Introduction
Lung cancer is the most common and deadly
malignancies worldwide (1). As a
heterogeneous class of malignant tumors, non-small-cell lung cancer
(NSCLC) accounts for 85% of lung cancer cases (2). Tobacco consumption remains the main
risk factor of NSCLC, however studies in recent years have shown
that air pollution and radon exposure also contribute to the
development of this disease (3).
Incidence of NSCLC in recent years has shown an increased trend in
developing countries, such as China (4). The majority of patients with NSCLC were
diagnosed at advanced stages with distant tumor metastasis, which
has been reported as the main cause of death (5). Therefore, early detection of tumor
distant metastasis remains crucial for the treatment of NSCLC.
Metastasis of NSCLC requires the involvement of
multiple signaling pathways (6,7), in
which TGF-β signaling transduction plays a central role (6). Activation of TGF-β signal in NSCLC
tissues promotes epithelial mesenchymal transition, thereby
inducing the invasion and migration of cancer cells (8). Long (>200 nt) non-coding RNAs, or
lncRNAs, are a group of non-protein coding RNA transcripts with
oncogenic or tumor suppression functions in cancer development
(9). The cross-talks between lncRNAs
and TGF-β pathway have been reported (10). LncRNA AWPPH is a novel lncRNA with
the oncogenic role in hepatocellular carcinoma (11), bladder cancer (12) and triple-negative breast cancer
(13). AWPPH is located on
chromosome 2 and is the host of MIR4435-2 (12). In hepatocellular carcinoma, lncRNA
AWPPH interacts with Y-box binding protein 1 to promote disease
progression (11). In bladder
cancer, lncRNA AWPPH regulates proliferation, autophagy, and
migration, but inhibits apoptosis of bladder cancer cells by
inhibiting SMAD4 via enhancer of zeste 2 polycomb repressive
complex 2 subunit (12). However, to
the best of our knowledge, the involvement of AWPPH in NSCLC and
its interactions with TGF-β signaling remains unknown. In the
present study, it was observed that lncRNA AWPPH may promote the
metastasis, but not the growth of NSCLC by upregulating TGF-β1
expression.
Materials and methods
Patients
This study included 138 patients with NSCLC, who
were pathologically diagnosed at the Affiliated Xing Tai People
Hospital of Hebei Medical University between January 2015 and
January 2018. Inclusion criteria were as follows: Patients
pathologically diagnosed with NSCLC; new NSCLC cases and patients
with normal functions of other major organs. Exclusion criteria
were as follows: Patients suffering from other malignancies;
patients with other lung diseases; patients with mental disorders
and patients with an education level below elementary high school
that would inhibit cooperation with researchers. This study's
patients included 88 males and 50 females, and the age ranged
between 26 and 72 years, with a mean age of 50±5.6 years. According
to computed tomography scanning results, 28 patients presented with
a primary tumor diameter between 0 and 1 cm, 21 patients were
between 1 and 2 cm, 25 patients were between 2 and 3 cm, 20
patients were between 3 and 4 cm, 23 patients were between 4 and 5
cm, and 21 patients were >5 cm. Tumor metastasis was observed in
72 patients, including 14 cases of brain metastasis, 13 cases of
bone metastasis, 20 cases of liver metastasis and 25 cases of
lymphatic metastasis. Tumor metastasis was not found in the
remaining 66 patients. At the same time, 32 healthy volunteers,
including 20 male and 12 female patients (range, 29–69 years, mean
48.3±5.1 years) served as the control group. Control group,
different tumor size groups and different metastasis groups showed
similar sex and age distributions. The Ethics Committee of
Affiliated Xing Tai People Hospital of Hebei Medical University
approved the present study and all participants signed an informed
consent.
Specimen collection
Lung biopsies were obtained from patients with NSCLC
and healthy controls. Healthy controls received biopsies to detect
potential lung lesions, however any suspected lung diseases were
excluded. Blood was also extracted from each participant on the day
of admission. Blood samples were stored at room temperature for 20
min and were centrifuged at 1,200 × g for 20 min at room
temperature to collect serum. All specimens were stored in liquid
nitrogen before use.
ELISA
TGF-β1 in serum was detected using a human TGF-β1
ELISA kit (cat. no., ab100647, Abcam), according to the
manufacturer's protocols. Plasma levels of TGF-β1 were normalized
to ng/ml.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from in vitro
cultvated cells, biopsies and plasma using TRIzol reagent
(Sigma-Aldrich; Merck KGaA) and subjected to reverse transcription
to synthesize cDNA. In cases of TGF-β1 treatment, cells were
cultviated in medium containing 5, 10 and 20 ng/ml TGF-β1 (Abcam)
under aforementioned conditions for 24 h prior to use. PCR reaction
was performed using SYBR-Green PCR Master Mix (Thermo Fisher
Scientific, Inc.), using the primers: 5′-CTGGATGGTCGCTGCTTTTTA-3′
(forward) and 5′-AGGGGGATGAGTCGTGATTT-3′ (reverse) for human lncRNA
AWPPH (11);
5′-GACCTCTATGCCAACACAGT3′ (forward) and 5′-AGTACTTGCGCTCAGGAGGA3′
(reverse) for β-actin. The thermocycling PCR reaction conditions
were as follows: 95°C for 1 min, 40 cycles of 95°C for 10 sec and
56°C for 20 sec. Cq values were processed using 2−ΔΔCq
method (14) and AWPPH expression
was normalized to endogenous control β-actin.
