035 | 0.124±0.029 |
| t-value | – | 82.225 | 37.205 |
| P-value | – | <0.001 | <0.001 |
Association between TPX2 expression
and clinicopatho-logical features
TPX2 levels in esophageal cancer patients with
different sex, age, tumor diameter, tumor location and
histopathological typing were not significantly different
(P>0.05). Patients with different TNM stages had significantly
different TPX2 levels (P<0.05). The relative expression levels
of TPX2 in different levels of infiltration were significantly
different (P<0.001). The TPX2 levels in tumor tissues with lymph
node metastasis were significantly higher than those in tissues
without lymph node metastasis (P<0.001) (Table V).
 | Table V.Relationship between
clinicopathological parameters and relative expression of TPX2 in
cancer tissues (mean±SD). |
Table V.
Relationship between
clinicopathological parameters and relative expression of TPX2 in
cancer tissues (mean±SD).
| Clinicopathological
parameters | No. | TPX2 relative
expression | t/F | P-value |
|---|
| Sex |
| 0.287 | 0.775 |
|
|
Male | 168 | 1.454±0.076 |
|
|
|
Female | 82 | 1.451±0.081 |
|
|
| Age (years) |
| 0.387 | 0.699 |
|
|
<58 | 52 | 1.448±0.071 |
|
|
|
≥58 | 198 | 1.452±0.065 |
|
|
| Tumor diameter
(cm) |
| 0.355 | 0.723 |
|
|
<5.0 | 183 | 1.455±0.056 |
|
|
|
≥5.0 | 67 | 1.458±0.067 |
|
|
| Tumor location |
| 0.913 | 0.403 |
|
| Upper
thoracic portion | 68 | 1.445±0.055 |
|
|
| Middle
thoracic portion | 128 | 1.452±0.068 |
|
|
|
Posterior thoracic
portion | 54 | 1.461±0.069 |
|
|
| TNM stage |
| 25.410 | <0.001 |
|
| I | 110 | 1.416±0.073 |
|
|
| II | 88 |
1.445±0.043a |
|
|
|
III | 32 |
1.494±0.067a,b |
|
|
| IV | 20 |
1.524±0.060a–c |
|
|
| Lymph node
metastasis |
| 4.261 | <0.001 |
|
|
Yes | 92 | 1.457±0.098 |
|
|
| No | 158 | 1.413±0.065 |
|
|
| Histopathological
typing |
|
| 0.563 | 0.642 |
|
Squamous cell carcinoma | 198 | 1.475±0.051 |
|
|
|
Adenocarcinoma | 29 | 1.468±0.046 |
|
|
|
Undifferentiated
carcinoma | 13 | 1.487±0.061 |
|
|
|
Adenosquamous carcinoma | 10 | 1.464±0.053 |
|
|
| Degree of
infiltration |
|
| 22.597 | <0.001 |
| Mucous
layer | 45 | 1.428±0.061 |
|
|
|
Muscular layer | 38 | 1.436±0.057 |
|
|
| Fibrous
membranes | 150 |
1.508±0.076d,e |
|
|
|
Surrounding tissue | 17 |
1.518±0.066d,e |
|
|
Association between NIBP expression
and clinico-pathological features
NIBP levels in esophageal cancer patients with
different sex, age, tumor diameter, tumor location, and
histopathological typing were not significantly different
(P>0.05). Patients with different TNM stages had significantly
different NIBP levels (P<0.001). The relative expression of NIBP
in different levels of infiltration was significantly different
(P<0.05). The NIBP levels in tumor tissues with lymph node
metastasis were significantly higher than those in tissues without
lymph node metastasis (P<0.001) (Table VI).
 | Table VI.Relationship between
clinicopathological parameters and relative expression of NIBP in
cancer tissues (mean ± SD). |
Table VI.