Cell line, cell culture and
transfection
Normal lung epithelial cell line, NuLi-1, and human
NSCLC cell lines NCI-H1581 (H1581), as well as NCI-H1993 (H1993),
were purchased from the American Type Culture Collection (ATCC).
RPMI-1640 medium (ATCC) containing 10% fetal bovine serum (FBS;
cat. no., ATCC 30-2020; ATCC) was used as cell culture medium and
cell culture conditions were at 37°C with 5% CO2.
Full-length AWPPH cDNA (accession no.: NR_015395.2) was inserted
into pIRSE2-EGFP vector (Clontech Laboratories, Inc.) to make AWPPH
expression vector. AWPPH short hairpin (sh)RNA
(GGTCTGGTCGGTTTCCCATTT) and scrambled shControl
(TCCTAAGGTTAAGTCGCCCTC) were synthesized by GenePharma, Inc. AWPPH
expression vectors (10 nM) and shRNA (20 nM) were transfected into
6×105 cells using Lipofectamine 2000 reagent (cat. no.,
11668-019; Invitrogen; Thermo Fisher Scientific, Inc.). Empty
vector transfection and transfection with scrambled shControl were
also performed to serve as negative controls. Cells without
transfection were used as controls. Overexpression and knockdown
were confirmed by RT-qPCR before subsequent experiments. Subsequent
experiments were performed when the overexpression and knockdown
rates were >200 and <50%, respectively, compared with control
cells. The interval between transfection and following experiments
was 24 h.
Transwell migration and invasion
assay
Cells were collected by centrifugation at 1,200 × g
for 10 min at room temperature and mixed with RPMI-1640 medium (1%
FBS) to prepare single cell suspesions (4×104 cells/ml).
In cases of TGF-β signaling inhibition, cells were treated with
TGF-β receptor inhibitor SB431542 (SB; 10 nM, Sigma-Aldrich; Merck
KGaA) at 37°C for 24 h before use. Regarding migration assay, 0.1
ml cell suspension (non-serum RPMI-1640 medium) were added to the
upper Transwell Inserts (Corning), while the low Transwell chamber
was filled with RPMI-1640 medium containing 20% FBS. After cell
culture for 24 h, Transwell chamber membranes were cleaned and
stained with 0.5% crystal violet (Sigma-Aldrich; Merck KGaA) for 15
min at 22°C. For the invasion assay the upper transwell chamber was
precoated with Matrigel (cat. no., 356234; EMD Millipore) before
experiments. Cell migration and invasion were observed under an
optical microscope (magnification, ×40). Cell migration and
invasion rates were normalized to the cell proliferation rate at
the same time point.
Western blot analysis
RIPA solution (Thermo Fisher Scientific, Inc.) was
used to extract protein and protein concentration was determined
through BCA assay (Sigma-Aldrich; Merck KGaA). Protein (20 µg) was
mixed with loading buffer, denatured and added into each lane of
10% SDS-PAGE gel. After electrophoresis, proteins transferred to
PVDF membranes were blocked with 5% skimmed milk at 22°C for 1 h,
followed by incubation with TGF-β1 (cat. no., ab9758; dilution
1:1,200; Abcam) GAPDH (rabbit anti human; cat. no., ab9485;
dilution, 1:1,400; Abcam) rabbit anti-human primary antibodies at
4°C overnight. The next day, the membranes were washed with TBST
(0.3% Tween-20), followed by incubation with IgG-HRP (dilution,
1:1,000; cat. no., MBS435036; MyBioSource, Inc.) secondary antibody
at room temperature for 3 h. Immobilon® ECL Ultra
Western HRP Substrate (cat. no., WBULS0100; Sigma-Aldrich; Merck
KGaA) was subsequently spread onto the membranes to develop
signals, and the grey band of TGF-β1 was normalized to that of
GAPDH using Image J v.148 software (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
Graphpad Prism 6 software (GraphPad Software, Inc.)
was used. Data were expressed as mean ± standard deviation. Three
biological replicates were included in each experiment. Mean values
were compared by one-way ANOVA and Tukey's post hoc test.
Correlations were analyzed by linear regression. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of AWPPH in lung biopsies
and serum of healthy controls and patients with different tumor
sizes
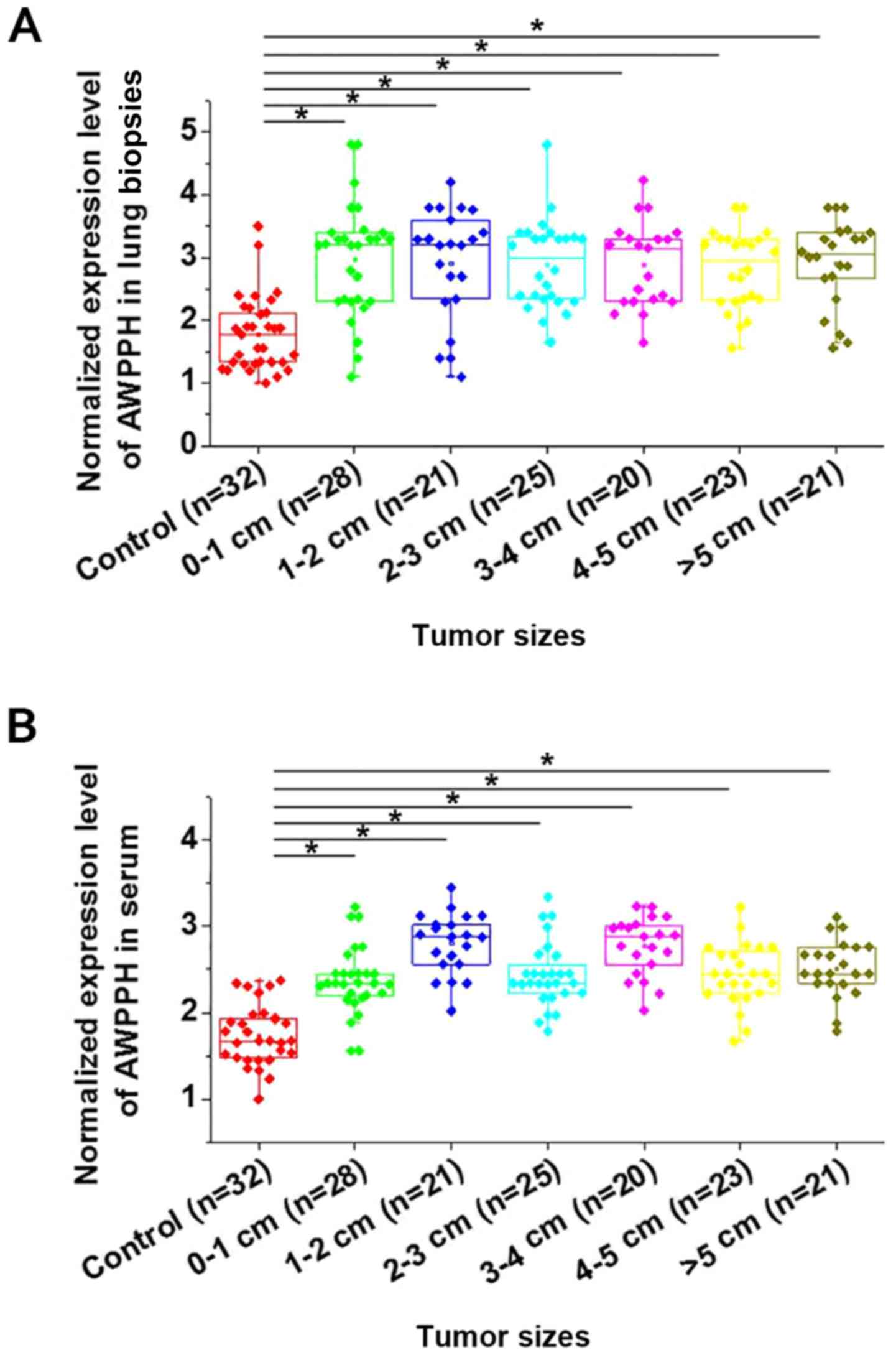
As shown in Fig. 1,
expression of AWPPH was found to be significantly upregulated in
patients with NSCLC with different primary tumor diameters compared
with healthy controls in lung biopsies (Fig. 1A) and serum (Fig. 1B) (P<0.05). However, no
significant differences in AWPPH expression were found among
patients with different tumor diameters. Correlations were analyzed
by linear regression. It was observed that expression levels of
AWPPH were significantly correlated with expression levels in serum
(R2=0.67; P<0.0001). In addition, no significant
differences in AWPPH expression were found among patients with
different T stages (3; 3–5; >5 cm; data not shown).
Expression of AWPPH in lung biopsies
and serum of healthy controls and patients with different
metastasis status
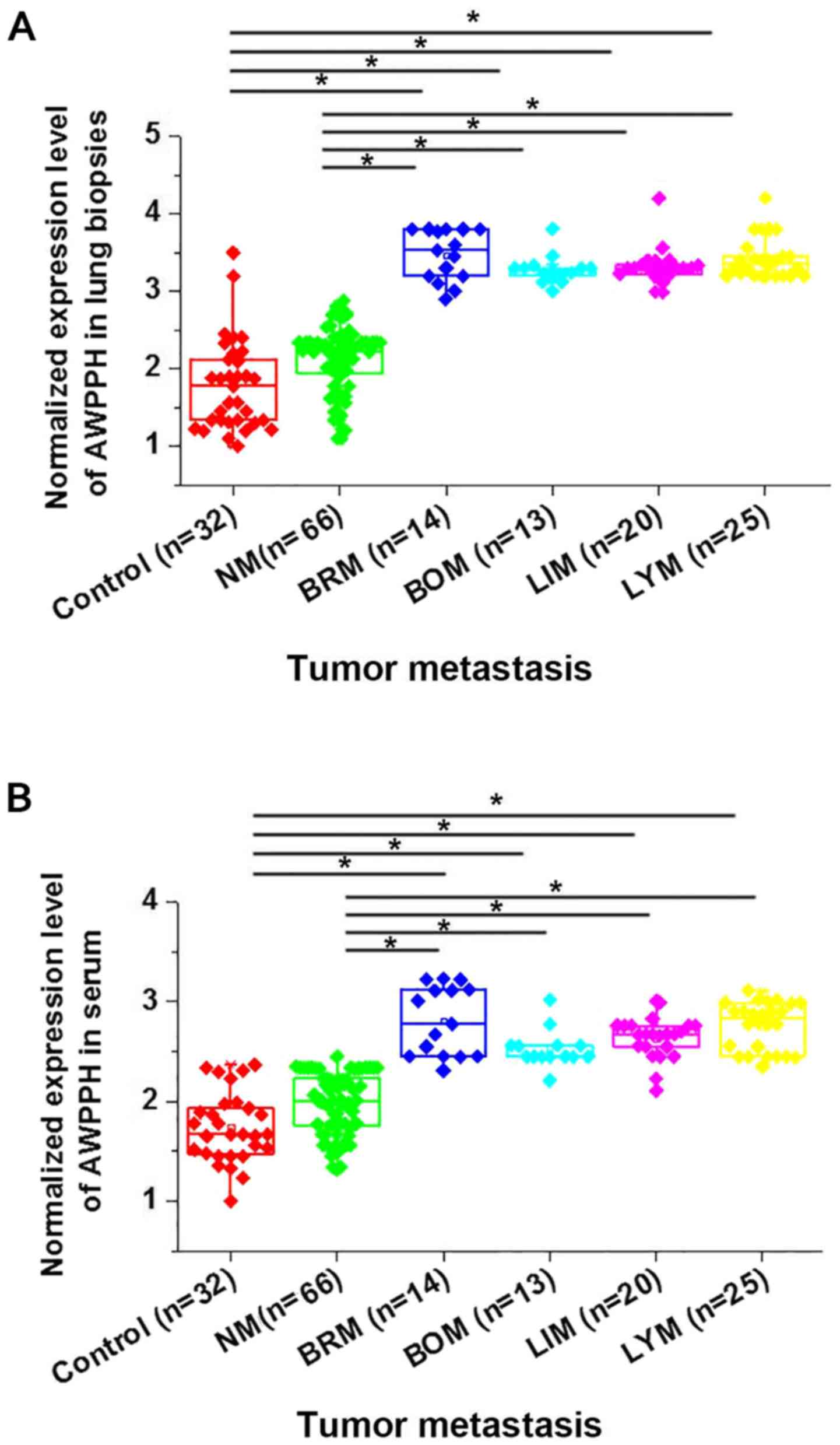
This study further compared the expression of AWPPH