Relationship between
clinicopathological parameters and relative expression of NIBP in
cancer tissues (mean ± SD).
| Clinicopathological
parameters | No. | NIBP relative
expression | t/F | P-value |
|---|
| Sex |
| 0.399 | 0.690 |
|
|
Male | 168 | 0.478±0.076 |
|
|
|
Female | 82 | 0.482±0.071 |
|
|
| Age (years) |
| 0.333 | 0.739 |
|
|
<58 | 52 | 0.474±0.081 |
|
|
|
≥58 | 198 | 0.478±0.076 |
|
|
| Tumor diameter
(cm) |
| 0.085 | 0.933 |
|
|
<5.0 | 183 | 0.480±0.092 |
|
|
|
≥5.0 | 67 | 0.475±0.086 |
|
|
| Tumor location |
| 0.066 | 0.936 |
|
| Upper
thoracic portion | 68 | 0.475±0.054 |
|
|
| Middle
thoracic portion | 128 | 0.473±0.051 |
|
|
|
Posterior thoracic
portion | 54 | 0.478±0.059 |
|
|
| TNM stage |
| 38.900 | <0.001 |
|
| I | 110 | 0.438±0.043 |
|
|
| II | 88 |
0.469±0.053a |
|
|
|
III | 32 |
0.501±0.068a,b |
|
|
| IV | 20 |
0.558±0.042a–c |
|
|
| Lymph node
metastasis |
| 6.828 | <0.001 |
|
|
Yes | 92 | 0.512±0.068 |
|
|
| No | 158 | 0.455±0.061 |
|
|
| Histopathological
typing |
|
| 2.062 | 0.106 |
|
Squamous cell carcinoma | 198 | 0.489±0.067 |
|
|
|
Adenocarcinoma | 29 | 0.466±0.056 |
|
|
|
Undifferentiated
carcinoma | 13 | 0.455±0.043 |
|
|
|
Adenosquamous carcinoma | 10 | 0.478±0.053 |
|
|
| Degree of
infiltration |
|
| 13.876 | <0.001 |
| Mucous
layer | 45 | 0.467±0.044 |
|
|
|
Muscular layer | 38 | 0.478±0.051 |
|
|
| Fibrous
membranes | 150 |
0.502±0.048d,e |
|
|
|
Surrounding tissue | 17 |
0.545±0.052d–f |
|
|
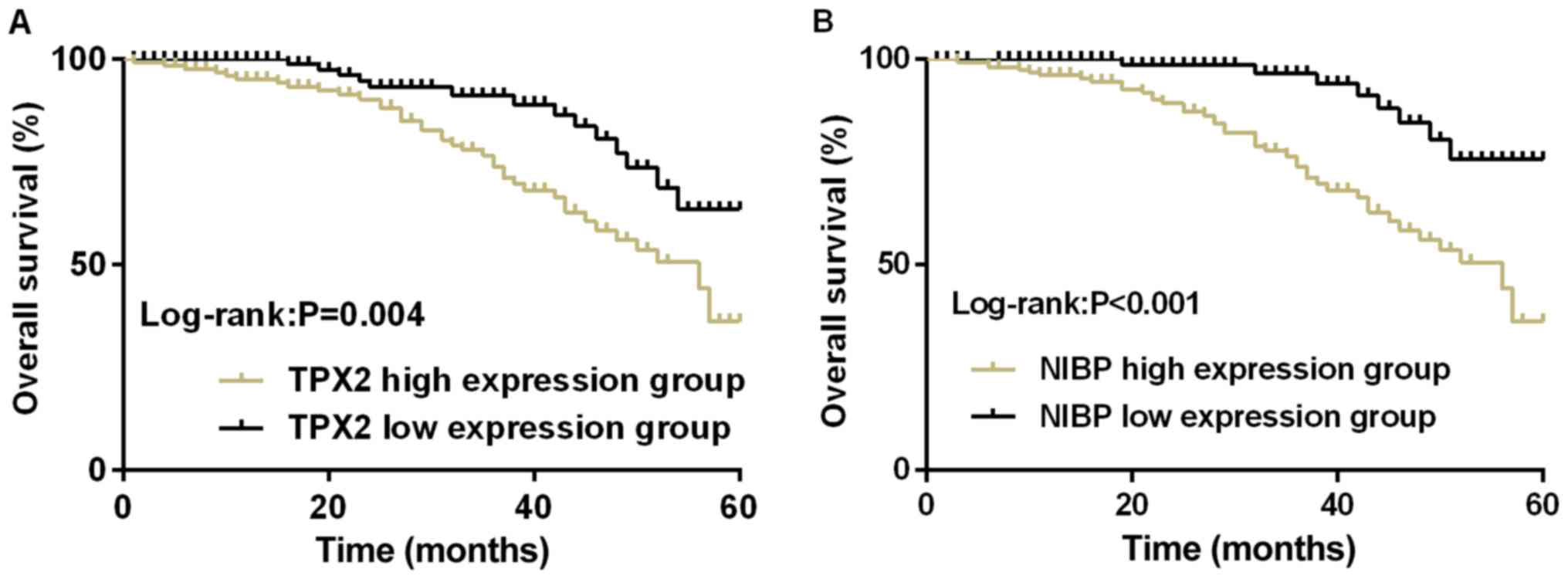
Survival of patients with esophageal
cancer
According to the average values of relative
expression of TPX2 (1.465) and NIBP (0.498) in patients with
esophageal cancer, 131 patients with a TPX2 level ≥1.465 were
divided into TPX2 high expression group, 119 patients with a TPX2