in lung biopsies and serum of healthy controls and patients with
different metastasis status to investigate its potential
involvement in cancer metastasis. As shown in Fig. 2, no significant differences in AWPPH
expression in lung biopsies (Fig.
2A) and serum (Fig. 2B) were
found between heathy controls and patients with NSCLC without
metastasis. Significant upregulated expression of AWPPH in lung
biopsies (Fig. 2A) and serum
(Fig. 2B) were found in patients
with 4 different types of cancer metastasis (P<0.05) compared to
heathy controls and non-metastatic patients with NSCLC. Therefore,
AWPPH is likely to be involved in the metastasis of NSCLC.
Potential interactions between AWPPH
and TGF-β1 in NSCLC cells and normal lung cancer cells
TGF-β signaling plays a pivotal role in the
metastasis of different types of human malignancies, including lung
cancer (6). Therefore, the potential
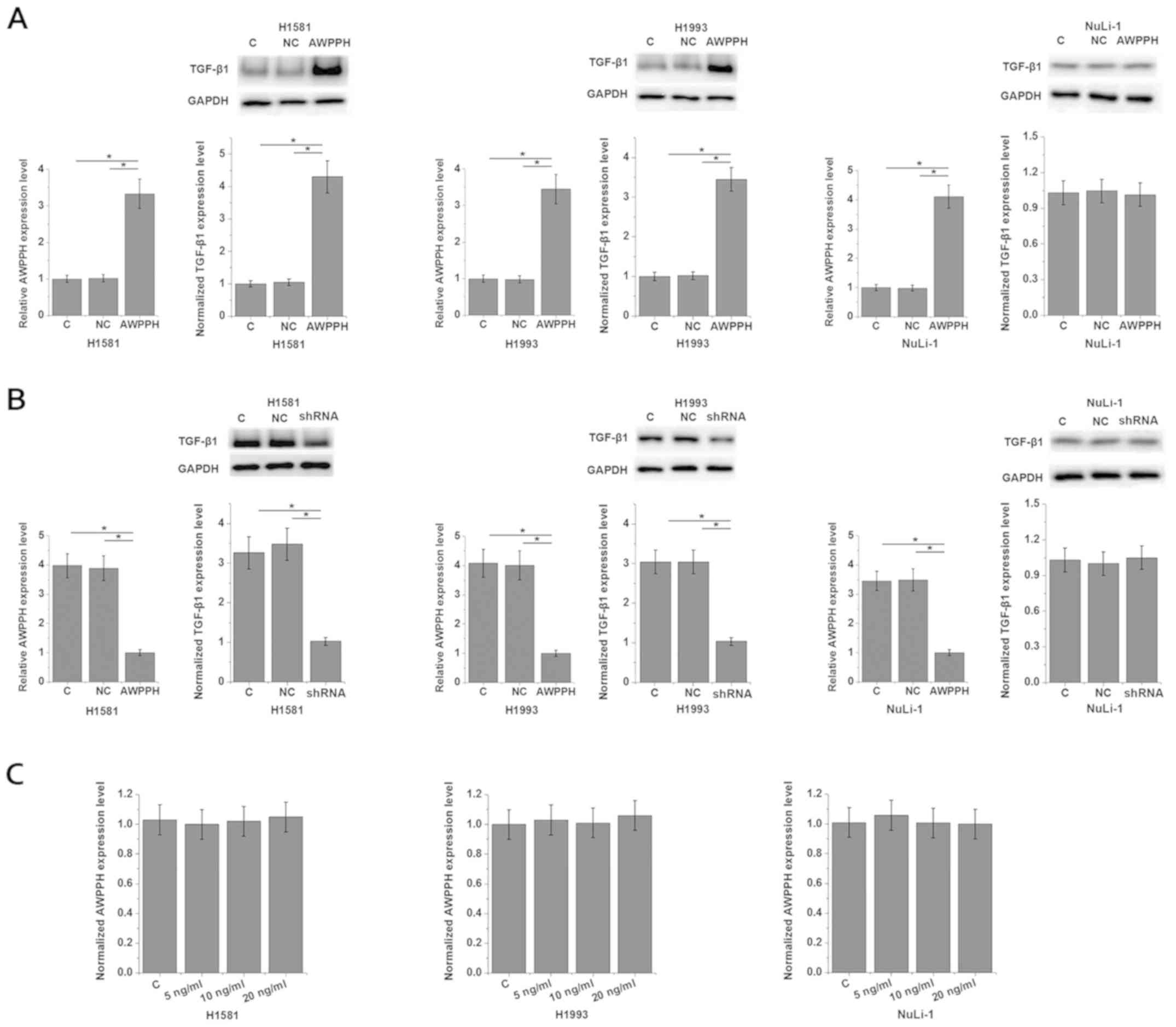
interactions between AWPPH and TGF-β1 were investigated. Analysis
of AWPPH expression in cells of different cell lines showed that
the expression level of AWPPH was significantly higher in cells of