level <1.465 were divided into TPX2 low expression group; 156
patients with a NIBP level ≥0.498 were divided into NIBP high
expression group, 94 patients with a NIBP level <0.498 were
divided into NIBP low expression group. The 5-year overall survival
rate of TPX2 high expression group was 36.64% (48/131),
statistically lower than that of TPX2 low expression group which
was 68.91% (82/119) (P=0.004, log-rank test); NIBP high expression
group had a 5-year overall survival rate of 37.18% (58/156),
statistically lower than that of TPX2 low expression group at
76.60% (72/94) (P<0.001, log-rank test) (Fig. 2).
Analysis of factors related to
prognosis of esophageal cancer
Univariate analysis of general factors and
clinicopathological factors showed that different age, sex, tumor
diameter, tumor location and pathological type were not prognostic
factors affecting the overall survival of patients with esophageal
cancer (P>0.05). TNM staging, degree of infiltration, TPX2
expression and NIBP expression may be the prognostic factors
affecting the overall survival of patients with esophageal cancer
(P<0.001) (Table VII). The
meaningful indicators in the univariate analysis were included in
the Cox proportional hazard model, and the multivariate analysis
was performed by the stepwise regression method. The variable entry
criterion was 0.05 and the rejection criterion was 0.1. The results
showed that TPX2, NIBP, TNM staging, lymph node metastasis, and
degree of infiltration were independent prognostic factors
(P<0.05) (Table VIII).
 | Table VII.Results of single factor analysis of
prognosis in patients with esophageal cancer. |
Table VII.
Results of single factor analysis of
prognosis in patients with esophageal cancer.
| Groups | No. | 5-year survival
case (no.) | χ2 | P-value |
|---|
| Age (years) |
|
| 2.118 | 0.146 |
|
<58 | 52 | 26 |
|
|
|
≥58 | 198 | 104 |
|
|
| Sex |
|
| 0.030 | 0.863 |
|
Male | 168 | 88 |
|
|
|
Female | 82 | 42 |
|
|
| Tumor diameter
(cm) |
|
| 1.205 | 0.272 |
|
<5 | 183 | 99 |
|
|
| ≥5 | 67 | 31 |
|
|
| Tumor location |
|
| 0.829 | 0.661 |
| Upper
thoracic portion | 68 | 37 |
|
|
| Middle
thoracic portion | 128 | 63 |
|
|
|
Posterior thoracic
portion | 54 | 30 |
|
|
| TNM stage |
|
| 10.963 | 0.012 |
| I | 110 | 68 |
|
|
| II | 88 | 44 |
|
|
|
III | 32 | 12 |
|
|
| IV | 20 | 6 |
|
|
| Degree of
infiltration |
|
| 13.676 | 0.003 |
| Mucous
layer | 45 | 31 |
|
|
|
Muscular layer | 38 | 25 |
|
|
| Fibrous
membranes | 150 | 69 |
|
|
|
Surrounding tissue | 17 | 5 |
|
|
| TPX2
expression |
|
| 26.010 | <0.001 |
|
High | 131 | 48 |
|
|
|
Low | 119 | 82 |
|
|
| NIBP
expression |
|
| 36.511 | <0.001 |
|
High | 156 | 58 |
|
|
|
Low | 94 | 72 |
|
|
| Histopathological
typing |
|
| 1.835 | 0.607 |
|
Squamous cell carcinoma | 198 | 107 |
|
|
|
Adenocarcinoma | 29 | 12 |
|
|
|
Undifferentiated
carcinoma | 13 | 6 |
|
|
|
Adenosquamous carcinoma | 10 | 5 |
|
|
 | Table VIII.Multivariate analysis of prognosis in
patients with esophageal cancer. |
Table VIII.
Multivariate analysis of prognosis in
patients with esophageal cancer.
|
| Multiple
variables |
|---|
|
|
|
|---|
| Factors | HR (95% CI) | P-value |
|---|
| TPX2 (high
expression vs. low expression) | 2.945
(1.344–4.575) | 0.012 |
| NIBP (high
expression vs. low expression) | 2.391
(1.358–3.830) | 0.009 |
| TNM stage | 1.369
(1.037–1.807) | 0.026 |
| Lymph node
metastasis (yes vs. no) | 2.097
(1.536–3.502) | 0.076 |
| Degree of
infiltration | 1.286
(1.057–1.564) | 0.012 |
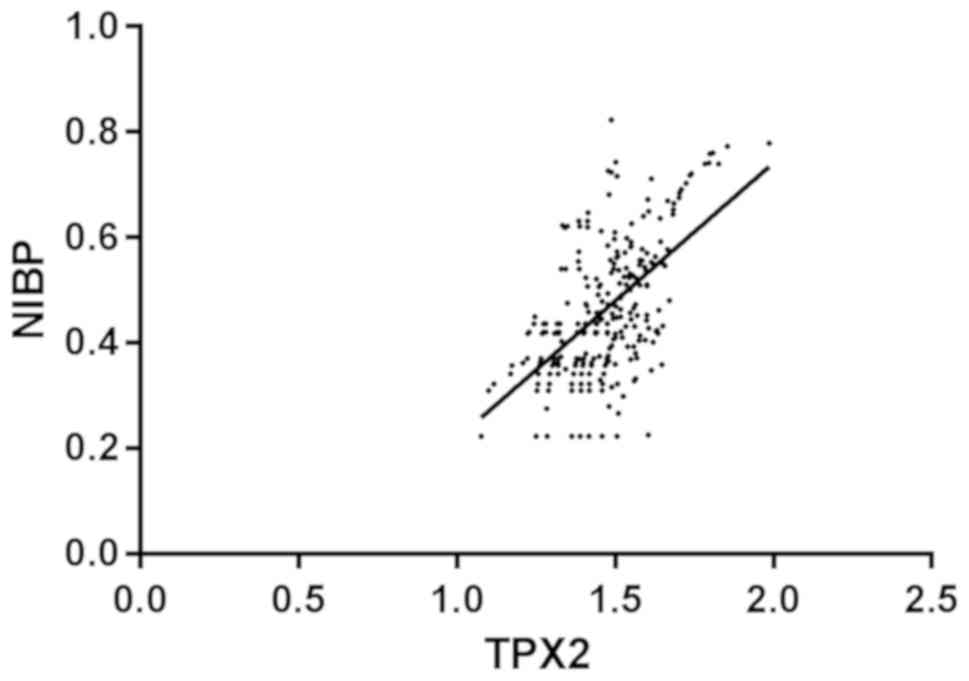
Correlation analysis of TPX2 and
NIBP
Pearsons analysis showed that there was a positive
correlation between TPX2 expression and NIBP expression in
esophageal cancer tissues, with significant difference (r=0.575,
P<0.001) (Fig. 3).