NSCLC cell lines H1581 (4.32-fold) and H1993 (3.37-fold) than in
cells of the normal human lung cell line NuLi-1 (Fig. S1). As shown in Fig. 3A, AWPPH overexpression significantly
promoted the expression of TGF-β1 in the two human NSCLC cell
lines, H1581 and H1993 (P<0.05), however not in NuLi-1 cells
(P>0.05). By contrast, AWPPH-knockdown significantly inhibited
the expression of TGF-β1 in the two human NSCLC cell lines, H1581
and H1993 (P<0.05), but not in cells of the normal human lung
cell line NuLi-1 (Fig. 3B;
P>0.05). In addition, TGF-β1 (Abcam) treatment at concentrations
of 5, 10 and 20 ng/ml showed no significant effects on AWPPH
expression (Fig. 3C). Therefore,
AWPPH is likely an upstream activator of TGF-β signaling
specifically in NSCLC cells, however not in normal lung cells. In
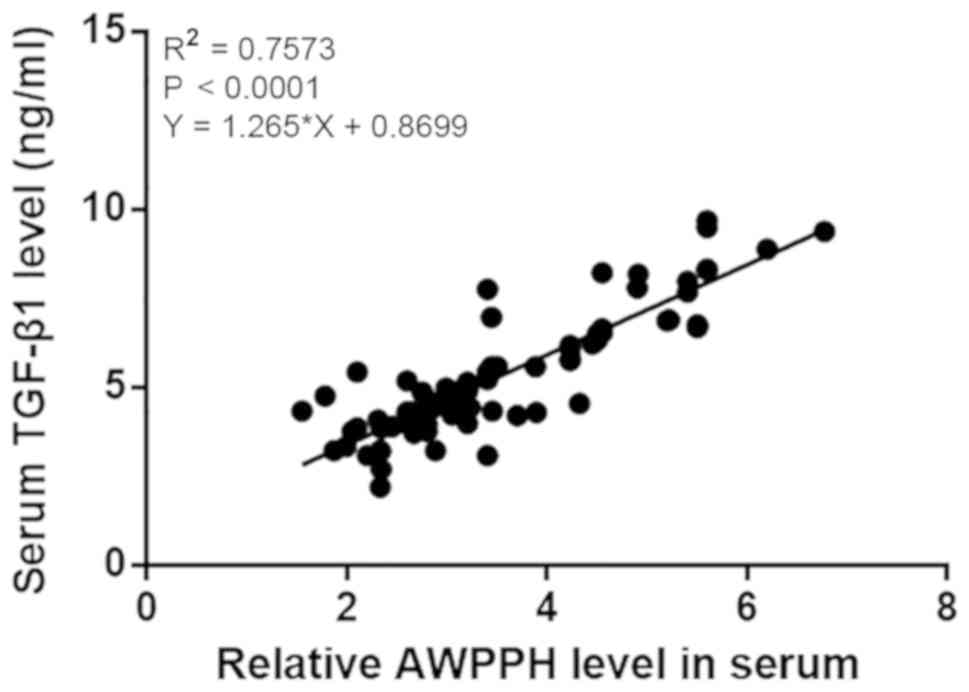
addition, the correlation between serum levels of TGF-β1 and AWPPH
in patients with NSCLC was analyzed by linear regression. It was
observed that TGF-β1 and AWPPH were significantly and positively
correlated (R2=0.7573; P<0.0001; Fig. 4).