Discussion
Due to only few clinical symptoms, low screening
rate for esophageal cancer, and patients' poor awareness of medical
care, patients with esophageal cancer are not correctly diagnosed
until they reach the middle or advanced cancer stage, and thus miss
the treatment opportunity of surgical resection (16). Owing to individual differences,
patients with esophageal cancer of the same clinical pathology type
sometimes have different conditions and survival rates after
treatment. Therefore, the search for effective prognostic markers
is of great significance for the stratified treatment of esophageal
cancer patients, and it has important clinical value to improve the
postoperative survival time (17).
In recent years in-depth studies on TPX2 and NIBP
have been conducted. Increasingly, abnormal expression of TPX2 and
NIBP has been identified in various types of cancer such as
non-small cell lung cancer (18),
liver cancer (19), and gastric
cancer (20), which has certain
value in the prognosis evaluation. In this study, TPX2 also
presented a high expression in tissues with lymph node metastasis.
The upregulated TPX2 expression indicates more active tumor cell
growth and division and stronger ability of invasion; therefore the
tumor development may be contained through the control of TPX2
expression. Hsu et al (21)
found that high expression of TPX2 is an independent prognostic
factor for patients with esophageal cancer, and knocking down TPX2
levels can significantly suppress cancer cell proliferation. Liu
et al (22) discovered that
high expression of TPX2 is a risk factor for lymph node metastasis
of esophageal carcinoma, and TPX2 can be used as a biomarker for
early diagnosis and prognosis of esophageal cancer. In this study,
TPX2 expression was upregulated in cancer tissues, and TPX2
expression was closely related to tumor TNM grade, lymph node
metastasis and degree of infiltration; TPX2 was a risk factor for
lymph node metastasis of esophageal cancer, similar to the above
results. This indicates that TPX2 can be used as a biomarker for
the diagnosis and prognosis of esophageal cancer. Liu et al
(23) showed that inhibition of TPX2
gene expression can effectively inhibit the invasion and metastasis
of esophageal cancer cell line EC9706, promote tumor cell
apoptosis, and may be a new way to treat esophageal cancer. This
further validates that the suppression on tumor progression can be
achieved via the control of TPX2 expression. Many studies
demonstrated that the positive expression of NIBP in gastric cancer
(10), colon cancer (9), lung cancer (24) is significantly higher than that in
normal tissues. Overexpression of NIBP in tumor tissues proved by
such studies is consistent with the results of these studies,
suggesting that NIBP is excessively expressed in cancer tissues and
is involved in the occurrence and development of esophageal cancer.
The study of NIBP in colon cancer cells by Qin et al
(25) suggested that NIBP may
accelerate the secretion of MMP-2 and MMP-9 by activating NF-κB
signaling pathway, thereby promoting the invasion and metastasis of
colon cancer cells. In the present study, the 5-year survival
analysis of esophageal cancer patients revealed that patient with
low expression of TPX2 and NIBP enjoyed a higher survival rate than
patients with high TPX2 and NIBP expression. The univariate Cox
regression analysis showed that TPX2, NIBP, TNM staging, lymph node
metastasis and the degree of infiltration were prognostic factors
that may affect the overall survival of patients with esophageal
cancer. The results of the multivariate analysis of the Cox model
showed that TPX2, NIBP, TNM typing, lymph node metastasis and
degree of infiltration were the prognostic factors affecting the
overall survival of patients with esophageal cancer. According to
the results of this study, the high expression of TPX2 and NIBP
promotes the development of esophageal cancer. TNM staging is a
related factor affecting the prognosis of patients with esophageal
cancer and can represent the degree of tumor deterioration. The
occurrence of lymph node metastasis implies further spread of tumor
cells. A more severe cancer results in a poorer prognosis. The
findings of this study suggest that TPX2 and NIBP are potential
predictors of prognosis of patients.