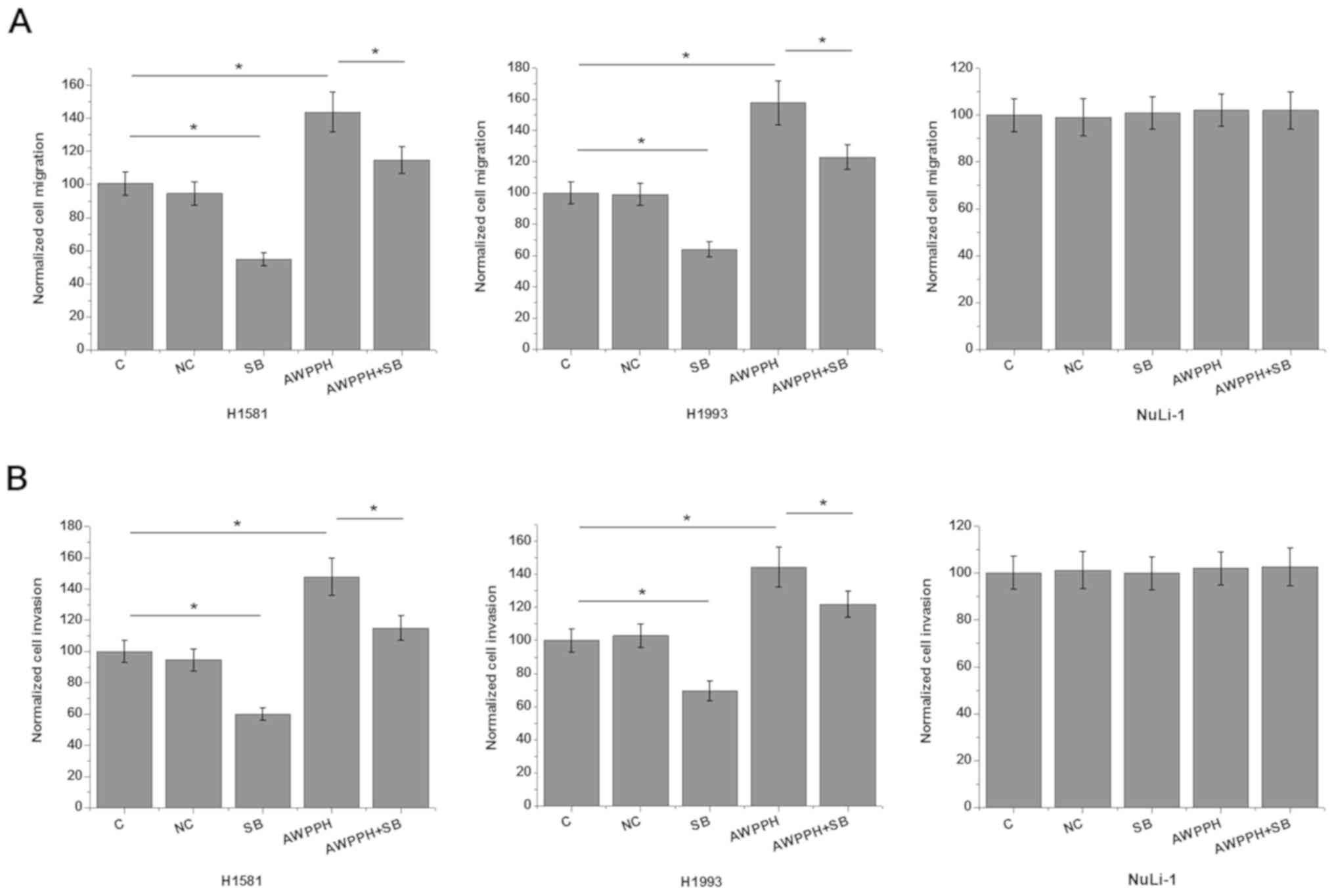
Effects of AWPPH overexpression and
TGF-β inhibition on cell migration and invasion
In vitro cell migration and invasion assay
was performed to further investigate the involvement of
AWPPH-TGF-β1 signaling in the regulation of NSCLC metastasis. As
showed in Fig. 4, AWPPH
overexpression significantly promoted (P<0.05), while treatment
with TGF-β inhibitor SB431542 significantly inhibited the migration
(Fig. 5A) and invasion (Fig. 5B) of the two human NSCLC cell lines
(P<0.05). In addition, treatment with TGF-β inhibitor SB431542
significantly reduced the enhancing effects of AWPPH overexpression
on NSCLC migration (Fig. 4A) and
invasion (Fig. 4B) (P<0.05). In
addition, AWPPH overexpression and treatment with TGF-β inhibitor
SB431542 showed no significant effects on cells of the normal human
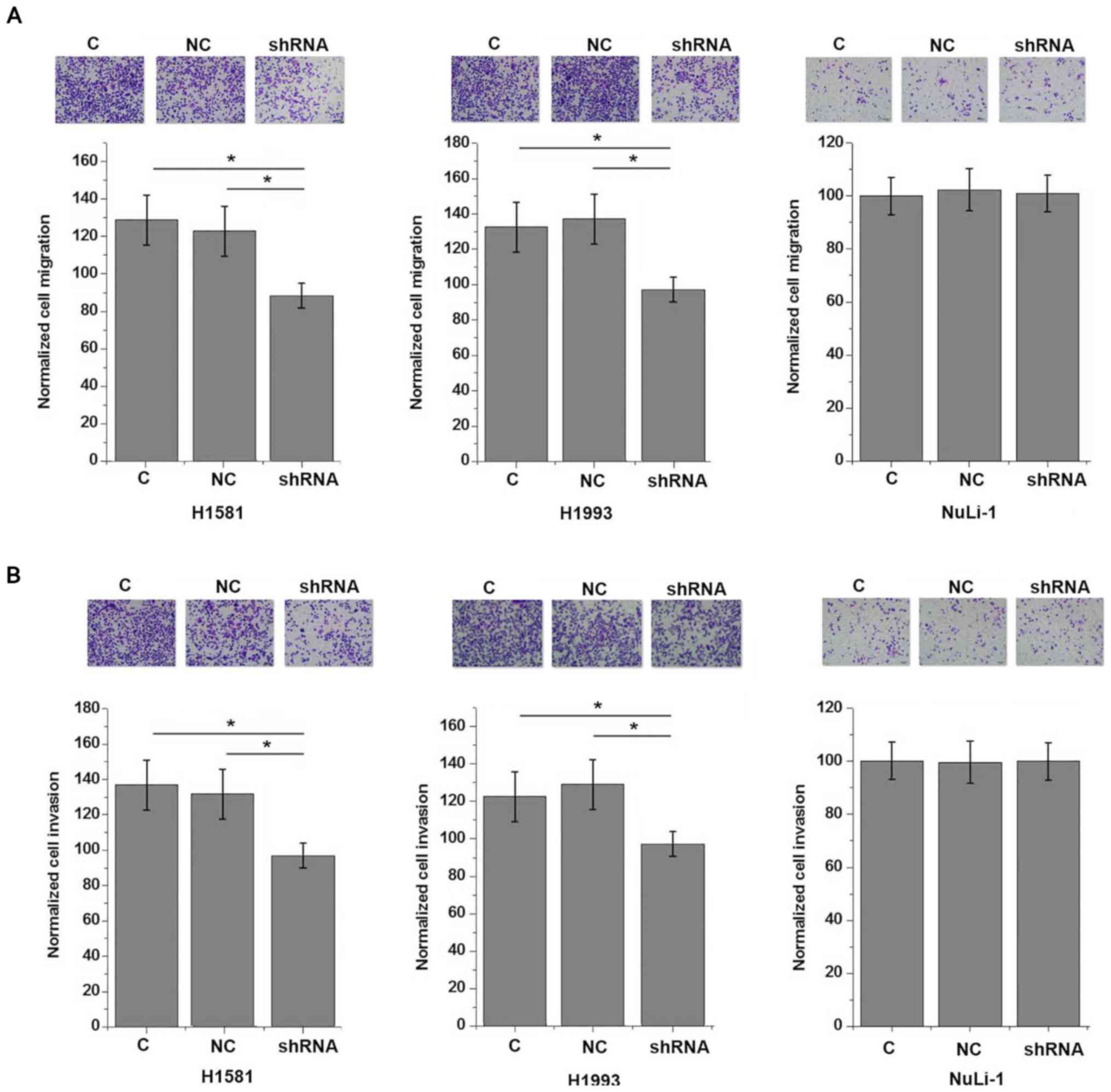
lung cell line NuLi-1 (P>0.05). By contrast, AWPPH
shRNA-knockdown significantly inhibited the migration (Fig. 6A) and invasion (Fig. 6B) of the two human NSCLC cell lines
(P<0.05).
Discussion
LncRNA AWPPH is a newly identified lncRNA with
characterized oncogenic role in hepatocellular carcinoma (11), bladder cancer (12) and breast cancer (13). The main finding of this study
indicated that AWPPH may participate in metastasis, but not growth
of NSCLC. It was also found that the action of AWPPH in NSCLC is
likely to be achieved through the upregulation of TGF-β1.
Genetic alterations participate in NSCLC (15). Genetic alterations not only define
the subtypes of NSCLC, but also provide guidance for therapeutic
treatments (16). It has also been
reported that the onset, development, progression and almost all
pathological changes in NSCLC are accompanied with changes in the
expression patterns of a large set of lncRNAs (17). The involvement and functionality of
those lncRNAs in NSCLC have been gradually identified (18,19).
Upregulation of lncRNA PVT1 is frequently observed in patients with
NSCLC, and overexpression of lncRNA plays a role as an oncogene to
promote tumorigenesis in this disease (18). By contrast, lncRNA maternally
expressed 3 (MEG3) was downregulated in NSCLC, and overexpression
of this lncRNA inhibits cancer cell proliferation and induces cell
apoptosis, indicating the potential application of MEG3 as a
therapeutic target for NSCLC (19).
Besides those two lncRNAs, functionality of a considerable number
of lncRNAs has been characterized in NSCLC (16). However, the aforementioned lncRNAs
have been suggested to participate in the whole procedure of the
development of NSCLC (16–18) and therefore may not be able to
predict a specific stage of NSCLC, such as tumor metastasis.
Functionality of lncRNA AWPPH has been characterized
in hepatocellular carcinoma (11),
bladder cancer (12) and triple
negative breast cancer (13). AWPPH
was upregulated in hepatocellular carcinoma and overexpression of
AWPPH, not only promoted hepatocellular carcinoma cell
proliferation and migration in vitro, but also accelerated
tumor growth and metastasis in vivo (11). A previous study that focused on the
involvement of AWPPH in bladder cancer also showed that this lncRNA
may participate in both tumor growth and metastasis (12). However, this study observed no
significant differences in AWPPH expression levels among patients
with NSCLC with different tumor sizes, even after patients were
grouped according to largest diameter. In contrast, all patients
with different types of distant tumor metastasis showed upregulated
expression of AWPPH compared with patients with non-tumor
metastasis. The in vitro cell migration and invasion assay
showed that AWPPH expression is an enhancer of NSCLC cell migration
and invasion. These results suggest that, instead of participating
in the entire process of NSCLC, AWPPH may only serve an important
role in metastasis of NSCLC, or that metastasis of NSCLC induces
the upregulation of AWPPH.