Pearsons correlation analysis was used to analyze
the correlation between TPX2 expression level and NIBP expression
level in esophageal cancer tissues. The results showed that TPX2
expression level was positively correlated with NIBP expression
level in esophageal cancer tissues. There may be a synergistic
effect between TPX2 and NIBP to promote the development of
esophageal cancer.
The present study confirmed that the high expression
of NIBP and TPX2 can promote the development and progression of
esophageal cancer. However, there are limitations in this study.
The relationship between NIBP and TPX2 was not observed from the
viewpoint of basic research, and the mechanism of NIBP and TPX2 in
esophageal cancer was not explored.
In conclusion, NIBP and TPX2 are highly expressed in
esophageal cancer tissues, and they may have the capability of
predicting the prognosis of esophageal cancer. At present, research
is scarce on the mechanism of TPX2 and NIBP in esophageal cancer.
TPX2 and NIBP are expected to become therapeutic targets for
esophageal cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CS wrote the manuscript. CS and ZS performed PCR. HY
analyzed and interpreted the patients' data. HW helped with
statistical analysis. All the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Weihai Central Hospital (Weihai, China). Patients who
participated in this study, had complete clinical data. Signed
informed consents were obtained from the patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arnold M, Laversanne M, Brown LM, Devesa
SS and Bray F: Predicting the future burden of esophageal cancer by
histological subtype: International trends in incidence up to 2030.
Am J Gastroenterol. 112:1247–1255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mao A: Interventional therapy of
esophageal cancer. Gastrointest Tumors. 3:59–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sunpaweravong S, Ruangsin S,
Laohawiriyakamol S, Mahattanobon S and Geater A: Prediction of
major postoperative complications and survival for locally advanced
esophageal carcinoma patients. Asian J Surg. 35:104–109. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schneider MA, Christopoulos P, Muley T,
Warth A, Klingmueller U, Thomas M, Herth FJ, Dienemann H, Mueller
NS, Theis F and Meister M: AURKA, DLGAP5, TPX2, KIF11 and CKAP5:
Five specific mitosis-associated genes correlate with poor
prognosis for non-small cell lung cancer patients. Int J Oncol.
50:365–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang H, Zhang T, You Z and Zhang Y:
Positive surgical margin, HPV persistence, and expression of both
TPX2 and PD-L1 are associated with persistence/recurrence of
cervical intraepithelial neoplasia after cervical conization. PLoS
One. 10:e01428682015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao X, Sun S, Zeng X and Cui L:
Expression profiles analysis identifies a novel three-mRNA
signature to predict overall survival in oral squamous cell
carcinoma. Am J Cancer Res. 8:450–461. 2018.PubMed/NCBI
|
|
7
|
Chang H, Wang J, Tian Y, Xu J, Gou X and
Cheng J: The TPX2 gene is a promising diagnostic and therapeutic
target for cervical cancer. Oncol Rep. 27:1353–1359.
2012.PubMed/NCBI
|
|
8
|
Jiang T, Sui D, You D, Yao S, Zhang L,
Wang Y, Zhao J and Zhang Y: MiR-29a-5p inhibits proliferation and
invasion and induces apoptosis in endometrial carcinoma via
targeting TPX2. Cell Cycle. 17:1268–1278. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu CY, Qin MB, Tan L, Liu SQ and Huang JA:
NIBP impacts on the expression of E-cadherin, CD44 and vimentin in
colon cancer via the NF-κB pathway. Mol Med Rep. 13:5379–5385.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu ZH, Liu SQ, Qin MB, Huang JA, Xu CY, Wu
WH, Zhu LY, Qin N and Lai MY: NIK and IKKβ binding protein
contributes to gastric cancer chemoresistance by promoting
epithelial mesenchymal transition through the NFκB signaling
pathway. Oncol Rep. 39:2721–2730. 2018.PubMed/NCBI
|
|
11
|
Cheng C, Zhou Y, Li H, Xiong T, Li S, Bi
Y, Kong P, Wang F, Cui H, Li Y, et al: Whole-genome sequencing
reveals diverse models of structural variations in esophageal
squamous cell carcinoma. Am J Hum Genet. 98:256–274. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Liu S, Wang H, Yang W, Li F, Yang
F, Yu D, Ramsey FV, Tuszyski GP and Hu W: Elevated NIBP/TRAPPC9
mediates tumorigenesis of cancer cells through NFκB signaling.