An increasing number of studies have confirmed that
TGF-β is the master regulator of epithelial mesenchymal transition
(20), which is the key marker of
the progression of metastasis in different malignancies (21). Consistently, this study also observed
reduced migration and invasion abilities of NSCLC cells after TGF-β
inhibitor treatment. This study indicated that AWPPH is likely an
upstream regulator of TGF-β1, due to the fact that AWPPH
overexpression upregulated TGF-β1. The addition of exogenous TGF-β1
however, did not affect AWPPH, and TGF-β1 inhibition attenuated the
effects of AWPPH overexpression on NSCLC cell behavior. However,
these data only supported an AWPPH-TGF-β1 sequential signaling
transduction. Whether this regulation is direct or indirect remains
unclear. AWPPH is host to MIR4435-2, while the function of
MIR4435-2, to the best of our knowledge, has yet to be revealed.
Further studies investigating whether MIR4435-2 may mediate the
interaction between AWPPH and TGF-β1 are to be performed.
In addition, this study indicated that AWPPH
overexpression showed no significant effect on the biological
behaviors of cells of normal human lung cell line NuLi-1.
Therefore, AWPPH may serve as a safe target for the prevention and
treatment of NSCLC. In conclusion, AWPPH is overexpressed in NSCLC.
Overexpression of AWPPH promotes the migration and invasion of
NSCLC cells possibly through the activation of TGF-β signaling.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH and AL designed experiments. YH, HA and ZW
performed experiments. YH and ZW analyzed data. AL drafted the
manuscript. All authors have read and approved the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Affiliated Xing Tai People
Hospital of Hebei Medical University approved the present study and
all participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gridelli C, Rossi A, Carbone DP, Guarize
J, Karachaliou N, Mok T, Petrella F, Spaggiari L and Rosell R:
Non-small-cell lung cancer. Nat Rev Dis Primers. 1:150092015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yeo CD and Joo H: P1.01–011 Roflumilast
Attenuates Benzo(a)Pyrene-Induced Lung Cancer via Suppression of
Airway Inflammation in Murine Model. J Thorac Oncol. 12
(Suppl):S4542017. View Article : Google Scholar
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Temel JS, Greer JA, Muzikansky A,
Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD,
Jacobsen J, Pirl WF, et al: Early palliative care for patients with
metastatic non-small-cell lung cancer. N Engl J Med. 363:733–742.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao M, Seike M, Soeno C, Mizutani H,
Kitamura K, Minegishi Y, Noro R, Yoshimura A, Cai L and Gemma A:
MiR-23a regulates TGF-β-induced epithelial-mesenchymal transition
by targeting E-cadherin in lung cancer cells. Int J Oncol.
41:869–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J, Wang J, Zhao F, Liu Q, Jiang K
and Yang GH: MicroRNA-21 (miR-21) represses tumor suppressor PTEN
and promotes growth and invasion in non-small cell lung cancer
(NSCLC). Clin Chim Acta. 411:846–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao D and He J: Epithelial mesenchymal
transition and lung cancer. J Thorac Dis. 2:154–159.
2010.PubMed/NCBI
|
|
9
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao X, Liu Y and Yu S: Long noncoding RNA
AWPPH promotes hepatocellular carcinoma progression through YBX1
and serves as a prognostic biomarker. Biochim Biophys Acta Mol
Basis Dis. 1863:1805–1816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu F, Zhang X, Yu Q, Han G, Diao F, Wu C
and Zhang Y: LncRNA AWPPH inhibits SMAD4 via EZH2 to regulate
bladder cancer progression. J Cell Biochem. 119:4496–4505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang K, Li X, Song C and Li M: LncRNA
AWPPH promotes the growth of triple-negative breast cancer by
up-regulating frizzled homolog 7 (FZD7). Biosci Rep. 28(pii):
BSR201812232018. View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scheffler M, Bos M, Gardizi M, König K,
Michels S, Fassunke J, Heydt C, Künstlinger H, Ihle M, Ueckeroth F,
et al: PIK3CA mutations in non-small cell lung cancer (NSCLC):
Genetic heterogeneity, prognostic impact and incidence of prior
malignancies. Oncotarget. 6:1315–1326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pikor LA, Ramnarine VR, Lam S and Lam WL:
Genetic alterations defining NSCLC subtypes and their therapeutic
implications. Lung Cance. 82:179–189. 2013. View Article : Google Scholar
|
|
17
|
Cheng N, Li X, Zhao C, Ren S, Chen X, Cai
W, Zhao M, Zhang Y, Li J, Wang Q and Zhou C: Microarray expression
profile of long non-coding RNAs in EGFR-TKIs resistance of human
non-small cell lung cancer. Oncol Rep. 33:833–839. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang YR, Zang SZ, Zhong CL, Li YX, Zhao SS
and Feng XJ: Increased expression of the lncRNA PVT1 promotes
tumorigenesis in non-small cell lung cancer. Int J Clin Exp Pathol.
7:6929–6935. 2014.PubMed/NCBI
|
|
19
|
Lu KH, Li W, Liu XH, Sun M, Zhang ML, Wu
WQ, Xie WP and Hou YY: Long non-coding RNA MEG3 inhibits NSCLC
cells proliferation and induces apoptosis by affecting p53
expression. BMC Cancer. 13:4612013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Katsuno Y, Lamouille S and Derynck R:
TGF-β signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|