Oncotarget. 6:6160–6178. 2015.PubMed/NCBI
|
|
13
|
Phillips WA, Russell SE, Ciavarella ML,
Choong DY, Montgomery KG, Smith K, Pearson RB, Thomas RJ and
Campbell IG: Mutation analysis of PIK3CA and PIK3CB in esophageal
cancer and Barrett's esophagus. Int J Cancer. 118:2644–2646. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao HH, Zhang SY, Shen JH, Wu ZY, Wu JY,
Wang SH, Li EM and Xu LY: A three-protein signature and clinical
outcome in esophageal squamous cell carcinoma. Oncotarget.
6:5435–5448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davis JN, Medbery C, Sharma S, Pablo J,
Kimsey F, Perry D, Muacevic A and Mahadevan A: Stereotactic body
radiotherapy for centrally located early-stage non-small cell lung
cancer or lung metastases from the RSSearch(®) patient
registry. Radiat Oncol. 10:1132015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yodying H, Matsuda A, Miyashita M,
Matsumoto S, Sakurazawa N, Yamada M and Uchida E: Prognostic
significance of neutrophil-to-lymphocyte ratio and
platelet-to-lymphocyte ratio in oncologic outcomes of esophageal
cancer: A systematic review and meta-analysis. Ann Surg Oncol.
23:646–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi YL, Takeuchi K, Soda M, Inamura K,
Togashi Y, Hatano S, Enomoto M, Hamada T, Haruta H, Watanabe H, et
al: Identification of novel isoforms of the EML4-ALK transforming
gene in non-small cell lung cancer. Cancer Res. 68:4971–4976. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Liu Z, Dou C, Han S, Li C, Tu K
and Yang W: miR-491 inhibits the proliferation, invasion and
migration of hepatocellular carcinoma cell via down-regulating TPX2
expression. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 32:512–517.
2016.(In Chinese). PubMed/NCBI
|
|
20
|
Sasaki N, Morisaki T, Hashizume K, Yao T,
Tsuneyoshi M, Noshiro H, Nakamura K, Yamanaka T, Uchiyama A, Tanaka
M and Katano M: Nuclear factor-kappaB p65 (RelA) transcription
factor is constitutively activated in human gastric carcinoma
tissue. Clin Cancer Res. 7:4136–4142. 2001.PubMed/NCBI
|
|
21
|
Hsu PK, Chen HY, Yeh YC, Yen CC, Wu YC,
Hsu CP, Hsu WH and Chou TY: TPX2 expression is associated with cell
proliferation and patient outcome in esophageal squamous cell
carcinoma. J Gastroenterol. 49:1231–1240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu HC, Zhang Y, Wang XL, Qin WS, Liu YH,
Zhang L and Zhu CL: Upregulation of the TPX2 gene is associated
with enhanced tumor malignance of esophageal squamous cell
carcinoma. Biomed Pharmacother. 67:751–755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu HC, Zhang GH, Liu YH, Wang P, Ma JF,
Su LS, Li SL, Zhang L and Liu JW: TPX2 siRNA regulates growth and
invasion of esophageal cancer cells. Biomed Pharmacother.
68:833–839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pazarentzos E and Bivona TG: Adaptive
stress signaling in targeted cancer therapy resistance. Oncogene.
34:5599–5606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qin M and Liu S, Li A, Xu C, Tan L, Huang
J and Liu S: NIK- and IKKβ-binding protein promotes colon cancer
metastasis by activating the classical NF-κB pathway and MMPs.
Tumour Biol. 37:5979–5990. 2016. View Article : Google Scholar : PubMed/NCBI
|